Synthesis, Characterization and Nanoformulation of Novel Sulfonamide-1,2,3-triazole Molecular Conjugates as Potent Antiparasitic Agents
Abstract
1. Introduction
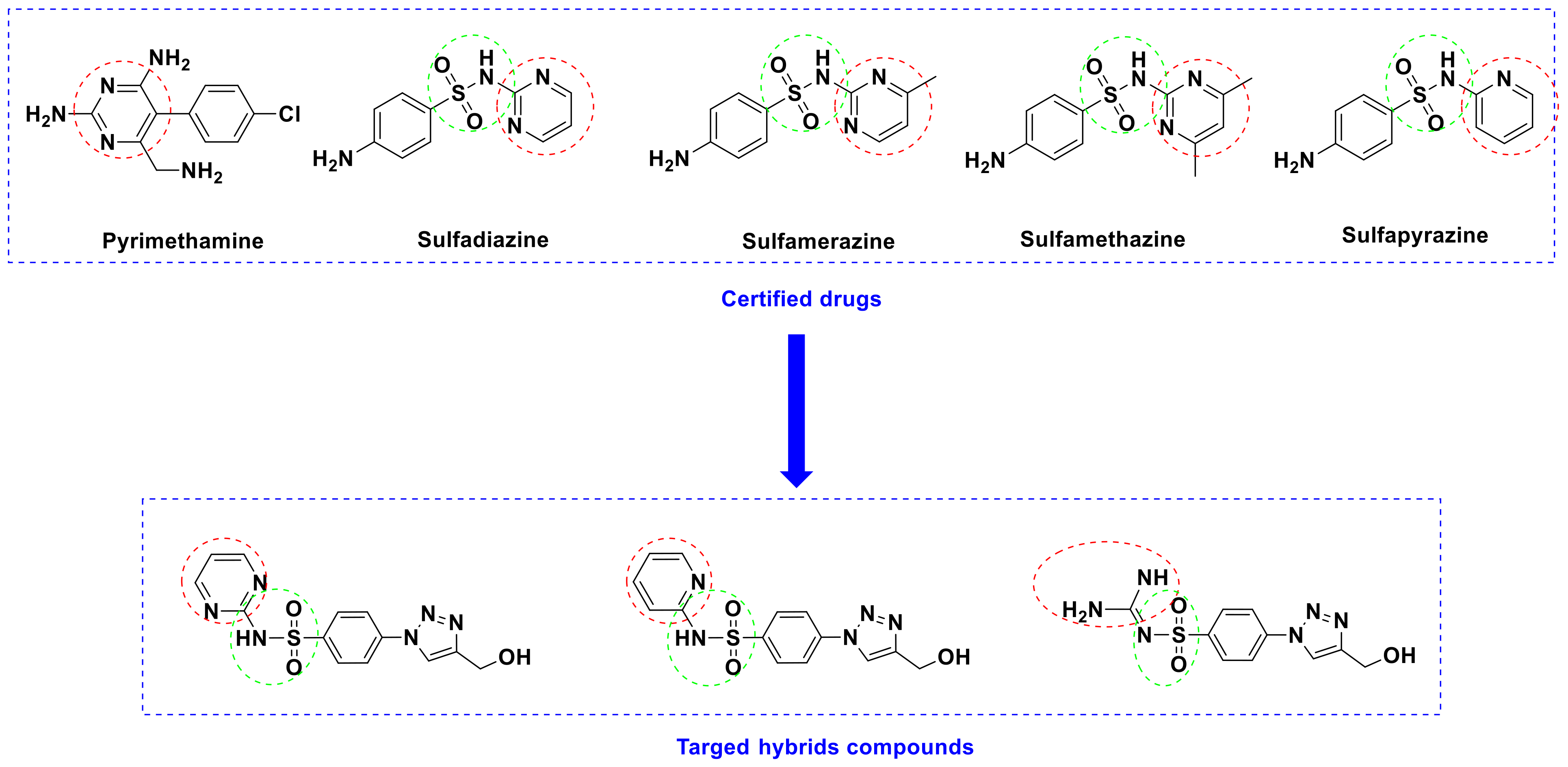
Rationale Study
2. Results and Discussion
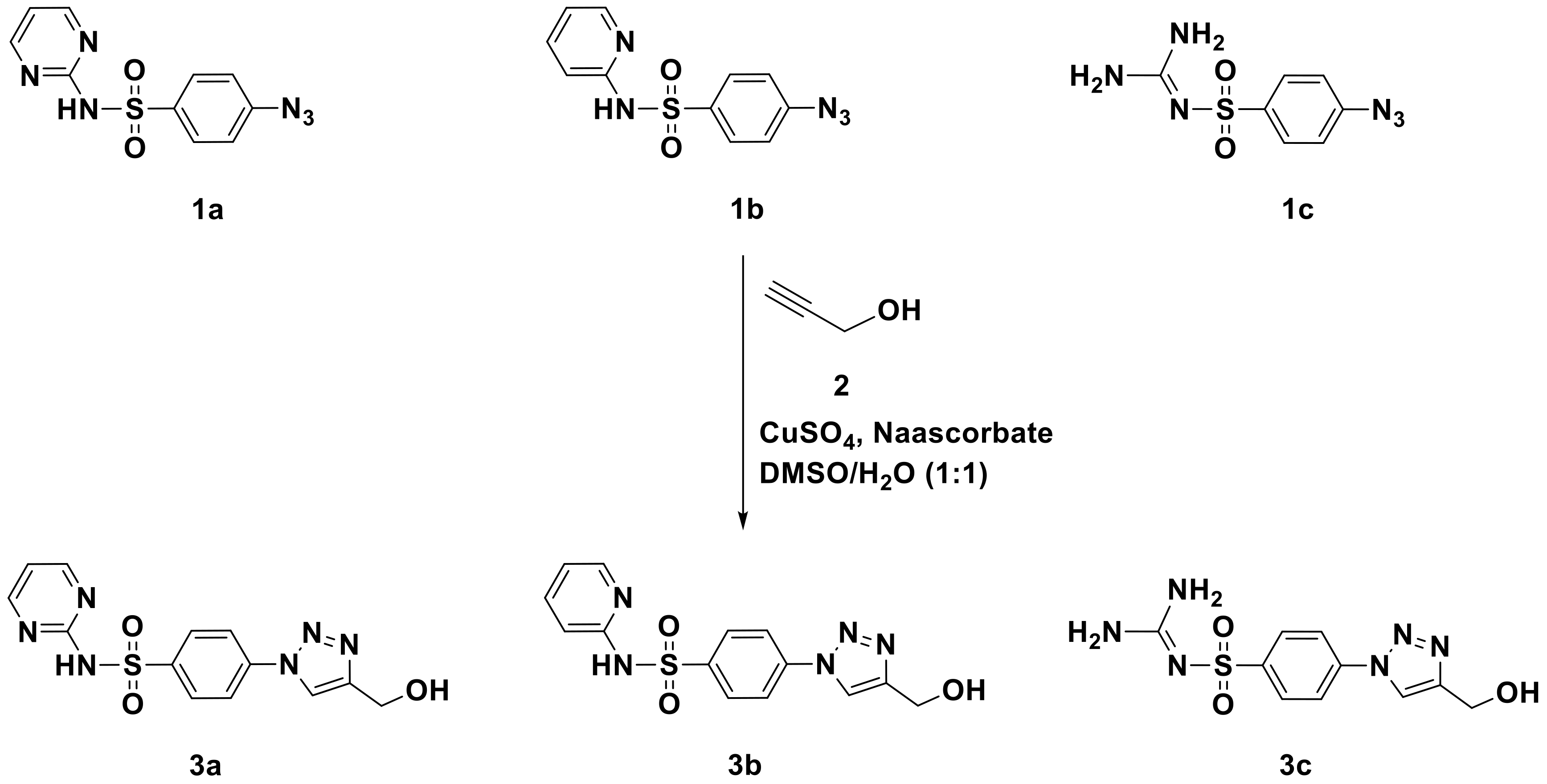
2.1. Chemistry
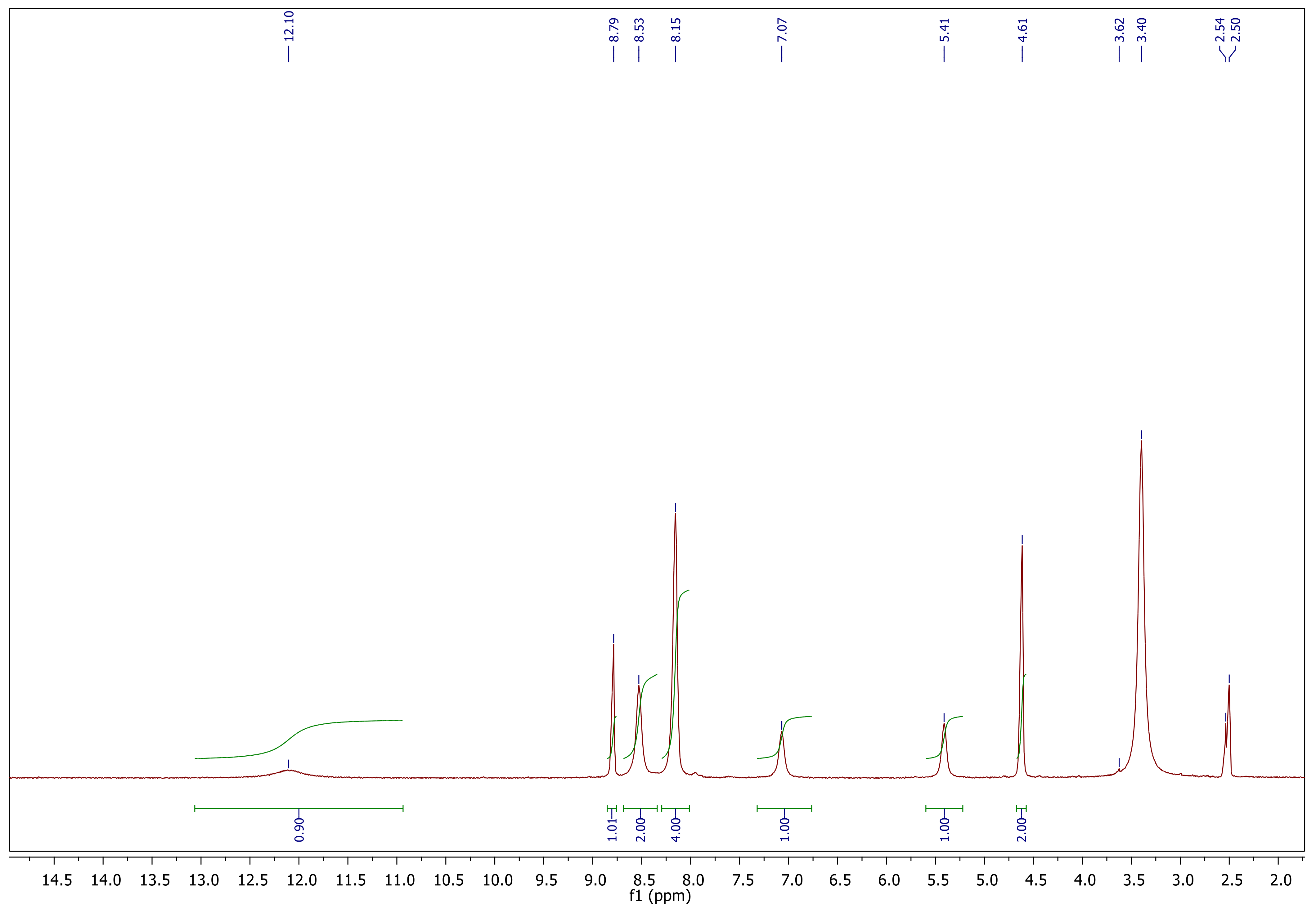
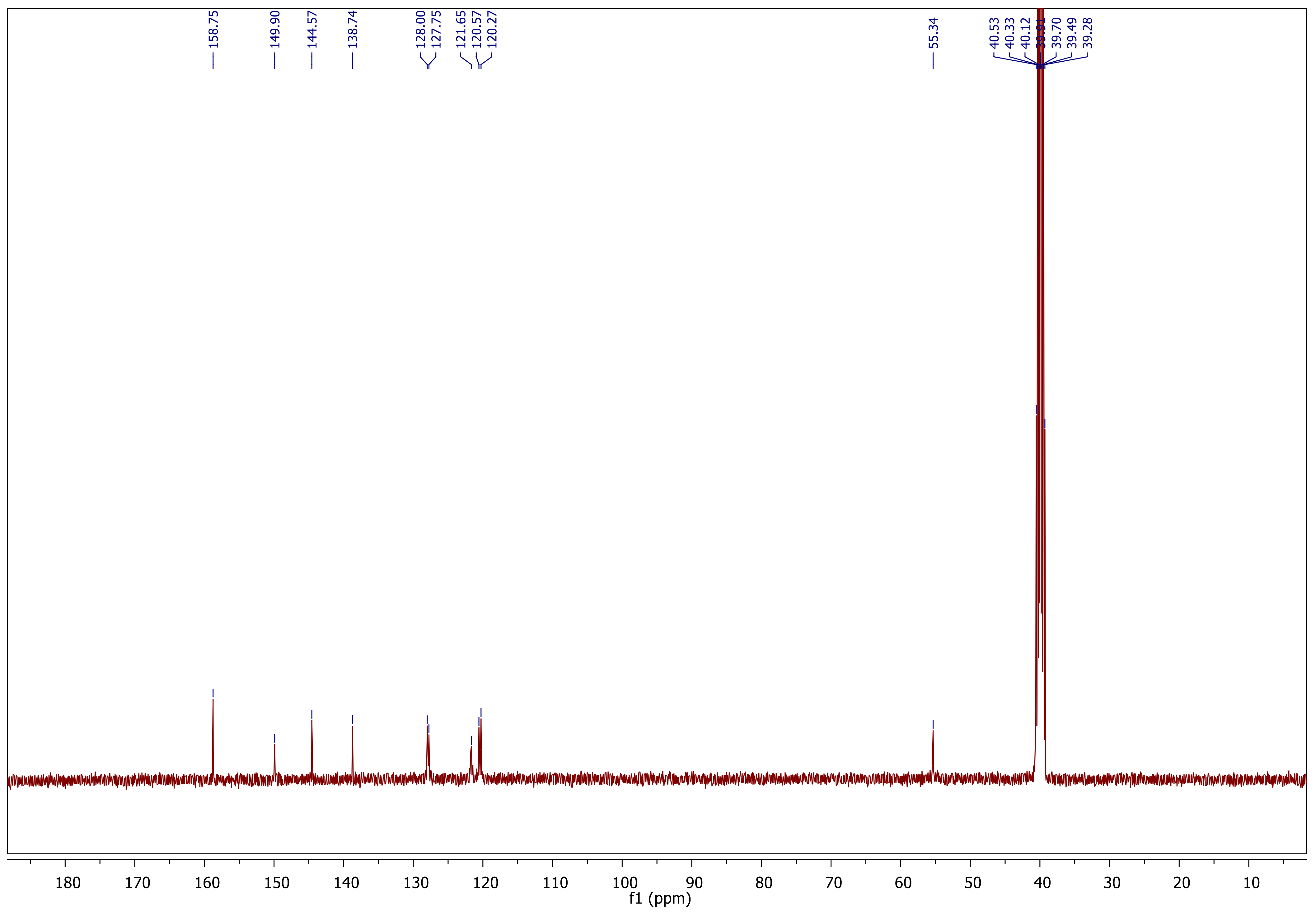
Synthesis and Characterization
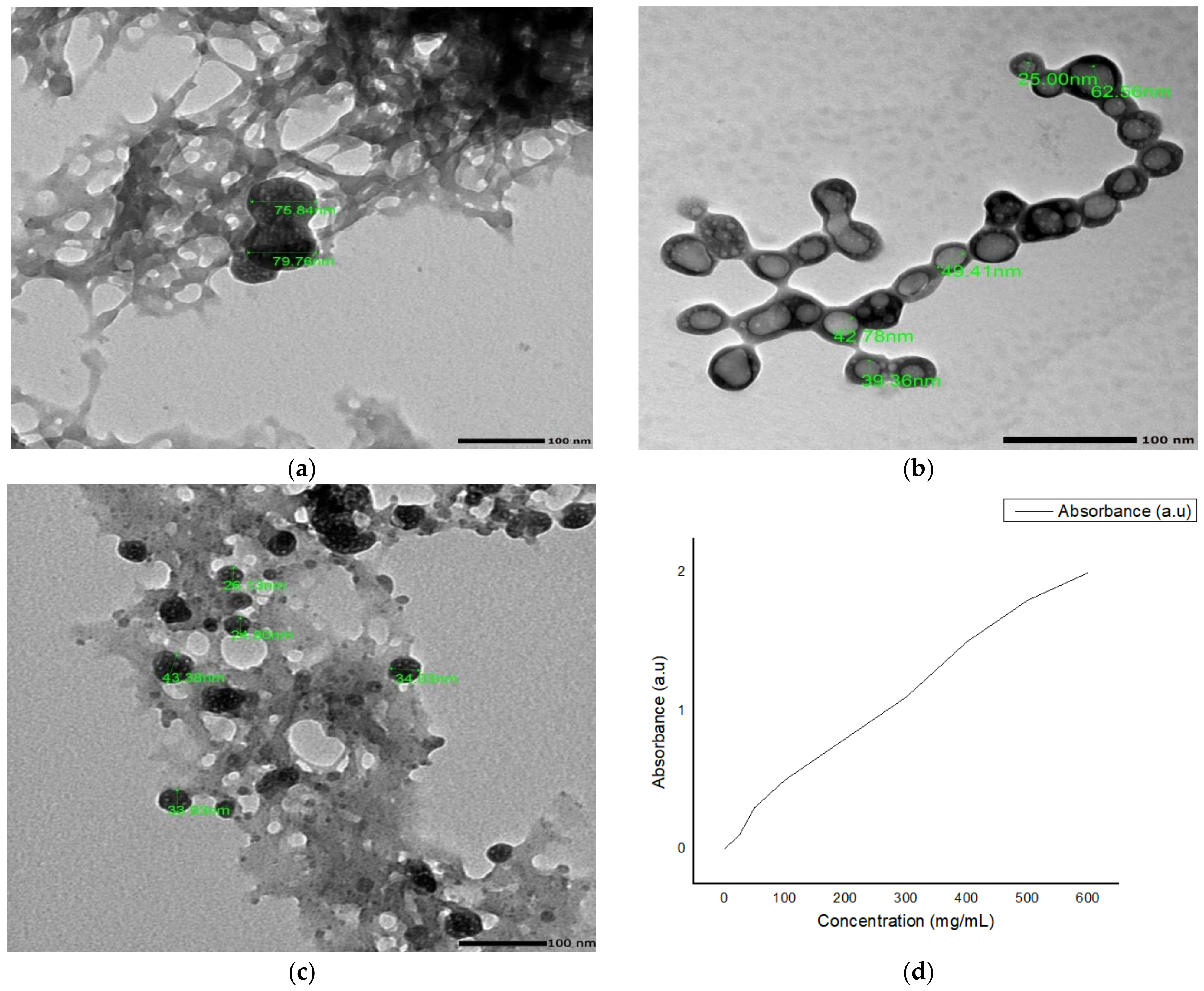
2.2. Formulation
Nanoparticles Characterization
2.3. Biological Assessment of the Novel Sulfonamide Derivatives
2.3.1. Clinical Behavior of Infected Mice
2.3.2. Parasitological Study
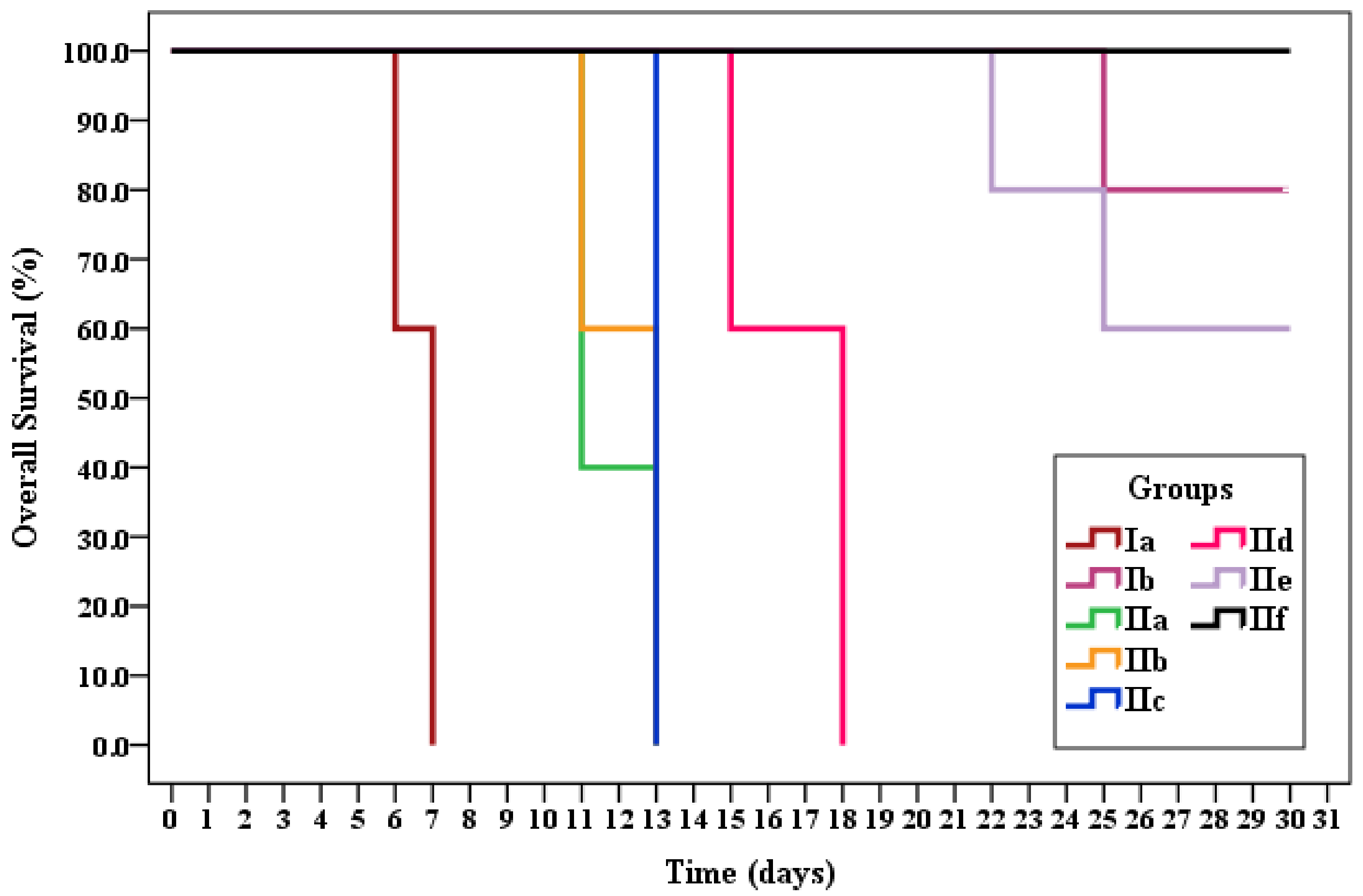
Survival Time
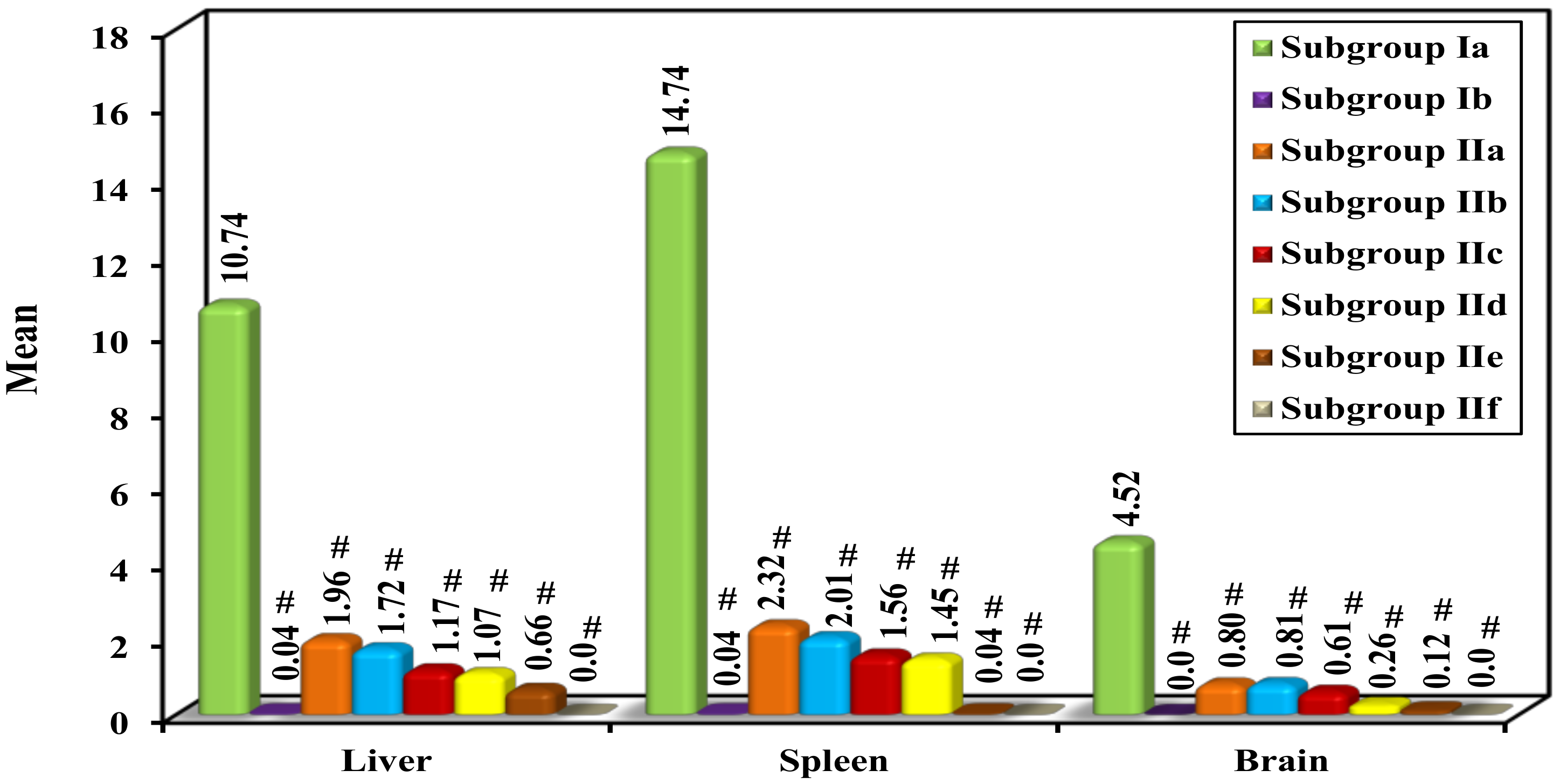
Parasite Load and Percent Reduction (%R)
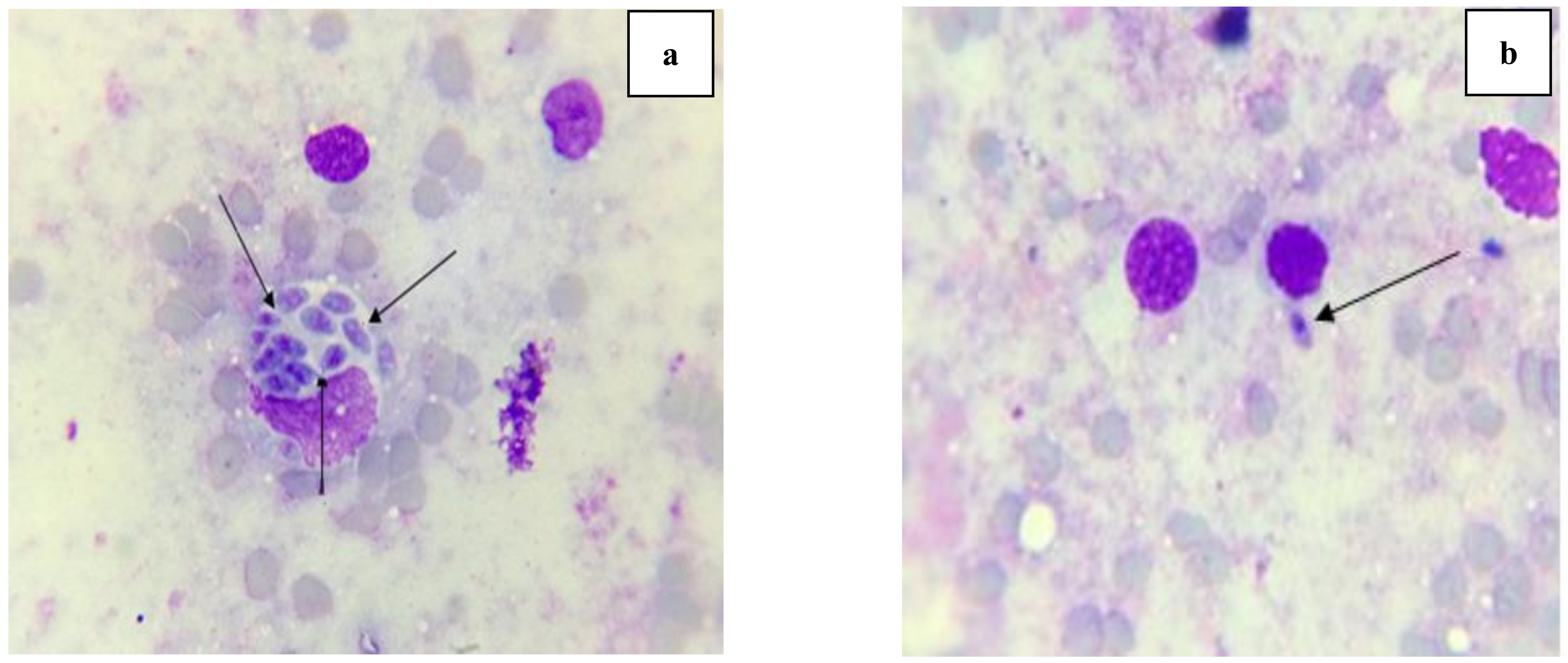
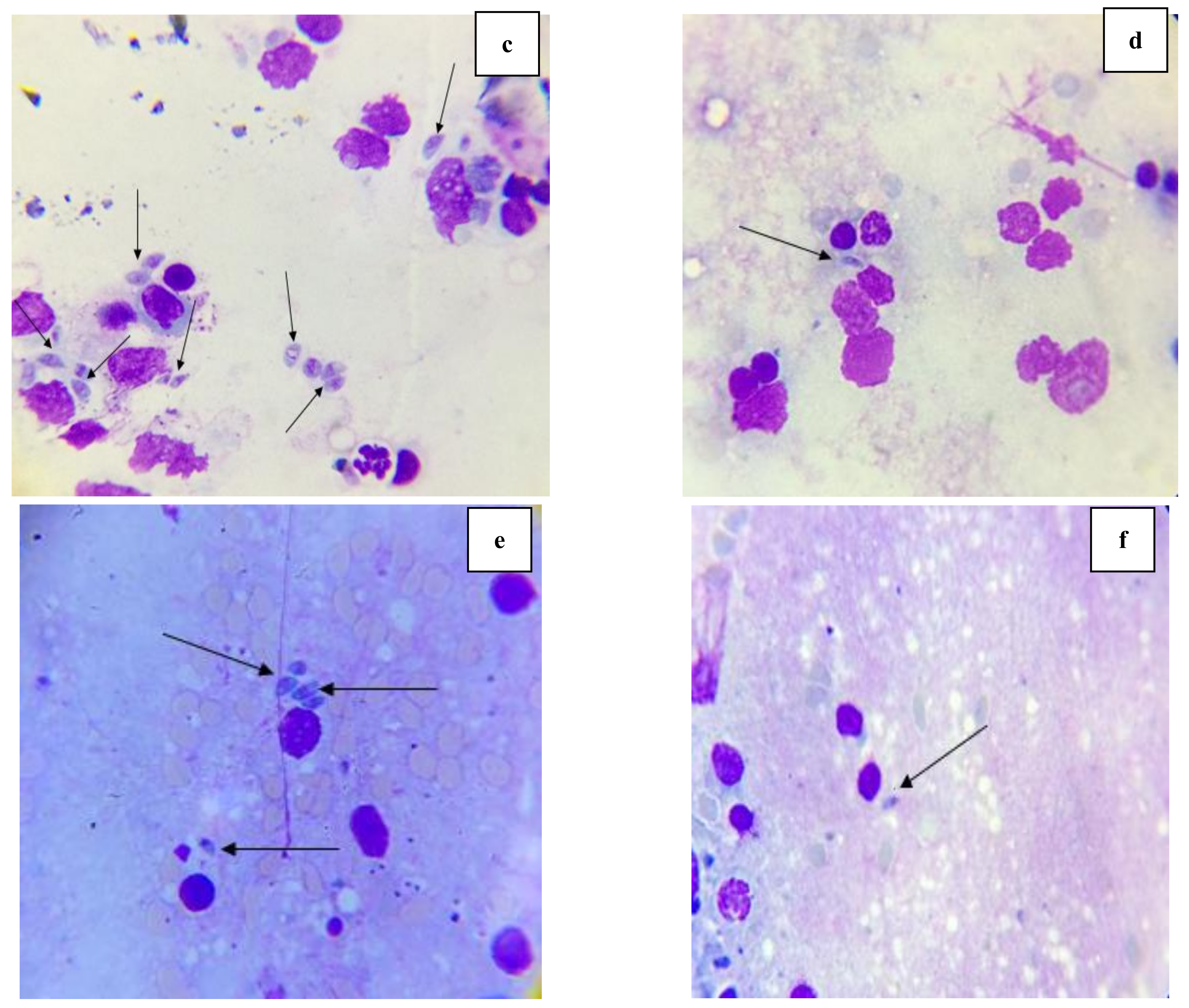
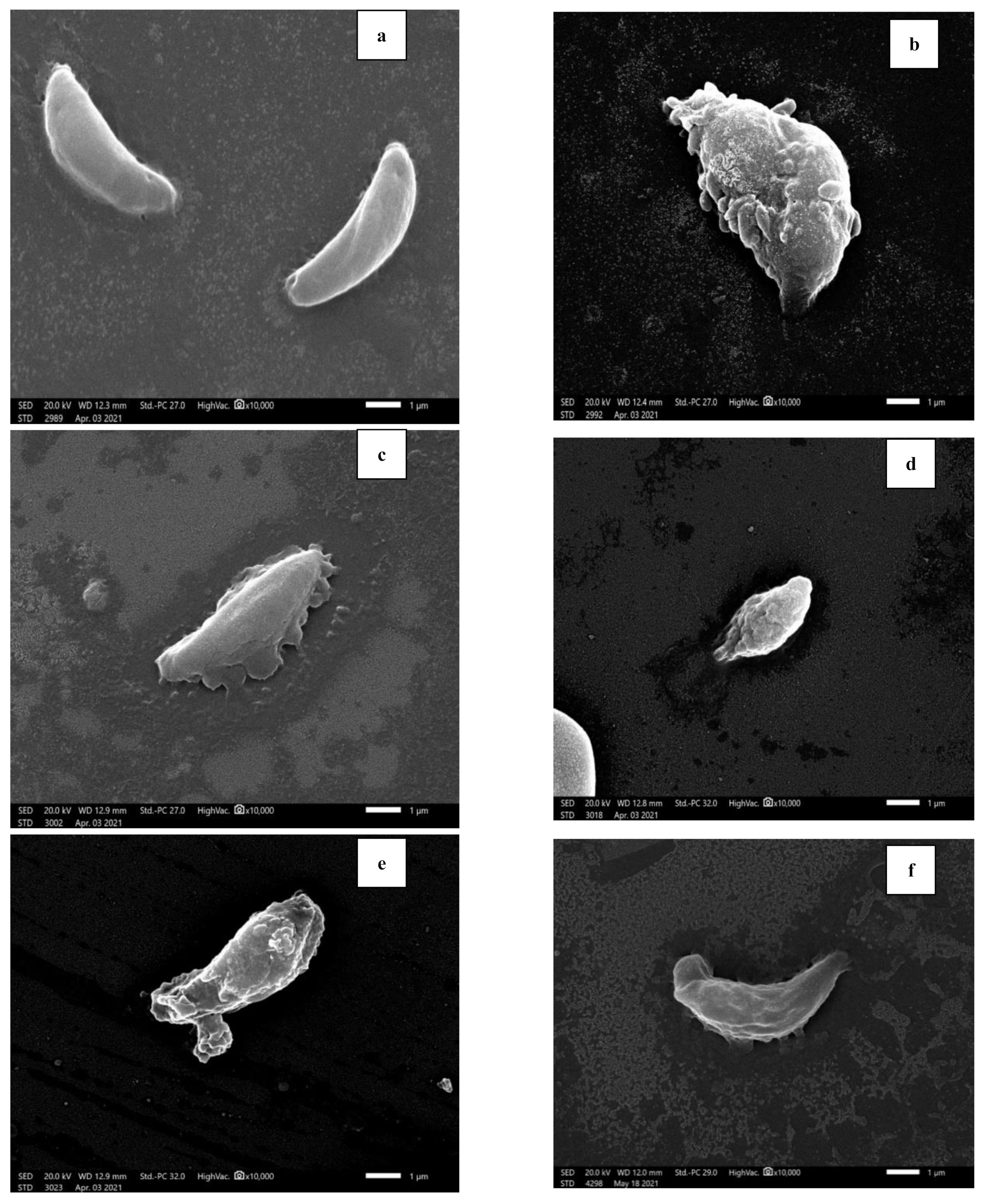
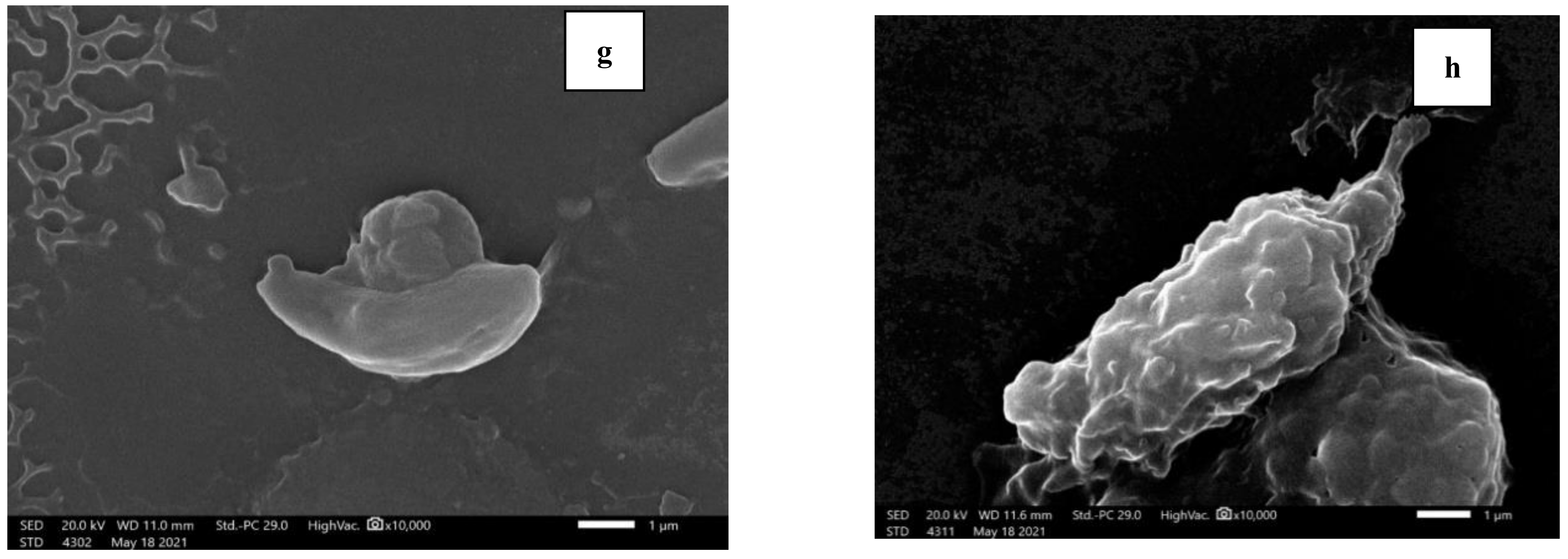
2.3.3. Morphological Study of Tachyzoites by Light Microscope and Scanning Electron Microscope (SEM)
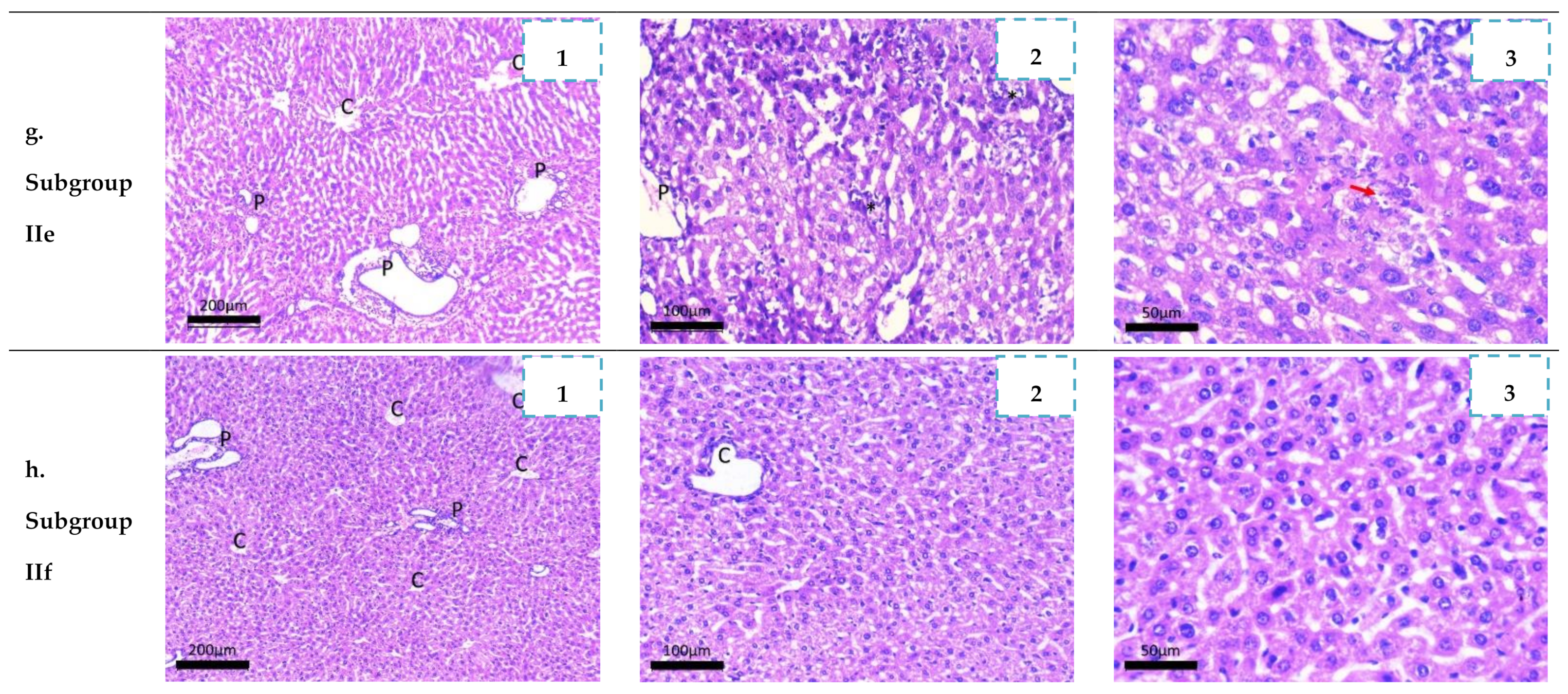
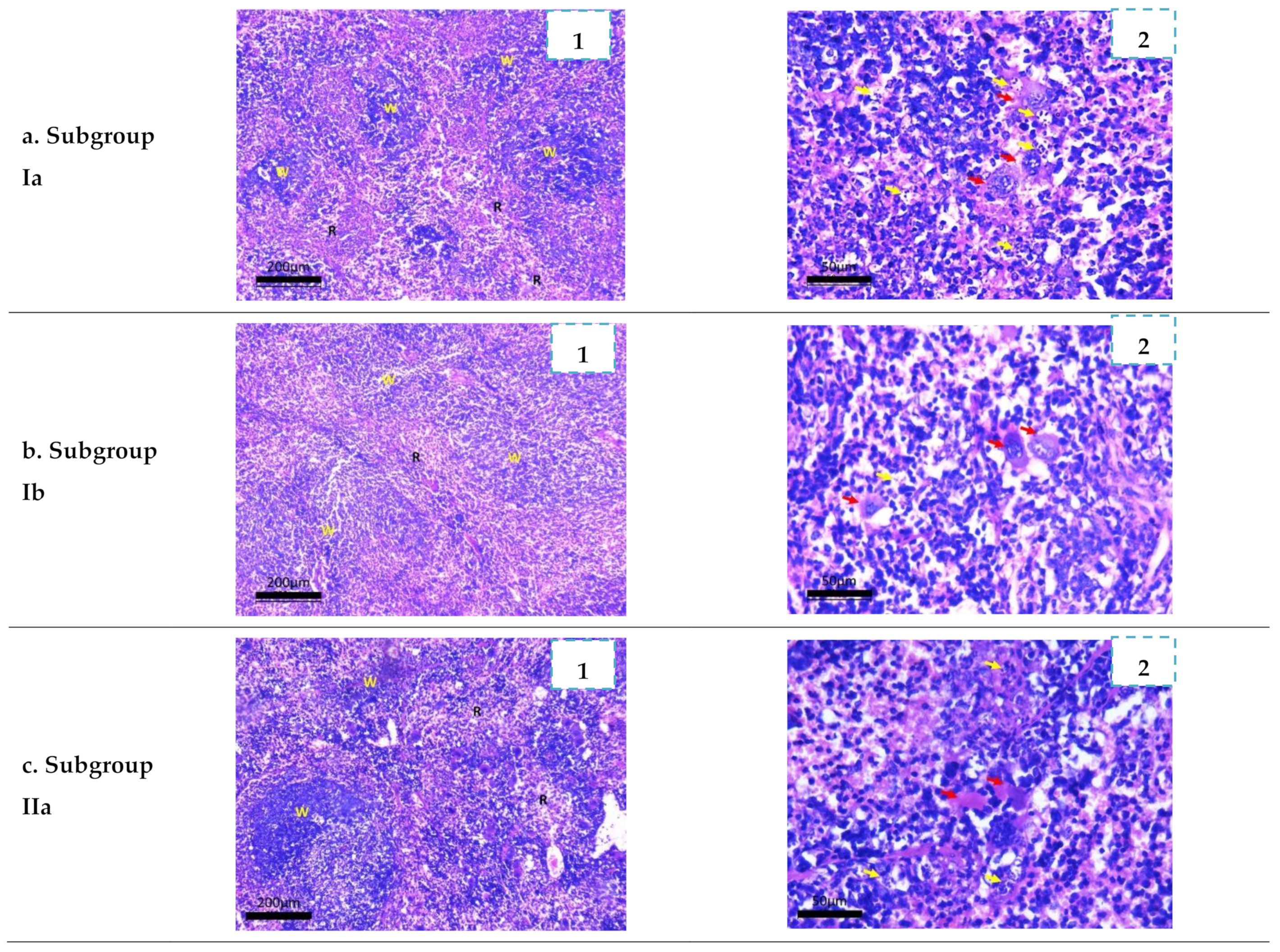
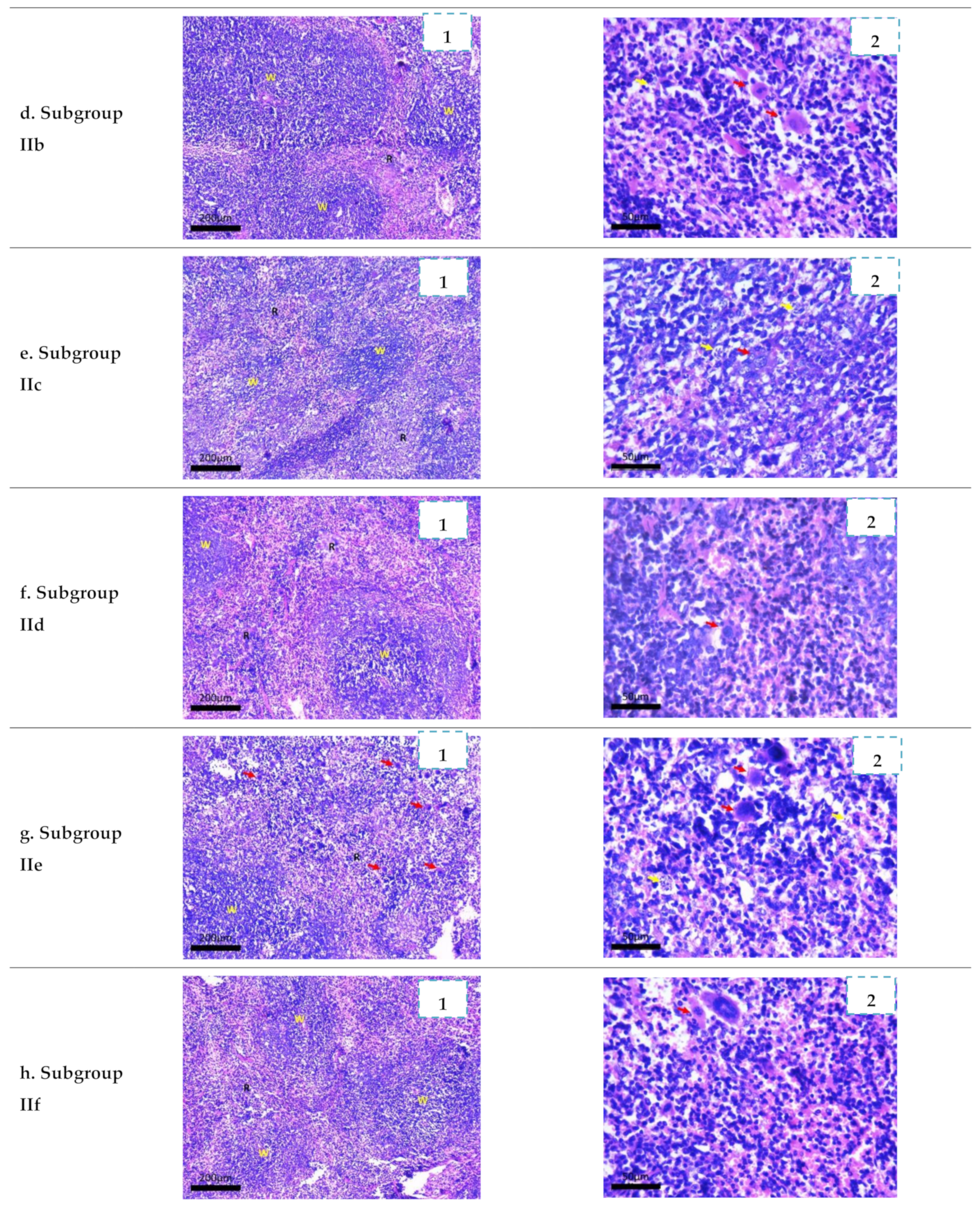
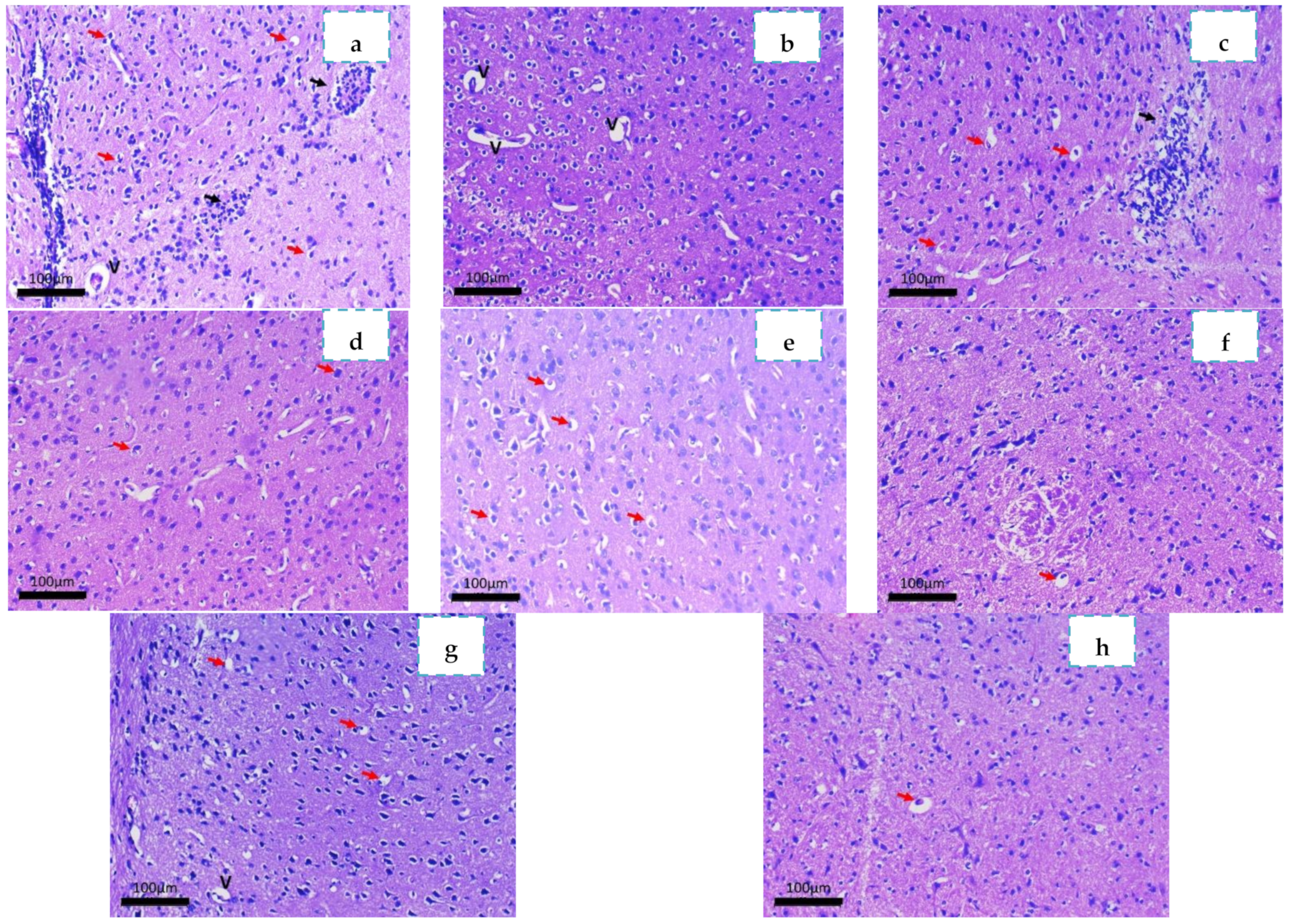
2.3.4. Histopathological Study
3. Materials and Methods
3.1. Chemistry
Synthesis and Characterization of Sulfonamide-Based 1,2,3-Triazoles 3a–c
3.2. Bioassay
3.2.1. Nanoparticles Preparation
3.2.2. Characterization of the Nanoformulations
3.2.3. Determination of the Loading and Entrapment Efficiencies
3.3. Experminal Design and Animal Grouping
3.3.1. Parasite
3.3.2. Drugs
3.3.3. Grouping of Animals and Experimental Design
- Group I: Control group (20 mice):
- Group II: Experimental group (60 mice):
- Five mice of each subgroup (either control or experimental) were observed daily for early detection of abnormal feeding behavior. Survival time was recorded for thirty days post-infection.
- The other five mice were anesthetized and sacrificed by cervical dislocation on the 8th day post-infection (24 h after the last dose of treatment). Liver, spleen and brain were obtained from each mouse for further parasitological assessment. Peritoneal exudate from each mouse was fixed in glutaraldehyde for further morphological studies using scanning electron microscope. Specimens from liver, spleen and brain tissues were fixed in formalin for histopathological studies.
3.4. Evaluation of Drug Efficacy as Anti-Parasitic Agent
3.4.1. Clinical Study
3.4.2. Parasitological Study
Survival Rate
Parasite Load
Percent Reduction (%R) in Parasite Load
3.4.3. Morphological Study by Light Microscopy and Scanning Electron Microscopy (SEM)
3.4.4. Histopathological Study
3.5. Statistical Analysis of the Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyrosdick, H.M.; Schaefer, J.J. Toxoplasma gondii: History and diagnostic test development. Anim. Health Res. Rev. 2015, 16, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Hagras, N.A.-E.; Allam, A.F.; Farag, H.F.; Osman, M.M.; Shalaby, T.I.; Mogahed, N.M.F.H.; Tolba, M.M.; Shehab, A.Y. Successful treatment of acute experimental toxoplasmosis by spiramycin-loaded chitosan nanoparticles. Exp. Parasitol. 2019, 204, 107717. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-Y.; Remington, J.S. Biology of Toxoplasma gondii. AIDS 1993, 7, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.E.; Remington, J.S.; Jacobs, L. Toxoplasmosis: The nature of virulence. Am. J. Ophthalmol. 1958, 46, 255–261. [Google Scholar] [CrossRef]
- Kaufman, H.E.; Melton, M.L.; Remington, J.S.; Jacobs, L. Strain Differences of Toxoplasma gondii. J. Parasitol. 1959, 45, 189–190. [Google Scholar] [CrossRef]
- Frenkel, J.; Dubey, J.; Miller, N.L. Toxoplasma gondii in cats: Fecal stages identified as coccidian oocysts. Science 1970, 167, 893–896. [Google Scholar] [CrossRef]
- Frenkel, J. Toxoplasma in and around us. Bioscience 1973, 23, 343–352. [Google Scholar] [CrossRef]
- Dubey, J. Toxoplasmosis—A waterborne zoonosis. Vet. Parasitol. 2004, 126, 57–72. [Google Scholar] [CrossRef]
- Blader, I.J.; Saeij, J.P. Communication between Toxoplasma gondii and its host: Impact on parasite growth, development, immune evasion, and virulence. Apmis 2009, 117, 458–476. [Google Scholar] [CrossRef]
- Baatz, H.; Mirshahi, A.; Puchta, J.; Gümbel, H.; Hattenbach, L.-O. Reactivation of toxoplasma retinochoroiditis under atovaquone therapy in an immunocompetent patient. Ocul. Immunol. Inflamm. 2006, 14, 185–187. [Google Scholar] [CrossRef]
- Briones, E.; Colino, C.I.; Lanao, J.M. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J. Control. Release 2008, 125, 210–227. [Google Scholar] [CrossRef]
- Smith, C.L.; Powell, K.R. Review of the sulfonamides and trimethoprim. Pediatr. Rev. 2000, 21, 368–371. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Solubility advantage of sulfanilamide and sulfacetamide in natural deep eutectic systems: Experimental and theoretical investigations. Drug Dev. Ind. Pharm. 2019, 45, 1120–1129. [Google Scholar] [CrossRef]
- Kodide, K.; Asadi, P.; Thati, J. Solubility and thermodynamic modeling of sulfanilamide in 12 mono solvents and 4 binary solvent mixtures from 278.15 to 318.15 K. J. Chem. Eng. Data 2019, 64, 5196–5209. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1, 2, 3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Faidallah, H.M.; Girgis, A.S.; Tiwari, A.D.; Honkanadavar, H.H.; Thomas, S.J.; Samir, A.; Kalmouch, A.; Alamry, K.A.; Khan, K.A.; Ibrahim, T.S. Synthesis, antibacterial properties and 2D-QSAR studies of quinolone-triazole conjugates. Eur. J. Med. Chem. 2018, 143, 1524–1534. [Google Scholar] [CrossRef]
- Jordão, A.K.; Ferreira, V.F.; Souza, T.M.; de Souza Faria, G.G.; Machado, V.; Abrantes, J.L.; de Souza, M.C.; Cunha, A.C. Synthesis and anti-HSV-1 activity of new 1,2,3-triazole derivatives. Bioorg. Med. Chem. 2011, 19, 1860–1865. [Google Scholar] [CrossRef]
- Dai, Z.-C.; Chen, Y.-F.; Zhang, M.; Li, S.-K.; Yang, T.-T.; Shen, L.; Wang, J.-X.; Qian, S.-S.; Zhu, H.-L.; Ye, Y.-H. Synthesis and antifungal activity of 1,2,3-triazole phenylhydrazone derivatives. Org. Biomol. Chem. 2015, 13, 477–486. [Google Scholar] [CrossRef]
- Boechat, N.; Ferreira, M.d.L.G.; Pinheiro, L.C.; Jesus, A.M.; Leite, M.M.; Júnior, C.C.; Aguiar, A.C.; de Andrade, I.M.; Krettli, A.U. New compounds hybrids 1H-1,2,3-triazole-quinoline against Plasmodium falciparum. Chem. Biol. Drug Des. 2014, 84, 325–332. [Google Scholar] [CrossRef]
- Whiting, M.; Muldoon, J.; Lin, Y.C.; Silverman, S.M.; Lindstrom, W.; Olson, A.J.; Kolb, H.C.; Finn, M.; Sharpless, K.B.; Elder, J.H. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. 2006, 118, 1463–1467. [Google Scholar] [CrossRef]
- Buckle, D.R.; Outred, D.J.; Rockell, C.J.; Smith, H.; Spicer, B.A. Studies on v-triazoles. 7. Antiallergic 9-oxo-1H, 9H-benzopyrano [2,3-d]-v-triazoles. J. Med. Chem. 1983, 26, 251–254. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Gao, C.; Ren, Q.-C.; Chang, L.; Lv, Z.-S.; Feng, L.-S. Triazole derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 138, 501–513. [Google Scholar] [CrossRef]
- Shukla, J.D.; Khan, P.; Deo, K. Synthesis and pharmacological evaluation of novel 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole derivatives for CNS depressant and anticonvulsant profile. Am. J. Pharm. Tech. Res. 2015, 5, 423–433. [Google Scholar]
- Rajasekaran, A.; Rajagopal, K. Synthesis of some novel triazole derivatives as anti-nociceptive and anti-inflammatory agents. Acta Pharm. 2009, 59, 355. [Google Scholar] [CrossRef]
- Nassar, E.M.; Abdelrazek, F.M.; Ayyad, R.R.; El-Farargy, A.F. Synthesis and some reactions of 1-aryl-4-acetyl-5-methyl-1,2,3-triazole derivatives with anticonvulsant activity. Mini Rev. Med. Chem. 2016, 16, 926–936. [Google Scholar] [CrossRef]
- Ji, C.; Egolfopoulos, F.N. Flame propagation of mixtures of air with binary liquid fuel mixtures. Proc. Combust. Inst. 2011, 33, 955–961. [Google Scholar] [CrossRef]
- Penthala, N.R.; Madhukuri, L.; Thakkar, S.; Madadi, N.R.; Lamture, G.; Eoff, R.L.; Crooks, P.A. Synthesis and anti-cancer screening of novel heterocyclic-(2H)-1,2,3-triazoles as potential anti-cancer agents. Medchemcomm 2015, 6, 1535–1543. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Tittal, R.K.; Ghule, V.D.; Kumar, N.; Kumar, L.; Lal, K.; Kumar, A. Design, synthesis, biological activity, molecular docking and computational studies on novel 1, 4-disubstituted-1,2,3-Triazole-Thiosemicarbazone hybrid molecules. J. Mol. Struct. 2020, 1209, 127951. [Google Scholar]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Aouad, M.R. Efficient eco-friendly solvent-free click synthesis and antimicrobial evaluation of new fluorinated 1,2,3-triazoles and their conversion into Schiff Bases. J. Braz. Chem. Soc. 2015, 26, 2105–2115. [Google Scholar] [CrossRef]
- Aouad, M.R. Synthesis and antimicrobial screening of novel thioglycosides and acyclonucleoside analogs carrying 1,2,3-triazole and 1,3,4-oxadiazole moieties. Nucleosides Nucleotides Nucleic Acids 2016, 35, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N.; Mayaba, M.M.; Al-blewi, F.F.; Aouad, M.R.; El Ashry, E.S.H. Click 1,4-regioselective synthesis, characterization, and antimicrobial screening of novel 1,2,3-triazoles tethering fluorinated 1,2,4-triazole and lipophilic side chain. Res. Chem. Intermed. 2017, 43, 995–1011. [Google Scholar] [CrossRef]
- Aouad, M.R. Click Synthesis and antimicrobial screening of novel isatin-1,2,3-triazoles with piperidine, morpholine, or piperazine moieties. Org. Prep. Proced. Int. 2017, 49, 216–227. [Google Scholar] [CrossRef]
- Aouad, M.R.; Mayaba, M.M.; Naqvi, A.; Bardaweel, S.K.; Al-Blewi, F.F.; Messali, M.; Rezki, N. Design, synthesis, in silico and in vitro antimicrobial screenings of novel 1,2,4-triazoles carrying 1,2,3-triazole scaffold with lipophilic side chain tether. Chem. Cent. J. 2017, 11, 117–219. [Google Scholar] [CrossRef]
- Rezki, N. Green microwave synthesis and antimicrobial evaluation of novel triazoles. Org. Prep. Proced. Int. 2017, 49, 525–541. [Google Scholar] [CrossRef]
- Rezki, Z.; Aouad, M.R. Green ultrasound-assisted three-component click synthesis of novel 1H-1,2,3-triazole carrying benzothiazoles and fluorinated-1,2,4-triazole conjugates and their antimicrobial evaluation. Acta Pharm. 2017, 67, 309–324. [Google Scholar] [CrossRef]
- Aouad, M.R.; Soliman, M.A.; Alharbi, M.O.; Bardaweel, S.K.; Sahu, P.K.; Ali, A.A.; Messali, M.; Rezki, N.; Al-Soud, Y.A. Design, synthesis and anticancer screening of novel benzothiazole-piperazine-1,2,3-triazole hybrids. Molecules 2018, 23, 2788. [Google Scholar] [CrossRef]
- Al-Blewi, F.F.; Almehmadi, M.A.; Aouad, M.R.; Bardaweel, S.K.; Sahu, P.K.; Messali, M.; Rezki, N.; El Sayed, H. Design, synthesis, ADME prediction and pharmacological evaluation of novel benzimidazole-1,2,3-triazole-sulfonamide hybrids as antimicrobial and antiproliferative agents. Chem. Cent. J. 2018, 12, 110–225. [Google Scholar] [CrossRef]
- Rezki, N.; Almehmadi, M.A.; Ihmaid, S.; Shehata, A.M.; Omar, A.M.; Ahmed, H.E.; Aouad, M.R. Novel scaffold hopping of potent benzothiazole and isatin analogues linked to 1,2,3-triazole fragment that mimic quinazoline epidermal growth factor receptor inhibitors: Synthesis, antitumor and mechanistic analyses. Bioorg. Chem. 2020, 103, 104133. [Google Scholar] [CrossRef]
- Almehmadi, M.A.; Aljuhani, A.; Alraqa, S.Y.; Ali, I.; Rezki, N.; Aouad, M.R.; Hagar, M. Design, synthesis, DNA binding, modeling, anticancer studies and DFT calculations of Schiff bases tethering benzothiazole-1,2,3-triazole conjugates. J. Mol. Struct. 2021, 1225, 129148. [Google Scholar] [CrossRef]
- Alraqa, S.Y.; Alharbi, K.; Aljuhani, A.; Rezki, N.; Aouad, M.R.; Ali, I. Design, click conventional and microwave syntheses, DNA binding, docking and anticancer studies of benzotriazole-1,2,3-triazole molecular hybrids with different pharmacophores. J. Mol. Struct. 2021, 1225, 129192. [Google Scholar] [CrossRef]
- Ihmaid, S.K.; Alraqa, S.Y.; Aouad, M.R.; Aljuhani, A.; Elbadawy, H.M.; Salama, S.A.; Rezki, N.; Ahmed, H.E. Design of molecular hybrids of phthalimide-triazole agents with potent selective MCF-7/HepG2 cytotoxicity: Synthesis, EGFR inhibitory effect, and metabolic stability. Bioorg. Chem. 2021, 111, 104835. [Google Scholar] [CrossRef]
- Aouad, M.R.; Khan, D.J.; Said, M.A.; Al-Kaff, N.S.; Rezki, N.; Ali, A.A.; Bouqellah, N.; Hagar, M. Novel 1,2,3-Triazole Derivatives as Potential Inhibitors against Covid-19 Main Protease: Synthesis, Characterization, Molecular Docking and DFT Studies. ChemistrySelect 2021, 6, 3468. [Google Scholar] [CrossRef]
- Assolini, J.P.; Concato, V.M.; Gonçalves, M.D.; Carloto, A.C.M.; Conchon-Costa, I.; Pavanelli, W.R.; Melanda, F.N.; Costa, I.N. Nanomedicine advances in toxoplasmosis: Diagnostic, treatment, and vaccine applications. Parasitol. Res. 2017, 116, 1603–1615. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Said, D.; Elsamad, L.; Gohar, Y. Validity of silver, chitosan, and curcumin nanoparticles as anti-Giardia agents. Parasitol. Res. 2012, 111, 545–554. [Google Scholar] [CrossRef]
- Teimouri, A.; Azami, S.J.; Keshavarz, H.; Esmaeili, F.; Alimi, R.; Mavi, S.A.; Shojaee, S. Anti-Toxoplasma activity of various molecular weights and concentrations of chitosan nanoparticles on tachyzoites of RH strain. Int. J. Nanomed. 2018, 13, 1341. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Khan, I.A.; Casciotti, L. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J. Immunol. 1999, 163, 4503–4509. [Google Scholar] [PubMed]
- El Temsahy, M.M.; El Kerdany, E.D.; Eissa, M.M.; Shalaby, T.I.; Talaat, I.M.; Mogahed, N.M. The effect of chitosan nanospheres on the immunogenicity of Toxoplasma lysate vaccine in mice. J. Parasit. Dis. 2016, 40, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Vitor, R. Efficacy of atovaquone and sulfadiazine in the treatment of mice infected with Toxoplasma gondii strains isolated in Brazil. Parasite 2005, 12, 171–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Zawawy, L.A.; El-Said, D.; Mossallam, S.F.; Ramadan, H.S.; Younis, S.S. Triclosan and triclosan-loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Exp. Parasitol. 2015, 149, 54–64. [Google Scholar] [CrossRef]
- Gaafar, M.; Mady, R.; Diab, R.; Shalaby, T.I. Chitosan and silver nanoparticles: Promising anti-toxoplasma agents. Exp. Parasitol. 2014, 143, 30–38. [Google Scholar] [CrossRef]
- Allam, A.F.; Hagras, N.A.-E.; Farag, H.F.; Osman, M.M.; Shalaby, T.I.; Kazem, A.H.; Shehab, A.Y.; Mogahed, N.M.F.H. Remarkable histopathological improvement of experimental toxoplasmosis after receiving spiramycin-chitosan nanoparticles formulation. J. Parasit. Dis. 2022, 46, 166–177. [Google Scholar] [CrossRef]
- Lee, S.-H.; Chu, K.-B.; Quan, F.-S. Parasite infiltration and apoptosis in spleen upon toxoplasma gondii infection. Korean J. Parasitol. 2019, 57, 537. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Elwakil, B.H.; Elshewemi, S.S.; El-Naggar, M.Y.; Bekhit, A.A.; Olama, Z.A. Novel Siwa propolis and colistin-integrated chitosan nanoparticles: Elaboration; in vitro and in vivo appraisal. Nanomedicine 2020, 15, 1269–1284. [Google Scholar] [CrossRef]
- Hagras, N.A.; Mogahed, N.; Sheta, E.; Darwish, A.A.; El-Hawary, M.A.; Hamed, M.T.; Elwakil, B.H. The powerful synergistic effect of spiramycin/propolis loaded chitosan/alginate nanoparticles on acute murine toxoplasmosis. PLoS Negl. Trop. Dis. 2022, 16, e0010268. [Google Scholar] [CrossRef]
- Zhao, G.; Song, X.; Kong, X.; Zhang, N.; Qu, S.; Zhu, W.; Yang, Y.; Wang, Q. Immunization with Toxoplasma gondii aspartic protease 3 increases survival time of infected mice. Acta Trop. 2017, 171, 17–23. [Google Scholar] [CrossRef]
- Eissa, M.M.; El-Azzouni, M.Z.; Mady, R.F.; Fathy, F.M.; Baddour, N.M. Initial characterization of an autoclaved Toxoplasma vaccine in mice. Exp. Parasitol. 2012, 131, 310–316. [Google Scholar] [CrossRef]
- Kavitha, N.; Noordin, R.; Kit-Lam, C.; Sasidharan, S. Real time anti-Toxoplasma gondii activity of an active fraction of Eurycoma longifolia root studied by in situ scanning and transmission electron microscopy. Molecules 2012, 17, 9207–9219. [Google Scholar] [CrossRef]
- James, P.M.; Coltman, D.W.; Murray, B.W.; Hamelin, R.C.; Sperling, F.A. Spatial genetic structure of a symbiotic beetle-fungal system: Toward multi-taxa integrated landscape genetics. PLoS ONE 2011, 6, e25359. [Google Scholar] [CrossRef]
- Titford, M. A short history of histopathology technique. J. Histotechnol. 2006, 29, 99–110. [Google Scholar] [CrossRef]
- Musumeci, G. Past, present and future: Overview on histology and histopathology. J. Histol. Histopathol. 2014, 1, 5. [Google Scholar] [CrossRef]
- Kotz, S.; Balakrishnan, N.; Read, C.B.; Vidakovic, B. Encyclopedia of Statistical Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 1. [Google Scholar]
- Kirkpatrick, L.A. A Simple Guide to IBM SPSS Statistics-Version 23.0; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]

| Nano Formulations | PS (nm) | ζ Potential (mV) | PDI | Entrapment Efficiency % (EE%) | Loading Efficiency (LE%) |
|---|---|---|---|---|---|
| NCs-3a | 76.3 ± 10.9 | +36.4 ± 2.7 | 0.34 ± 0.1 | 78.11 ± 1.70 | 38.42 ± 0.8 |
| NCs-3b | 50.4 ± 7.3 | +37.2 ± 0.8 | 0.36 ± 0.03 | 80.15 ± 1.10 | 38.70 ± 0.5 |
| NCs-3c | 36.0 ± 10.4 | +39.3 ± 1.9 | 0.35 ± 0.1 | 89.27 ± 1.23 | 43.31 ± 1.0 |
| Subgroup | Mean Survival | % End of Study | Log Rank | |
|---|---|---|---|---|
| χ2 | p | |||
| Ia | 6.6 | 0.0 | 81.343 * | <0.001 * |
| Ib | 29.0 | 80.0 | ||
| IIa | 11.8 | 0.0 | ||
| IIb | 12.2 | 0.0 | ||
| IIc | 13.0 | 0.0 | ||
| IId | 16.8 | 0.0 | ||
| IIe | 27.4 | 60.0 | ||
| IIf | 30.0 | 100.0 | ||
| Subgroup | Ib | IIa | IIb | IIc | IId | IIe | IIf |
|---|---|---|---|---|---|---|---|
| Ia | 0.003 * | 0.003 * | 0.003 * | 0.003 * | 0.003 * | 0.003 * | 0.003 * |
| Ib | 0.002 * | 0.003 * | 0.003 * | 0.003 * | 0.459 | 0.317 | |
| IIa | 0.549 | 0.049 * | 0.002 * | 0.002 * | 0.002 * | ||
| IIb | 0.134 | 0.003 * | 0.003 * | 0.003 * | |||
| IIc | 0.003 * | 0.003 * | 0.003 * | ||||
| IId | 0.003 * | 0.003 * | |||||
| IIe | 0.134 |
| Subgroup Ia (n = 5) | Subgroup Ib (n = 5) | Subgroup IIa (n = 5) | Subgroup IIb (n = 5) | Subgroup IIc (n = 5) | Subgroup IId (n = 5) | Subgroup IIe (n = 5) | Subgroup IIf (n = 5) | |
|---|---|---|---|---|---|---|---|---|
| Liver | ||||||||
| Mean | 10.74 | 0.04 # | 1.96 # | 1.72 # | 1.17 # | 1.07 # | 0.66 # | 0.0 # |
| ±SD. | 1.84 | 0.07 | 0.80 | 0.54 | 0.69 | 1.84 | 0.81 | 0.0 |
| F (p) | 56.471 * (<0.001 *) | |||||||
| % Reduction | 99.63 | 81.75 | 83.99 | 89.11 | 90.04 | 93.85 | 100.0 | |
| Spleen | ||||||||
| Mean | 14.74 | 0.04 # | 2.32 # | 2.01 # | 1.56 # | 1.45 # | 0.04 # | 0.0 # |
| ±SD. | 2.61 | 0.09 | 0.49 | 2.38 | 0.70 | 3.16 | 0.09 | 0.0 |
| F (p) | 41.833 * (<0.001 *) | |||||||
| % Reduction | 99.73 | 84.26 | 86.36 | 89.42 | 90.16 | 99.73 | 100.0 | |
| Brain | ||||||||
| Mean | 4.52 | 0.0 # | 0.80 # | 0.81 # | 0.61 # | 0.26 # | 0.12 # | 0.0 # |
| ±SD. | 1.08 | 0.0 | 0.67 | 0.83 | 0.65 | 0.44 | 0.22 | 0.0 |
| F (p) | 30.448 * (<0.001 *) | |||||||
| % Reduction | 100.0 | 82.30 | 82.08 | 86.50 | 94.25 | 97.35 | 100.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljohani, F.S.; Rezki, N.; Aouad, M.R.; Elwakil, B.H.; Hagar, M.; Sheta, E.; Hussein Mogahed, N.M.F.; Bardaweel, S.K.; Hagras, N.A.-e. Synthesis, Characterization and Nanoformulation of Novel Sulfonamide-1,2,3-triazole Molecular Conjugates as Potent Antiparasitic Agents. Int. J. Mol. Sci. 2022, 23, 4241. https://doi.org/10.3390/ijms23084241
Aljohani FS, Rezki N, Aouad MR, Elwakil BH, Hagar M, Sheta E, Hussein Mogahed NMF, Bardaweel SK, Hagras NA-e. Synthesis, Characterization and Nanoformulation of Novel Sulfonamide-1,2,3-triazole Molecular Conjugates as Potent Antiparasitic Agents. International Journal of Molecular Sciences. 2022; 23(8):4241. https://doi.org/10.3390/ijms23084241
Chicago/Turabian StyleAljohani, Faizah S., Nadjet Rezki, Mohamed R. Aouad, Bassma H. Elwakil, Mohamed Hagar, Eman Sheta, Nermine Mogahed Fawzy Hussein Mogahed, Sanaa K. Bardaweel, and Nancy Abd-elkader Hagras. 2022. "Synthesis, Characterization and Nanoformulation of Novel Sulfonamide-1,2,3-triazole Molecular Conjugates as Potent Antiparasitic Agents" International Journal of Molecular Sciences 23, no. 8: 4241. https://doi.org/10.3390/ijms23084241
APA StyleAljohani, F. S., Rezki, N., Aouad, M. R., Elwakil, B. H., Hagar, M., Sheta, E., Hussein Mogahed, N. M. F., Bardaweel, S. K., & Hagras, N. A.-e. (2022). Synthesis, Characterization and Nanoformulation of Novel Sulfonamide-1,2,3-triazole Molecular Conjugates as Potent Antiparasitic Agents. International Journal of Molecular Sciences, 23(8), 4241. https://doi.org/10.3390/ijms23084241









