Osteocytes: Their Lacunocanalicular Structure and Mechanoresponses
Abstract
:1. Introduction
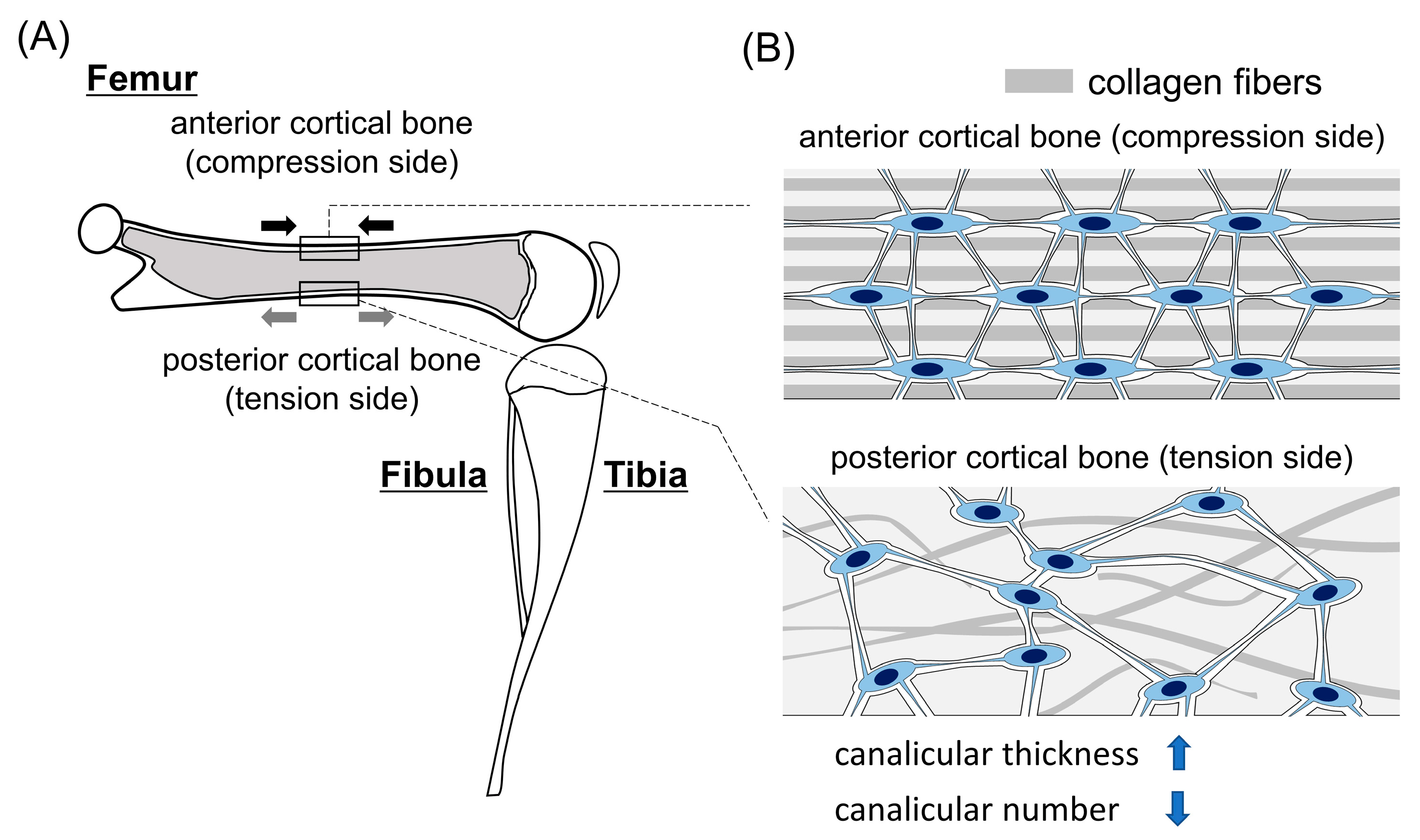
2. Differences in the Lacunocanalicular Structure between the Compression and Tension Sides
3. Osteocyte Death and Bone Resorption
4. Animal Models for the Investigation of the Relationship between Lacunocanalicular Structure and Mechanoresponses
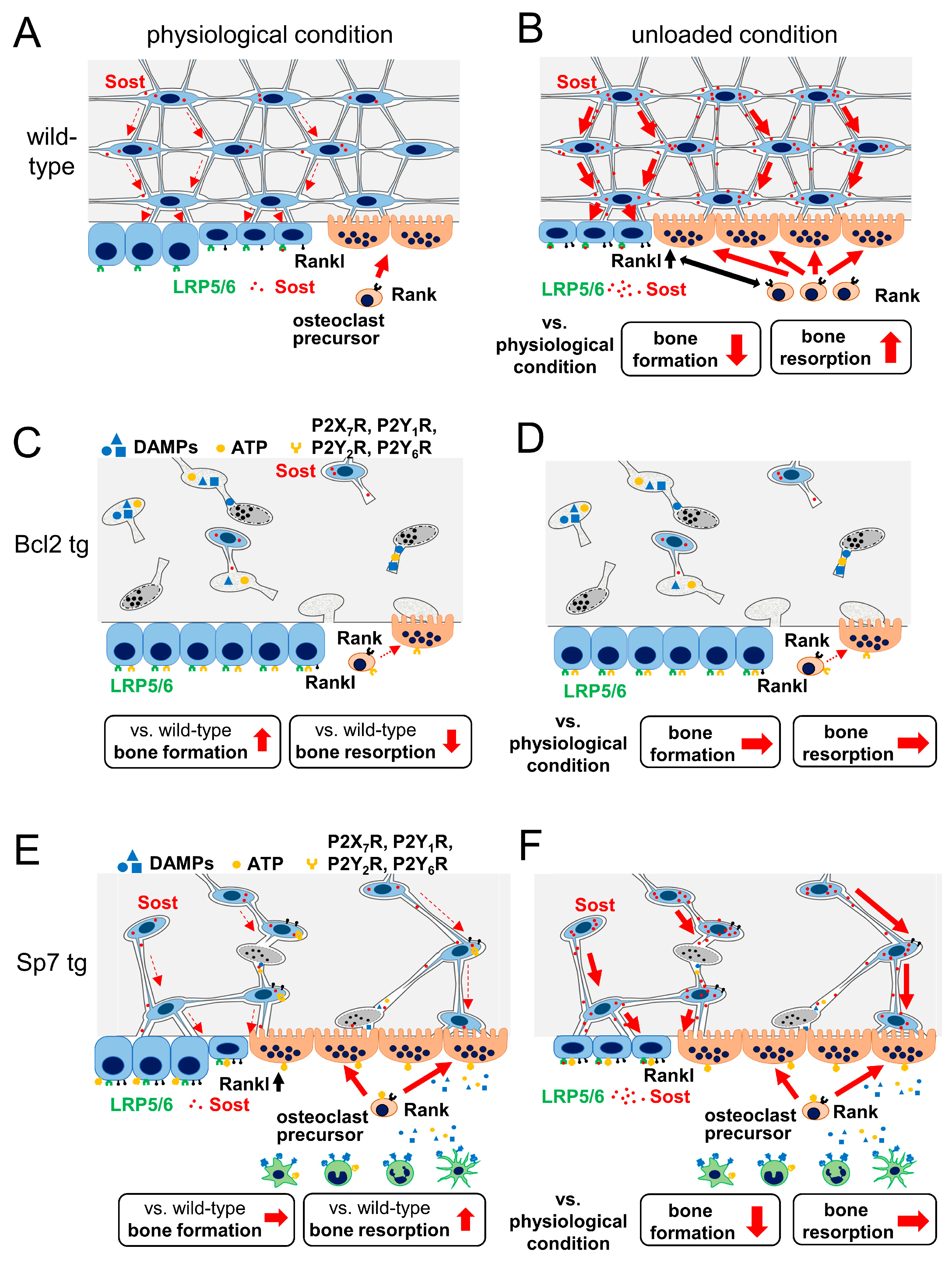
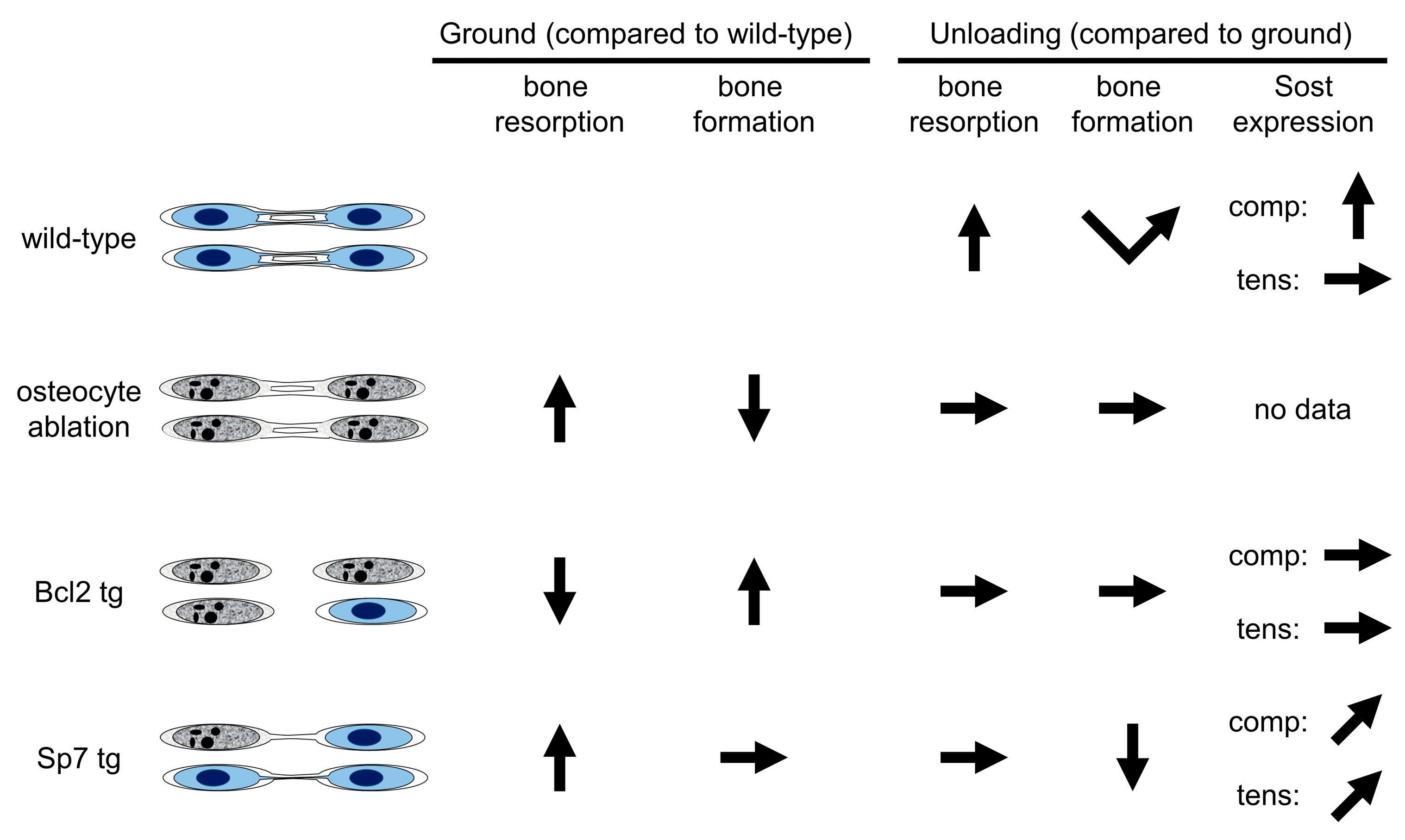
4.1. Ablation of Osteocytes by Diphtheria Toxin
4.2. Bcl2 tg Mice
4.3. Sp7 tg Mice
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marks, S.C.; Odgren, P.R. Structure and Development of the Skeleton. In Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: New York, NY, USA, 2002; Volume 1, pp. 3–15. [Google Scholar]
- Martin, R.B. Does osteocyte formation cause the nonlinear refilling of osteons? Bone 2000, 26, 71–78. [Google Scholar] [CrossRef]
- Ehrlich, P.J.; Lanyon, L.E. Mechanical strain and bone cell function: A review. Osteoporos. Int. 2002, 13, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Knothe Tate, M.L. “Whither flows the fluid in bone?” An osteocyte’s perspective. J. Biomech. 2003, 36, 1409–1424. [Google Scholar] [CrossRef]
- Burger, E.; Klein-Nulend, J. Mechanotransduction in bone—Role of the lacunocanalicular network. FASEB J. 1999, 13, S101–S112. [Google Scholar] [CrossRef]
- Bonewald, L.; Johnson, M. Osteocytes, mechanosensing and Wnt signaling. Bone 2008, 42, 606–615. [Google Scholar] [CrossRef] [Green Version]
- Noble, B. The osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 106–111. [Google Scholar] [CrossRef]
- Komori, T. Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res. 2013, 352, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Kerschnitzki, M.; Wagermaier, W.; Roschger, P.; Seto, J.; Shahar, R.; Duda, G.N.; Mundlos, S.; Fratzl, P. The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. J. Struct. Biol. 2011, 173, 303–311. [Google Scholar] [CrossRef]
- Genthial, R.; Beaurepaire, E.; Schanne-Klein, M.C.; Peyrin, F.; Farlay, D.; Olivier, C.; Bala, Y.; Boivin, G.; Vial, J.C.; Débarre, D.; et al. Label-free imaging of bone multiscale porosity and interfaces using third-harmonic generation microscopy. Sci. Rep. 2017, 7, 3419. [Google Scholar] [CrossRef]
- Ishimoto, T.; Kawahara, K.; Matsugaki, A.; Kamioka, H.; Nakano, T. Quantitative Evaluation of Osteocyte Morphology and Bone Anisotropic Extracellular Matrix in Rat Femur. Calcif. Tissue Int. 2021, 109, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Moriishi, T.; Ito, T.; Fukuyama, R.; Qin, X.; Komori, H.; Kaneko, H.; Matsuo, Y.; Yoshida, N.; Komori, T. Sp7 transgenic mice with a markedly impaired lacunocanalicular network induced Sost and reduced bone mass by unloading. Int. J. Mol. Sci. 2022, 23, 3173. [Google Scholar] [CrossRef] [PubMed]
- Skedros, J.G.; Dayton, M.R.; Sybrowsky, C.L.; Bloebaum, R.D.; Bachus, K.N. The influence of collagen fiber orientation and other histocompositional characteristics on the mechanical properties of equine cortical bone. J. Exp. Biol. 2006, 209 Pt 15, 3025–3042. [Google Scholar] [CrossRef] [Green Version]
- Portigliatti-Barbos, M.; Carando, S.; Ascenzi, A.; Boyde, A. On the structural symmetry of human femurs. Bone 1987, 8, 165–169. [Google Scholar] [CrossRef]
- van Tol, A.F.; Schemenz, V.; Wagermaier, W.; Roschger, A.; Razi, H.; Vitienes, I.; Fratzl, P.; Willie, B.M.; Weinkamer, R. The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. Proc. Natl. Acad. Sci. USA 2020, 117, 32251–32259. [Google Scholar] [CrossRef] [PubMed]
- Qing, H.; Ardeshirpour, L.; Pajevic, P.D.; Dusevich, V.; Jahn, K.; Kato, S.; Wysolmerski, J.; Bonewald, L.F. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 2012, 27, 1018–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, S.; Basta-Pljakic, J.; Seref-Ferlengez, Z.; Majeska, R.J.; Cardoso, L.; Bromage, T.G.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Yakar, S.; et al. Lactation-Induced Changes in the Volume of Osteocyte Lacunar-Canalicular Space Alter Mechanical Properties in Cortical Bone Tissue. J. Bone Miner. Res. 2017, 32, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Moriishi, T.; Maruyama, Z.; Fukuyama, R.; Ito, M.; Miyazaki, T.; Kitaura, H.; Ohnishi, H.; Furuichi, T.; Kawai, Y.; Masuyama, R.; et al. Overexpression of Bcl2 in osteoblasts inhibits osteoblast differentiation and induces osteocyte apoptosis. PLoS ONE 2011, 6, e27487. [Google Scholar] [CrossRef]
- Moriishi, T.; Fukuyama, R.; Ito, M.; Miyazaki, T.; Maeno, T.; Kawai, Y.; Komori, H.; Komori, T. Osteocyte network; a negative regulatory system for bone mass augmented by the induction of rankl in osteoblasts and sost in osteocytes at unloading. PLoS ONE 2012, 7, e40143. [Google Scholar] [CrossRef]
- Noble, B.S.; Reeve, J. Osteocyte function, osteocyte death and bone fracture resistance. Mol. Cell. Endocrinol. 2000, 159, 7–13. [Google Scholar] [CrossRef]
- Cardoso, L.; Herman, B.C.; Verborgt, O.; Laudier, D.; Majeska, R.J.; Schaffler, M.B. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J. Bone Miner. Res. 2009, 24, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerton, K.B.; Hu, B.; Woo, A.A.; Sinofsky, A.; Hernandez, C.; Majeska, R.J.; Jepsen, K.J.; Schaffler, M.B. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone 2010, 46, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, O.D.; Laudier, D.M.; Majeska, R.J.; Sun, H.B.; Schaffler, M.B. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 2014, 64, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messmer, D.; Yang, H.; Telusma, G.; Knoll, F.; Li, J.; Messmer, B.; Tracey, K.J.; Chiorazzi, N. High mobility group box protein 1: An endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 2004, 173, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Svetkauskaite, D.; He, Q.; Kim, J.Y.; Strassheim, D.; Ishizaka, A.; Abraham, E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004, 279, 7370–7377. [Google Scholar] [CrossRef] [Green Version]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- O’Brien, C.A. Control of RANKL gene expression. Bone 2010, 46, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Cell Death in Chondrocytes, Osteoblasts, and Osteocytes. Int. J. Mol. Sci. 2016, 17, 2045. [Google Scholar] [CrossRef] [Green Version]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ’find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef] [Green Version]
- Buckley, K.A.; Hipskind, R.A.; Gartland, A.; Bowler, W.B.; Gallagher, J.A. Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-kappa B ligand. Bone 2002, 31, 582–590. [Google Scholar] [CrossRef]
- Korcok, J.; Raimundo, L.N.; Du, X.; Sims, S.M.; Dixon, S.J. P2Y6 nucleotide receptors activate NF-kappaB and increase survival of osteoclasts. J. Biol. Chem. 2005, 280, 16909–16915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaire, I.; Falzoni, S.; Zhang, B.; Pellegatti, P.; Di Virgilio, F. The P2X7 receptor and Pannexin-1 are both required for the promotion of multinucleated macrophages by the inflammatory cytokine GM-CSF. J. Immunol. 2011, 187, 3878–3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, W.Y.; Fritton, J.C.; Morgan, S.A.; Seref-Ferlengez, Z.; Basta-Pljakic, J.; Thi, M.M.; Suadicani, S.O.; Spray, D.C.; Majeska, R.J.; Schaffler, M.B. Pannexin-1 and P2X7-Receptor Are Required for Apoptotic Osteocytes in Fatigued Bone to Trigger RANKL Production in Neighboring Bystander Osteocytes. J. Bone Miner. Res. 2016, 31, 890–899. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, S.; Ishii, K.; Amizuka, N.; Li, M.; Kobayashi, T.; Kohno, K.; Ito, M.; Takeshita, S.; Ikeda, K. Targeted Ablation of Osteocytes Induces Osteoporosis with Defective Mechanotransduction. Cell Metab. 2007, 5, 464–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, L.; He, X.; Farmer, P.; Boden, S.; Kozlowski, M.; Rubin, J.; Nanes, M.S. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 2000, 141, 3956–3964. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Z.; Tang, E.; Fan, Z.; McCauley, L.; Franceschi, R.; Guan, K.; Krebsbach, P.H.; Wang, C.Y. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat. Med. 2009, 15, 682–689. [Google Scholar] [CrossRef]
- Alles, N.; Soysa, N.S.; Hayashi, J.; Khan, M.; Shimoda, A.; Shimokawa, H.; Ritzeler, O.; Akiyoshi, K.; Aoki, K.; Ohya, K. Suppression of NF-kappaB increases bone formation and ameliorates osteopenia in ovariectomized mice. Endocrinology 2010, 151, 4626–4634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.; Liu, F.; Lee, M.; Wu, B.; Ting, K.; Zara, J.N.; Soo, C.; Al Hezaimi, K.; Zou, W.; Chen, X.; et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 9469–9474. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef]
- Ke, H.; Parron, V.I.; Reece, J.; Zhang, J.Y.; Akiyama, S.K.; French, J.E. BCL2 inhibits cell adhesion, spreading, and motility by enhancing actin polymerization. Cell Res. 2010, 20, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Sugiyama, T.; Prasad, J.; Zaman, G.; Gross, T.S.; Lanyon, L.E.; Price, J.S. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos. Int. 2012, 23, 1225–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, X.; Rhee, Y.; Condon, K.W.; Bivi, N.; Allen, M.R.; Dwyer, D.; Stolina, M.; Turner, C.H.; Robling, A.G.; Plotkin, L.I.; et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 2012, 50, 209–217. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriishi, T.; Komori, T. Osteocytes: Their Lacunocanalicular Structure and Mechanoresponses. Int. J. Mol. Sci. 2022, 23, 4373. https://doi.org/10.3390/ijms23084373
Moriishi T, Komori T. Osteocytes: Their Lacunocanalicular Structure and Mechanoresponses. International Journal of Molecular Sciences. 2022; 23(8):4373. https://doi.org/10.3390/ijms23084373
Chicago/Turabian StyleMoriishi, Takeshi, and Toshihisa Komori. 2022. "Osteocytes: Their Lacunocanalicular Structure and Mechanoresponses" International Journal of Molecular Sciences 23, no. 8: 4373. https://doi.org/10.3390/ijms23084373
APA StyleMoriishi, T., & Komori, T. (2022). Osteocytes: Their Lacunocanalicular Structure and Mechanoresponses. International Journal of Molecular Sciences, 23(8), 4373. https://doi.org/10.3390/ijms23084373





