Mebendazole-Induced Blood-Testis Barrier Injury in Mice Testes by Disrupting Microtubules in Addition to Triggering Programmed Cell Death
Abstract
:1. Introduction
2. Results
2.1. Subsection
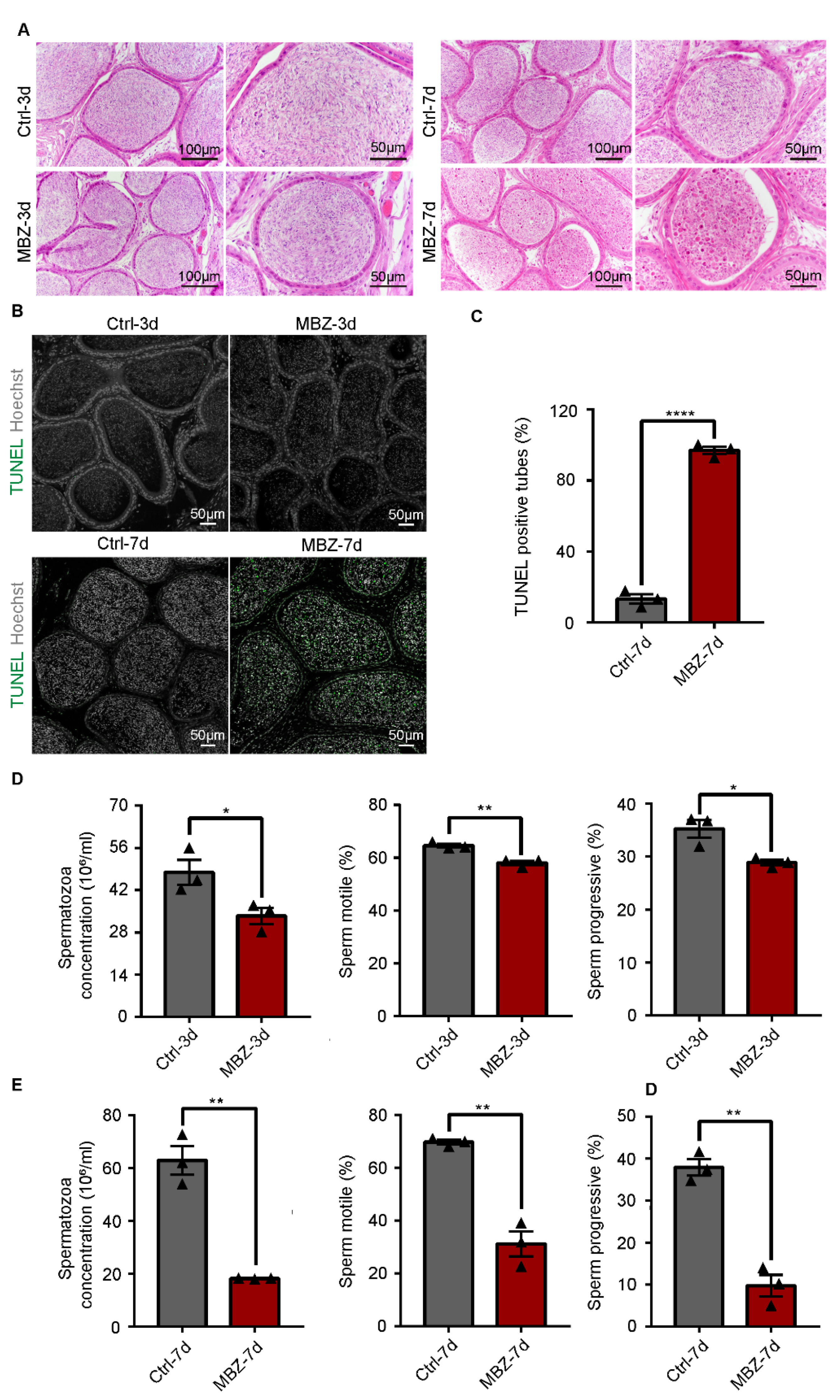
2.1.1. Mebendazole Triggers Testicular Damage in Mice Testes
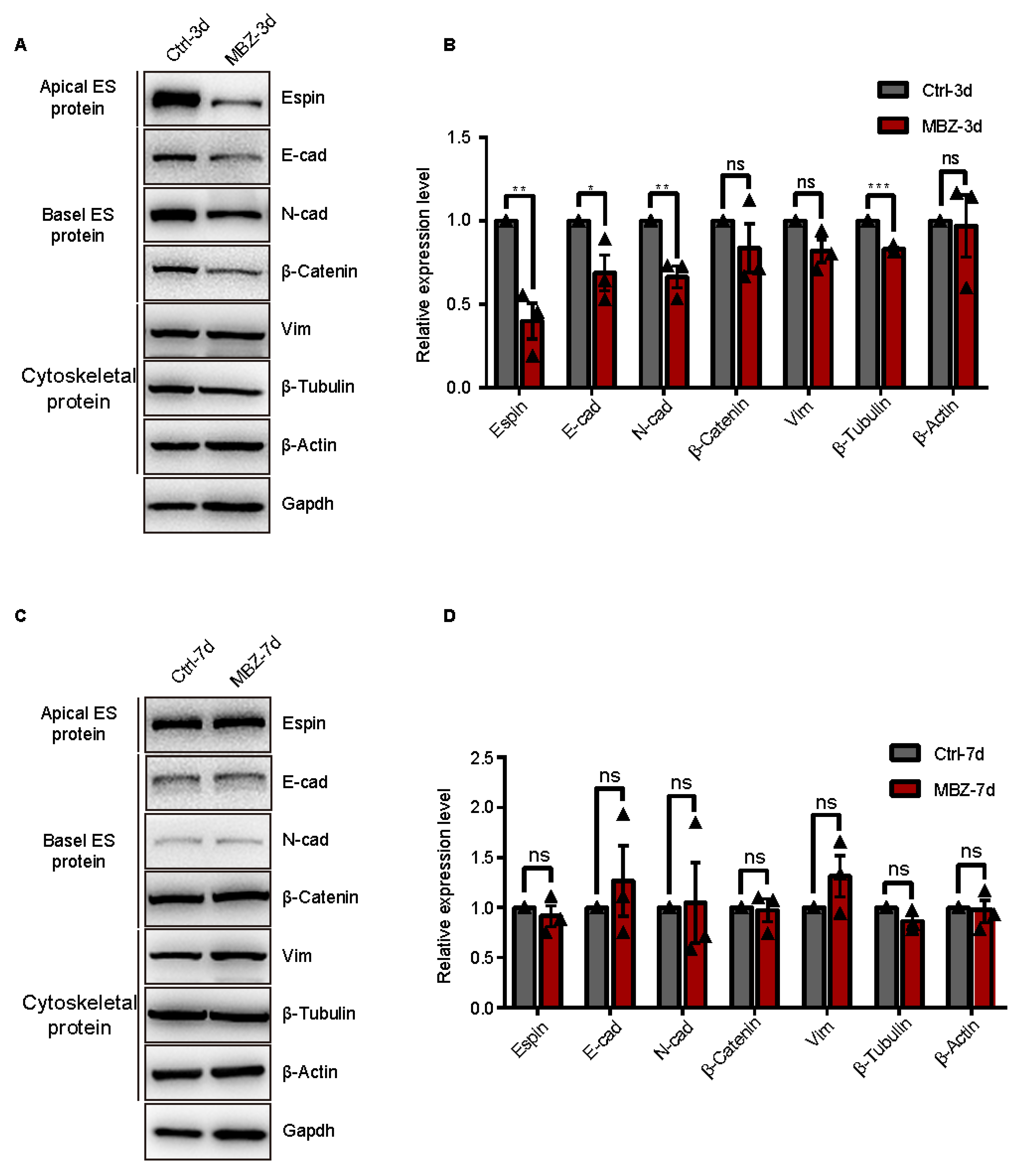
2.1.2. BTB-Related Junction Proteins Exhibit Destruction in Mebendazole-Treated Mice
2.1.3. Mebendazole Treatment Initiates Cell Apoptosis in Mice Testes and Epididymis
2.1.4. Mebendazole Leads to Significant Abnormalities in the Sperm Cells in Mice Epididymis
3. Discussion
4. Materials and Methods
4.1. Animal and Treatment
4.2. Histological Analysis
4.3. Epididymal Sperm Analysis
4.4. Protein Isolation and Western Blot
4.5. Antibodies
4.6. Statistical Analysis
4.7. Quantitative Analysis of Immunofluorescence Staining Sections
4.8. Quantitative Analysis of Sperm Abnormalities
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kudo, N.; Kubota, H.; Gotoh, H.; Ishida, H.; Ikadai, H.; Oyamada, T. Efficacy of thiabendazole, mebendazole, levamisole and ivermectin against gullet worm, Gongylonema pulchrum: In vitro and in vivo studies. Vet. Parasitol. 2008, 151, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Horton, J. Albendazole: A review of anthelmintic efficacy and safety in humans. Parasitology 2000, 121, S113–S132. [Google Scholar] [CrossRef] [PubMed]
- Guerini, A.E.; Triggiani, L.; Maddalo, M.; Bonù, M.L.; Frassine, F.; Baiguini, A.; Alghisi, A.; Tomasini, D.; Borghetti, P.; Pasinetti, N.; et al. Mebendazole as a Candidate for Drug Repurposing in Oncology: An Extensive Review of Current Literature. Cancers 2019, 11, 1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Witt, M.; Gamble, A.; Hanson, D.; Markowitz, D.; Powell, C.; Al Dimassi, S.; Atlas, M.; Boockvar, J.; Ruggieri, R.; Symons, M. Repurposing Mebendazole as a Replacement for Vincristine for the Treatment of Brain Tumors. Mol. Med. 2017, 23, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Lou, K.; Song, X.; Ma, H.; Zhou, X.; Xu, H.; Wang, W. Mebendazole is a potent inhibitor to chemoresistant T cell acute lymphoblastic leukemia cells. Toxicol. Appl. Pharmacol. 2020, 396, 115001. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, L.; Zhang, J.; He, F.; Wang, H.; Liu, Q.; Shi, D.; Ni, N.; Wagstaff, W.; Chen, C.; et al. Antiparasitic mebendazole (MBZ) effectively overcomes cisplatin resistance in human ovarian cancer cells by inhibiting multiple cancer-associated signaling pathways. Aging 2021, 13, 17407–17427. [Google Scholar] [CrossRef]
- Nath, J.; Paul, R.; Ghosh, S.K.; Paul, J.; Singha, B.; Debnath, N. Drug repurposing and relabeling for cancer therapy: Emerging benzimidazole antihelminthics with potent anticancer effects. Life Sci. 2020, 258, 118189. [Google Scholar] [CrossRef]
- Younis, N.S.; Ghanim, A.M.H.; Saber, S. Mebendazole augments sensitivity to sorafenib by targeting MAPK and BCL-2 signalling in n-nitrosodiethylamine-induced murine hepatocellular carcinoma. Sci. Rep. 2019, 9, 19095. [Google Scholar] [CrossRef] [Green Version]
- Jahnukainen, K.; Ehmcke, J.; Hou, M.; Schlatt, S. Testicular function and fertility preservation in male cancer patients. Best practice & research. Clin. Endocrinol. Metab. 2011, 25, 287–302. [Google Scholar] [CrossRef]
- Allen, C.M.; Lopes, F.; Mitchell, R.T.; Spears, N. How does chemotherapy treatment damage the prepubertal testis? Reproduction 2018, 156, R209–R233. [Google Scholar] [CrossRef] [Green Version]
- Geary, T.G.; Mackenzie, C.D.; Silber, S.A. Flubendazole as a macrofilaricide: History and background. PLoS Negl. Trop. Dis. 2019, 13, e0006436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachau-Durand, S.; Lammens, L.; van der Leede, B.J.; Van Gompel, J.; Bailey, G.; Engelen, M.; Lampo, A. Preclinical toxicity and pharmacokinetics of a new orally bioavailable flubendazole formulation and the impact for clinical trials and risk/benefit to patients. PLoS Negl. Trop. Dis. 2019, 13, e0007026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Xie, Y.; Yu, T.; Liu, N.; Wang, Z.; Woolsey, R.J.; Tang, Y.; Zhang, X.; Qin, W.; Zhang, Y.; et al. m(6)A-dependent biogenesis of circular RNAs in male germ cells. Cell Res. 2020, 30, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.E.I.; Xiang-Liang, T.; Yu, Z.; Bin, L.; Lian-Ju, S.; Chun-Lan, L.; Tao, L.I.N.; Da-Wei, H.E.; Sheng-de, W.U.; Guang-Hui, W.E.I. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes Dis. 2018, 5, 263–274. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef]
- Huang, W.; Liu, M.; Xiao, B.; Zhang, J.; Song, M.; Li, Y.; Cao, Z. Aflatoxin B1 disrupts blood-testis barrier integrity by reducing junction protein and promoting apoptosis in mice testes. Food Chem. Toxicol. 2021, 148, 111972. [Google Scholar] [CrossRef] [PubMed]
- Carreau, S.; Hess, R.A. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2010, 365, 1517–1535. [Google Scholar] [CrossRef] [Green Version]
- Tang, E.I.; Mruk, D.D.; Cheng, C.Y. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin. Cell Dev. Biol. 2016, 59, 35–45. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, L.; O’Bryan, M.K. Microtubules and spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 45–54. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Q.; Cao, J.; Huang, Q.; Zhu, X. The microtubule plus end-binding protein EB1 is involved in Sertoli cell plasticity in testicular seminiferous tubules. Exp. Cell Res. 2008, 314, 213–226. [Google Scholar] [CrossRef]
- Song, H.W.; Wilkinson, M.F. Transcriptional control of spermatogonial maintenance and differentiation. Semin. Cell Dev. Biol. 2014, 30, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celeste, A.; Petersen, S.; Romanienko, P.J.; Fernandez-Capetillo, O.; Chen, H.T.; Sedelnikova, O.A.; Reina-San-Martin, B.; Coppola, V.; Meffre, E.; Difilippantonio, M.J.; et al. Genomic instability in mice lacking histone H2AX. Science 2002, 296, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Chang, H.; Chaboissier, M.C.; Schedl, A.; Behringer, R.R. Sox9 in testis determination. Ann. N. Y. Acad. Sci. 2005, 1061, 9–17. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, B.; Akhmanova, A.; Straube, A. Regulation of microtubule dynamic instability. Biochem. Soc. Trans. 2009, 37, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, E.I.; Mok, K.W.; Lee, W.M.; Cheng, C.Y. EB1 regulates tubulin and actin cytoskeletal networks at the sertoli cell blood-testis barrier in male rats: An in vitro study. Endocrinology 2015, 156, 680–693. [Google Scholar] [CrossRef] [Green Version]
- Thimon, V.; Koukoui, O.; Calvo, E.; Sullivan, R. Region-specific gene expression profiling along the human epididymis. Mol. Hum. Reprod. 2007, 13, 691–704. [Google Scholar] [CrossRef]
- Dayan, A.D. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003, 86, 141–159. [Google Scholar] [CrossRef]
- Kumar, S.; Fairmichael, C.; Longley, D.B.; Turkington, R.C. The Multiple Roles of the IAP Super-family in cancer. Pharmacol. Ther. 2020, 214, 107610. [Google Scholar] [CrossRef]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Büsselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, Y.; Shen, L.; Wei, Y.; Long, C.; Fu, Y.; Wu, H.; Wang, J.; Wu, Y.; Wu, S.; et al. Exposure to DEHP induces testis toxicity and injury through the ROS/mTOR/NLRP3 signaling pathway in immature rats. Ecotoxicol. Environ. Saf. 2021, 227, 112889. [Google Scholar] [CrossRef]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2016, 2016, 2183026. [Google Scholar] [CrossRef] [PubMed]
- Antonuccio, P.; Micali, A.G.; Romeo, C.; Freni, J.; Vermiglio, G.; Puzzolo, D.; Squadrito, F.; Irrera, N.; Marini, H.R.; Rana, R.A.; et al. NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele. Int. J. Mol. Sci. 2021, 22, 1319. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.Y.; Staedtke, V.; Aprhys, C.M.; Gallia, G.L.; Riggins, G.J. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro-Oncology 2011, 13, 974–982. [Google Scholar] [CrossRef]
- Johnson, K.J. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis 2014, 4, e979106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Barrionuevo, F.; Bagheri-Fam, S.; Klattig, J.; Kist, R.; Taketo, M.M.; Englert, C.; Scherer, G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol. Reprod. 2006, 74, 195–201. [Google Scholar] [CrossRef]
- Lardenois, A.; Chalmel, F.; Barrionuevo, F.; Demougin, P.; Scherer, G.; Primig, M. Profiling spermatogenic failure in adult testes bearing Sox9-deficient Sertoli cells identifies genes involved in feminization, inflammation and stress. Reprod. Biol. Endocrinol. 2010, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Malki, S.; Berta, P.; Poulat, F.; Boizet-Bonhoure, B. Cytoplasmic retention of the sex-determining factor SOX9 via the microtubule network. Exp. Cell Res. 2005, 309, 468–475. [Google Scholar] [CrossRef]
- Knower, K.C.; Kelly, S.; Harley, V.R. Turning on the male—SRY, SOX9 and sex determination in mammals. Cytogenet. Genome Res. 2003, 101, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bishop, C.E. Sox9 is sufficient for functional testis development producing fertile male mice in the absence of Sry. Hum. Mol. Genet. 2005, 14, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Sim, H.; Argentaro, A.; Harley, V.R. Boys, girls and shuttling of SRY and SOX9. Trends Endocrinol. Metab. 2008, 19, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.K.; Mattiske, D.M.; Pask, A.J. Oestrogen regulates SOX9 bioavailability by rapidly activating ERK1/2 and stabilising microtubules in a human testis-derived cell line. Exp. Cell Res. 2021, 398, 112405. [Google Scholar] [CrossRef] [PubMed]
- Amlani, S.; Vogl, A.W. Changes in the Distribution of Microtubules and Intermediate Filaments in Mammalian Sertoli Cells during Spermatogenesis. Anat. Rec. 1988, 220, 143. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Wang, C.; Yao, Y.; Li, H.; Yao, Y.; Zhu, Y.; Cui, Y.; Yuan, Y.; Sha, J. Mebendazole-Induced Blood-Testis Barrier Injury in Mice Testes by Disrupting Microtubules in Addition to Triggering Programmed Cell Death. Int. J. Mol. Sci. 2022, 23, 4220. https://doi.org/10.3390/ijms23084220
Huang M, Wang C, Yao Y, Li H, Yao Y, Zhu Y, Cui Y, Yuan Y, Sha J. Mebendazole-Induced Blood-Testis Barrier Injury in Mice Testes by Disrupting Microtubules in Addition to Triggering Programmed Cell Death. International Journal of Molecular Sciences. 2022; 23(8):4220. https://doi.org/10.3390/ijms23084220
Chicago/Turabian StyleHuang, Mingqian, Chang Wang, Ying Yao, Huiling Li, Yejin Yao, Yunfei Zhu, Yiqiang Cui, Yan Yuan, and Jiahao Sha. 2022. "Mebendazole-Induced Blood-Testis Barrier Injury in Mice Testes by Disrupting Microtubules in Addition to Triggering Programmed Cell Death" International Journal of Molecular Sciences 23, no. 8: 4220. https://doi.org/10.3390/ijms23084220
APA StyleHuang, M., Wang, C., Yao, Y., Li, H., Yao, Y., Zhu, Y., Cui, Y., Yuan, Y., & Sha, J. (2022). Mebendazole-Induced Blood-Testis Barrier Injury in Mice Testes by Disrupting Microtubules in Addition to Triggering Programmed Cell Death. International Journal of Molecular Sciences, 23(8), 4220. https://doi.org/10.3390/ijms23084220





