CD40–CD40L in Neurological Disease
Abstract
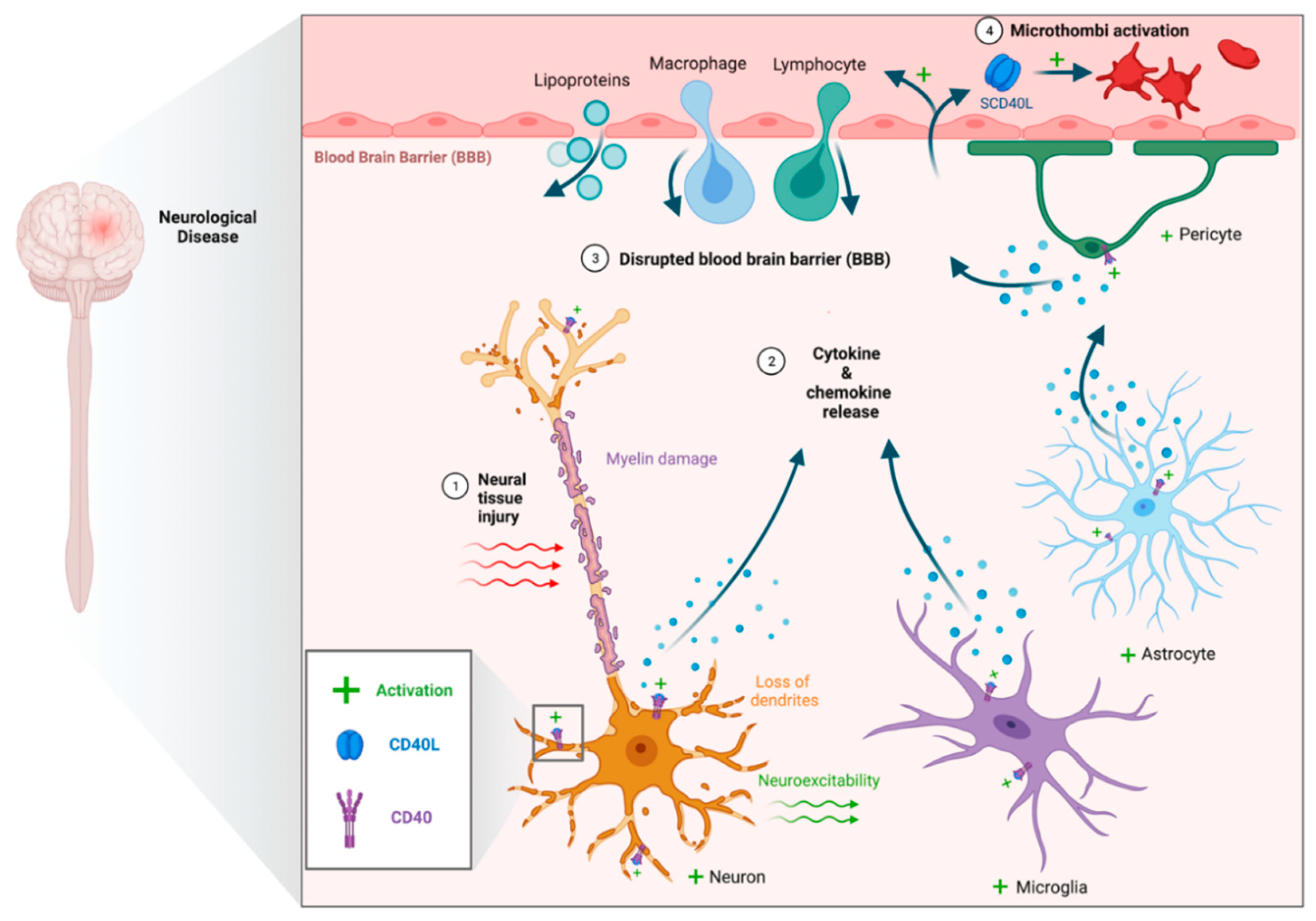
1. Introduction
2. CD40–CD40L Molecular Signaling Overview
3. CD40–CD40L in Neurological Diseases
3.1. Traumatic Brain Injury
3.2. Aging and Alzheimer’s Disease
3.3. Parkinson’s Disease
3.4. Ischemic Stroke
3.5. Epilepsy
3.6. Central and Peripheral Nerve Injury
3.7. Multiple Sclerosis
3.8. Amyotrophic Lateral Sclerosis
3.9. Myasthenia Gravis
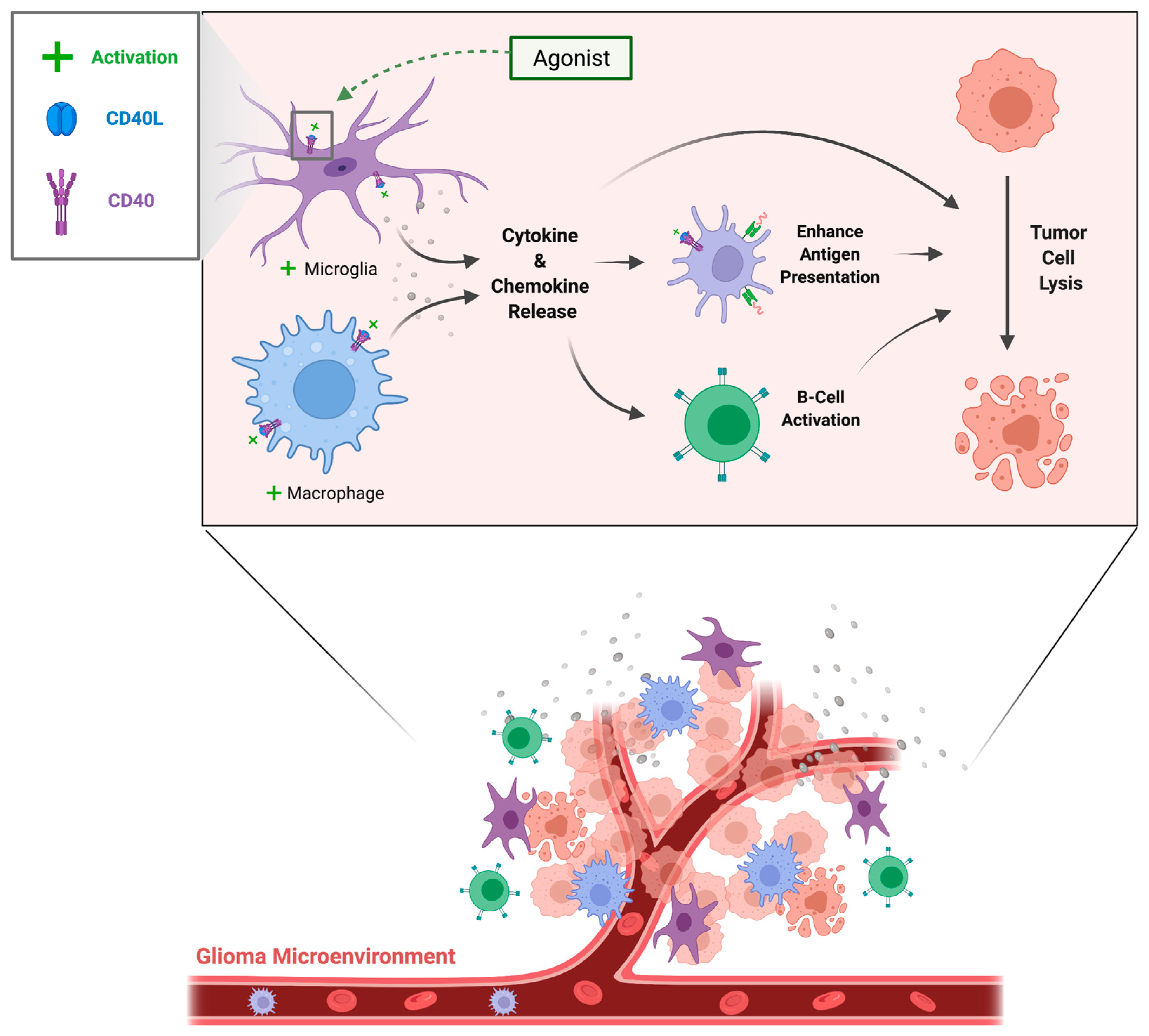
3.10. Brain Tumors
| Target | Therapy | Format | Disease | Phase | Date of Trial Completion |
|---|---|---|---|---|---|
| CD40 AGONIST | Mitazalimab [60,61,62] | Anti-CD40 mAb | PDAC [60], solid tumors [61,62] | Ib/II | 8/2025 [60], 2/2023 [61], 3/2017 [62] |
| RO7300490 [63] | FAP-α targeted CD40 agonist | Solid tumors | I | 8/2026 | |
| CD40.HIVRI.Env Vaccine [64] | Anti-CD40 mAb fused to HIV-1 envelope protein | HIV prevention | I | 12/2023 | |
| LVGN7409 [65] | Anti-CD40 mAb | Metastasis | I | 4/2023 | |
| CDX-1140 [66,67,68,69,70] | Anti-CD40 mAb | Solid and hematological malignancies [66,67,68,69,70] | I | 5/2025 [66], 12/2024 [67], 8/2023 [68], 7/2023 [69], 11/2021 [70] | |
| 2141-V11 [71,72] | Anti-CD40 mAb | Cancer lesions to the skin [71], malignant glioma [72] | I | 7/2025 [71], 12/2025 [72] | |
| Sotigalimab [73,74,75,76,77,78,79,80,81,82,83,84] | Anti-CD40 mAb | Melanoma [73,74,76,78,82,83,84], RCC [73,74], sarcoma [75], NSCLC [74,82,84], adenocarcinoma [77,81], PDAC [79], CNS tumors [80] | I/II | 2/2025 [73], 10/2024 [74], 12/2023 [75], 12/2022 [76], 11/2022 [77,78], 9/2022 [79,80], 12/2021 [81], 11/2020 [82], 8/2020 [83], 6/2018 [84] | |
| Selicrelumab [85,86,87,88,89] | Anti-CD40 mAb | BCL [85], solid tumors [86,87,89], PDAC [88] | I | 04/2021 [85], 10/2019 [86], 11/2019 [87], 11/2018 [88], 4/2018 [89] | |
| NG-350A [90] | Adenovirus expressing anti-CD40 antibody | Epithelial cancers | I | 12/2021 | |
| SEA-CD40 [91] | Anti-CD40 mAb derived from dacetuzumab | Solid tumors, lymphomas, PDAC | I | 2/2024 | |
| CP-870,893 [92,93,94,95,96] | Anti-CD40 mAb | Melanoma [92,93], mesothelioma [94], PDAC [95,96] | I | 5/2016 [92], 9/2015 [93], 1/2014 [94], 4/2013 [95], 1/2011 [96] | |
| Chi Lob 7/4 [97] | Anti-CD40 mAb | Advanced malignancies | I | 10/2014 | |
| CD40L AGONIST | AdcuCD40L [98] | Adenovirus encoding CD40L | Esophageal Carcinoma | II | 7/2011 |
| CMN-001 [99,100,101,102,103,104,105,106,107] | Dendritic cells with RNA from tumor specimen and CD40L RNA | RCC [99,100,101,103,105,106,107], NSCLC [38], genitourinary cancer [103,104] | II/III | 3/2022 [99], 5/2018 [100], 4/2018 [101], 3/2018 [102], 9/2017 [103,104], 3/2017 [105], 5/2012 [106], 2/2012 [107] | |
| Ad-sig-hMUC-1/ecdCD40L vector vaccine [108] | Adenoviral vector encoding hMUC-1 and CD40L | Epithelial cancer of the lung, breast, ovary, prostate, colon | I | 6/2017 | |
| AdCD40L [109] | Adenoviral vector encoding CD40L | Solid tumors | II | 1/2016 | |
| B-CLL vaccine [110,111] | Tumor cells expressing CD40L, IL-2 | B-CLL | I | 4/2015 [110], 8/2013 [111] | |
| rAd.CD40L [112] | Adenoviral vector encoding CD40L | Metastatic melanoma | I/II | 3/2022 | |
| GM.CD40L [113,114,115,116] | Irradiated tumor cells transduced with GM-CSF and CD40L | Mantle cell lymphoma [113], adenocarcinoma of the lung [114,116], MDS [115] | II | 7/2021 [113], 7/2020 [114], 12/2019 [115], 2/2019 [116] | |
| LOAd703 [117,118,119,120] | Adenovirus encoding TMZ-CD40L and 4-1BBL | Melanoma [117], CRC [118,119], PDAC [120], ovarian cancer [120], biliary carcinoma [120] | I/II | 6/2024 [117], 10/2023 [118], 12/2022 [119], 12/2021 [120] | |
| SL-172154 [121,122] | Fusion protein SIRPα -Fc-CD40L | Ovarian cancer [121], SCC [122] | I | 7/2022 [121,122] | |
| CD40 ANTAGONIST | CFZ533 (iscalimab) [123,124,125,126,127,128,129,130,131,132] | CD40 mAb | Kidney/liver transplant [123,124,130,132], SLE [125], SS [126,128], LN [127], MG [129], graves’ disease [131], RA [132] | II | 3/2027 [123], 1/2027 [124], 10/2024 [125], 2/2024 [125], 2/2023 [126], 9/2022 [127], 6/2018 [128], 12/2017 [129], 11/2017 [130], 4/2017 [131], 2/2017 [132] |
| BI 655064 [133,134,135,136,137,138,139] | CD40 mAb | LN [133,134], ITP [136], RA [137] | II | 8/2021 [133], 8/2020 [134], 5/2016 [135], 4/2016 [136], 4/2015 [137], 5/2014 [138], 9/2012 [139] | |
| FFP104 [140,141] | CD40 mAb | PBC [140], CD [141] | II | 12/2017 [140,141] | |
| Lucatumumab [142,143] | CD40 mAb | Lymphoma | I/II | 2/2013 [142], 5/2012 [143] | |
| Bleselumab [144,145,146,147,148] | CD40 mAb | Kidney transplant [144,145,148], psoriasis [147] | II | 10/2021 [144], 1/2017 [145], 1/2015 [146], 9/2012 [147], 1/2012 [148] | |
| CD40L ANTAGONIST | SAR441344 [149,150] | CD40L mAb | Relapsing MS [149], SS [150] | II | 1/2023 [149], 10/2022 [150] |
| AT-1501 [151,152] | CD40L mAb | T1DM patients undergoing islet cell transplantation [151], ALS [152] | II | 6/2026 [151], 10/2021 [152] | |
| VIB4920 [153,154,155,156,157] | CD40L binding protein lacking Fc domain | SS [153], kidney transplant [154], RA [155,156] | I/II | 4/2022 [153], 8/2021 [154], 7/2021 [155], 8/2018 [156], 5/2016 [157] | |
| Letolizumab [158,159] | Fc-silent anti-CD40L dAb | GVHD [158], ITP [159] | I/II | 1/2024 [158], 1/2018 [159] |
4. Therapies Targeting CD40 and CD40L
- (1)
- Attenuating CD40-mediated neuroinflammation: CD40–CD40L signaling potentiates neuroinflammatory damage in the CNS. The majority of CD40 therapies used in the treatment of autoimmune and neuroinflammatory disorders such as MG, MS, and ALS, exist in the form of antagonistic monoclonal antibodies against CD40 or CD40L and are administered either as a single agent or in combination with other antibodies, chemotherapeutic agents, and/or corticosteroids (Table 1).Using this treatment strategy, CD40 antagonists have the potential not only to limit edema, demyelination, BBB permeability, and neural tissue damage, but also to limit the disease-specific mechanisms that CD40 activation typically exacerbates; for example, the dysregulation of amyloid precursor protein (APP) processing and tau phosphorylation that contributes to the formation of neurofibrillary tangles and β-amyloid plaques in AD (Section 3.2).
- (2)
- Employing CD40-mediated recruitment of inflammatory cells to enhance tumor lysis: Motivations for targeting the CD40 axis in cancer treatment include (1) CD40 ligation initiates antigen-specific activation of B and T cells (2) the CD40 axis bridges innate and adaptive immunity as it activates natural killer cells for tumor killing and (3) CD40 expression by antigen presenting cells such as macrophages enhances their antigen presentation and co-stimulatory capacity, allowing for activation of cytotoxic T cells even without CD4+ helper T-cell signaling [160].CD40-based therapies tested in in vivo tumor models include recombinant CD40L molecules, intratumor adenoviral vectors which lead to CD40L expression, and agonistic monoclonal CD40 antibodies [160]. CD40 ligation on the surface of neoplastic cells resulted in direct cytotoxic effects, even in the absence of immune accessory cells [161,162,163]. CD40 agonism and resulting tumor cell death was shown to be synergic with chemotherapy in murine models: when combined with gemcitabine and administered to mice with established implanted tumors, most mice were cured and resistant to tumor rechallenge [164].Regarding the safety of agonistic CD40 antibodies, clinical trials have noted that adverse events such as cytokine storm, hepatotoxicity, and thromboembolic events were transient and clinically manageable [160]. Trials are underway for the treatment of solid and hematological malignancies both within and outside of the CNS. CD40-agonistic immunotherapies under investigation for the treatment of brain tumors include Sotigalimab and 2141-V11, with expected completion by the end of 2022 and 2025, respectively (Table 1).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiù, V.; et al. The Atlas of Inflammation Resolution (AIR). Mol. Asp. Med. 2020, 74, 100894. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Tone, M.; Tone, Y.; Fairchild, P.J.; Wykes, M.; Waldmann, H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc. Natl. Acad. Sci. USA 2001, 98, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Ma, C.A.; Lopez-Granados, E.; Means, G.; Brady, W.; Orange, J.; Liu, S.; Holland, S.; Derry, J.M. Specific NEMO mutations impair CD40-mediated c-Rel activation and B cell terminal differentiation. J. Clin. Investig. 2004, 114, 1593–1602. [Google Scholar] [CrossRef]
- Nair, A.; Chakraborty, S.; Banerji, L.A.; Srivastava, A.; Navare, C.; Saha, B. Ras isoforms: Signaling specificities in CD40 pathway. Cell Commun. Signal. 2020, 18, 3. [Google Scholar] [CrossRef]
- Karnell, J.L.; Rieder, S.A.; Ettinger, R.; Kolbeck, R. Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Adv. Drug Deliv. Rev. 2019, 141, 92–103. [Google Scholar] [CrossRef]
- Phipps Ying Zhang, R.P.; James Cao, H.; Graf, B.; Meekins, H. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J. Immunol. 1998, 160, 1053–1057. Available online: http://www.jimmunol.org/content/160/3/1053 (accessed on 1 December 2021).
- Michel, N.A.; Zirlik, A.; Wolf, D. CD40L and Its Receptors in Atherothrombosis—An Update. Front. Cardiovasc. Med. 2017, 4, 40. [Google Scholar] [CrossRef]
- Aloui, C.; Prigent, A.; Sut, C.; Tariket, S.; Hamzeh-Cognasse, H.; Pozzetto, B.; Richard, Y.; Cognasse, F.; Laradi, S.; Garraud, O. The Signaling Role of CD40 Ligand in Platelet Biology and in Platelet Component Transfusion. Int. J. Mol. Sci. 2014, 15, 22342–22364. [Google Scholar] [CrossRef]
- Yacoub, D.; Benslimane, N.; Al-Zoobi, L.; Hassan, G.; Nadiri, A.; Mourad, W. CD154 Is Released from T-cells by a Disintegrin and Metalloproteinase Domain-containing Protein 10 (ADAM10) and ADAM17 in a CD40 Protein-dependent Manner. J. Biol. Chem. 2013, 288, 36083–36093. [Google Scholar] [CrossRef] [PubMed]
- Heeschen, C.; Dimmeler, S.; Hamm, C.W.; Brand, M.J.V.D.; Boersma, E.; Zeiher, A.M.; Simoons, M.L. Soluble CD40 Ligand in Acute Coronary Syndromes. N. Engl. J. Med. 2003, 348, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Esposito, P.; Rampino, T.; Gregorini, M.; Gabanti, E.; Bianzina, S.; Canton, A.D. Mechanisms underlying sCD40 production in hemodialysis patients. Cell. Immunol. 2012, 278, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, M.; Rodionova, E.; Hess, M.; Giese, T.; Ho, A.D.; Dreger, P.; Luft, T. Complementary JAK/STAT Signalling Is Required for the Pro-Inflammatory Effects of CD40 Ligation: Differential Effects in Human Myeloid and B Cells. Blood 2007, 110, 2413. [Google Scholar] [CrossRef]
- Luft, T.; Jefford, M.; Luetjens, P.; Hochrein, H.; Masterman, K.-A.; Maliszewski, C.; Shortman, K.; Cebon, J.; Maraskovsky, E. IL-1β Enhances CD40 Ligand-Mediated Cytokine Secretion by Human Dendritic Cells (DC): A Mechanism for T Cell-Independent DC Activation. J. Immunol. 2002, 168, 713–722. [Google Scholar] [CrossRef]
- Masel, B.E.; DeWitt, D.S. Traumatic Brain Injury: A Disease Process, Not an Event. J. Neurotrauma 2010, 27, 1529–1540. [Google Scholar] [CrossRef]
- Turtzo, L.C.; Lescher, J.; Janes, L.; Dean, D.D.; Budde, M.D.; Frank, J.A. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J. Neuroinflamm. 2014, 11, 82. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Jiménez, A.; Borreguero-León, J.M.; García-Marín, V. Nonsurviving Patients with Severe Traumatic Brain Injury Have Maintained High Serum sCD40L Levels. World Neurosurg. 2019, 126, e1537–e1541. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Serrano, N.; Rodríguez, S.T.; Jiménez, A.; et al. Serum soluble CD40 Ligand levels are associated with severity and mortality of brain trauma injury patients. Thromb. Res. 2014, 134, 832–836. [Google Scholar] [CrossRef]
- Lorente, L. New Prognostic Biomarkers in Patients with Traumatic Brain Injury. Arch. Trauma Res. 2015, 4, e30165. [Google Scholar] [CrossRef]
- DU, A.L.; Ji, T.L.; Yang, B.; Cao, J.F.; Zhang, X.G.; Li, Y.; Pan, S. Neuroprotective effect of AG490 in experimental traumatic brain injury of rats. Chin. Med. J. 2013, 126, 2934–2937. [Google Scholar] [PubMed]
- Rezai-Zadeh, K.; Tan, J. Impact of the CD40-CD40L Dyad in Alzheimers Disease. CNS Neurol. Disord. Drug Targets 2010, 9, 149–155. [Google Scholar] [CrossRef]
- Howard, L.; McWilliams, T.G.; Wyatt, S.; Davies, A.M. CD40 forward signaling is a physiological regulator of early sensory axon growth. Development 2019, 146, dev176495. [Google Scholar] [CrossRef] [PubMed]
- Laporte, V.; Ait-Ghezala, G.; Volmar, C.-H.; Mullan, M. CD40 deficiency mitigates Alzheimer’s disease pathology in transgenic mouse models. J. Neuroinflamm. 2006, 3, 3. [Google Scholar] [CrossRef]
- Calingasan, N.Y.; Erdely, H.A.; Altar, C.A. Identification of CD40 ligand in Alzheimer’s disease and in animal models of Alzheimer’s disease and brain injury. Neurobiol. Aging 2002, 23, 31–39. [Google Scholar] [CrossRef]
- Singh, N.; Pillay, V.; Choonara, Y. Advances in the treatment of Parkinson’s disease. Prog. Neurobiol. 2007, 81, 29–44. [Google Scholar] [CrossRef]
- Okuno, T.; Nakatsuji, Y.; Kumanogoh, A.; Moriya, M.; Ichinose, H.; Sumi, H.; Fujimura, H.; Kikutani, H.; Sakoda, S. Loss of dopaminergic neurons by the induction of inducible nitric oxide synthase and cyclooxygenase-2 via CD40: Relevance to Parkinson’s disease. J. Neurosci. Res. 2005, 81, 874–882. [Google Scholar] [CrossRef]
- Schönbeck, U.; Libby, P. CD40 Signaling and Plaque Instability. Circ. Res. 2001, 89, 1092–1103. [Google Scholar] [CrossRef]
- Garlichs, C.; Kozina, S.; Fateh-Moghadam, S.; Tomandl, B.; Stumpf, C.; Eskafi, S.; Raaz, D.; Schmeißer, A.; Yilmaz, A.; Ludwig, J.; et al. Upregulation of CD40-CD40 Ligand (CD154) in Patients with Acute Cerebral Ischemia. Stroke 2003, 34, 1412–1418. [Google Scholar] [CrossRef]
- Mach, F.; Schönbeck, U.; Sukhova, G.K.; Bourcier, T.; Bonnefoy, J.-Y.; Pober, J.S.; Libby, P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40–CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA 1997, 94, 1931–1936. [Google Scholar] [CrossRef]
- Ramos-Cabrer, P.; Campos, F.; Sobrino, T.; Castillo, J. Targeting the Ischemic Penumbra. Stroke 2011, 42, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Vowinkel, T.; Stokes, K.Y.; Arumugam, T.V.; Yilmaz, G.; Nanda, A.; Granger, D.N. CD40/CD40 Ligand Signaling in Mouse Cerebral Microvasculature After Focal Ischemia/Reperfusion. Circulation 2005, 111, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J. Neuroinflamm. 2018, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, M.; Yang, H.; Wu, T.; Song, C.; Guo, R. Evidence for involvement of the CD40/CD40L system in post-stroke epilepsy. Neurosci. Lett. 2014, 567, 6–10. [Google Scholar] [CrossRef]
- Pototskiy, E.; Vinokuroff, K.; Ojeda, A.; Major, C.K.; Sharma, D.; Anderson, T.; Howard, K.; Borenstein, R.; Musto, A.E. Downregulation of CD40L–CD40 attenuates seizure susceptibility and severity of seizures. Sci. Rep. 2021, 11, 17262. [Google Scholar] [CrossRef]
- Cao, L.; Palmer, C.D.; Malon, J.T.; De Leo, J.A. Critical role of microglial CD40 in the maintenance of mechanical hypersensitivity in a murine model of neuropathic pain. Eur. J. Immunol. 2009, 39, 3562–3569. [Google Scholar] [CrossRef]
- Malon, J.T.; Maddula, S.; Bell, H.; Cao, L. Involvement of calcitonin gene-related peptide and CCL2 production in CD40-mediated behavioral hypersensitivity in a model of neuropathic pain. Neuron Glia Biol. 2011, 7, 117–128. [Google Scholar] [CrossRef]
- Brown, D.L.; Bishop, D.K.; Wood, S.Y.; Cederna, P.S. Short-Term Anti-CD40 Ligand Costimulatory Blockade Induces Tolerance to Peripheral Nerve Allografts, Resulting in Improved Skeletal Muscle Function. Plast. Reconstr. Surg. 2006, 117, 2250–2258. [Google Scholar] [CrossRef]
- Malon, J.T.; Cao, L. Calcitonin gene-related peptide contributes to peripheral nerve injury-induced mechanical hypersensitivity through CCL5 and p38 pathways. J. Neuroimmunol. 2016, 297, 68–75. [Google Scholar] [CrossRef][Green Version]
- Kan, H.-W.; Hsieh, J.-H.; Chien, H.-F.; Lin, Y.-H.; Yeh, T.-Y.; Chao, C.-C.; Hsieh, S.-T. CD40-mediated HIF-1α expression underlying microangiopathy in diabetic nerve pathology. Dis. Model. Mech. 2018, 11, dmm033647. [Google Scholar] [CrossRef]
- Aarts, S.A.B.M.; Seijkens, T.; Van Dorst, K.J.F.; Dijkstra, C.D.; Kooij, G.; Lutgens, E. The CD40–CD40L Dyad in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Front. Immunol. 2017, 8, 1791. [Google Scholar] [CrossRef]
- Laman, J.D.; De Boer, M.; Hart, B.A. CD40 in Clinical Inflammation: From Multiple Sclerosis to Atherosclerosis. Dev. Immunol. 1998, 6, 215–222. [Google Scholar] [CrossRef]
- Salou, M.; Nicol, B.; Garcia, A.; Laplaud, D.-A. Involvement of CD8+ T Cells in Multiple Sclerosis. Front. Immunol. 2015, 6, 604. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Mori, M.; Uchida, T.; Uzawa, A.; Ohtani, R.; Kuwabara, S. Soluble CD40 ligand contributes to blood–brain barrier breakdown and central nervous system inflammation in multiple sclerosis and neuromyelitis optica spectrum disorder. J. Neuroimmunol. 2017, 305, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Karni, A.; Abraham, M.; Monsonego, A.; Cai, G.; Freeman, G.J.; Hafler, D.; Khoury, S.J.; Weiner, H.L. Innate immunity in multiple sclerosis: Myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 2006, 177, 4196–4202. [Google Scholar] [CrossRef]
- Okada, Y.; Ochi, H.; Fujii, C.; Hashi, Y.; Hamatani, M.; Ashida, S.; Kawamura, K.; Kusaka, H.; Matsumoto, S.; Nakagawa, M.; et al. Signaling via toll-like receptor 4 and CD40 in B cells plays a regulatory role in the pathogenesis of multiple sclerosis through interleukin-10 production. J. Autoimmun. 2018, 88, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, D.; Mausner-Fainberg, K.; Urshansky, N.; Fahoum, F.; Karni, A. Interferon-β therapy up-regulates BDNF secretion from PBMCs of MS patients through a CD40-dependent mechanism. J. Neuroimmunol. 2009, 211, 114–119. [Google Scholar] [CrossRef]
- Lincecum, J.M.; Vieira, F.; Wang, M.Z.; Thompson, K.; De Zutter, G.S.; Kidd, J.; Moreno, A.; Sanchez, R.; Carrion, I.J.; Levine, B.A.; et al. From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat. Genet. 2010, 42, 392–399. [Google Scholar] [CrossRef]
- Behin, A.; Le Panse, R. New Pathways and Therapeutic Targets in Autoimmune Myasthenia Gravis. J. Neuromuscul. Dis. 2018, 5, 265–277. [Google Scholar] [CrossRef]
- Cruz, P.M.R.; Cossins, J.; Beeson, D.; Vincent, A. The Neuromuscular Junction in Health and Disease: Molecular Mechanisms Governing Synaptic Formation and Homeostasis. Front. Mol. Neurosci. 2020, 13, 226. [Google Scholar] [CrossRef]
- Shi, F.D.; He, B.; Li, H.; Matusevicius, D.; Link, H.; Ljunggren, H.G. Differential requirements for CD28 and CD40 ligand in the in-duction of experimental autoimmune myasthenia gravis. Eur. J. Immunol. 1998, 28, 3587–3593. [Google Scholar] [CrossRef]
- Alabbad, S.; AlGaeed, M.; Sikorski, P.; Kaminski, H.J. Monoclonal Antibody-Based Therapies for Myasthenia Gravis. BioDrugs 2020, 34, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-H.; Barchan, D.; Maiti, P.K.; Fuchs, S.; Souroujon, M.C. Blockade of CD40 Ligand Suppresses Chronic Experimental Myasthenia Gravis by Down-Regulation of Th1 Differentiation and Up-Regulation of CTLA. J. Immunol. 2001, 166, 6893–6898. [Google Scholar] [CrossRef] [PubMed]
- Van Hooren, L.; Vaccaro, A.; Ramachandran, M.; Vazaios, K.; Libard, S.; van de Walle, T.; Georganaki, M.; Huang, H.; Pietilä, I.; Lau, J.; et al. Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma. Nat. Commun. 2021, 12, 4127. [Google Scholar] [CrossRef]
- Chonan, M.; Saito, R.; Shoji, T.; Shibahara, I.; Kanamori, M.; Sonoda, Y.; Watanabe, M.; Kikuchi, T.; Ishii, N.; Tominaga, T. CD40/CD40L expression correlates with the survival of patients with glioblastomas and an augmentation in CD40 signaling enhances the efficacy of vaccinations against glioma models. Neuro-Oncol. 2015, 17, 1453–1462. [Google Scholar] [CrossRef]
- Lee-Chang, C.; Miska, J.; Hou, D.; Rashidi, A.; Zhang, P.; Burga, R.A.; Jusué-Torres, I.; Xiao, T.; Arrieta, V.A.; Zhang, D.Y.; et al. Activation of 4-1BBL+ B cells with CD40 agonism and IFNγ elicits potent immunity against glioblastoma. J. Exp. Med. 2021, 218, e20200913. [Google Scholar] [CrossRef]
- Werner, J.-M.; Kuhl, S.; Ulrich, K.; Krischek, B.; Stavrinou, P.; Goldbrunner, R.; Timmer, M. Expression of CD40 Correlates Negatively with Overall and Progression-Free Survival of Low- and High-Grade Gliomas. World Neurosurg. 2019, 130, e17–e25. [Google Scholar] [CrossRef]
- Berberich, A.; Bartels, F.; Tang, Z.; Knoll, M.; Pusch, S.; Hucke, N.; Kessler, T.; Dong, Z.; Wiestler, B.; Winkler, F.; et al. LAPTM5–CD40 Crosstalk in Glioblastoma Invasion and Temozolomide Resistance. Front. Oncol. 2020, 10, 747. [Google Scholar] [CrossRef]
- Xie, F.; Shi, Q.; Wang, Q.; Ge, Y.; Chen, Y.; Zuo, J.; Gu, Y.; Deng, H.; Mao, H.; Hu, Z. CD40 is a regulator for vascular endothelial growth factor in the tumor microenvironment of glioma. J. Neuroimmunol. 2010, 222, 62–69. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). An Open-label Phase 1b/2 Study Assessing the Safety and Efficacy of Mitazalimab in Combination with Chemotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma. (17 September 2021). Identifier: NCT04888312. Available online: https://clinicaltrials.gov/ct2/show/NCT04888312 (accessed on 1 October 2021).
- Janssen Research & Development, LLC. A Phase 1, Open-Label Study of the Safety, Pharmacokinetics and Pharmacodynamics of JNJ-64457107, an Agonistic Human Monoclonal Antibody Targeting CD40 in Patients with Advanced Stage Solid Tumors. (21 September 2016–29 July 2021). Identifier: NCT02829099. Available online: https://clinicaltrials.gov/ct2/show/NCT02829099 (accessed on 1 October 2021).
- Irenaeus, S.M.M.; Nielsen, D.; Ellmark, P.; Yachnin, J.; Deronic, A.; Nilsson, A.; Norlén, P.; Veitonmäki, N.; Wennersten, C.S.; Ullenhag, G.J. First-in-human study with intratumoral administration of a CD40 agonistic antibody, ADC-1013, in advanced solid malignancies. Int. J. Cancer 2019, 145, 1189–1199. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). An Open-Label, Multicenter, Dose-Escalation and Expansion, Phase I Study to Evaluate Safety, Pharmacokinetics, and Anti-Tumor Activity of RO7300490, A Fibroblast Activation Protein-alpha (FAP) Targeted CD40 Agonist, as Single Agent or in Combination with Atezolizumab in Participants with Advanced and/or Metastatic Solid Tumors. (18 May 2021). Identifier: NCT04857138. Available online: https://clinicaltrials.gov/ct2/show/NCT04857138 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase I Multicenter Double-blind Placebo Controlled Dose Escalation Trial of an Adjuvanted Anti-CD40 mAb Fused to Env GP140 HIV Clade C ZM-96 (CD40.HIVRI.Env) Vaccine Combined or Not with a DNA-HIV-PT123 HIV-1 Vaccine in Healthy Participants. (29 March 2021). Identifier: NCT04842682. Available online: https://clinicaltrials.gov/ct2/show/NCT04842682 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). An Open-Label, First in Human (FIH), Phase 1a/1b Trial of LVGN7409 (CD40 Agonist Antibody) as a Single Agent, in Combination with LVGN3616 (Anti-PD-1 Antibody), and in Combination with LVGN3616 and LVGN6051 (CD137 Agonist Antibody) in Patients with Locally Advanced, Relapsed, Refractory, or Metastatic Malignancy. (11 December 2020). Identifier: NCT04635995. Available online: https://clinicaltrials.gov/ct2/show/NCT04635995 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Enhanced Melanoma Vaccine Against Neoantigen and Shared Antigens by CD40 Activation and TLR Agonists in Patients with Melanoma. (28 September 2020). Identifier: NCT04364230. Available online: https://clinicaltrials.gov/ct2/show/NCT04364230 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Hotspot TCR-T: A Phase I/Ib Study of Adoptively Transferred T-cell Receptor Gene-engineered T Cells (TCR-T) Targeting Tumor-Specific Antigens, with in Vivo CD40 Activation and PD-1 Blockade, for Patients with Incurable Cancers. (8 February 2022). Identifier: NCT04520711. Available online: https://clinicaltrials.gov/ct2/show/NCT04520711 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). FLT3 Ligand (CDX-301), CD40 Agonist Antibody (CDX-1140), and Stereotactic Radiotherapy versus Standard Therapy for Advanced Non-small Cell Lung Cancer: A Phase I/II Randomized Trial. (1 January 2021). Identifier: NCT04491084. Available online: https://clinicaltrials.gov/ct2/show/NCT04491084 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase I Study of In Situ Immunomodulation with CDX-301, Radiotherapy, CDX-1140, and Poly-ICLC in Unresectable and Metastatic Breast Cancer Patients with Injectable Palpable Disease. (15 January 2022). Identifier: NCT04616248. Available online: https://clinicaltrials.gov/ct2/show/NCT04616248 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 1 Study of CDX-1140 as Monotherapy or in Combination in Patients with Advanced Malignancies. (1 December 2017). Identifier: NCT03329950. Available online: https://clinicaltrials.gov/ct2/show/NCT03329950 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase I, Dose-escalation Study Investigating the Safety and Tolerability of Intratumoral Injection of an Fc-engineered Anti-CD40 Monoclonal Antibody (2141-V11) in Patients with Cancer. (16 January 2020). Identifier: NCT04059588. Available online: https://clinicaltrials.gov/ct2/show/NCT04059588 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 1 Trial of D2C7-IT in Combination with an Fc-engineered Anti-CD40 Monoclonal Antibody (2141-V11) Administered Intratumorally via Convection-Enhanced Delivery for Adult Patients with Recurrent Malignant Glioma. (9 July 2021). Identifier: NCT04547777. Available online: https://clinicaltrials.gov/ct2/show/NCT04547777 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase I Study of APX005M in Combination with Nivolumab and Ipilimumab in Treatment Naïve patients with Advanced Melanoma or Renal Cell Carcinoma. (14 September 2020). Identifier: NCT04495257. Available online: https://clinicaltrials.gov/ct2/show/NCT04495257 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase I/Ib Study of APX005M in Combination with Nivolumab and Cabiralizumab in Patients with Advanced Melanoma, Non-small Cell Lung Cancer or Renal Cell Carcinoma Whose Disease Has Progressed on Anti-PD-1/PD-L1 Therapy. (9 June 2018). Identifier: NCT03502330. Available online: https://clinicaltrials.gov/ct2/show/NCT03502330 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase II Trial Evaluating APX005M (a CD40 Agonistic Monoclonal Antibody) in Combination with Standard-of-Care Doxorubicin for the Treatment of Advanced Sarcomas. (20 March 2019). Identifier: NCT03719430. Available online: https://clinicaltrials.gov/ct2/show/NCT03719430 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Phase I/II Dose Escalation and Cohort Expansion of Safety and Tolerability Study of Intratumoral CD40 Agonistic Monoclonal Antibody APX005M in Combination with Systemic Pembrolizumab in Patients with Metastatic Melanoma. (2 June 2017). Identifier: NCT02706353. Available online: https://clinicaltrials.gov/ct2/show/NCT02706353 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). INNATE: Immunotherapy During Neoadjuvant Therapy for Rectal Cancer, a Phase II Randomized Multi-Center Trial with and without APX005M, an Anti-CD40 Agonist. (24 April 2020). Identifier: NCT04130854. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04130854 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase II Multicenter, Open-label Study to Evaluate the Safety and Efficacy of the CD40 Agonistic Antibody APX005M with or without Stereotactic Body Radiation in Adults with Unresectable or Metastatic Melanoma. (16 December 2019). Identifier: NCT04337931. Available online: https://clinicaltrials.gov/ct2/show/NCT04337931 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Open-label, Multicenter, Phase 1b/2 Clinical Study to Evaluate the Safety and Efficacy of CD40 Agonistic Monoclonal Antibody (APX005M) Administered with Gemcitabine and Nab-Paclitaxel with or without PD-1 Blocking Antibody (Nivolumab) in Patients with Previously Untreated Metastatic Pancreatic Adenocarcinoma. (21 July 2017). Identifier: NCT03214250. Available online: https://clinicaltrials.gov/ct2/show/NCT03214250 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Phase I Study to Evaluate the Safety and Tolerability of the CD40 Agonistic Monoclonal Antibody APX005M in Pediatric Subjects with Recurrent/Refractory Brain Tumors and Newly Diagnosed Brain Stem Glioma. (2 February 2018). Identifier: NCT03389802. Available online: https://clinicaltrials.gov/ct2/show/NCT03389802 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 2 Study of APX005M in Combination with Concurrent Chemoradiation as Neoadjuvant Therapy for Resectable Esophageal and Gastroesophageal Junction Cancers. (6 October 2017). Identifier: NCT03165994. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03165994 (accessed on 1 October 2021).
- Apexigen, Inc. A Study to Evaluate the Safety and Efficacy of the CD40 Agonistic Antibody APX005M Administered in Combination with Nivolumab in Subjects with Non-Small Cell Lung Cancer and Subjects with Metastatic Melanoma. (10 July 2017–16 November 2020). Identifier: NCT03123783. Available online: https://clinicaltrials.gov/ct2/show/NCT03123783 (accessed on 1 October 2021).
- DeMario, M. An Open-label, Phase 1B Study of NEO-PV-01 + CD40 Agonist Antibody (APX005M) or Ipilimumab with Nivolumab in Patients with Advanced or Metastatic Melanoma. (8 October 2018–11 August 2020). Identifier: NCT03597282. Available online: https://clinicaltrials.gov/ct2/show/NCT03597282 (accessed on 1 October 2021).
- Apexigen, Inc. Phase 1 Study to Evaluate the Safety and Tolerability of the CD40 Agonistic Monoclonal Antibody APX005M in Subjects with Solid Tumors. (May 2015–19 June 2018). Identifier: NCT02482168. Available online: https://clinicaltrials.gov/ct2/show/NCT02482168 (accessed on 1 October 2021).
- Roch HOUOT & CHU Rennes. Phase Ib Study Evaluating the Safety and Efficacy of Intratumoral Agonistic Anti-CD40 (Selicrelumab) in Combination with Anti-PDL1 (Atezolizumab) in Patients with Refractory or Relapsed B Cell Lymphoma. (4 July 2019–7 April 2021). Identifier: NCT03892525. Available online: https://clinicaltrials.gov/ct2/show/NCT03892525 (accessed on 1 October 2021).
- Hoffmann-La Roche. An Open-Label, Multicenter, Dose Escalation Phase Ib Study with Expansion Cohorts to Evaluate the Safety, Pharmacokinetics, Pharmacodynamics, and Therapeutic Activity of RO7009789 (CD40 Agonistic Monoclonal Antibody) in Combination with Vanucizumab (Anti-Ang2 and Anti-VEGF Bi-Specific Monoclonal Antibody, Part I) or Bevacizumab (Anti-VEGF Monoclonal Antibody, Part II) in patients with Metastatic Solid Tumors. (25 January 2016–30 October 2019). Identifiers: NCT02665416. Available online: https://clinicaltrials.gov/ct2/show/NCT02665416 (accessed on 1 October 2021).
- Hoffmann-La Roche. An Open-Label, Multicenter, Dose-Escalation Phase IB Study to Investigate the Safety, Pharmacokinetics, Pharmacodynamics, and Therapeutic Activity of Selicrelumab (CD40 Agonist) in Combination with Atezolizumab (Anti PD-L1) in Patients with Locally Advanced and/or Metastatic Solid Tumors. (12 December 2014–7 November 2019). Identifiers: NCT02304393. Available online: https://clinicaltrials.gov/ct2/show/NCT02304393 (accessed on 1 October 2021).
- Vonderheide, R. Phase I Study of Neo-adjuvant RO7009789 Alone or Neo-adjuvant RO7009789 Plus Nab-Paclitaxel and Gemcitabine Followed by Adjuvant RO7009789 Plus Nab-Paclitaxel and Gemcitabine for Patients with Newly Diagnosed Resectable Pancreatic Carcinoma. (October 2015–November 2018). Identifier: NCT02588443. Available online: https://clinicaltrials.gov/ct2/show/NCT02588443 (accessed on 1 October 2021).
- Machiels, J.P.; Gomez-Roca, C.; Michot, J.M.; Zamarin, D.; Mitchell, T.; Catala, G.; Eberst, L.; Jacob, W.; Jegg, A.M.; Cannarile, M.A.; et al. Phase Ib study of anti-CSF-1R antibody emactuzumab in combination with CD40 agonist selicrelumab in advanced solid tumor patients. J. Immunother. Cancer 2020, 8, e001153. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Multicentre, Open Label, Non-randomised First in Human Study of NG-350A (Monotherapy), and NG-350A With a Check Point Inhibitor in Patients with Metastatic or Advanced Epithelial Tumours. (19 February 2019). Identifier: NCT03852511. Available online: https://clinicaltrials.gov/ct2/show/NCT03852511 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 1, Open-label, Dose-escalation Study of SEA-CD40 in Adult Patients with Advanced Malignancies. (28 February 2015). Identifier: NCT02376699. Available online: https://clinicaltrials.gov/ct2/show/NCT02376699 (accessed on 1 October 2021).
- Vonderheide, R. A Phase 1 Dose-Escalation Trial to Evaluate Safety, Tolerability and Immune Pharmacodynamics of Combined Administration of Tremelimumab (Blocking Anti-CTLA-4 Antibody) And CP-870,893 (Agonist Anti-CD40 Antibody) In Patients With Metastatic Melanoma. (February 2010–May 2016). Identifier: NCT0113635. Available online: https://clinicaltrials.gov/ct2/show/NCT01103635 (accessed on 1 October 2021).
- Weber, J. A Phase I Study of Poly IC:LC and NY-ESO-1/gp100 Peptides Either Emulsified with Montanide ISA 51 or in Aqueous Solution with Escalating Doses of CP 870,893 in the Treatment of Subjects With Resected Stage III or Stage IV Melanoma. (October 2009–September 2015). Identifier: NCT01008527. Available online: https://clinicaltrials.gov/ct2/show/NCT01008527 (accessed on 1 October 2021).
- Nowak, A.K.; Cook, A.M.; McDonnell, A.M.; Millward, M.J.; Creaney, J.; Francis, R.J.; Hasani, A.; Segal, A.; Musk, A.W.; Turlach, B.A.; et al. A phase 1b clinical trial of the CD40-activating antibody CP-870,893 in combination with cisplatin and pemetrexed in malignant pleural mesothelioma. Ann. Oncol. 2015, 26, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Rose, S. Immunotherapy network launches first trial. Cancer Discov. 2012, 2, 760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Challis, R.; Chowdhury, F.; Gao, Y.; Harvey, M.; Geldart, T.; Kerr, P.; Chan, C.; Smith, A.; Steven, N.; et al. Clinical and biological effects of an agonist anti-CD40 antibody: A Cancer Research UK phase I study. Clin. Cancer Res. 2015, 21, 1321–1328. [Google Scholar] [CrossRef]
- Crystal, R.G. Phase II, Randomized, Double-blinded, Placebo-Control, Toxicity/Efficacy Study on the Transfer of Adenovirus with the CD40 Ligand Gene (AdcuCD40L) to Patients with Stage I, II or III Esophageal Carcinoma. (July 2011). Identifier: NCT00504322. Available online: https://clinicaltrials.gov/ct2/show/NCT00504322 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 2b Randomized Trial of Autologous Dendritic Cell Immunotherapy (CMN-001) Plus Standard Treatment of Advanced Renal Cell Carcinoma. (22 July 2020). Identifier: NCT04203901. Available online: https://clinicaltrials.gov/ct2/show/NCT04203901 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Rollover Phase II Study Testing the Biologic Activity and Safety of AGS-003 in Renal Cell Carcinoma Subjects with Prolonged Response or Stable Disease and Ongoing AGS-003 Treatment in Protocol AGS-003-004 or AGS-003-006. (27 June 2018). Identifier: NCT01482949. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01482949 (accessed on 1 October 2021).
- Figlin, R.A.; Tannir, N.M.; Uzzo, R.G.; Tykodi, S.S.; Chen, D.Y.T.; Master, V.; Kapoor, A.; Vaena, D.; Lowrance, W.; Bratslavsky, G.; et al. Results of the ADAPT Phase 3 Study of Rocapuldencel-T in Combination with Sunitinib as First-Line Therapy in Patients with Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2020, 26, 2327–2336. [Google Scholar] [CrossRef]
- Nordquist, L.T. A Safety and Feasibility Study of AGS-003-LNG for the Treatment of Stage 3 Non-Small Cell Lung Cancer. (March 2016–March 2018). Identifier: NCT02662634. Available online: https://clinicaltrials.gov/ct2/show/NCT02662634 (accessed on 1 October 2021).
- Harrison, M. RNA Extraction and Amplification from Biopsy Specimens in Subjects with Metastatic Renal Cell Carcinoma (AGS-NTS-017). (March 2014–7 September 2017). Identifier: NCT 02026960. Available online: https://clinicaltrials.gov/ct2/show/NCT02026960 (accessed on 1 October 2021).
- Costello, B. Pilot Study of Gemcitabine and Cisplatin Plus AGS-003-BLD in Patients with Muscle-Invasive Bladder Cancer Undergoing Neoadjuvant Cisplatin-Based Chemotherapy. (November 2016–5 September 2017). Identifier: NCT02944357. Available online: https://clinicaltrials.gov/ct2/show/NCT02944357 (accessed on 1 October 2021).
- Schwaab, T. Neoadjuvant AGS-003 Immunotherapy in Patients with Localized Kidney Cancer. (14 October 2014–17 March 2017). Identifier: NCT02170389. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02170389 (accessed on 1 October 2021).
- Amin, A.; Dudek, A.Z.; Logan, T.F.; Lance, R.S.; Holzbeierlein, J.M.; Knox, J.J.; Master, V.A.; Pal, S.K.; Miller, W.H., Jr.; Karsh, L.I.; et al. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J. Immunother. Cancer. 2015, 3, 14. [Google Scholar] [CrossRef]
- Chew, T. A Phase I/II Study Testing the Biologic Activity and Safety of AGS-003 as an Immunotherapeutic in Subjects with Newly Diagnosed Stage IV Renal Cell Carcinoma. (January 2006–February 2012). Identifier: NCT00272649. Available online: https://clinicaltrials.gov/ct2/show/NCT00272649 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Ad-sig-hMUC-1/ecdCD40L Vector Vaccine for Immunotherapy of Epithelial Cancers. (September 2014). Identifier: NCT02140996. Available online: https://clinicaltrials.gov/ct2/show/NCT02140996 (accessed on 1 October 2021).
- Schiza, A.; Wenthe, J.; Mangsbo, S.; Eriksson, E.; Nilsson, A.; Tötterman, T.H.; Loskog, A.; Ullenhag, G. Adenovirus-mediated CD40L gene transfer increases Teffector/Tregulatory cell ratio and upregulates death receptors in metastatic melanoma patients. J. Transl. Med. 2017, 15, 79. [Google Scholar] [CrossRef]
- Mims, M.; Brenner, M.B. Treatment of B-CLL With Autologous IL2 and CD40 Ligand-Expressing Tumor Cells + Lenalidomide. (February 2013–April 2015). Identifier: NCT01604031. Available online: https://clinicaltrials.gov/ct2/show/NCT01604031 (accessed on 1 October 2021).
- Brenner, M.K. Prolonged Immunization with Autologous CD40 Ligand and IL-2-Expressing Tumor Cells for Treatment of B-Chronic Lymphocytic Leukemia (B-CLL). (December 2006–August 2013). Identifier: NCT00458679. Available online: https://clinicaltrials.gov/ct2/show/NCT00458679 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Phase I/II Dose Escalation and Cohort Expansion of Safety and Tolerability Study of Intratumoral rAd.CD40L (ISF35) in Combination of Systemic Pembrolizumab in Patients with Refractory Metastatic Melanoma. (March 2018). Identifier: NCT02719015. Available online: https://clinicaltrials.gov/ct2/show/NCT02719015 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase II Trial Using a Universal GM-CSF-Producing and CD40L-Expressing Bystander Cell Line (GM.CD40L) in the Formulation of Autologous Tumor Cell-Based Vaccines for Patients with Mantle Cell Lymphoma. (July 2004). Identifier: NCT00101101. Available online: https://clinicaltrials.gov/ct2/show/NCT00101101 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Randomized Phase I/II Study of Nivolumab in Combination With GM.CD40L Vaccine in Adenocarcinoma of the Lung. (July 2018). Identifier: NCT02466568. Available online: https://clinicaltrials.gov/ct2/show/NCT02466568 (accessed on 1 October 2021).
- Pinilla, J. A Phase I Pilot Study of Immunotherapy Using Lenalidomide Plus “Bystander” Vaccine in Patients with High-Risk Myelodysplastic Syndrome (MDS).(2 February 2009–December 2019). Identifier: NCT00840931. Available online: https://clinicaltrials.gov/ct2/show/NCT00840931 (accessed on 1 October 2021).
- Gray, J.E.; Chiappori, A.; Williams, C.C.; Tanvetyanon, T.; Haura, E.B.; Creelan, B.C.; Kim, J.; Boyle, T.A.; Pinder-Schenck, M.; Khalil, F.; et al. A phase I/randomized phase II study of GM.CD40L vaccine in combination with CCL21 in patients with advanced lung adenocarcinoma. Cancer Immunol. Immunother. 2018, 67, 1853–1862. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Phase I/II Trial Investigating LOAd703 in Combination with Atezolizumab in Malignant Melanoma. (28 January 2020). Identifier: NCT04123470. Available online: https://clinicaltrials.gov/ct2/show/NCT04123470 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase Ib/II, Open-Label, Multicenter, Randomized Umbrella Study Evaluating the Efficacy and Safety of Multiple Immunotherapy-Based Treatment Combinations in Patients with Metastatic Colorectal Cancer (Morpheus-CRC). (27 September 2018). Identifier: NCT03555149. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03555149 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Phase I/II Trial Investigating an Immunostimulatory Oncolytic Adenovirus for Cancer. (01 March 2018). Identifier: NCT03225989. Available online: https://clinicaltrials.gov/ct2/show/NCT03225989 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Phase I/IIa Trial Evaluating Safety of LOAd703, an Armed Oncolytic Adenovirus for Pancreatic Cancer. (November 2016). Identifier: NCT02705196. Available online: https://clinicaltrials.gov/ct2/show/NCT02705196 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Phase 1 Dose Escalation Study of the Agonist Redirected Checkpoint, SL-172154 (SIRPα-Fc-CD40L) Administered Intravenously in Subjects with Ovarian Cancer. (29 June 2020). Identifier: NCT04406623. Available online: https://clinicaltrials.gov/ct2/show/NCT04406623 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). Phase 1 Dose Escalation Study of the Agonist Redirected Checkpoint, SL-172154 (SIRPα-Fc-CD40L), Administered Intratumorally in Subjects with Cutaneous Squamous Cell Carcinoma or Squamous Cell Carcinoma of the Head and Neck. (17 September 2020). Identifier: NCT04502888. Available online: https://clinicaltrials.gov/ct2/show/NCT04502888 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Partially-blinded, Active-controlled, Multicenter, Randomized Study Evaluating Efficacy, Safety, Tolerability, Pharmacokinetic (PK) and Pharmacodynamic (PD) of an Anti-CD40 Monoclonal Antibody, CFZ533, in de Novo and Maintenance Kidney Transplant Recipients (CIRRUS I). (28 November 2018). Identifier: NCT03663335. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03663335 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A 12-month, Open-label, Multicenter, Randomized, Safety, Efficacy, Pharmacokinetic (PK) and Pharmacodynamic (PD) Study of an Anti-CD40 Monoclonal Antibody, CFZ533 vs. Standard of Care Control, in Adult de Novo Liver Transplant Recipients With a 12-month Additional Follow-up (CONTRAIL I). (7 October 2019). Identifier: NCT03781414. Available online: https://clinicaltrials.gov/ct2/show/NCT03781414 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Placebo-controlled, Patient and Investigator Blinded, Randomized Parallel Cohort Study to Assess Pharmacodynamics, Pharmacokinetics, Safety, Tolerability and Preliminary Clinical Efficacy of VAY736 and CFZ533 in Patients with Systemic Lupus Erythematosus (SLE). (19 December 2018). Identifier: NCT03656562. Available online: https://clinicaltrials.gov/ct2/show/NCT03656562 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A 48-week, 6-arm, Randomized, Double-blind, Placebo-controlled Multicenter Trial to Assess the Safety and Efficacy of Multiple CFZ533 Doses Administered Subcutaneously in Two Distinct Populations of Patients with Sjögren’s Syndrome (TWINSS). (1 October 2019). Identifier: NCT03905525. Available online: https://clinicaltrials.gov/ct2/show/NCT03905525 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Randomized, Placebo-controlled, Patient and Investigator Blinded, Study Investigating the Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of Multiple Doses of CFZ533 in Patients with Moderately Active Proliferative Lupus Nephritis. (12 September 2018). Identifier: NCT03610516. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03610516 (accessed on 1 October 2021).
- Novartis Pharmaceuticals. A Multi-center, Randomized, Double-blind, Placebo-controlled, Parallel Group Study to Assess the Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of CFZ533 in Patients with Primary Sjögren’s Syndrome. 22 (October 2014–29 June 2018). Identifier: NCT02291029. Available online: https://clinicaltrials.gov/ct2/show/NCT02291029 (accessed on 1 October 2021).
- Novartis Pharmaceuticals. A Multi-center, Randomized, Double-blind, Placebo-controlled, Parallel Group Study to Preliminarily Evaluate the Safety, Tolerability, Pharmacokinetics and Efficacy of CFZ533 in Patients with Moderate to Severe Myasthenia Gravis. (29 September 2015–19 December 2017). Identifier: NCT02562276. Available online: https://clinicaltrials.gov/ct2/show/NCT02565576 (accessed on 1 October 2021).
- Novartis Pharmaceuticals. A 12-month Randomized, Multiple Dose, Open-label, Study Evaluating Safety, Tolerability, Pharmacokinetics/Pharmacodynamics (PK/PD) and Efficacy of an Anti-CD40 Monoclonal Antibody, CFZ533, in Combination with Mycophenolate Mofetil (MMF) and Corticosteroids (CS), With and Without Tacrolimus (Tac), in de Novo Renal Transplant Recipients. (5 February 2015–29 November 2017). Identifier: NCT02217410. Available online: https://clinicaltrials.gov/ct2/show/NCT02217410 (accessed on 1 October 2021).
- Kahaly, G.J.; Stan, M.N.; Frommer, L.; Gergely, P.; Colin, L.; Amer, A.; Schuhmann, I.; Espie, P.; Rush, J.S.; Basson, C.; et al. A Novel Anti-CD40 Monoclonal Antibody, Iscalimab, for Control of Graves Hyperthyroidism-A Proof-of-Concept Trial. J. Clin. Endocrinol. Metab. 2020, 105, dgz013. [Google Scholar] [CrossRef]
- Espié, P.; He, Y.; Koo, P.; Sickert, D.; Dupuy, C.; Chokoté, E.; Schuler, R.; Mergentaler, H.; Ristov, J.; Milojevic, J.; et al. First-in-human clinical trial to assess pharmacokinetics, pharmacodynamics, safety, and tolerability of iscalimab, an anti-CD40 monoclonal antibody. Am. J. Transplant. 2020, 20, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Boehringer Ingelheim. An Exploratory Maintenance Trial Evaluating the Effect of BI 655064 in Lupus Nephritis Patients Who Have Achieved a Meaningful Response Either at the End of 1293.10 or After an Induction Treatment Outside of 1293.10. (9 January 2018–27 July 2021). Identifier: NCT03385564. Available online: https://clinicaltrials.gov/ct2/show/NCT03385564 (accessed on 1 October 2021).
- Boehringer Ingelhiem. A Double-blind, Randomised, Placebo-controlled Trial Evaluating the Effect of BI 655064 Administered as Sub-cutaneous Injections, on Renal Response After One Year of Treatment, in Patients with Active Lupus Nephritis. (16 May 2016–18 August 2020). Identifier: NCT02770170. Available online: https://clinicaltrials.gov/ct2/show/NCT02770170 (accessed on 1 October 2021).
- Boehringer Ingelheim. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Multiple Subcutaneous Doses of BI 655064 in Healthy Chinese Male Volunteers (Randomised, Double-blind, Placebo-controlled, Clinical Phase I Study). (September 2015–May 2016). Identifier: NCT02331277. Available online: https://clinicaltrials.gov/ct2/show/NCT02331277 (accessed on 1 October 2021).
- Boehringer Ingelheim. Safety, Efficacy, Tolerability, Pharmacokinetics and Pharmacodynamics of Open Label Study with Multiple (and for Non-Responders) Escalating Subcutaneous Doses of BI 655064 Once a Week in Patients with Chronic Primary Immune Thrombocytopenic Purpura. (December 2013–April 2016). Identifier: NCT02009761. Available online: https://clinicaltrials.gov/ct2/show/NCT02009761 (accessed on 1 October 2021).
- Visvanathan, S.; Daniluk, S.; Ptaszyński, R.; Müller-Ladner, U.; Ramanujam, M.; Rosenstock, B.; Eleftheraki, A.G.; Vinisko, R.; Petříková, A.; Kellner, H.; et al. Effects of BI 655064, an antagonistic anti-CD40 antibody, on clinical and biomarker variables in patients with active rheumatoid arthritis: A randomised, double-blind, placebo-controlled, phase IIa study. Ann. Rheum. Dis. 2019, 78, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Grimaldi, C.; Huang, F.; Benediktus, E.; Yagi, N.; Padula, S.J.; Jang, I.-J.; Steffgen, J. Safety, pharmacokinetics and pharmacodynamics of BI 655064 in phase 1 clinical trials in healthy Chinese and Japanese subjects. Br. J. Clin. Pharmacol. 2021, 87, 2000–2013. [Google Scholar] [CrossRef] [PubMed]
- Albach, F.N.; Wagner, F.; Hüser, A.; Igel, J.; Joseph, D.; Hilbert, J.; Schoelch, C.; Padula, S.J.; Steffgen, J. Safety, pharmacokinetics and pharmacodynamics of single rising doses of BI 655064, an antagonistic anti-CD40 antibody in healthy subjects: A potential novel treatment for autoimmune diseases. Eur. J. Clin. Pharmacol. 2018, 74, 161–169. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Phase I/II, Open Label, Multicenter, Pilot Dose Escalation Study to Evaluate the Safety, Tolerability and Pharmacodynamics of FFP104 in Subjects Previously Diagnosed with Primary Biliary Cirrhosis (PBC). (May 2015). Identifier: NCT02193360. Available online: https://clinicaltrials.gov/ct2/show/NCT02193360 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase II, Double-blind, Randomised, Placebo-controlled, Parallel Group Pilot Study to Evaluate the Safety and Efficacy of FFP104 in the Treatment of Subjects with Moderate to Severely Active Crohn’s Disease. (January 2016). Identifier: NCT02465944. Available online: https://clinicaltrials.gov/ct2/show/NCT02465944 (accessed on 1 October 2021).
- Novartis Pharmaceuticals. A Phase IA/II, Multi-center, Open-label Study of HCD122 Administered Intravenously Once Weekly for Four Weeks in Adult Patients with Advanced Non-Hodgkin’s or Hodgkin’s Lymphoma Who Have Progressed After at Least Two Prior Therapies (CHIR-12.12-LYM-01). (March 2008–February 2013). Identifier: NCT00670592. Available online: https://clinicaltrials.gov/ct2/show/NCT00670592 (accessed on 1 October 2021).
- Novartis Pharmaceuticals. A Phase Ib, Multicenter, Open-label Study of HCD122 Administered Intravenously in Combination with Bendamustine in Patients with CD40+ Follicular Lymphoma Who Are Refractory to Rituximab. (February 2011–May 2012). Identifier: NCT01275209. Available online: https://clinicaltrials.gov/ct2/show/NCT01275209 (accessed on 1 October 2021).
- Astellas Pharma Global Development, Inc. A Phase 2a, Randomized, Open-Label, Active Control, Multi-Center Study to Assess the Efficacy and Safety of Bleselumab in Preventing the Recurrence of Focal Segmental Glomerulosclerosis in de Novo Kidney Transplant Recipients. (22 May 2017–18 May 2021). Identifier: NCT02921789. Available online: https://clinicaltrials.gov/ct2/show/NCT02921789 (accessed on 1 October 2021).
- Harland, R.C.; Klintmalm, G.; Jensik, S.; Yang, H.; Bromberg, J.; Holman, J.; Kumar, M.S.A.; Santos, V.; Larson, T.J.; Wang, X. Efficacy and safety of bleselumab in kidney transplant recipients: A phase 2, randomized, open-label, noninferiority study. Am. J. Transplant. 2020, 20, 159–171. [Google Scholar] [CrossRef]
- Astellas Pharma Global Development. A Phase 2a, Randomized, Double-Blind, Placebo-Controlled, Sequential, Multiple Dose Escalation Study to Evaluate the Safety, Efficacy, Pharmacokinetics and Pharmacodynamics of ASKP1240 in Subjects with Moderate to Severe Plaque Psoriasis. (April 2012–January 2015). Identifier: NCT01585233. Available online: https://clinicaltrials.gov/ct2/show/NCT01585233 (accessed on 1 October 2021).
- Astellas Pharma Global Development. A Phase 1 Single-Dose, Parallel Group, Randomized, Open-Label Study to Determine the Absolute Bioavailability of ASKP1240 After Intravenous and Subcutaneous Administration in Healthy Subjects. (February 2012–September 2012). Identifier: NCT01582399. Available online: https://clinicaltrials.gov/ct2/show/NCT01582399 (accessed on 1 October 2021).
- Vincenti, F.; Klintmalm, G.; Yang, H.; Ram Peddi, V.; Blahunka, P.; Conkle, A.; Santos, V.; Holman, J. A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti-CD40 monoclonal antibody, in kidney transplantation. Am. J. Transplant. 2020, 20, 172–180. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Phase 2, Double-blind, Randomized, Placebo-controlled Study Assessing Efficacy and Safety of SAR441344, a CD40L-antagonist Monoclonal Antibody, in Participants with Relapsing Multiple Sclerosis. (7 June 2021). Identifier: NCT04879628. Available online: https://clinicaltrials.gov/ct2/show/NCT04879628 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Randomized, Double-blind, Placebo-controlled, Parallel-group Study of the Safety, Tolerability, Pharmacokinetics, and Therapeutic Efficacy of SAR441344 in Adult Patients with Primary Sjögren’s Syndrome (pSjS). (12 November 2020). Identifier: NCT04572841. Available online: https://clinicaltrials.gov/ct2/show/NCT04572841 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). An Open-Label Study to Evaluate the Safety, Tolerability and Efficacy of Immunomodulation With AT-1501 in Adults with Type 1 Diabetes Undergoing Islet Cell Transplant. (19 February 2021). Identifier: NCT04711226. Available online: https://clinicaltrials.gov/ct2/show/NCT04711226 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 2a Open-Label, Multi-Center Study to Evaluate the Safety and Tolerability of Multiple Doses of AT-1501 in Adults With ALS. (16 October 2020). Identifier: NCT04322149. Available online: https://clinicaltrials.gov/ct2/show/NCT04322149 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 2 Randomized, Double-blind, Placebo-controlled, Proof of Concept Study to Evaluate the Efficacy and Safety of VIB4920 in Subjects with Sjögren’s Syndrome (SS). (16 October 2019). Identifier: NCT04129164. Available online: https://clinicaltrials.gov/ct2/show/NCT04129164 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 2a Single-arm, Prospective, Open-label Pilot Study to Evaluate the Safety and Efficacy of Dual Costimulation Blockade with VIB4920 and Belatacept for Prophylaxis of Allograft Rejection in Adults Receiving a Kidney Transplant. (30 October 2019). Identifier: NCT04046549. Available online: https://clinicaltrials.gov/ct2/show/NCT04046549 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Mechanistic Insight and Dosage Optimization Study of the Efficacy and Safety of VIB4920 in Patients with Rheumatoid Arthritis (RA). (9 December 2019). Identifier: NCT04163991. Available online: https://clinicaltrials.gov/ct2/show/NCT04163991 (accessed on 1 October 2021).
- Karnell, J.L.; Albulescu, M.; Drabic, S.; Wang, L.; Moate, R.; Baca, M.; Oganesyan, V.; Gunsior, M.; Thisted, T.; Yan, L.; et al. A CD40L-targeting protein reduces autoantibodies and improves disease activity in patients with autoimmunity. Sci. Transl. Med. 2019, 11, eaar6584. [Google Scholar] [CrossRef]
- MedImmune LLC. A Phase 1, Randomized, Blinded, Placebo-controlled, Single-ascending Dose Study to Evaluate the Safety and Tolerability of MEDI4920 in Healthy Adults. (27 May 2014–9 May 2016). Identifier: NCT02151110. Available online: https://clinicaltrials.gov/ct2/show/NCT02151110 (accessed on 1 October 2021).
- National Library of Medicine (U.S.). CD40-L Blockade for Prevention of Acute Graft-Versus-Host Disease. 25 February 2019. Identifier: NCT03605927. Available online: https://clinicaltrials.gov/ct2/show/NCT03605927 (accessed on 1 October 2021).
- Squibb, B.-M. Open Label, Adaptive Design, Ascending, Multiple-Dose Study to Evaluate Safety and Efficacy of BMS-986004 in Adult Subjects with Primary Immune Thrombocytopenia (ITP). (17 November 2014–22 January 2018). Identifier: NCT02273960. Available online: https://clinicaltrials.gov/ct2/show/NCT02273960 (accessed on 1 October 2021).
- Piechutta, M.; Berghoff, A.S. New emerging targets in cancer immunotherapy: The role of Cluster of Differentiation 40 (CD40/TNFR5). ESMO Open 2019, 4, e000510. [Google Scholar] [CrossRef]
- Vonderheide, R.H. Prospect of Targeting the CD40 Pathway for Cancer Therapy. Clin. Cancer Res. 2007, 13, 1083–1088. [Google Scholar] [CrossRef]
- Richards, D.M.; Sefrin, J.P.; Gieffers, C.; Hill, O.; Merz, C. Concepts for agonistic targeting of CD40 in immuno-oncology. Hum. Vaccines Immunother. 2020, 16, 377–387. [Google Scholar] [CrossRef]
- Elmetwali, T.; Salman, A.; Wei, W.; Hussain, S.A.; Young, L.S.; Palmer, D.H. CD40L membrane retention enhances the immunostimulatory effects of CD40 ligation. Sci. Rep. 2020, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.K.; Robinson, B.W.S.; Lake, R.A. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003, 63, 4490–4496. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ots, H.D.; Tracz, J.A.; Vinokuroff, K.E.; Musto, A.E. CD40–CD40L in Neurological Disease. Int. J. Mol. Sci. 2022, 23, 4115. https://doi.org/10.3390/ijms23084115
Ots HD, Tracz JA, Vinokuroff KE, Musto AE. CD40–CD40L in Neurological Disease. International Journal of Molecular Sciences. 2022; 23(8):4115. https://doi.org/10.3390/ijms23084115
Chicago/Turabian StyleOts, Heather D., Jovanna A. Tracz, Katherine E. Vinokuroff, and Alberto E. Musto. 2022. "CD40–CD40L in Neurological Disease" International Journal of Molecular Sciences 23, no. 8: 4115. https://doi.org/10.3390/ijms23084115
APA StyleOts, H. D., Tracz, J. A., Vinokuroff, K. E., & Musto, A. E. (2022). CD40–CD40L in Neurological Disease. International Journal of Molecular Sciences, 23(8), 4115. https://doi.org/10.3390/ijms23084115






