Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration
Abstract
:1. Introduction
2. Mesenchymal Stem/Stromal Cells from Adipose Tissue (AT-MSCs)
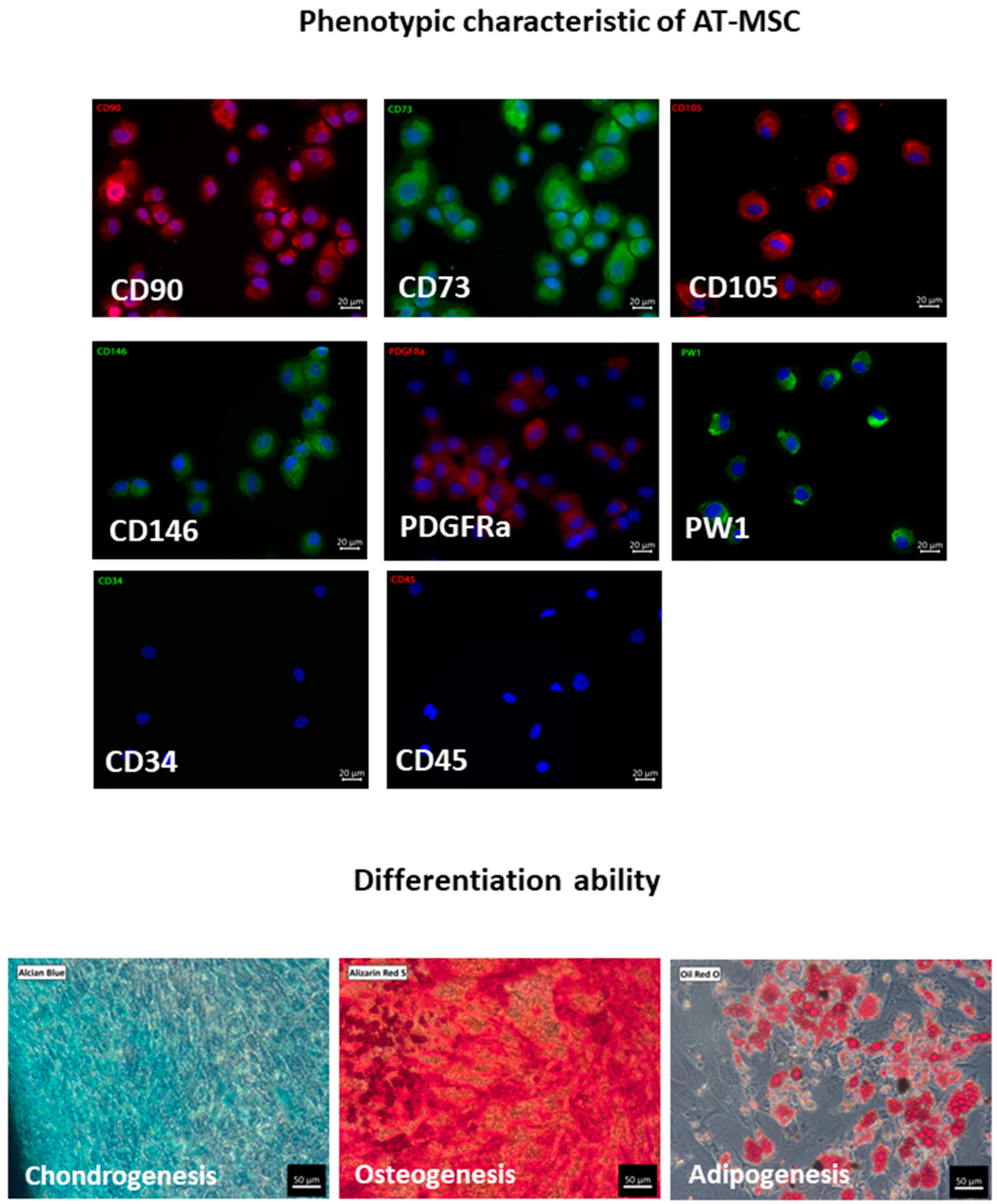
2.1. Biology of AT-MSCs
2.2. Paracrine Activity and Secretome of AT-MSC
3. AT-MSC-Derived Extracellular Vesicles (EVs): Exosomes (Exo) and Microvesicles (MVs)
3.1. Mode of Formation
3.2. Biological Content
3.3. Intercellular Communication
3.4. AT-MSC-Derived Extracellular Vesicles (EVs)
4. Effect of AT-MSCs on Angiogenic Processes
5. AT-MSCs and Their Derivatives: Contribution to Regenerative Processes
5.1. Preclinical Studies on AT-MSCs in Tissue Regeneration
5.2. Clinical Application of AT-MSCs and Their Derivatives in Tissue Regeneration
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPs | antimicrobial peptides/proteins |
| ANG | angiogenin |
| ANGPTL4 | angiopoietin-related protein 4 |
| Ang-1 | angiopoietin 1 |
| ASCs | adipose tissue-derived stromal/stem cells |
| AT-MSCs | adipose tissue-derived mesenchymal stem cells |
| BDNF | brain-derived neurotrophic factor |
| BM-MSCs | bone marrow derived mesenchymal stem cells |
| CCL2 | C-C Motif chemokine ligand 2, MCP-1 |
| CCL5 | C-C motif chemokine ligand 5, RANTES |
| CCL7 | C-C Motif chemokine ligand 7 |
| CDH5 | VE-cadherin (vascular endothelial cadherin) |
| CM | conditioned medium |
| c-Myc | transcription factor c-Myc |
| CXCR4 | C-X-C chemokine receptor type 4 |
| CXCR7 | C-X-C chemokine receptor type 7 |
| DMD | Duchenne muscular dystrophy |
| ENA-78 | epithelial neutrophil activating peptide |
| EPCs | endothelial progenitor cells |
| EVs | extracellular vesicles |
| Exo | exosomes |
| FGF | fibroblast growth factor |
| GDNF | glial-derived neurotrophic factor |
| GRO | growth-regulated protein |
| HATMSC1 | human adipose tissue MSCs cell line |
| hCAP18/LL37 | human cationic antimicrobial protein |
| HGF | hepatocyte growth factor |
| HIF1α | hypoxia-inducible factor 1-alpha |
| HLA ABC | human leukocyte antigen, major histocompatibility complex, class I |
| HLA DR | human leukocyte antigen, major histocompatibility complex, class II |
| HO-1 | heme oxygenase-1 |
| HUVEC | human umbilical vein endothelial cells |
| ICAM-1 | intercellular adhesion molecule 1 |
| IDO | indoleamine 2,3-dioxygenase |
| IFN-γ | interferon gamma |
| IGF | insulin-like growth factor |
| IL | interleukin |
| KDR | kinase insert domain receptor, FLK1, vascular endothelial growth factor receptor 2 |
| LPS | lipopolysaccharides |
| MCP-1 | monocyte chemoattractant protein-1, CCL2 |
| miR | microRNA |
| MSCs | mesenchymal stem cells |
| MMPs | matrix metalloproteinases |
| MVs | microvesicles |
| NANOG | homeobox protein NANOG, transcriptional factor |
| NOTCH | a transmembrane receptor in a signaling pathway |
| Oct4 | octamer-binding transcription factor 4 |
| PDGFA | platelet derived growth factor A |
| PDGF-BB | platelet derived growth factor-BB |
| PDGFRα | platelet-derived growth factor receptor A |
| PW1 | Peg3, paternally expressed gene 3, a marker of adult stem cells |
| RANTES | regulated on activation, normal T cell expressed and secreted protein, CCL5 |
| SDF-1 | stromal cell-derived factor 1, C-X-C motif chemokine 12, CXCL12 |
| Sox2 | (sex determining region Y)-box 2, transcription factor |
| SVF | stromal vascular fraction |
| TGF-β | transforming growth factor β |
| TLR3 | toll-like receptor 3 |
| TNFα | tumor necrosis factor alpha |
| uPA | urokinase-type plasminogen activator |
| uPAR | urokinase-type plasminogen activator receptor |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef]
- Klimczak, A.; Kozlowska, U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor Cells: Their Role in Tissue Homeostasis. Stem Cells Int. 2016, 2016, 4285215. [Google Scholar] [CrossRef] [Green Version]
- Gimble, J.M.; Bunnell, B.A.; Frazier, T.; Rowan, B.; Shah, F.; Thomas-Porch, C.; Wu, X. Adipose-derived stromal/stem cells: A primer. Organogenesis 2013, 9, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Al-Nbaheen, M.; Vishnubalaji, R.; Ali, D.; Bouslimi, A.; Al-Jassir, F.; Megges, M.; Prigione, A.; Adjaye, J.; Kassem, M.; Aldahmash, A. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. Rep. 2013, 9, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem Cells 2019, 11, 347–374. [Google Scholar] [CrossRef]
- BINWIT Database. Available online: https://db.binwit.pl/pl (accessed on 29 January 2022).
- Park, S.B.; Seo, K.W.; So, A.Y.; Seo, M.S.; Yu, K.R.; Kang, S.K.; Kang, K.S. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2012, 19, 534–545. [Google Scholar] [CrossRef] [Green Version]
- Pitrone, M.; Pizzolanti, G.; Tomasello, L.; Coppola, A.; Morini, L.; Pantuso, G.; Ficarella, R.; Guarnotta, V.; Perrini, S.; Giorgino, F.; et al. NANOG Plays a Hierarchical Role in the Transcription Network Regulating the Pluripotency and Plasticity of Adipose Tissue-Derived Stem Cells. Int. J. Mol. Sci. 2017, 18, 1107. [Google Scholar] [CrossRef] [Green Version]
- Riekstina, U.; Cakstina, I.; Parfejevs, V.; Hoogduijn, M.; Jankovskis, G.; Muiznieks, I.; Muceniece, R.; Ancans, J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. Rep. 2009, 5, 378–386. [Google Scholar] [CrossRef]
- Dave, S.D.; Patel, C.N.; Vanikar, A.V.; Trivedi, H.L. In vitro differentiation of neural cells from human adipose tissue derived stromal cells. Neurol. India 2018, 66, 716–721. [Google Scholar] [CrossRef]
- Schaffler, A.; Buchler, C. Concise review: Adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.; Kwon, Y.; Park, K.S.; Jeong, J.H.; Choi, S.J.; Bang, S.I.; Chang, J.W.; Lee, C. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 845. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front Immunol. 2017, 8, 339. [Google Scholar] [CrossRef]
- Chow, L.; Johnson, V.; Impastato, R.; Coy, J.; Strumpf, A.; Dow, S. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl. Med. 2020, 9, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Hosseiniyan Khatibi, S.M.; Kheyrolahzadeh, K.; Barzegari, A.; Rahbar Saadat, Y.; Zununi Vahed, S. Medicinal signaling cells: A potential antimicrobial drug store. J. Cell Physiol. 2020, 235, 7731–7746. [Google Scholar] [CrossRef]
- Marrazzo, P.; Pizzuti, V.; Zia, S.; Sargenti, A.; Gazzola, D.; Roda, B.; Bonsi, L.; Alviano, F. Microfluidic Tools for Enhanced Characterization of Therapeutic Stem Cells and Prediction of Their Potential Antimicrobial Secretome. Antibiotics 2021, 10, 750. [Google Scholar] [CrossRef]
- Sutton, M.T.; Fletcher, D.; Ghosh, S.K.; Weinberg, A.; van Heeckeren, R.; Kaur, S.; Sadeghi, Z.; Hijaz, A.; Reese, J.; Lazarus, H.M.; et al. Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int. 2016, 2016, 5303048. [Google Scholar] [CrossRef] [Green Version]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Deng, Y.H.; Tsai, F.C.; Deng, W.P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef] [Green Version]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Xu, A. Adipose Extracellular Vesicles in Intercellular and Inter-Organ Crosstalk in Metabolic Health and Diseases. Front Immunol. 2021, 12, 608680. [Google Scholar] [CrossRef]
- Hsiao, S.T.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef] [Green Version]
- Kraskiewicz, H.; Paprocka, M.; Bielawska-Pohl, A.; Krawczenko, A.; Panek, K.; Kaczynska, J.; Szyposzynska, A.; Psurski, M.; Kuropka, P.; Klimczak, A. Can supernatant from immortalized adipose tissue MSC replace cell therapy? An in vitro study in chronic wounds model. Stem Cell Res. Ther. 2020, 11, 29. [Google Scholar] [CrossRef]
- Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Kraskiewicz, H.; Szyposzynska, A.; Wojdat, E.; Klimczak, A. Microvesicles from Human Immortalized Cell Lines of Endothelial Progenitor Cells and Mesenchymal Stem/Stromal Cells of Adipose Tissue Origin as Carriers of Bioactive Factors Facilitating Angiogenesis. Stem Cells Int. 2020, 2020, 1289380. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, J.G.; Frobert, O.; Pilgaard, L.; Kastrup, J.; Simonsen, U.; Zachar, V.; Fink, T. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy 2011, 13, 318–328. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-gamma. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Jo, C.H.; Kim, H.R.; Hwang, Y.I. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018, 2018, 8429042. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [Green Version]
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019, 13, 569–586. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, X.; Qu, C.; Chang, P.; Zhang, C.; Qu, Y.; Liu, Y. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy 2015, 17, 560–570. [Google Scholar] [CrossRef]
- Qin, H.H.; Filippi, C.; Sun, S.; Lehec, S.; Dhawan, A.; Hughes, R.D. Hypoxic preconditioning potentiates the trophic effects of mesenchymal stem cells on co-cultured human primary hepatocytes. Stem Cell Res. Ther. 2015, 6, 237. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.; Hu, Q.; Ma, Y.; Xiong, W.; Wu, T.; Cao, J.; Wu, D. Human adipose-derived mesenchymal stem cells repair cisplatin-induced acute kidney injury through antiapoptotic pathways. Exp. Ther. Med. 2015, 10, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Kraskiewicz, H.; Hinc, P.; Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Witkowska, D.; Mohd Isa, I.L.; Pandit, A.; Klimczak, A. HATMSC Secreted Factors in the Hydrogel as a Potential Treatment for Chronic Wounds-In Vitro Study. Int. J. Mol. Sci. 2021, 22, 12241. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Di Vizio, D.; Kim, J.; Hager, M.H.; Morello, M.; Yang, W.; Lafargue, C.J.; True, L.D.; Rubin, M.A.; Adam, R.M.; Beroukhim, R.; et al. Oncosome formation in prostate cancer: Association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009, 69, 5601–5609. [Google Scholar] [CrossRef] [Green Version]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Aatonen, M.T.; Ohman, T.; Nyman, T.A.; Laitinen, S.; Gronholm, M.; Siljander, P.R. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles 2014, 3, 24692. [Google Scholar] [CrossRef]
- Gudbergsson, J.M.; Johnsen, K.B.; Skov, M.N.; Duroux, M. Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology 2016, 68, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, G.; Liu, M.L. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genom. Proteom. Bioinform. 2018, 16, 50–62. [Google Scholar] [CrossRef]
- de Freitas, R.C.C.; Hirata, R.D.C.; Hirata, M.H.; Aikawa, E. Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases. Biomolecules 2021, 11, 388. [Google Scholar] [CrossRef]
- Lane, R.E.; Korbie, D.; Hill, M.M.; Trau, M. Extracellular vesicles as circulating cancer biomarkers: Opportunities and challenges. Clin. Transl. Med. 2018, 7, 14. [Google Scholar] [CrossRef]
- Povero, D.; Yamashita, H.; Ren, W.; Subramanian, M.G.; Myers, R.P.; Eguchi, A.; Simonetto, D.A.; Goodman, Z.D.; Harrison, S.A.; Sanyal, A.J.; et al. Characterization and Proteome of Circulating Extracellular Vesicles as Potential Biomarkers for NASH. Hepatol. Commun. 2020, 4, 1263–1278. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Yang, S.; Siaw-Debrah, F.; Hu, J.; Wu, K.; He, Z.; Yang, J.; Pan, S.; Lin, X.; Ye, H.; et al. Exosomes Derived From Bone Mesenchymal Stem Cells Ameliorate Early Inflammatory Responses Following Traumatic Brain Injury. Front. Neurosci. 2019, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xue, S.; Zhang, S.; Cheng, K.; Ye, Q. Exosomes from donor-derived adipose mesenchymal stem cells prolong the survival of vascularized composite allografts. J. Cell Physiol. 2021, 236, 5895–5905. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, 1197. [Google Scholar] [CrossRef] [Green Version]
- Lo Furno, D.; Mannino, G.; Giuffrida, R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J. Cell Physiol. 2018, 233, 3982–3999. [Google Scholar] [CrossRef]
- Lelek, J.; Zuba-Surma, E.K. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int. J. Mol. Sci. 2020, 21, 799. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Wang, J.; Li, H.; Gao, S.; Shi, R.; Yang, D.; Wang, X.; Wang, X.; Zhu, L.; Wang, X.; et al. Extracellular Vesicles Secreted by Human Adipose-derived Stem Cells (hASCs) Improve Survival Rate of Rats with Acute Liver Failure by Releasing lncRNA H19. EBioMedicine 2018, 34, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lou, G.; Li, A.; Zhang, T.; Qi, J.; Ye, D.; Zheng, M.; Chen, Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 2018, 36, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, H.; Yang, Y.; Fang, L.; Wu, Q.; Li, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Roles in Tumor Growth, Progression, and Drug Resistance. Stem Cells Int. 2017, 2017, 1758139. [Google Scholar] [CrossRef]
- Mannino, G.; Gennuso, F.; Giurdanella, G.; Conti, F.; Drago, F.; Salomone, S.; Furno, D.L.; Bucolo, C.; Giuffrida, R. Pericyte-like differentiation of human adipose-derived mesenchymal stem cells: An in vitro study. World J. Stem Cells 2020, 12, 1152–1170. [Google Scholar] [CrossRef]
- Mannino, G.; Longo, A.; Gennuso, F.; Anfuso, C.D.; Lupo, G.; Giurdanella, G.; Giuffrida, R.; Lo Furno, D. Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 4604. [Google Scholar] [CrossRef]
- Beloglazova, I.; Stepanova, V.; Zubkova, E.; Dergilev, K.; Koptelova, N.; Tyurin-Kuzmin, P.A.; Dyikanov, D.; Plekhanova, O.; Cines, D.B.; Mazar, A.P.; et al. Mesenchymal stromal cells enhance self-assembly of a HUVEC tubular network through uPA-uPAR/VEGFR2/integrin/NOTCH crosstalk. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119157. [Google Scholar] [CrossRef]
- Bi, H.; Li, H.; Zhang, C.; Mao, Y.; Nie, F.; Xing, Y.; Sha, W.; Wang, X.; Irwin, D.M.; Tan, H. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res. Ther. 2019, 10, 302. [Google Scholar] [CrossRef] [Green Version]
- Ratushnyy, A.; Ezdakova, M.; Buravkova, L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020, 21, 1802. [Google Scholar] [CrossRef] [Green Version]
- Trinh, N.T.; Yamashita, T.; Tu, T.C.; Kato, T.; Ohneda, K.; Sato, F.; Ohneda, O. Microvesicles enhance the mobility of human diabetic adipose tissue-derived mesenchymal stem cells in vitro and improve wound healing in vivo. Biochem. Biophys. Res. Commun. 2016, 473, 1111–1118. [Google Scholar] [CrossRef]
- Zomer, H.D.; Varela, G.; Delben, P.B.; Heck, D.; Jeremias, T.D.S.; Trentin, A.G. In vitro comparative study of human mesenchymal stromal cells from dermis and adipose tissue for application in skin wound healing. J. Tissue Eng. Regen. Med. 2019, 13, 729–741. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.L.; Garcia-Posadas, L.; Diebold, Y. Extracellular Vesicles from Human Adipose-Derived Mesenchymal Stem Cells: A Review of Common Cargos. Stem Cell Rev. Rep. 2021, 1–48. [Google Scholar] [CrossRef]
- Lopatina, T.; Bruno, S.; Tetta, C.; Kalinina, N.; Porta, M.; Camussi, G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. 2014, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Zubkova, E.S.; Beloglazova, I.B.; Makarevich, P.I.; Boldyreva, M.A.; Sukhareva, O.Y.; Shestakova, M.V.; Dergilev, K.V.; Parfyonova, Y.V.; Menshikov, M.Y. Regulation of Adipose Tissue Stem Cells Angiogenic Potential by Tumor Necrosis Factor-Alpha. J. Cell Biochem. 2016, 117, 180–196. [Google Scholar] [CrossRef]
- Zhong, Z.; Gu, H.; Peng, J.; Wang, W.; Johnstone, B.H.; March, K.L.; Farlow, M.R.; Du, Y. GDNF secreted from adipose-derived stem cells stimulates VEGF-independent angiogenesis. Oncotarget 2016, 7, 36829–36841. [Google Scholar] [CrossRef]
- Pu, C.M.; Liu, C.W.; Liang, C.J.; Yen, Y.H.; Chen, S.H.; Jiang-Shieh, Y.F.; Chien, C.L.; Chen, Y.C.; Chen, Y.L. Adipose-Derived Stem Cells Protect Skin Flaps against Ischemia/Reperfusion Injury via IL-6 Expression. J. Investig. Dermatol. 2017, 137, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, S.L.; Hsiao, S.T.; Peshavariya, H.M.; Lim, S.Y.; Dusting, G.J.; Dilley, R.J. Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev. 2012, 21, 1887–1896. [Google Scholar] [CrossRef]
- Hsiao, S.T.; Lokmic, Z.; Peshavariya, H.; Abberton, K.M.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Hypoxic conditioning enhances the angiogenic paracrine activity of human adipose-derived stem cells. Stem Cells Dev. 2013, 22, 1614–1623. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Shen, Y.; Li, X.; Li, B.; Zhao, S.; Gu, J.; Chen, Y.; Ma, B.; Wei, J.; Han, Q.; et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018, 27, 456–465. [Google Scholar] [CrossRef]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Huang, L.F.; Zhao, L.; Zeng, Z.; Wang, X.; Cao, D.; Yang, L.; Ye, Z.; Chen, X.; Liu, B.; et al. Microvesicles (MIVs) secreted from adipose-derived stem cells (ADSCs) contain multiple microRNAs and promote the migration and invasion of endothelial cells. Genes Dis. 2020, 7, 225–234. [Google Scholar] [CrossRef]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, X.; Zhang, Y.L.; Lu, Y.; Zhang, W.; Lu, S.; Fu, Y.; Zhou, Y.; Zhang, J.; Zhang, J. Human Exosomes Accelerate Cutaneous Wound Healing by Promoting Collagen Synthesis in A Diabetic Mice Model. Stem Cells Dev. 2021. [Google Scholar] [CrossRef] [PubMed]
- de la Garza-Rodea, A.S.; van der Velde-van Dijke, I.; Boersma, H.; Goncalves, M.A.; van Bekkum, D.W.; de Vries, A.A.; Knaan-Shanzer, S. Myogenic properties of human mesenchymal stem cells derived from three different sources. Cell Transplant. 2012, 21, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Abd Elaziz, A.M.; Moussa, H.; Hamam, G.G.; El-Waseef, D.A.A. Effect of Allogenic Bone Marrow- Versus Adipose Tissue Derived Mesenchymal Stem Cells in Treatment of Experimental Skeletal Muscle Injury in Adult Female Albino Rats: A Comparative Study. J. Stem Cell. Biol. Transplant. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Moussa, M.H.; Hamam, G.G.; Abd Elaziz, A.E.; Rahoma, M.A.; Abd El Samad, A.A.; El-Waseef, D.A.A.; Hegazy, M.A. Comparative Study on Bone Marrow-Versus Adipose-Derived Stem Cells on Regeneration and Re-Innervation of Skeletal Muscle Injury in Wistar Rats. Tissue Eng. Regen. Med. 2020, 17, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Assoni, A.; Coatti, G.; Valadares, M.C.; Beccari, M.; Gomes, J.; Pelatti, M.; Mitne-Neto, M.; Carvalho, V.M.; Zatz, M. Different Donors Mesenchymal Stromal Cells Secretomes Reveal Heterogeneous Profile of Relevance for Therapeutic Use. Stem Cells Dev. 2017, 26, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Renaud, A.; Guilloton, F.; Mebarki, M.; Trichet, V.; Sensebe, L.; Deschaseaux, F.; Chevallier, N.; Layrolle, P. Inferior In Vivo Osteogenesis and Superior Angiogenesis of Human Adipose-Derived Stem Cells Compared with Bone Marrow-Derived Stem Cells Cultured in Xeno-Free Conditions. Stem Cells Transl. Med. 2017, 6, 2160–2172. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Y.; Dunstan, C.; Roohani-Esfahani, S.; Zreiqat, H. Priming Adipose Stem Cells with Tumor Necrosis Factor-Alpha Preconditioning Potentiates Their Exosome Efficacy for Bone Regeneration. Tissue Eng. Part A 2017, 23, 1212–1220. [Google Scholar] [CrossRef]
- Ciervo, Y.; Gatto, N.; Allen, C.; Grierson, A.; Ferraiuolo, L.; Mead, R.J.; Shaw, P.J. Adipose-derived stem cells protect motor neurons and reduce glial activation in both in vitro and in vivo models of ALS. Mol. Ther. Methods Clin. Dev. 2021, 21, 413–433. [Google Scholar] [CrossRef]
- Masgutov, R.; Masgutova, G.; Mullakhmetova, A.; Zhuravleva, M.; Shulman, A.; Rogozhin, A.; Syromiatnikova, V.; Andreeva, D.; Zeinalova, A.; Idrisova, K.; et al. Adipose-Derived Mesenchymal Stem Cells Applied in Fibrin Glue Stimulate Peripheral Nerve Regeneration. Front Med. 2019, 6, 68. [Google Scholar] [CrossRef]
- Sanchez, D.N.R.; Bertanha, M.; Fernandes, T.D.; Resende, L.A.L.; Deffune, E.; Amorim, R.M. Effects of Canine and Murine Mesenchymal Stromal Cell Transplantation on Peripheral Nerve Regeneration. Int. J. Stem Cells 2017, 10, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Bucan, V.; Vaslaitis, D.; Peck, C.T.; Strauss, S.; Vogt, P.M.; Radtke, C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef] [Green Version]
- Marconi, S.; Castiglione, G.; Turano, E.; Bissolotti, G.; Angiari, S.; Farinazzo, A.; Constantin, G.; Bedogni, G.; Bedogni, A.; Bonetti, B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng. Part A 2012, 18, 1264–1272. [Google Scholar] [CrossRef]
- Brini, A.T.; Amodeo, G.; Ferreira, L.M.; Milani, A.; Niada, S.; Moschetti, G.; Franchi, S.; Borsani, E.; Rodella, L.F.; Panerai, A.E.; et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep. 2017, 7, 9904. [Google Scholar] [CrossRef] [Green Version]
- Sumarwoto, T.; Suroto, H.; Mahyudin, F.; Utomo, D.N.; Romaniyanto; Tinduh, D.; Notobroto, H.B.; Sigit Prakoeswa, C.R.; Rantam, F.A.; Rhatomy, S. Role of adipose mesenchymal stem cells and secretome in peripheral nerve regeneration. Ann. Med. Surg. 2021, 67, 102482. [Google Scholar] [CrossRef]
- Carstens, M.H.; Quintana, F.J.; Calderwood, S.T.; Sevilla, J.P.; Rios, A.B.; Rivera, C.M.; Calero, D.W.; Zelaya, M.L.; Garcia, N.; Bertram, K.A.; et al. Treatment of chronic diabetic foot ulcers with adipose-derived stromal vascular fraction cell injections: Safety and evidence of efficacy at 1 year. Stem Cells Transl. Med. 2021, 10, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials Database. Available online: https://clinicaltrials.gov/ (accessed on 28 January 2022).
- Maslowski, L.; Paprocka, M.; Czyzewska-Buczynska, A.; Bielawska-Pohl, A.; Dus, D.; Grendziak, R.; Witkiewicz, W.; Czarnecka, A. Autotransplantation of the Adipose Tissue-Derived Mesenchymal Stromal Cells in Therapy of Venous Stasis Ulcers. Arch. Immunol. Ther. Exp. 2020, 68, 5. [Google Scholar] [CrossRef] [PubMed]
- del Moral, L.R.; Salazar, A.A.; Stefanov Kiuri, S.; Tong, H.; de Cubas, L.R.; García-Olmo, D.; García-Arranz, M. Phase Ib Open Clinical Trial to Assess the Safety of Autologous Mesenchymal Stem Cells for the Treatment of Nonrevascularizable Critical Lower Limb Ischemia. Stem Cell Res. Ther. 2017, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.C.; An, S.G.; Lee, H.W.; Park, J.S.; Cha, K.S.; Hong, T.J.; Park, J.H.; Lee, S.Y.; Kim, S.P.; Kim, Y.D.; et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 2012, 76, 1750–1760. [Google Scholar] [CrossRef] [Green Version]
- Lonardi, R.; Leone, N.; Gennai, S.; Trevisi Borsari, G.; Covic, T.; Silingardi, R. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: A randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res. Ther. 2019, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Gennai, S.; Leone, N.; Covic, T.; Migliari, M.; Lonardi, R.; Silingardi, R. Health-related quality of life outcomes and hospitalization length of stay after micro-fragmented autologous adipose tissue injection in minor amputations for diabetic foot ulceration (MiFrAADiF Trial): Results from a randomized controlled single-center clinical trial. Int. Angiol. 2021, 40, 512–519. [Google Scholar] [CrossRef]
- de Celis-Ruiz, E.; Fuentes, B.; Moniche, F.; Montaner, J.; Borobia, A.M.; Gutierrez-Fernandez, M.; Diez-Tejedor, E. Allogeneic adipose tissue-derived mesenchymal stem cells in ischaemic stroke (AMASCIS-02): A phase IIb, multicentre, double-blind, placebo-controlled clinical trial protocol. BMJ Open 2021, 11, e051790. [Google Scholar] [CrossRef]
- Kastrup, J.; Haack-Sorensen, M.; Juhl, M.; Harary Sondergaard, R.; Follin, B.; Drozd Lund, L.; Monsted Johansen, E.; Ali Qayyum, A.; Bruun Mathiasen, A.; Jorgensen, E.; et al. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Transl. Med. 2017, 6, 1963–1971. [Google Scholar] [CrossRef]
- Diez-Tejedor, E.; Gutierrez-Fernandez, M.; Martinez-Sanchez, P.; Rodriguez-Frutos, B.; Ruiz-Ares, G.; Lara, M.L.; Gimeno, B.F. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: A safety assessment: A phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J. Stroke Cerebrovasc. Dis. 2014, 23, 2694–2700. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Mathiasen, A.B.; Helqvist, S.; Jorgensen, E.; Haack-Sorensen, M.; Ekblond, A.; Kastrup, J. Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J. Transl. Med. 2019, 17, 360. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Mathiasen, A.B.; Mygind, N.D.; Kuhl, J.T.; Jorgensen, E.; Helqvist, S.; Elberg, J.J.; Kofoed, K.F.; Vejlstrup, N.G.; Fischer-Nielsen, A.; et al. Adipose-Derived Stromal Cells for Treatment of Patients with Chronic Ischemic Heart Disease (MyStromalCell Trial): A Randomized Placebo-Controlled Study. Stem Cells Int. 2017, 2017, 5237063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimczak, A. Perspectives on mesenchymal stem/progenitor cells and their derivates as potential therapies for lung damage caused by COVID-19. World J. Stem Cells 2020, 12, 1013–1022. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Generali, M.; Kehl, D.; Wanner, D.; Okoniewski, M.J.; Hoerstrup, S.P.; Cinelli, P. Heterogeneous expression of ACE2 and TMPRRS2 in mesenchymal stromal cells. J. Cell. Mol. Med. 2022, 26, 228–234. [Google Scholar] [CrossRef]
- Shi, M.M.; Yang, Q.Y.; Monsel, A.; Yan, J.Y.; Dai, C.X.; Zhao, J.Y.; Shi, G.C.; Zhou, M.; Zhu, X.M.; Li, S.K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef]

| Study Number/Status | Type of Disease | Type of Therapy | Patients | Results | Ref. |
|---|---|---|---|---|---|
| Not reported Completed | Nonhealing diabetic foot ulcers (>3 cm in diameter) | Local injections of autologous adipose-derived stromal vascular fraction (SVF) cells (EPCs and MSCs), phase 1 study; injection into the target foot of a total dose of 30 × 106 SVF cells | 63 patients with type 2 diabetes and underlying microangiopathy | Improved ulcer healing: closure response rates among evaluable patients between 86% and 93% at the 6- and 12-month endpoints; changes in the vascular bed beneath the ulcer and structural characteristics of the arteries supplying the foot | [94] |
| KB/27/2015 Bioethics committee at the Regional Specialist Hospital, Research and Development Center in Wroclaw, Poland Completed | Chronic venous stasis ulcers | Subcutaneous administration to the tissues surrounding the ulcers and under the ulcer bed of autologous AT-MSCs (3.0 × 105 to 2.3 × 107 cells) | 11 patients (12 ulcers) | Improvement in clinical condition observed in 75% of ulcers; complete healing occurred in 25% of ulcers | [96] |
| NCT04746599 Recruiting ClinicalTrials.gov | Critical limb ischemia | Autologous fat grafting | 20 participants | No results posted | |
| NCT04661644 Recruiting ClinicalTrials.gov | Critical limb ischemia | Clusters of adipose-derived mesenchymal stem cells (dose: 1 × 107 cells/1 mL/vial, phase 1; and 1 × 108 cells/1 mL/vial, phase 2), phase 1/2a clinical trial | 20 participants | No results posted | |
| NCT04466007 Recruiting ClinicalTrials.gov | Critical ischemia of the lower limbs in diabetic patients without the possibility of revascularization | Allogeneic mesenchymal stromal cells derived from adipose tissue administered intramuscularly (low and high doses) | 90 participants | No results posted | |
| NCT03968198 Recruiting ClinicalTrials.gov | Critical limb ischemia and peripheral artery disease | Autologous intramuscular administration of adipose tissue-derived mesenchymal stromal/stem cells (ASCs), phase 2 study | 43 participants | No results posted | |
| NCT01824069 Completed ClinicalTrials.gov | Nonrevascularizable critical ischemia of the lower limbs | Intramuscular injection of autologous adult mesenchymal stem cells derived from adipose tissue (1 × 106/kg), phase 1 and 2a study | 10 participants | 7 patients were followed-up after the treatment for 1 year (phase 1b study). A statistically significant improvement in health-related quality of life in the post-treatment period was observed. An ankle-brachial index and clinical behavior of the limb improved during the follow-up. | [97] |
| NCT01745744 Completed ClinicalTrials.gov | Critical chronic ischemic syndrome of the lower limb in nondiabetic patients | Infusion of mesenchymal stem cells derived from adipose tissue administered intraarterially: 0.5 × 106 cells/kg of patient weight and 1 × 106 cells/kg of patient weight, phase 2 study | 33 participants | No results posted | |
| NCT01663376 Completed | Critical Limb Ischemia | Intramuscular injection of autologous adipose derived mesenchymal stem cells. Dose: 1 × 108–3 × 108 cells | 20 participants | Autologous AT-MSC implantation effectively increases blood flow. Above 66% of patients with non-healing ulcers experienced ulcer healing, only in the cases with an initially necrotic foot, no observable tissue regeneration occurred. There was clinical improvement in 100% of patients with a diabetic foot (3 patients) and in 58.3% of patients with Buerger’s Disease (7 patients) | [98] |
| NCT01302015 Completed ClinicalTrials.gov | Buerger’s disease (thromboangiitis obliterans) | RNL-Vascostem (autologous adipose tissue-derived mesenchymal stem cells) dosage: intramuscular infusion, 5 × 106 cells/kg | 15 participants | ||
| NCT04569409 Recruiting ClinicalTrials.gov | Diabetic foot ulcer | Application of a hydrogel sheet (ALLO-ASC-DFU) containing allogenic adipose-derived mesenchymal stem cells to diabetic grade 2 foot ulcer, phase 3 study | 104 patients | No results available yet | |
| NCT04497805 Recruiting ClinicalTrials.gov | Diabetic foot ulcer | Application of a hydrogel sheet (ALLO-ASC-DFU) containing allogenic mesenchymal stem cells to diabetic grade 2 foot ulcer, phase 2 study | 64 participants | No results available yet | |
| NCT04457037 Completed ClinicalTrials.gov | Trophic ulcer | Patients with trophic ulcers received standard treatment and autologous adipose-derived mesenchymal stem cells | 18 participants | No results available yet | |
| NCT03276312 Completed ClinicalTrials.gov | Minor amputations of diabetic foot | Lipogems–local injection of autologous micro-fragmented adipose tissue | 112 participants | After 6 months, 80% of the micro-fragmented adipose tissue-treated feet healed and 20% failed compared to the control group. A significant improvement in terms of physical health-related quality of life and a significant reduction of the hospital length of stay was reported. | [99,100] |
| NCT03183648 Active, not recruiting ClinicalTrials.gov | Burn | Application of a hydrogel sheet (ALLO-ASC-DFU) containing allogenic adipose-derived mesenchymal stem cells | 30 participants | No results available yet | |
| NCT04280003 Recruiting ClinicalTrials.gov | Ischemic stroke | Intravenous treatment with allogenic adipose tissue-derived stem cells in a single dose of one million cells per kg, phase 2 study | 30 participants | No results available yet | [101] |
| NCT02387723 Completed ClinicalTrials.gov | Heart failure | Patients with heart failure treated with culture-expanded adipose tissue-derived mesenchymal stem cells from healthy donors. The cells were injected directly into the myocardium | 10 participants | Four out of ten patients developed donor-specific de novo HLA class I antibodies, and two other patients had donor-specific antibodies at baseline. None of the patients had any clinical symptoms or changes in biochemical or inflammatory parameters. The cardiac function tended to improve after AD MSC treatment at 6-month follow-up. | [102] |
| NCT01678534 Completed ClinicalTrials.gov | Ischemic Stroke | Intravenous treatment with allogeneic stem cells from adipose tissue, phase 2 study | 19 participants | No results posted | [103] |
| NCT01449032 Completed ClinicalTrials.gov | Chronic ischemic heart disease (coronary artery disease, CAD) | Intramyocardial injections of autologous VEGF-A165-stimulated adipose-derived stem cells (ASCs), phase 2 study | 60 participants | Intramyocardially delivered ASC treatment was safe but did not improve exercise capacity compared to placebo in a pilot study. After a 3-year follow-up, patients receiving ASCs had improved cardiac symptoms and unchanged exercise capacity, in contrast to deterioration in the placebo group | [104,105] |
| NCT04388761 Recruiting ClinicalTrials.gov | Ischemia reperfusion injury in patients with a kidney allograft | AMSC treatment via direct injection into the kidney parenchyma and intra-arterial infusion, phase 2 study | 15 participants | No results posted | |
| NCT01257776 Completed ClinicalTrials.gov | Critical limb ischemia (CLI) in diabetic patients | Intra-arterial administration of autologous adipose-derived mesenchymal stem cells, phase 1 study | 33 participants | No results posted | |
| NCT03865394 Completed ClinicalTrials.gov | Chronic wounds in diabetic foot syndrome | Application of allogeneic adipose-derived mesenchymal stem cells in fibrin gel | 46 participants | No results posted | |
| NCT03183726 Completed ClinicalTrials.gov | Diabetic foot ulcer | Application of ALLO-ASC-DFU (a hydrogel sheet containing allogenic adipose-derived mesenchymal stem cells), phase 1 study | 4 participants | No results posted | |
| NCT03754465 Recruiting ClinicalTrials.gov | Diabetic foot ulcer | Application of an ALLO-ASC-DFU sheet to diabetic foot ulcer (a hydrogel sheet containing allogenic adipose-derived mesenchymal stem cells), phase 2 study | 44 participants | No results posted | |
| NCT03370874 Active, not recruiting ClinicalTrials.gov | Diabetic foot ulcer | Application of an ALLO-ASC-DFU sheet to diabetic foot ulcer (a hydrogel sheet containing allogenic adipose-derived mesenchymal stem cells), phase 3 study | 164 participants | No results posted | |
| NCT02394873 Completed ClinicalTrials.gov | Deep second-degree burn wound | Application of an ALLO-ASC-DFU sheet (a hydrogel sheet containing allogenic adipose-derived mesenchymal stem cells), phase 1 study | 5 participants | No results posted | |
| NCT05165459 Recruiting ClinicalTrials.gov | Venous keg ulcer | Venous leg ulcer treatment with adipose SVF (autologous adipose stromal vascular fraction) administered locally into the target ulcer. | 10 participants | No results posted | |
| NCT04569409 Recruiting ClinicalTrials.gov | Diabetic Wagner grade 2 foot ulcers | Application of an ALLO-ASC-DFU sheet (a hydrogel sheet containing allogenic adipose-derived mesenchymal stem cells), phase 3 study | 104 participants | No results posted |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczenko, A.; Klimczak, A. Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 2425. https://doi.org/10.3390/ijms23052425
Krawczenko A, Klimczak A. Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. International Journal of Molecular Sciences. 2022; 23(5):2425. https://doi.org/10.3390/ijms23052425
Chicago/Turabian StyleKrawczenko, Agnieszka, and Aleksandra Klimczak. 2022. "Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration" International Journal of Molecular Sciences 23, no. 5: 2425. https://doi.org/10.3390/ijms23052425
APA StyleKrawczenko, A., & Klimczak, A. (2022). Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. International Journal of Molecular Sciences, 23(5), 2425. https://doi.org/10.3390/ijms23052425







