Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism
Abstract
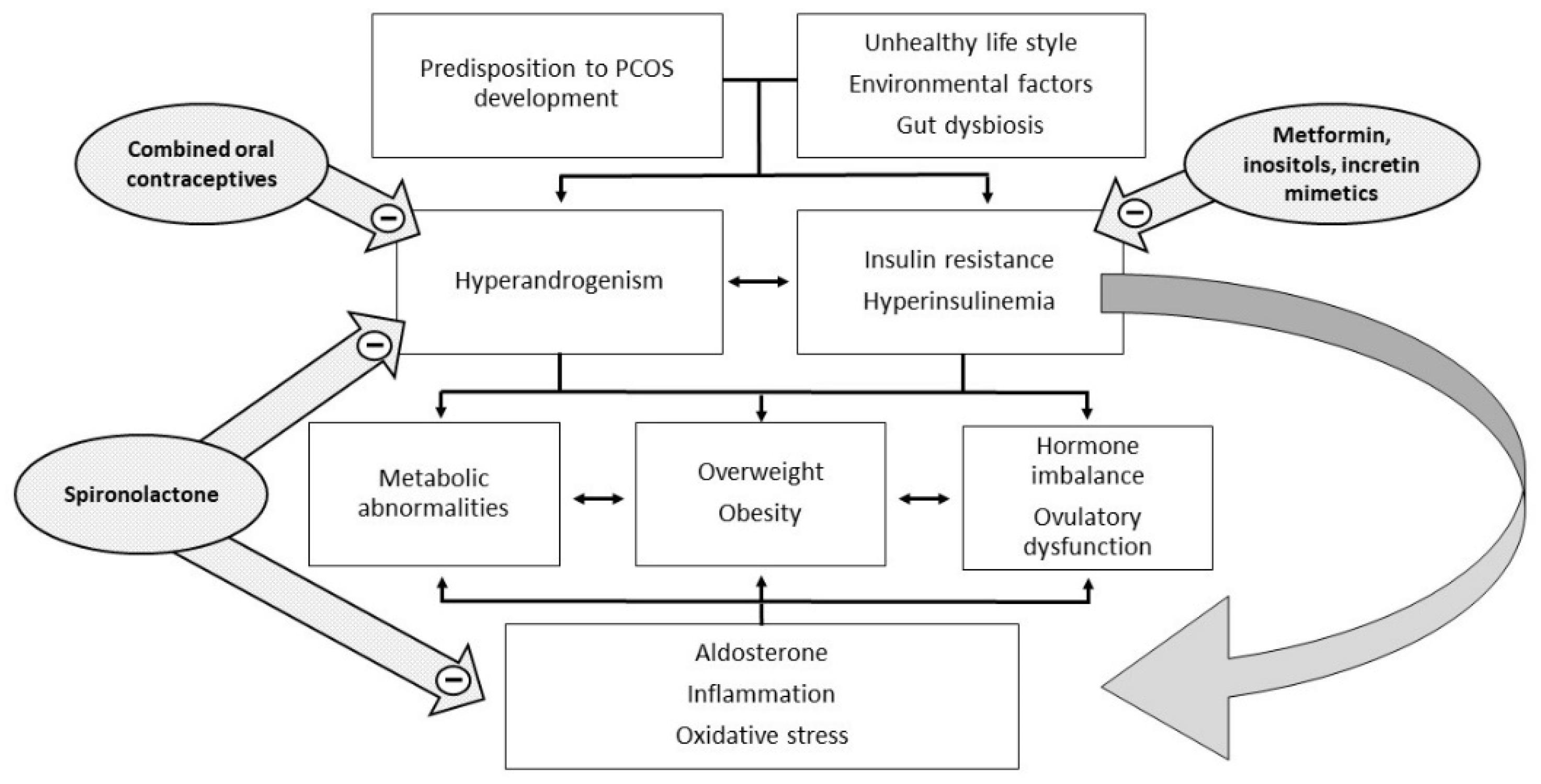
1. Introduction
2. The Pathogenetic Role of Insulin Resistance in PCOS
3. The Pathogenetic Role of Inflammation in PCOS
4. The Pathogenetic Role of Hyperandrogenism in PCOS
5. Controversies in the Diagnosis of PCOS
6. Controversies in the Treatment of PCOS
6.1. Insulin Sensitizers
6.2. Hormonal Contraceptives
6.3. Antiandrogens
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin. Endocrinol. 2018, 89, 251–268. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Abbott, D.H.; Sanchita, S.; Chazenbalk, G.D. Endocrine-metabolic dysfunction in polycystic ovary syndrome: An evolutionary perspective. Curr. Opin. Endocr. Metab. Res. 2020, 12, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Dunaif, A.; Segal, K.R.; Shelley, D.R.; Green, G.; Dobrjansky, A.; Licholai, T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 1992, 41, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Papavassiliou, A.G. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol. Med. 2006, 12, 324–332. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Hawrelak, J. A narrative review of the role of gastrointestinal dysbiosis in the pathogenesis of polycystic ovary syndrome. Obstet. Gynecol. Sci. 2022, 65, 14–28. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Knochenhauer, E.S.; Azziz, R. Impact of obesity on the risk for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 162–168. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E. Polycystic ovarian syndrome: Pathophysiology, molecular aspects and clinical implications. Expert Rev. Mol. Med. 2008, 10, e3. [Google Scholar] [CrossRef]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic low grade inflammation in pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Sabbadin, C.; Andrisani, A.; Ambrosini, G.; Bordin, L.; Donà, G.; Manso, J.; Ceccato, F.; Scaroni, C.; Armanini, D. Aldosterone in gynecology and its involvement on the risk of hypertension in pregnancy. Front. Endocrinol. 2019, 10, 575. [Google Scholar] [CrossRef]
- Armanini, D.; Bordin, L.; Donà, G.; Sabbadin, C.; Bakdounes, L.; Ragazzi, E.; Giorgino, F.L.; Fiore, C. Polycystic ovary syndrome: Implications of measurement of plasma aldosterone, renin activity and progesterone. Steroids 2012, 77, 655–658. [Google Scholar] [CrossRef]
- Armanini, D.; Strasser, T.; Weber, P.C. Binding of agonists and antagonists to mineralocorticoid receptors in human peripheral mononuclear leucocytes. J. Hypertens. Suppl. 1985, 3, S157–S159. [Google Scholar]
- Calò, L.A.; Zaghetto, F.; Pagnin, E.; Davis, P.A.; De Mozzi, P.; Sartorato, P.; Martire, G.; Fiore, C.; Armanini, D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 2004, 89, 1973–1976. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone evaluation study investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008, 14, 222–231. [Google Scholar] [CrossRef]
- Shorakae, S.; Ranasinha, S.; Abell, S.; Lambert, G.; Lambert, E.; de Courten, B.; Teede, H. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin. Endocrinol. 2018, 89, 628–633. [Google Scholar] [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.; Amer, S. The role of chronic inflammation in polycystic ovarian syndrome-A systematic review and meta-analysis. Int. J. Mol. Sci. 2021, 22, 2734. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Woods, K.S.; Stanczyk, F.; Bartolucci, A.; Azziz, R. Stability of adrenocortical steroidogenesis over time in healthy women and women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 5558–5562. [Google Scholar] [CrossRef][Green Version]
- Rosenfield, R.L. Ovarian and adrenal function in polycystic ovary syndrome. Endocrinol. Metab. Clin. N. Am. 1999, 28, 265–293. [Google Scholar] [CrossRef]
- Tsilchorozidou, T.; Honour, J.W.; Conway, G.S. Altered cortisol metabolism in polycystic ovary syndrome: Insulin enhances 5alpha-reduction but not the elevated adrenal steroid production rates. J. Clin. Endocrinol. Metab. 2003, 88, 5907–5913. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F.; Bonin, C.; Di Sarra, D.; Fiers, T.; Kaufman, J.M.; Giagulli, V.A.; Signori, C.; Zambotti, F.; Dall’Alda, M.; et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E628–E637. [Google Scholar] [CrossRef]
- Armanini, D.; Andrisani, A.; Bordin, L.; Sabbadin, C. Spironolactone in the treatment of polycystic ovary syndrome. Expert Opin. Pharmacother. 2016, 17, 1713–1715. [Google Scholar] [CrossRef]
- Corbould, A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J. Endocrinol. 2007, 192, 585–594. [Google Scholar] [CrossRef]
- Allemand, M.C.; Irving, B.A.; Asmann, Y.W.; Klaus, K.A.; Tatpati, L.; Coddington, C.C.; Nair, K.S. Effect of testosterone on insulin stimulated IRS1 Ser phosphorylation in primary rat myotubes—A potential model for PCOS-related insulin resistance. PLoS ONE 2009, 4, e4274. [Google Scholar] [CrossRef]
- Rao, P.; Bhide, P. Controversies in the diagnosis of polycystic ovary syndrome. Ther. Adv. Reprod. Health 2020, 14, 2633494120913032. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, challenges, and guiding treatment. J. Clin. Endocrinol. Metab. 2021, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Androgen excess society. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef]
- Moran, L.J.; Norman, R.J.; Teede, H.J. Metabolic risk in PCOS: Phenotype and adiposity impact. Trends Endocrinol. Metab. 2015, 26, 136–143. [Google Scholar] [CrossRef]
- Yang, R.; Yang, S.; Li, R.; Liu, P.; Qiao, J.; Zhang, Y. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: A meta-analysis. Reprod. Biol. Endocrinol. 2016, 14, 67. [Google Scholar] [CrossRef]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef]
- Martin, K.A.; Anderson, R.R.; Chang, R.J.; Ehrmann, D.A.; Lobo, R.A.; Murad, M.H.; Pugeat, M.M.; Rosenfield, R.L. Evaluation and treatment of hirsutism in premenopausal women: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1233–1257. [Google Scholar] [CrossRef]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; Kempegowda, P.; Jenkinson, C.; Taylor, A.E.; Quanson, J.L.; Storbeck, K.H.; Arlt, W. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 840–848. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic ovary syndrome: An evolutionary adaptation to lifestyle and the environment. Int. J. Environ. Res. Public Health 2022, 19, 1336. [Google Scholar] [CrossRef]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- So, A.; Sakaguchi, K.; Okada, Y.; Morita, Y.; Yamada, T.; Miura, H.; Otowa-Suematsu, N.; Nakamura, T.; Komada, H.; Hirota, Y.; et al. Relation between HOMA-IR and insulin sensitivity index determined by hyperinsulinemic-euglycemic clamp analysis during treatment with a sodium-glucose cotransporter 2 inhibitor. Endocr. J. 2020, 67, 501–507. [Google Scholar] [CrossRef]
- Tosi, F.; Bonora, E.; Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 2017, 32, 2515–2521. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Chiossi, G.; Muscogiuri, G.; Fornaciari, E.; Orio, F.; Tolino, A.; Colao, A.; La Sala, G.B.; Zullo, F. Lipid profile in nonobese pregnant women with polycystic ovary syndrome: A prospective controlled clinical study. Steroids 2014, 88, 36–43. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K.; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Dietz de Loos, A.; Jiskoot, G.; Beerthuizen, A.; Busschbach, J.; Laven, J. Metabolic health during a randomized controlled lifestyle intervention in women with PCOS. Eur. J. Endocrinol. 2021, 186, 53–64. [Google Scholar] [CrossRef]
- Cignarella, A.; Mioni, R.; Sabbadin, C.; Dassie, F.; Parolin, M.; Vettor, R.; Barbot, M.; Scaroni, C. Pharmacological approaches to controlling cardiometabolic risk in women with PCOS. Int. J. Mol. Sci. 2020, 21, 9554. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Zullo, F.; Orio, F., Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: A comprehensive review. Endocr. Rev. 2009, 30, 1–50. [Google Scholar] [CrossRef]
- Bharath, L.P.; Nikolajczyk, B.S. The intersection of metformin and inflammation. Am. J. Physiol. Cell Physiol. 2021, 320, C873–C879. [Google Scholar] [CrossRef]
- Elsenbruch, S.; Hahn, S.; Kowalsky, D.; Offner, A.H.; Schedlowski, M.; Mann, K.; Janssen, O.E. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5801–5807. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Chierchia, E.; Rattighieri, E.; Santagni, S.; Casarosa, E.; Luisi, M.; Genazzani, A.R. Metformin administration restores allopregnanolone response to adrenocorticotropic hormone (ACTH) stimulation in overweight hyperinsulinemic patients with PCOS. Gynecol. Endocrinol. 2010, 26, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From established knowledge to novel approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Sabbadin, C.; Fiore, C.; Bragadin, M.; Giorgino, F.L.; Ragazzi, E.; Clari, G.; Bordin, L.; Armanini, D. Inositol administration reduces oxidative stress in erythrocytes of patients with polycystic ovary syndrome. Eur. J. Endocrinol. 2012, 166, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Laganà, A.S.; Nestler, J.E.; Soulage, C.O.; Group of Inositol in PCOS and Reproduction. Inositols in polycystic ovary syndrome: An overview on the advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Siamashvili, M.; Davis, S.N. Update on the effects of GLP-1 receptor agonists for the treatment of polycystic ovary syndrome. Expert Rev. Clin. Pharmacol. 2021, 14, 1081–1089. [Google Scholar] [CrossRef]
- Jensterle, M.; Goricar, K.; Janez, A. Metformin as an initial adjunct to low-dose liraglutide enhances the weight-decreasing potential of liraglutide in obese polycystic ovary syndrome: Randomized control study. Exp. Ther. Med. 2016, 11, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Elkind-Hirsch, K.; Marrioneaux, O.; Bhushan, M.; Vernor, D.; Bhushan, R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 2670–2678. [Google Scholar] [CrossRef]
- Tentolouris, A.; Vlachakis, P.; Tzeravini, E.; Eleftheriadou, I.; Tentolouris, N. SGLT2 Inhibitors: A review of their antidiabetic and cardioprotective effects. Int. J. Environ. Res. Public Health 2019, 16, 2965. [Google Scholar] [CrossRef]
- Marinkovic-Radosevic, J.; Cigrovski Berkovic, M.; Kruezi, E.; Bilic-Curcic, I.; Mrzljak, A. Exploring new treatment options for polycystic ovary syndrome: Review of a novel antidiabetic agent SGLT2 inhibitor. World J. Diabetes 2021, 12, 932–938. [Google Scholar] [CrossRef]
- Montandon, S.A.; Jornayvaz, F.R. Effects of antidiabetic drugs on gut microbiota composition. Genes 2017, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Akopians, A.L.; Madrigal, V.K.; Ramirez, E.; Margolis, D.J.; Sarma, M.K.; Thomas, A.M.; Grogan, T.R.; Haykal, R.; Schooler, T.A.; et al. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J. Clin. Endocrinol. Metab. 2016, 101, 4178–4188. [Google Scholar] [CrossRef] [PubMed]
- Dokras, A. Cardiovascular disease risk in women with PCOS. Steroids 2013, 78, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Meyer, C.; Hutchison, S.K.; Zoungas, S.; McGrath, B.P.; Moran, L.J. Endothelial function and insulin resistance in polycystic ovary syndrome: The effects of medical therapy. Fertil. Steril. 2010, 93, 184–191. [Google Scholar] [CrossRef]
- Bird, S.T.; Delaney, J.A.; Etminan, M.; Brophy, J.M.; Hartzema, A.G. Drospirenone and non-fatal venous thromboembolism: Is there a risk difference by dosage of ethinyl-estradiol? J. Thromb. Haemost. 2013, 11, 1059–1068. [Google Scholar] [CrossRef]
- Cushman, M.; Kuller, L.H.; Prentice, R.; Rodabough, R.J.; Psaty, B.M.; Stafford, R.S.; Sidney, S.; Rosendaal, F.R.; Women’s Health Initiative Investigators. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004, 292, 1573–1580. [Google Scholar] [CrossRef]
- Gronich, N.; Lavi, I.; Rennert, G. Higher risk of venous thrombosis associated with drospirenone-containing oral contraceptives: A population-based cohort study. CMAJ 2011, 183, E1319–E1325. [Google Scholar] [CrossRef]
- Cascella, T.; Palomba, S.; Tauchmanovà, L.; Manguso, F.; Di Biase, S.; Labella, D.; Giallauria, F.; Vigorito, C.; Colao, A.; Lombardi, G.; et al. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 4395–4400. [Google Scholar] [CrossRef]
- Gonzalez, F. Adrenal involvement in polycystic ovary syndrome. Semin Reprod. Endocrinol. 1997, 15, 137–157. [Google Scholar] [CrossRef]
- Bastianelli, C.; Farris, M.; Rosato, E.; Brosens, I.; Benagiano, G. Pharmacodynamics of combined estrogen-progestin oral contraceptives: 1. Effects on metabolism. Expert Rev. Clin. Pharmacol. 2017, 10, 315–326. [Google Scholar] [CrossRef]
- Grandi, G.; Napolitano, A.; Cagnacci, A. Metabolic impact of combined hormonal contraceptives containing estradiol. Expert Opin. Drug Metab. Toxicol. 2016, 12, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Hage, M.; Plesa, O.; Lemaire, I.; Raffin Sanson, M.L. Estrogen and progesterone therapy and meningiomas. Endocrinology 2022, 163, bqab259. [Google Scholar] [CrossRef] [PubMed]
- Young, R.L.; Goldzieher, J.W.; Elkind-Hirsch, K. The endocrine effects of spironolactone used as an antiandrogen. Fertil. Steril. 1987, 48, 223–228. [Google Scholar] [CrossRef]
- Armanini, D.; Sabbadin, C.; Donà, G.; Clari, G.; Bordin, L. Aldosterone receptor blockers spironolactone and canrenone: Two multivalent drugs. Expert Opin. Pharmacother. 2014, 15, 909–912. [Google Scholar] [CrossRef]
- Sabbadin, C.; Andrisani, A.; Zermiani, M.; Donà, G.; Bordin, L.; Ragazzi, E.; Boscaro, M.; Ambrosini, G.; Armanini, D. Spironolactone and intermenstrual bleeding in polycystic ovary syndrome with normal BMI. J. Endocrinol. Invest. 2016, 39, 1015–1021. [Google Scholar] [CrossRef]
- Sabbadin, C.; Bordin, L.; Donà, G.; Manso, J.; Avruscio, G.; Armanini, D. Licorice: From pseudohyperaldosteronism to therapeutic uses. Front. Endocrinol. 2019, 10, 484. [Google Scholar] [CrossRef]
- Armanini, D.; Castello, R.; Scaroni, C.; Bonanni, G.; Faccini, G.; Pellati, D.; Bertoldo, A.; Fiore, C.; Moghetti, P. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 61–67. [Google Scholar] [CrossRef]
- Moghetti, P.; Castello, R.; Magnani, C.M.; Tosi, F.; Negri, C.; Armanini, D.; Bellotti, G.; Muggeo, M. Clinical and hormonal effects of the 5 alpha-reductase inhibitor finasteride in idiopathic hirsutism. J. Clin. Endocrinol. Metab. 1994, 79, 1115–1121. [Google Scholar] [CrossRef]
- Moghetti, P.; Castello, R.; Negri, C.; Tosi, F.; Magnani, C.M.; Fontanarosa, M.C.; Armanini, D.; Muggeo, M. Flutamide in the treatment of hirsutism: Long-term clinical effects, endocrine changes, and androgen receptor behavior. Fertil. Steril. 1995, 64, 511–517. [Google Scholar] [CrossRef]
- Brahm, J.; Brahm, M.; Segovia, R.; Latorre, R.; Zapata, R.; Poniachik, J.; Buckel, E.; Contreras, L. Acute and fulminant hepatitis induced by flutamide: Case series report and review of the literature. Ann. Hepatol. 2011, 10, 93–98. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. https://doi.org/10.3390/ijms23084110
Armanini D, Boscaro M, Bordin L, Sabbadin C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. International Journal of Molecular Sciences. 2022; 23(8):4110. https://doi.org/10.3390/ijms23084110
Chicago/Turabian StyleArmanini, Decio, Marco Boscaro, Luciana Bordin, and Chiara Sabbadin. 2022. "Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism" International Journal of Molecular Sciences 23, no. 8: 4110. https://doi.org/10.3390/ijms23084110
APA StyleArmanini, D., Boscaro, M., Bordin, L., & Sabbadin, C. (2022). Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. International Journal of Molecular Sciences, 23(8), 4110. https://doi.org/10.3390/ijms23084110






