Tadalafil and Steroid Hormones Interactions in Adipose, Bone and Prostate Tissues: Focus on Translational Perspectives
Abstract
1. Introduction
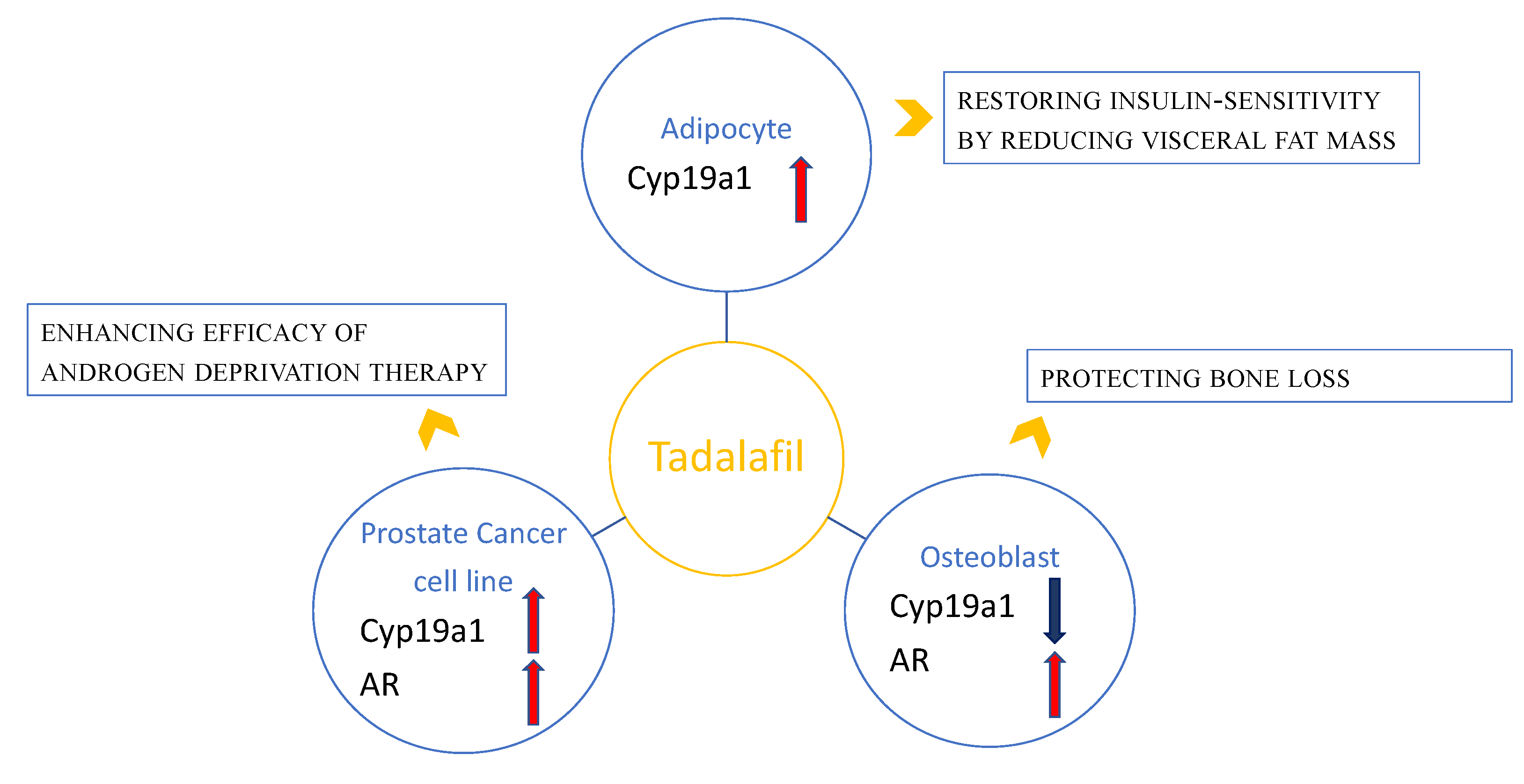
2. Tadalafil and Adipose Tissue: Clinical and Preclinical Observations
3. Tadalafil and Bone: Preclinical Observations
4. Tadalafil and Prostate Cancer: Clinical and Preclinical Observations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| AR | Androgen receptor |
| ARKO | Androgen receptor knock-out |
| ARO | Cyp19A1 (aromatase) |
| BCT | Bicalutamide |
| BPH | Benign prostate hyperplasia |
| cAMP | Cyclic adenosine monophosphate |
| cGMP | Cyclic guanosine monophosphate |
| CRPC | Castration-resistant prostate cancer |
| E2 | Estradiol |
| ED | Erectile dysfunction |
| ERα | Estrogen receptor alpha |
| ERβ | Estrogen receptor beta |
| LnCaP | Androgen-sensitive human PCa cell line |
| LUTS | Low urinary tract symptoms |
| NO | Nitric oxide |
| NO-cGMP-PKG | Nitric oxide-cyclic guanosine monophosphate-dependent protein kinase G |
| PCa | Prostate cancer |
| PDE | Phosphodiesterase |
| PDE5 | Type-5 phosphodiesterase |
| PDE5i | Type-5 phosphodiesterase inhibitors |
| SAOS-2 | Sarcoma osteogenic cell line (human osteoblasts) |
| T | Testosterone |
| T/E2 | Testosterone/estradiol ratio |
References
- Francis, S.H.; Turko, I.V.; Corbin, J.D. Cyclic nucleotide phosphodiesterases: Relating structure and function. Prog. Nucleic Acid Res. Mol. Biol. 2001, 65, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.A.; Ward, A.; Chen, X.; Maxuitenko, Y.; Coley, A.; Aboelella, N.S.; Buchsbaum, D.J.; Boyd, M.R.; Keeton, A.B.; Zhou, G. PDE5 and PDE10 inhibition activates cGMP/PKG signaling to block Wnt/β-catenin transcription, cancer cell growth, and tumor immunity. Drug Discov. Today 2020, 25, 1521–1527. [Google Scholar] [CrossRef]
- Aversa, A.; Caprio, M.; Antelmi, A.; Armani, A.; Brama, M.; Greco, E.A.; Francomano, D.; Calanchini, M.; Spera, G.; Di Luigi, L.; et al. Exposure to phosphodiesterase type-5 inhibitors stimulates aromatase expression in human adipocytes in vitro. J. Sex. Med. 2011, 8, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.A.; Pili, M.; Bruzziches, R.; Corona, G.; Spera, G.; Aversa, A. Testosterone:estradiol ratio changes associated with long-term tadalafil administration: A pilot study. J. Sex. Med. 2006, 3, 716–722. [Google Scholar] [CrossRef]
- Catalano, S.; Campana, A.; Giordano, C.; Gyorffy, B.; Tarallo, R.; Rinaldi, A.; Bruno, G.; Ferraro, A.; Romeo, F.; Lanzino, M.; et al. Expression and Function of Phosphodiesterase Type 5 in Human Breast Cancer Cell Lines and Tissues: Implications for Targeted Therapy. Clin. Cancer Res. 2016, 22, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.S.; Geethakumari, A.M.; Biswas, K.H. Phosphodiesterase 5 (PDE5): Structure-function regulation and therapeutic applications of inhibitors. Biomed. Pharmacother. 2021, 134, 111128. [Google Scholar] [CrossRef]
- Burnett, A.L. Phosphodiesterase 5 mechanisms and therapeutic applications. Am. J. Cardiol. 2005, 26, 29M–31M. [Google Scholar] [CrossRef]
- Morelli, A.; Filippi, S.; Mancina, R.; Luconi, M.; Vignozzi, L.; Marini, M.; Orlando, C.; Vannelli, G.V.; Aversa, A.; Natali, A.; et al. Androgens regulate phosphodiesterase. Type 5 expression and functional activity in corpora cavernosa. Endocriniogy 2004, 145, 2253–2263. [Google Scholar] [CrossRef]
- Maneschi, E.; Cellai, I.; Aversa, A.; Mello, T.; Filippi, S.; Comeglio, P.; Bani, D.; Guasti, D.; Sarchielli, E.; Salvatore, G.; et al. Tadalafil reduces visceral adipose tissue accumulation by promoting preadipocytes differentiation towards a metabolically healthy phenotype: Studies in rabbits. Mol. Cell. Endocrinol. 2016, 15, 50–70. [Google Scholar] [CrossRef]
- Aversa, A.; Greco, E.; Bruzziches, R.; Pili, M.; Rosano, G.; Spera, G. Relationship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: A pilot study. Int. J. Impot. Res. 2007, 19, 200–207. [Google Scholar] [CrossRef]
- Aversa, A.; Fittipaldi, S.; Francomano, D.; Bimonte, V.M.; Greco, E.A.; Crescioli, C.; Di Luigi, L.; Lenzi, A.; Migliaccio, S. Tadalafil improves lean mass and endothelial function in nonobese men with mild ED/LUTS: In vivo and in vitro characterization. Endocrine 2017, 56, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Antinozzi, C.; Greco, E.A.; Sgrò, P.; Dimauro, I.; Aversa, A.; Di Luigi, L. Effects of Tadalafil on skeletal muscle tissue: Exploring interactions and novel mechanisms of action. Minerva Endocrinol 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Campolo, F.; Pofi, R.; Venneri, M.A.; Isidori, A.M. Priming metabolism with the type 5 phosphodiesterase: The role of cGMP-hydrolyzing enzymes. Curr. Opin. Pharmacol. 2021, 60, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.E.; Bracy, D.P.; Julien, B.M.; Rottman, J.N.; Fueger, P.T.; Wasserman, D.H. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 2007, 56, 1025–1033. [Google Scholar] [CrossRef]
- Kim, S.M.; Taneja, C.; Perez-Pena, H.; Ryu, V.; Gumerova, A.; Li, W.; Ahmad, N.; Zhu, L.L.; Liu, P.; Natgew, M.; et al. Repurposing erectile dysfunction drugs tadalafil and vardenafil to increase bone mass. Proc. Natl. Acad. Sci. USA 2020, 117, 14386–14394. [Google Scholar] [CrossRef]
- Aguirre, J.; Buttery, L.; O’Shaughnessy, M.; Afzal, F.; Fernandez de Marticorena, I.; Hukkanen, M.; Huang, P.; MacIntyre, I.; Polak, J. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am. J. Pathol. 2001, 158, 247–257. [Google Scholar] [CrossRef]
- Kalyanaraman, H.; Ramdani, G.; Joshua, J.; Schall, N.; Boss, G.R.; Cory, E.; Sah, R.L.; Casteel, D.E.; Pilz, R.B. A novel, direct NO donor regulates osteoblast and osteoclast functions and increases bone mass in ovariectomized mice. J. Bone Miner. Res. 2017, 32, 46–59. [Google Scholar] [CrossRef]
- Jamal, S.A.; Browner, W.S.; Bauer, D.C.; Cummings, S.R. Intermittent use of nitrates increases bone mineral density: The study of osteoporotic fractures. J. Bone Miner. Res. 1998, 13, 1755–1759. [Google Scholar] [CrossRef]
- Rejnmark, L.; Vestergaard, P.; Mosekilde, L. Decreased fracture risk in users of organic nitrates: A nationwide case-control study. J. Bone Miner. Res. 2006, 21, 1811–1817. [Google Scholar] [CrossRef]
- Ramdani, G.; Schall, N.; Kalyanaraman, H.; Wahwah, N.; Moheize, S.; Lee, J.J.; Sah, R.L.; Pfeifer, A.; Casteel, D.E.; Pilz, R.B. cGMP-dependent protein kinase-2 regulates bone mass and pre- vents diabetic bone loss. J. Endocrinol. 2018, 238, 203–219. [Google Scholar] [CrossRef]
- Pfeifer, A.; Aszódi, A.; Seidler, U.; Ruth, P.; Hofmann, F.; Fässler, R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 1996, 274, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Florent Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Takeda, S.; Karsenty, G. Molecular basis of the sympathetic regulation of bone mass. Bone 2008, 42, 837–840. [Google Scholar] [CrossRef]
- Campion, J.M.; Maricic, M.J. Osteoporosis in men. Am. Fam. Physician 2003, 67, 1521–1526. [Google Scholar]
- Huyut, Z.; Bakan, N.; Yıldırım, S.; Alp, H.H. Effects of the Phosphodiesterase-5 (PDE-5) Inhibitors, Avanafil and Zaprinast, on Bone Remodeling and Oxidative Damage in a Rat Model of Glucocorticoid-Induced Osteoporosis. Med. Sci. Monit. Basic Res. 2018, 13, 47–58. [Google Scholar] [CrossRef]
- Hamit, H. The effect of PDE5 inhibitors on bone and oxidative damage in ovariectomy-induced osteoporosis. Exp. Biol. Med. 2017, 242, 1051–1061. [Google Scholar] [CrossRef]
- Pal, S.; Rashid, M.; Singh, S.K.; Porwal, K.; Singh, P.; Mohamed, R.; Gayen, J.R.; Wahajuddin, M.; Chattopadhyay, N. Skeletal restoration by phosphodiesterase 5 inhibitors in osteopenic mice: Evidence of osteoanabolic and osteoangiogenic effects of the drugs. Bone 2020, 135, 115305. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, H.; Tower, R.J.; Levine, M.A.; Qin, L. Analysis of short-term treatment with the phosphodiesterase type 5 inhibitor tadalafil on long bone development in young rats. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E446–E453. [Google Scholar] [CrossRef]
- Aversa, A.; Fittipaldi, S.; Bimonte, V.M.; Wannenes, F.; Papa, V.; Francomano, D.; Greco, E.A.; Lenzi, A.; Migliaccio, S. Tadalafil modulates aromatase activity and androgen receptor expression in a human osteoblastic cell in vitro model. J. Endocrinol. Investig. 2016, 39, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wisanwattana, W.; Wongkrajang, K.; Cao, D.Y.; Shi, X.K.; Zhang, Z.H.; Zhou, Z.Y.; Li, F.; Mei, Q.G.; Wang, C.; Suksamrarn, A.; et al. Inhibition of Phosphodiesterase 5 Promotes the Aromatase-Mediated Estrogen Biosynthesis in Osteoblastic Cells by Activation of cGMP/PKG/SHP2 Pathway. Front. Endocrinol. 2021, 12, 636784. [Google Scholar] [CrossRef]
- Kügler, R.; Mietens, A.; Seidensticker, M.; Tasch, S.; Wagenlehner, F.M.; Kaschtanow, A.; Tjahjono, Y.; Tomczyk, C.U.; Beyer, D.; Risbridger, G.P.; et al. Novel imaging of the prostate reveals spontaneous gland contraction and excretory duct quiescence together with different drug effects. FASEB J. 2018, 32, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zang, N.; Jiang, Y.; Chen, P.; Wang, X.; Zhang, X. Upregulation of phosphodiesterase type 5 in the hyperplastic prostate. Sci. Rep. 2015, 5, 17888. [Google Scholar] [CrossRef] [PubMed]
- Fibbi, B.; Morelli, A.; Vignozzi, L.; Filippi, S.; Chavalmane, A.; De Vita, G.; Marini, M.; Gacci, M.; Vannelli, G.B.; Sandner, P.; et al. Characterization of phosphodiesterase type 5 expression and functional activity in the human male lower urinary tract. J. Sex. Med. 2010, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ückert, S.; Kuthe, A.; Jonas, U.; Stief, C.G. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J. Urol. 2001, 166, 2484. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020, 77, 38. [Google Scholar] [CrossRef] [PubMed]
- Bisegna, C.; Gravina, G.L.; Pierconti, F.; Martini, M.; Larocca, L.; Rossi, P.; Grimaldi, P.; Di Stasi, S.; Jannini, E.A. Regulation of PDE5 expression in normal prostate, benign prostatic hyperplasia, and adenocarcinoma. Andrology 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, V.M.; Marampon, F.; Antonioni, A.; Fittipaldi, S.; Ferretti, E.; Pestell, R.G.; Curreli, M.; Lenzi, A.; Vitale, G.; Brumetti, A.; et al. Phosphodiesterase Type-5 inhibitor tadalafil modulates steroid hormones signaling in a prostate cancer cell line. Int. J. Mol. Sci. 2021, 22, 754. [Google Scholar] [CrossRef]
- Bonkhoff, H.; Berges, R. The evolving role of estrogens and their receptors in the development and progression of prostate cancer. Eur. Urol. 2009, 55, 533–542. [Google Scholar] [CrossRef]
- Bonkhoff, H. Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression. Prostate 2018, 78, 2–10. [Google Scholar] [CrossRef]
- Park, K.; Dalton, J.T.; Narayanan, R.; Barbieri, C.E.; Hancock, M.L.; Bostwick, D.G.; Steiner, M.S.; Rubin, M.A. TMPRSS2:ERG gene fusion predicts subsequent detection of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. J. Clin. Oncol. 2014, 32, 206–211. [Google Scholar] [CrossRef]
- Bianco, J.J.; McPherson, S.J.; Wang, H.; Prins, G.S.; Risbridger, G.P. Transient neonatal estrogen exposure to estrogen deficient mice (Aromatase knockout) reduces prostate weight and induces inflammation in late life. Am. J. Pathol. 2006, 168, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Ozten, N.; Vega, K.; Liehr, J.; Huang, X.; Horton, L.; Cavalieri, E.L.; Rogan, E.G.; Bosland, M.C. Role of Estrogen in Androgen-Induced Prostate Carcinogenesis in NBL Rats. Horm. Cancer 2019, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Andò, S.; Catalano, S. Phosphodiesterase Type 5 and Cancers: Progress and Challenges. Oncotarget 2017, 8, 99179–99202. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Mitchell, C.; Dent, P.; Batra, S.K.; Kukreja, R.C. Sildenafil (Viagra) sensitizes prostate cancer cells to doxorubicin-mediated apoptosis through CD95. Oncotarget 2016, 7, 4399–4441. [Google Scholar] [CrossRef]
- Zenzmaier, C.; Sampson, N.; Pernkopf, D.; Plas, E.; Untergasser, G.; Berger, P. Attenuated proliferation and trans-differentiation of prostatic stromal cells indicate suitability of phosphodiesterase type 5 inhibitors for prevention and treatment of benign prostatic hyperplasia. Endocrinology 2010, 151, 3975–3984. [Google Scholar] [CrossRef][Green Version]
- Chavez, A.H.; ScottCoffield, K.; Hasan Rajab, M.; Jo, C. Incidence rate of prostate cancer in men treated for erectile dysfunction with phosphodiesterase type 5 inhibitors: Retrospective analysis. Asian J. Androl. 2013, 15, 246–248. [Google Scholar] [CrossRef]
- Smith, M.R.; Kaufman, D.; George, D.; Oh, W.K.; Kazanis, M.; Manola, J.; Kantoff, P.W. Selective aromatase inhibition for patients with androgen-independent prostate carcinoma. Cancer 2002, 95, 1864. [Google Scholar] [CrossRef]
- Attia, D.M.A.; Ederveen, A.G.H. Opposing roles of ERα and ERβ in the genesis and progression of adenocarcinoma in the rat ventral prostate. Prostate 2012, 72, 1022. [Google Scholar] [CrossRef]
- Hankey, W.; Sunkel, B.; Yuan, F.; He, H.; Thomas-Ahner, J.M.; Chen, Z.; Clinton, S.K.; Huang, J.; Wang, Q. Prostate Cancer Cell Phenotypes Remain Stable Following PDE5 Inhibition in the Clinically Relevant Range. Transl. Oncol. 2020, 13, 100797. [Google Scholar] [CrossRef]
- Aversa, A.; Duca, Y.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Androgen Deficiency and Phosphodiesterase Type 5 Expression Changes in Aging Male: Therapeutic Implications. Front. Endocrinol. 2019, 10, 225. [Google Scholar] [CrossRef]
| Indication | Outcomes | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Improves:
| Phase 3 (completed) | NCT02595684 |
| Improves:
| Phase 3 (completed) | NCT01444651 |
| Improves:
| Phase 3 (completed) | NCT00750308 |
| Improves:
| Phase 3 (completed) | NCT02554045 |
| Indication | Outcomes | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|
| BPH | Improves:
| Phase 3 (completed) | NCT01460342 |
Improves:
| Phase 3 (completed) | NCT00386009 | |
Improves:
| Phase 3 (completed) | NCT01183650 | |
Improves:
| Phase 3 (completed) | NCT00827242 | |
Improves:
| Phase 3 (completed) | NCT00848081 | |
Improves:
| Phase 3 (completed) | NCT00783094 | |
Improves:
| Phase 3 (completed) | NCT00970632 | |
Improves:
| Phase 3 (completed) | NCT00861757 | |
Improves:
| Phase 3 (completed) | NCT00540124 | |
Improves:
| Phase 3 (completed) | NCT00384930 | |
Improves:
| Phase 3 (completed) | NCT01152190 | |
Improves:
| Phase 3 (completed) | NCT02431754 | |
Improves:
| Phase 3 (completed) | NCT00855582 | |
Improves:
| Phase 3 (completed) | NCT01937871 | |
Improves:
| Phase 3 (completed) | NCT01139762 | |
Improves:
| Phase 1 (recruiting) | NCT04947631 | |
Improves:
| Phase 3 (completed) | NCT00547625 | |
Improves:
| Phase 3 (completed) | NCT03246880 | |
Improves:
| Phase 3 (completed) | NCT02352311 | |
Improves:
| Phase 3 (completed) | NCT02252367 | |
Improves:
| Phase 3 (completed) | NCT04383093 | |
| Prostate cancer | Improves:
| Phase 3 (completed) | NCT00931528 |
Improves:
| Unknown | NCT00906269 | |
Improves:
| Phase 3 (completed) | NCT02103088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, E.A.; Antinozzi, C.; Di Luigi, L.; Aversa, A.; Sgrò, P. Tadalafil and Steroid Hormones Interactions in Adipose, Bone and Prostate Tissues: Focus on Translational Perspectives. Int. J. Mol. Sci. 2022, 23, 4191. https://doi.org/10.3390/ijms23084191
Greco EA, Antinozzi C, Di Luigi L, Aversa A, Sgrò P. Tadalafil and Steroid Hormones Interactions in Adipose, Bone and Prostate Tissues: Focus on Translational Perspectives. International Journal of Molecular Sciences. 2022; 23(8):4191. https://doi.org/10.3390/ijms23084191
Chicago/Turabian StyleGreco, Emanuela Alessandra, Cristina Antinozzi, Luigi Di Luigi, Antonio Aversa, and Paolo Sgrò. 2022. "Tadalafil and Steroid Hormones Interactions in Adipose, Bone and Prostate Tissues: Focus on Translational Perspectives" International Journal of Molecular Sciences 23, no. 8: 4191. https://doi.org/10.3390/ijms23084191
APA StyleGreco, E. A., Antinozzi, C., Di Luigi, L., Aversa, A., & Sgrò, P. (2022). Tadalafil and Steroid Hormones Interactions in Adipose, Bone and Prostate Tissues: Focus on Translational Perspectives. International Journal of Molecular Sciences, 23(8), 4191. https://doi.org/10.3390/ijms23084191








