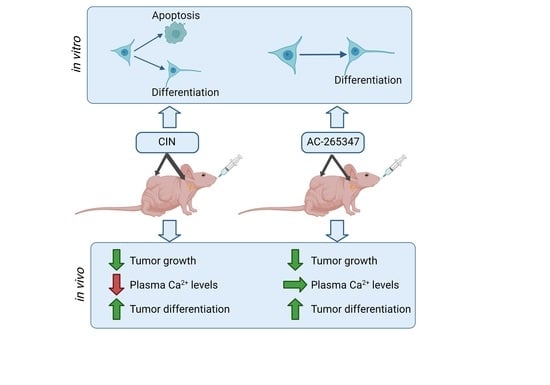
AC-265347 Inhibits Neuroblastoma Tumor Growth by Induction of Differentiation without Causing Hypocalcemia
Abstract
:1. Introduction
2. Results
2.1. AC-265347 Inhibits NB Tumor Growth While Keeping Normal Plasma Calcium Levels
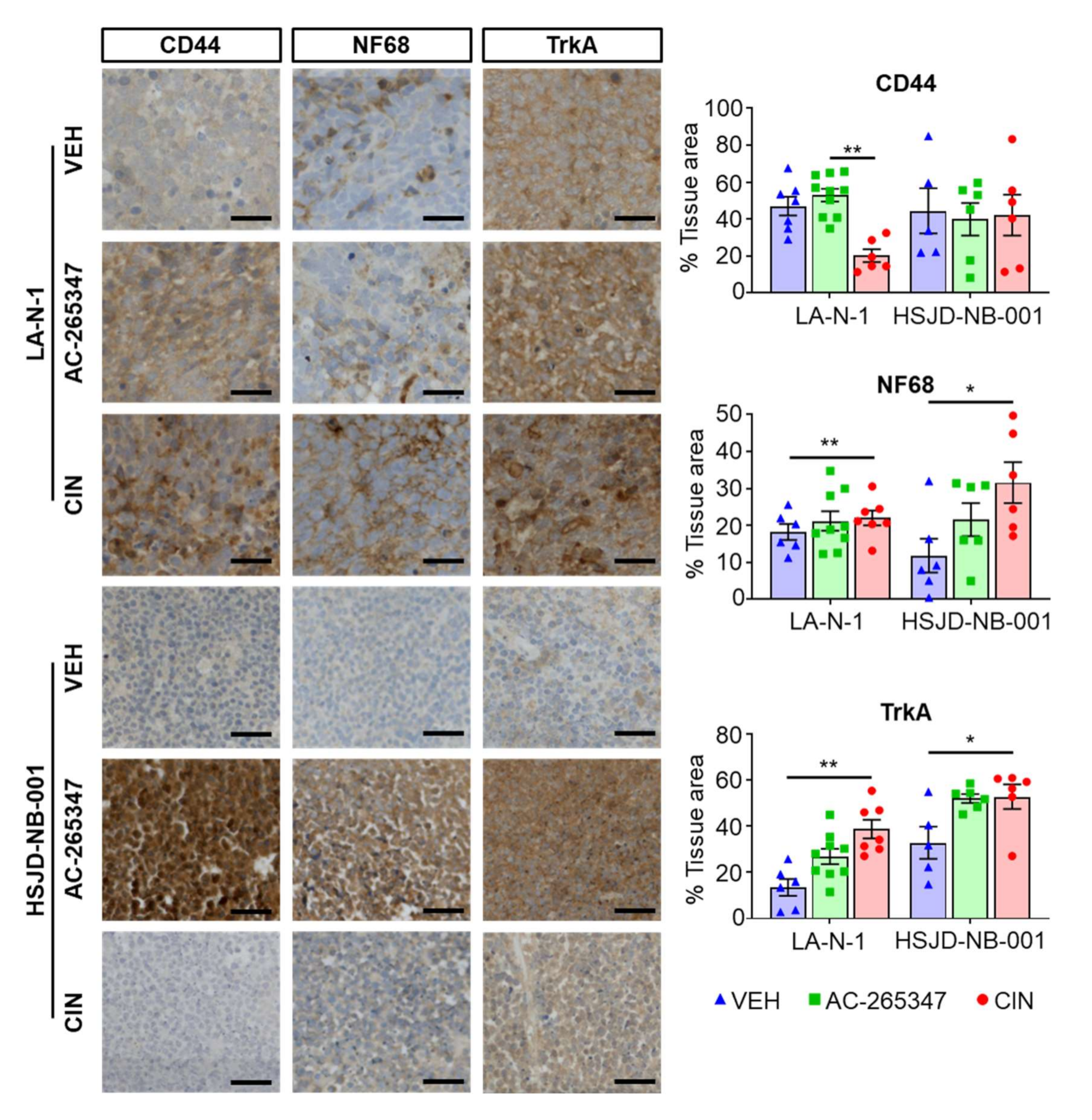
2.2. Expression of NB Differentiation Markers Is Increased by Exposure to Calcimimetics
2.3. AC-265347 and CIN Induce a Different Gene Expression Pattern in NB Xenograft Models
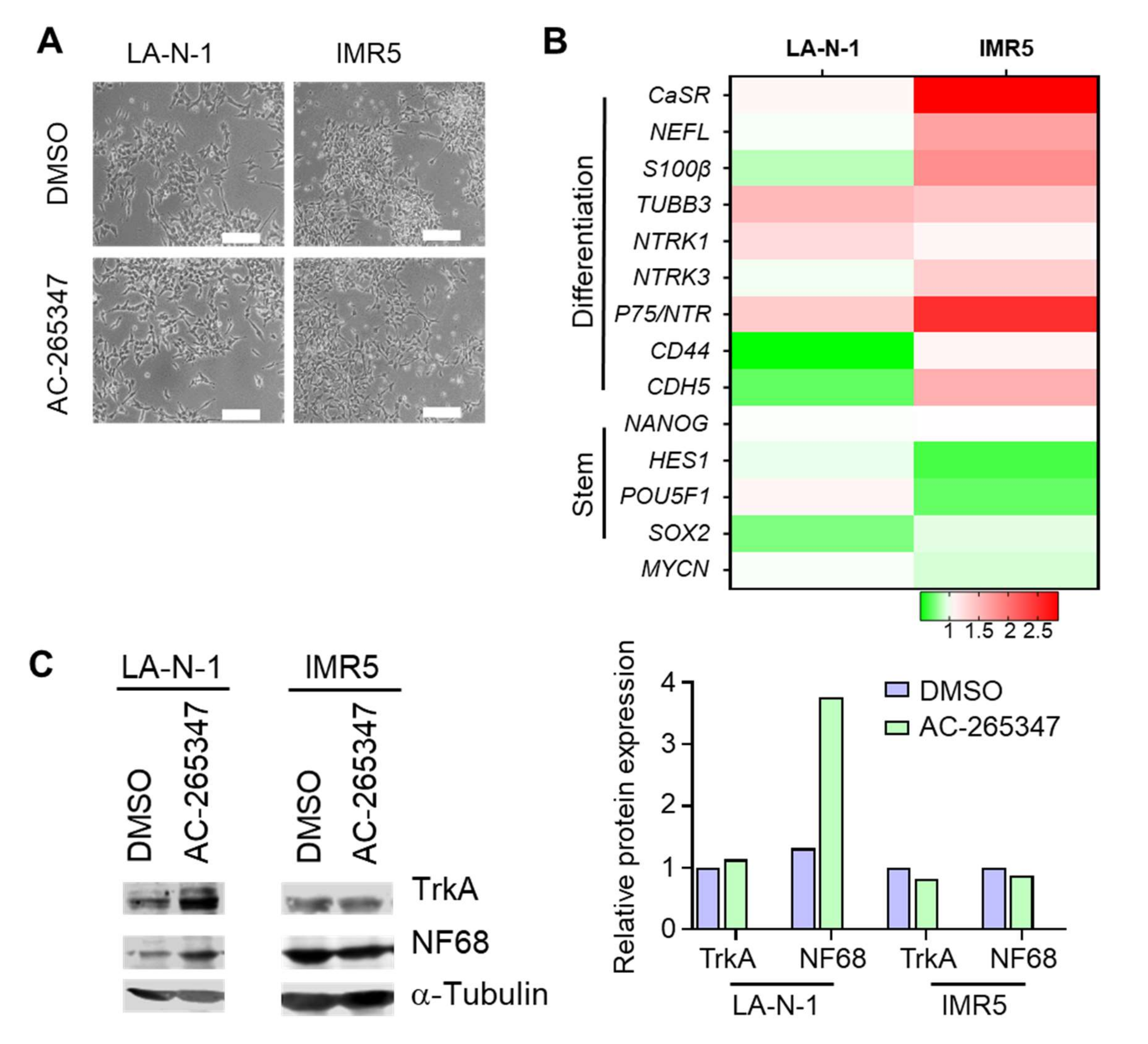
2.4. NB Cell Lines Respond Differently to Chronic Exposure of AC-265347
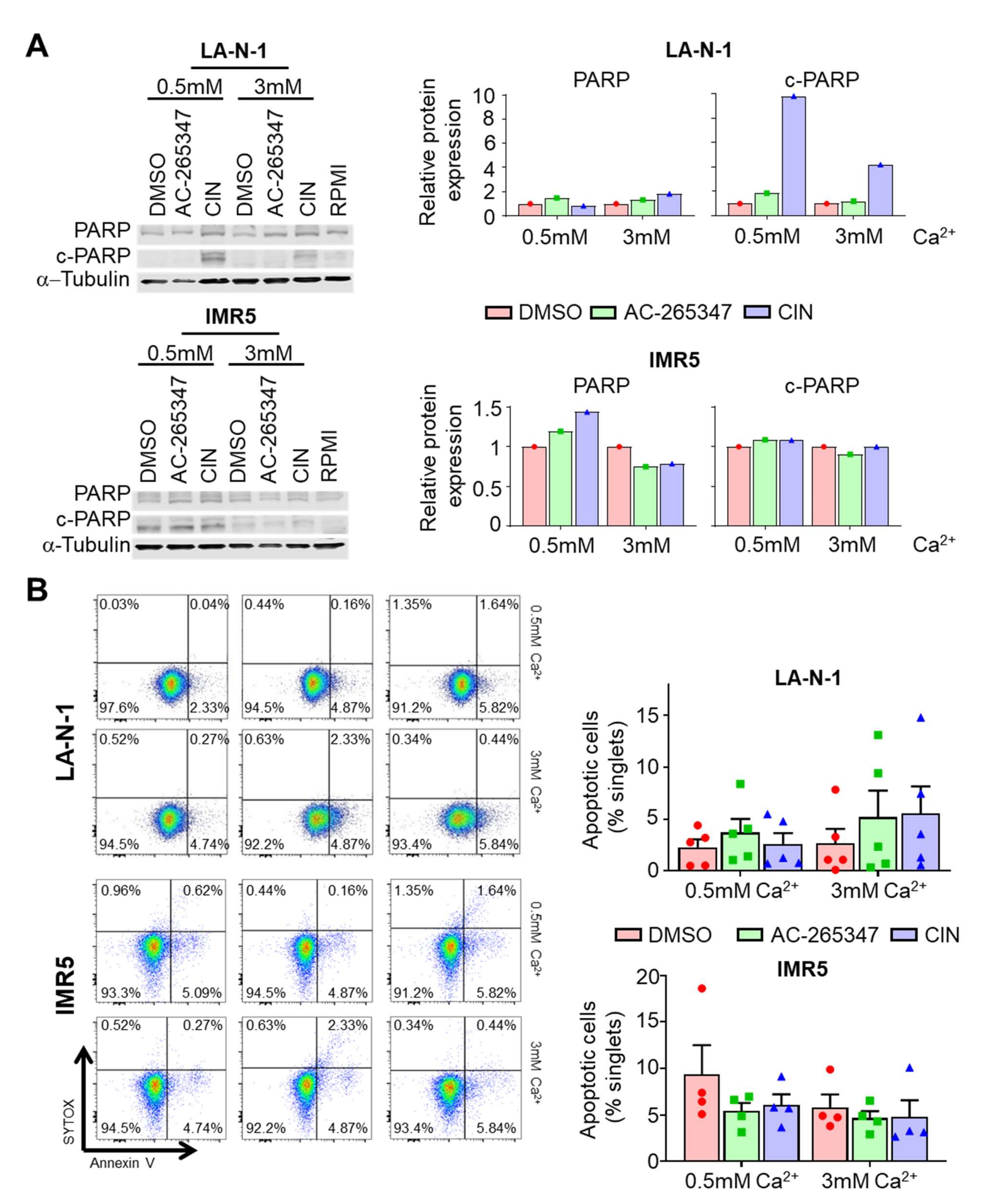
2.5. Acute Exposure to Calcimimetics Differently Affects NB Cells
2.6. Genome-Wide Analyses of CIN and AC-265347-Treated Neuroblastoma Xenografts Reveal Biased Signaling
3. Discussion
4. Material and Methods
4.1. Cell Lines
4.2. Reagents
4.3. Cell Viability Assays
4.4. Apoptosis Assays
4.5. RNA Extraction and Quantitative RT-PCR
4.6. Immunoblots
4.7. Histology and Immunohistochemistry (IHC)
4.8. Mouse Xenograft Models and Calcemia Study
4.9. Microarray Processing and Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brodeur, G.M.; Hogarty, M.; Bagatell, R.; Mosse, Y.; Maris, J. Neuroblastoma. In Principles and Practice of Pediatric Oncology, 7th ed.; Pizzo, P., Poplack, D., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2016; pp. 772–798. ISBN 978-1-4511-9423-4. [Google Scholar]
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.; Varmus, H.E.; Bishop, J.M. Amplification of N-myc in Untreated Human Neuroblastomas Correlates with Advanced Disease Stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Ambros, I.M.; Zellner, A.; Roald, B.; Amann, G.; Ladenstein, R.; Printz, D.; Gadner, H.; Ambros, P.F. Role of Ploidy, Chromosome 1p, and Schwann Cells in the Maturation of Neuroblastoma. N. Engl. J. Med. 1996, 334, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef]
- De Torres, C.; Beleta, H.; Díaz, R.; Toran, N.; Rodríguez, E.; Lavarino, C.; García, I.; Acosta, S.; Suñol, M.; Mora, J. The Calcium sensing Receptor and Parathyroid Hormone Related Protein Are Expressed in Differentiated, Favourable Neuroblastic Tumors. Cancer 2009, 115, 2792–2803. [Google Scholar] [CrossRef] [PubMed]
- Casalà, C.; Gil-Guiñón, E.; Ordóñez, J.L.; Miguel-Queralt, S.; Rodríguez, E.; Galván, P.; Lavarino, C.; Munell, F.; de Alava, E.; Mora, J.; et al. The Calcium-Sensing Receptor Is Silenced by Genetic and Epigenetic Mechanisms in Unfavorable Neuroblastomas and Its Reactivation Induces ERK1/2-Dependent Apoptosis. Carcinogenesis 2013, 34, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Brennan, S.; Thiem, U.; Roth, S.; Aggarwal, A.; Fetahu, I.; Tennakoon, S.; Gomes, A.R.; Brandi, M.L.; Bruggeman, F.; Mentaverri, R.; et al. Calcium sensing receptor signaling in physiology and cancer. Biochim. Biophys. Acta 2013, 1833, 1732–1744. [Google Scholar] [CrossRef] [Green Version]
- Leach, K.; Gregory, K.J.; Kufareva, I.; Khajehali, E.; Cook, A.E.; Abagyan, R.; Conigrave, A.D.; Sexton, P.; Christopoulos, A. Towards a Structural Understanding of Allosteric Drugs at the Human Calcium-Sensing Receptor. Cell Res. 2016, 26, 574–592. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Hernández, C.J.; Mateo-Lozano, S.; García, M.; Casalà, C.; Briansó, F.; Castrejón, N.; Rodríguez, E.; Suñol, M.; Carcaboso, A.M.; Lavarino, C.; et al. Cinacalcet Inhibits Neuroblastoma Tumor Growth and Upregulates Cancer-Testis Antigens. Oncotarget 2016, 7, 16112–16129. [Google Scholar] [CrossRef]
- Nemeth, E.F.; Steffey, M.E.; Hammerland, L.G.; Hung, B.C.P.; Van Wagenen, B.C.; DelMar, E.G.; Balandrin, M.F. Calcimimetics with Potent and Selective Activity on the Parathyroid Calcium Receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 4040–4045. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E.F.; Heaton, W.H.; Miller, M.; Fox, J.; Balandrin, M.F.; Van Wagenen, B.C.; Colloton, M.; Karbon, W.; Scherrer, J.; Shatzen, E.; et al. Pharmacodynamics of the Type II Calcimimetic Compound Cinacalcet HCl. J. Pharmacol. Exp. Ther. 2004, 308, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Cook, A.; Mistry, S.; Gregory, K.; Furness, S.; Sexton, P.; Scammells, P.; Conigrave, A.; Christopoulos, A.; Leach, K. Biased Allosteric Modulation at the CaS Receptor Engendered by Structurally Diverse Calcimimetics. Br. J. Pharmacol. 2015, 172, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.D.; De La Fuente, V.; Delgado, P.; Gil, M.T.; Gutiérrez, P.; Ribero, J.; Rodríguez, M.; Almadén, Y. Pharmacodynamics of Cinacalcet over 48 Hours in Patients with Controlled Secondary Hyperparathyroidism: Useful Data in Clinical Practice. J. Clin. Endocrinol. Metab. 2013, 98, 1718–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.-N.; Owens, M.; Gustafsson, M.; Jensen, J.; Tabatabaei, A.; Schmelzer, K.; Olsson, R.; Burstein, E.S. Characterization of Highly Efficacious Allosteric Agonists of the Human Calcium-Sensing Receptor. J. Pharmacol. Exp. Ther. 2011, 337, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, F.J.; Lopez, I.; Canalejo, R.; Almaden, Y.; Martin, D.; Aguilera-Tejero, E.; Rodriguez, M. Direct Upregulation of Parathyroid Calcium-Sensing Receptor and Vitamin D Receptor by Calcimimetics in Uremic Rats. Am. J. Physiol.-Ren. Physiol. 2009, 296, F605–F613. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E.F.; Goodman, W.G. Calcimimetic and Calcilytic Drugs: Feats, Flops, and Futures. Calcif. Tissue Int. 2016, 98, 341–358. [Google Scholar] [CrossRef]
- Schramm, A.; Schulte, J.H.; Astrahantseff, K.; Apostolov, O.; van Limpt, V.; Sieverts, H.; Kuhfittig-Kulle, S.; Pfeiffer, P.; Versteeg, R.; Eggert, A. Biological Effects of TrkA and TrkB Receptor Signaling in Neuroblastoma. Cancer Lett. 2005, 228, 143–153. [Google Scholar] [CrossRef]
- Eggert, A.; Ikegaki, N.; Liu, X.G.; Chou, T.T.; Lee, V.M.; Trojanowski, J.Q.; Brodeur, G.M. Molecular Dissection of TrkA Signal Transduction Pathways Mediating Differentiation in Human Neuroblastoma Cells. Oncogene 2000, 19, 2043–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, S.; Lavarino, C.; Paris, R.; Garcia, I.; de Torres, C.; Rodríguez, E.; Beleta, H.; Mora, J. Comprehensive Characterization of Neuroblastoma Cell Line Subtypes Reveals Bilineage Potential Similar to Neural Crest Stem Cells. BMC Dev. Biol. 2009, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Capasso, M.; Diskin, S.; Cimmino, F.; Acierno, G.; Totaro, F.; Petrosino, G.; Pezone, L.; Diamond, M.; McDaniel, L.; Hakonarson, H.; et al. Common Genetic Variants in NEFL Influence Gene Expression and Neuroblastoma Risk. Cancer Res. 2014, 74, 6913–6924. [Google Scholar] [CrossRef] [Green Version]
- Vega, F.M.; Colmenero-Repiso, A.; Gómez-Muñoz, M.A.; Rodríguez-Prieto, I.; Aguilar-Morante, D.; Ramírez, G.; Márquez, C.; Cabello, R.; Pardal, R. CD44-High Neural Crest Stem-like Cells Are Associated with Tumour Aggressiveness and Poor Survival in Neuroblastoma Tumours. EBioMedicine 2019, 49, 82–95. [Google Scholar] [CrossRef]
- Sorci, G.; Riuzzi, F.; Arcuri, C.; Tubaro, C.; Bianchi, R.; Giambanco, I.; Donato, R. S100B Protein in Tissue Development, Repair and Regeneration. World J. Biol. Chem. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, M.; Tsuneyoshi, M.; Enjoji, M. S-100 Positive Undifferentiated Neuroblastomas with a Special Reference to the Tumor Stroma Related to Favorable Prognosis. Pathol.-Res. Pract. 1992, 88, 273–283. [Google Scholar] [CrossRef]
- Koyama, H.; Zhuang, T.; Light, J.E.; Kolla, V.; Higashi, M.; McGrady, P.W.; London, W.B.; Brodeur, G.M. Mechanisms of CHD5 Inactivation in Neuroblastomas. Clin. Cancer Res. 2012, 18, 1588–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, I.; Mayol, G.; Rodríguez, E.; Suñol, M.; Gershon, T.R.; Ríos, J.; Cheung, N.-K.V.; Kieran, M.W.; George, R.E.; Perez-Atayde, A.R.; et al. Expression of the Neuron-Specific Protein CHD5 Is an Independent Marker of Outcome in Neuroblastoma. Mol. Cancer 2010, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Kholodenko, I.V.; Kalinovsky, D.V.; Doronin, I.I.; Deyev, S.M.; Kholodenko, R.V. Neuroblastoma Origin and Therapeutic Targets for Immunotherapy. J. Immunol. Res. 2018, 2018, 7394268. [Google Scholar] [CrossRef]
- Matsumoto, M.; Akiyama, T.; Miyatake, S.; Oda, Y.; Kikuchi, H.; Hanaoka, M.; Namba, Y. Expression of Proto-Oncogene Products during Drug-Induced Differentiation of a Neuroblastoma Cell Line SK-N-DZ. Acta Neuropathol. 1989, 79, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.D.; Patti, R.; Guttapalli, A.; Maris, J.M.; Yanamandra, N.; Rachamallu, A.; Sutton, L.N.; Phillips, P.C.; Posner, G.H. Anticancer Effects of the Novel 1α, 25-Dihydroxyvitamin D3 Hybrid Analog QW1624F2-2 in Human Neuroblastoma. J. Cell. Biochem. 2006, 97, 198–206. [Google Scholar] [CrossRef]
- Thiele, C.J.; Reynolds, C.P.; Israel, M.A. Decreased Expression of N-Myc Precedes Retinoic Acid-Induced Morphological Differentiation of Human Neuroblastoma. Nature 1985, 313, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Robert Parker, J.M.; Opas, M. Ca2+ Signaling and Calcium Binding Chaperones of the Endoplasmic Reticulum. Cell Calcium 2002, 32, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Mekahli, D.; Bultynck, G.; Parys, J.; De Smedt, H.; Missiaen, L. Endoplasmic-Reticulum Calcium Depletion and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a004317. [Google Scholar] [CrossRef]
- Santulli, G.; Pagano, G.; Sardu, C.; Xie, W.; Reiken, S.; D’Ascia, S.L.; Cannone, M.; Marziliano, N.; Trimarco, B.; Guise, T.A.; et al. Calcium Release Channel RyR2 Regulates Insulin Release and Glucose Homeostasis. J. Clin. Investig. 2015, 125, 1968–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratta, E.; Coral, S.; Covre, A.; Parisi, G.; Colizzi, F.; Danielli, R.; Nicolay, H.J.M.; Sigalotti, L.; Maio, M. The Biology of Cancer Testis Antigens: Putative Function, Regulation and Therapeutic Potential. Mol. Oncol. 2011, 5, 164–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnadas, D.K.; Shusterman, S.; Bai, F.; Diller, L.; Sullivan, J.E.; Cheerva, A.C.; George, R.E.; Lucas, K.G. A Phase I Trial Combining Decitabine/Dendritic Cell Vaccine Targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for Children with Relapsed or Therapy-Refractory Neuroblastoma and Sarcoma. Cancer Immunol. Immunother. 2015, 64, 1251–1260. [Google Scholar] [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iamartino, L.; Elajnaf, T.; Kallay, E.; Schepelmann, M. Calcium-Sensing Receptor in Colorectal Inflammation and Cancer: Current Insights and Future Perspectives. World J. Gastroenterol. 2018, 24, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Higashi, M.; Tajiri, T.; Kinoshita, Y.; Tatsuta, K.; Souzaki, R.; Maehara, Y.; Suita, S.; Taguchi, T. High Expressions of Neuronatin Isoforms in Favorable Neuroblastoma. J. Pediatr. Hematol./Oncol. 2007, 29, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Urgard, E.; Reigo, A.; Reinmaa, E.; Rebane, A.; Metspalu, A. Human Basonuclin 2 Up-Regulates a Cascade Set of Interferon-Stimulated Genes with Anti-Cancerous Properties in a Lung Cancer Model. Cancer Cell Int. 2017, 17, 18. [Google Scholar] [CrossRef] [Green Version]
- Roskoski, R., Jr. ERK1/2 MAP Kinases: Structure, Function, and Regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef]
- Perron, J.C.; Bixby, J. Distinct Neurite Outgrowth Signaling Pathways Converge on ERK Activation. Mol. Cell. Neurosci. 1999, 13, 362–378. [Google Scholar] [CrossRef]
- Singh, U.S.; Pan, J.; Kao, Y.-L.; Joshi, S.; Young, K.L.; Baker, K.M. Tissue Transglutaminase Mediates Activation of RhoA and MAP Kinase Pathways during Retinoic Acid-Induced Neuronal Differentiation of SH-SY5Y Cells. J. Biol. Chem. 2003, 278, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, Z.G.; Dhanasekaran, D.N. G Protein Regulation of MAPK Networks. Oncogene 2007, 26, 3122–3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monterrubio, C.; Paco, S.; Vila-Ubach, M.; Rodríguez, E.; Glisoni, R.; Lavarino, C.; Schaiquevich, P.; Sosnik, A.; Mora, J.; Carcaboso, A.M. Combined Microdialysis-Tumor Homogenate Method for the Study of the Steady State Compartmental Distribution of a Hydrophobic Anticancer Drug in Patient-Derived Xenografts. Pharm. Res. 2015, 32, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’Ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| LA-N-1 | HSJD-NB001 | ||||
|---|---|---|---|---|---|
| AC-265347 | CIN | AC-265347 | CIN | ||
| Differentiation | CASR | 2.51 | 2.21 | 17.33 | 15.46 |
| NEFL | 1.47 | 2.31 | 1.23 | 1.98 | |
| NTRK1 | 1.76 | 3.67 | 0.69 | 0.9 | |
| NTRK2 | 1.25 | 1.81 | 1.12 | 0.95 | |
| S100B | 1.31 | 1.51 | 1.23 | 1.98 | |
| TUBB3 | 1.41 | 5.24 | 0.94 | 0.82 | |
| CHD5 | 1.1 | 11.91 | 1.5 | 1.07 | |
| MYCN | 1 | 0.93 | 0.68 | 0.9 | |
| Ca2+ signaling | TGFB1 | 1.56 | 1.44 | 0.44 | 0.92 |
| RYR | 1.15 | 2.26 | 1.16 | 1.75 | |
| ER-stress | CHOP | 0.78 | 1.58 | 1.18 | 1.14 |
| BID | 0.91 | 2.15 | 1.31 | 0.87 | |
| CTAs | CTCFL | 1.9 | 2.47 | 1.22 | 1 |
| GAGE12J | 3.56 | 9.9 | 0.32 | 0.13 | |
| MAGEA2 | 1.01 | 0.98 | 0.92 | 0.45 | |
| MAGEA3 | 1.16 | 2.86 | 3.5 | 0.49 | |
| MAGEA6 | 1.4 | 2.48 | 2.36 | 0.43 | |
| NY-ESO-1 | 1.68 | 5.24 | 1.32 | 0.43 | |
| SSX4/4B | 3.05 | 10.64 | 2.76 | 0.69 | |
 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves-Alves, E.; Garcia, M.; Rodríguez-Hernández, C.J.; Gómez-González, S.; Ecker, R.C.; Suñol, M.; Muñoz-Aznar, O.; Carcaboso, A.M.; Mora, J.; Lavarino, C.; et al. AC-265347 Inhibits Neuroblastoma Tumor Growth by Induction of Differentiation without Causing Hypocalcemia. Int. J. Mol. Sci. 2022, 23, 4323. https://doi.org/10.3390/ijms23084323
Gonçalves-Alves E, Garcia M, Rodríguez-Hernández CJ, Gómez-González S, Ecker RC, Suñol M, Muñoz-Aznar O, Carcaboso AM, Mora J, Lavarino C, et al. AC-265347 Inhibits Neuroblastoma Tumor Growth by Induction of Differentiation without Causing Hypocalcemia. International Journal of Molecular Sciences. 2022; 23(8):4323. https://doi.org/10.3390/ijms23084323
Chicago/Turabian StyleGonçalves-Alves, Eliana, Marta Garcia, Carlos J. Rodríguez-Hernández, Soledad Gómez-González, Rupert C. Ecker, Mariona Suñol, Oscar Muñoz-Aznar, Angel M. Carcaboso, Jaume Mora, Cinzia Lavarino, and et al. 2022. "AC-265347 Inhibits Neuroblastoma Tumor Growth by Induction of Differentiation without Causing Hypocalcemia" International Journal of Molecular Sciences 23, no. 8: 4323. https://doi.org/10.3390/ijms23084323
APA StyleGonçalves-Alves, E., Garcia, M., Rodríguez-Hernández, C. J., Gómez-González, S., Ecker, R. C., Suñol, M., Muñoz-Aznar, O., Carcaboso, A. M., Mora, J., Lavarino, C., & Mateo-Lozano, S. (2022). AC-265347 Inhibits Neuroblastoma Tumor Growth by Induction of Differentiation without Causing Hypocalcemia. International Journal of Molecular Sciences, 23(8), 4323. https://doi.org/10.3390/ijms23084323