2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule
Abstract
1. Introduction
2. Results
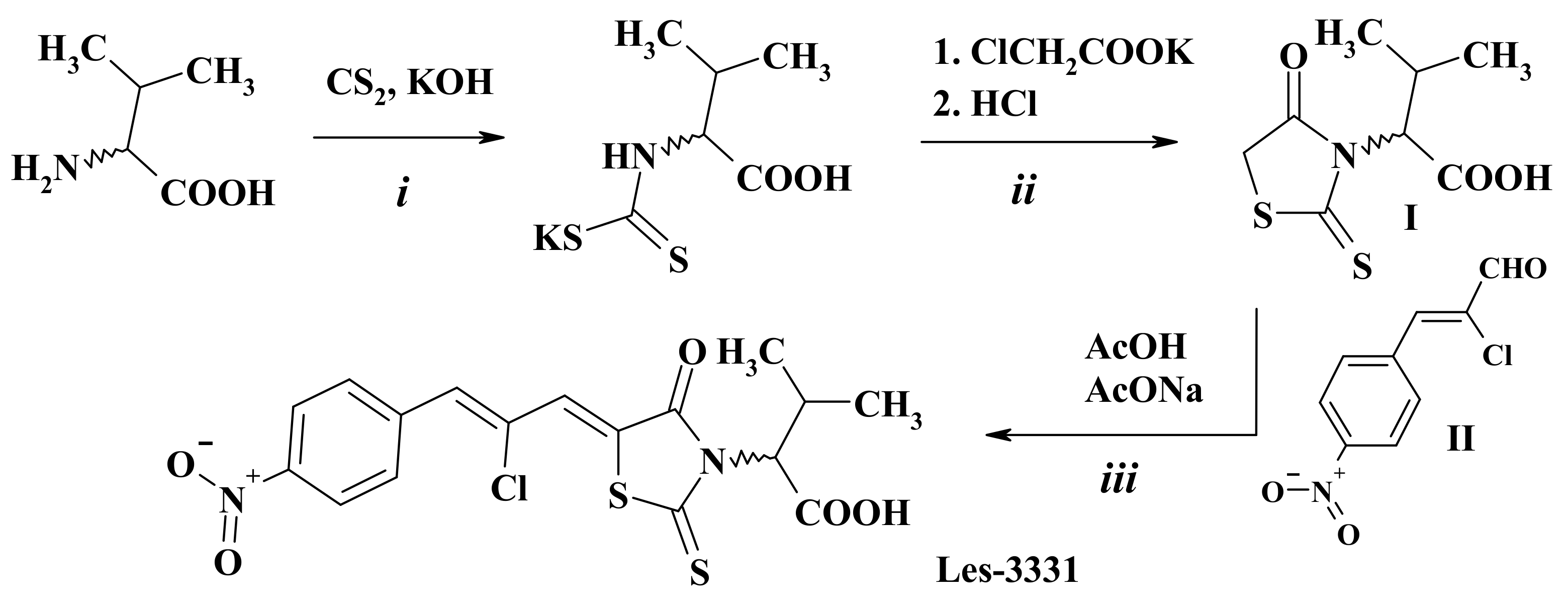
2.1. Chemistry
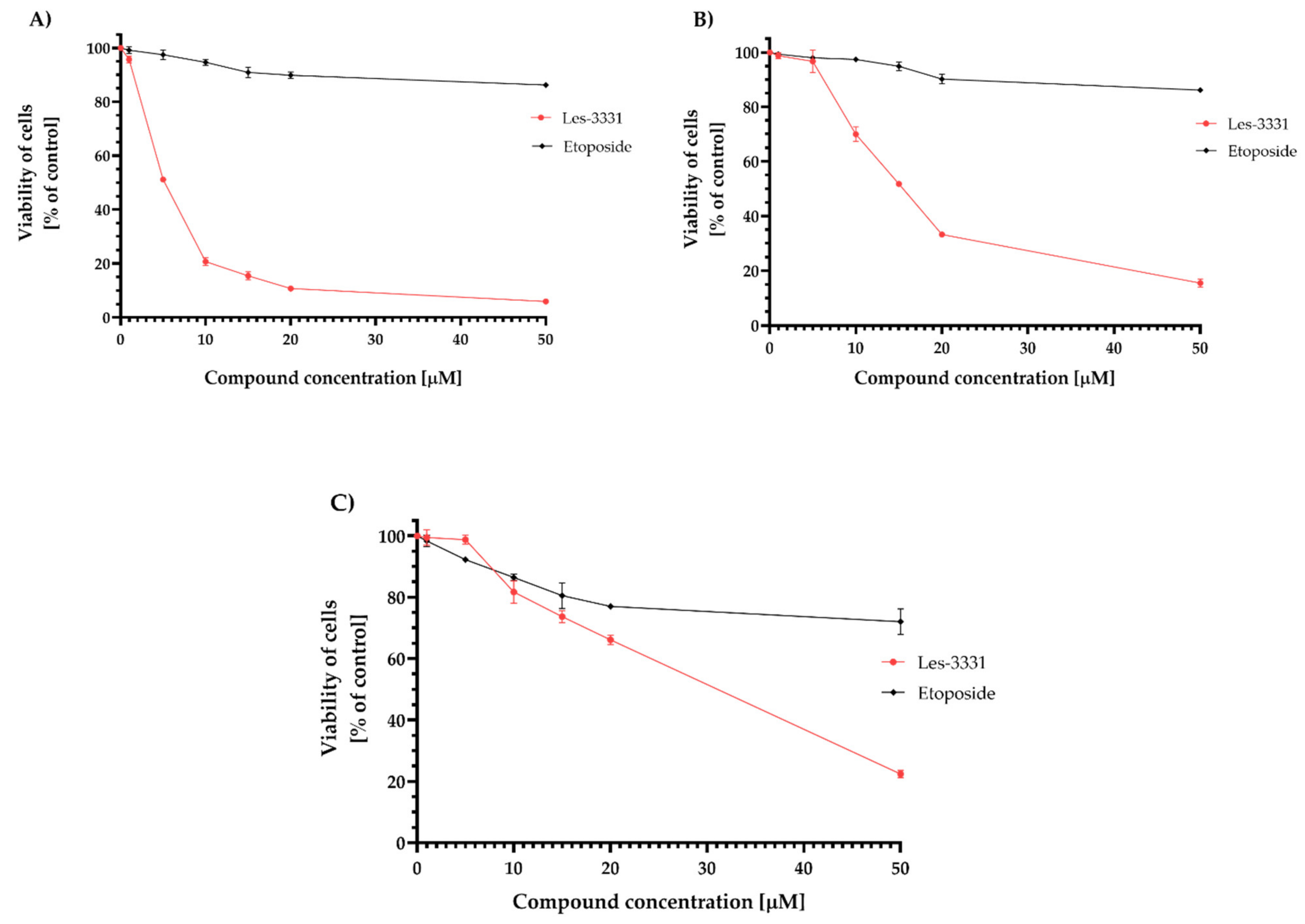
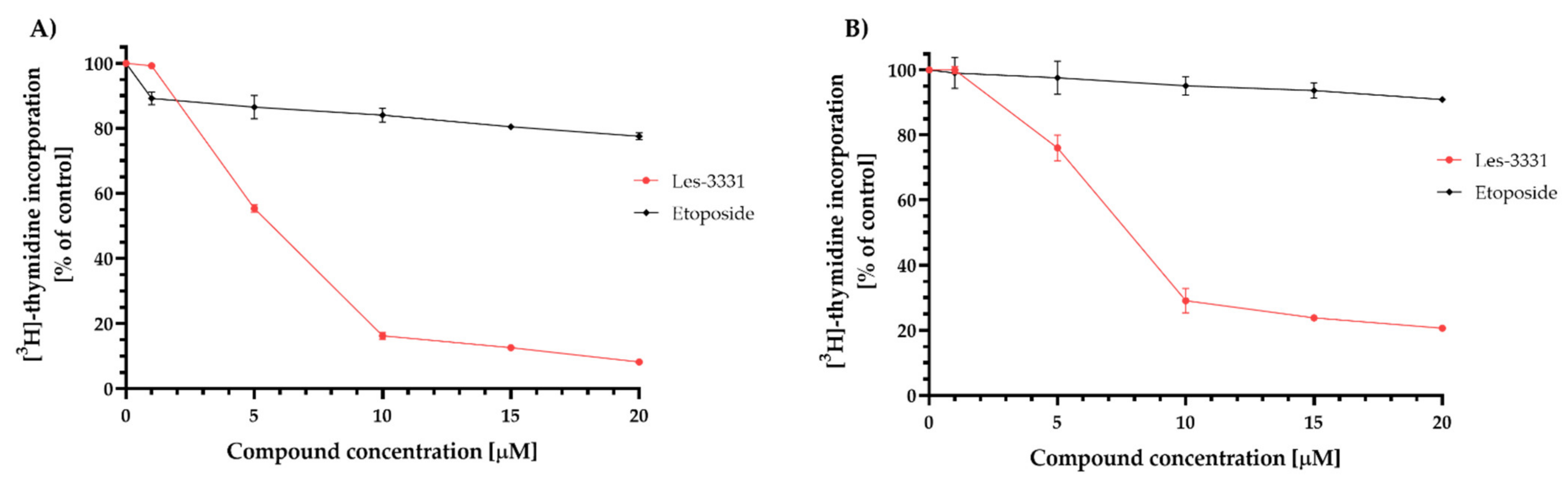
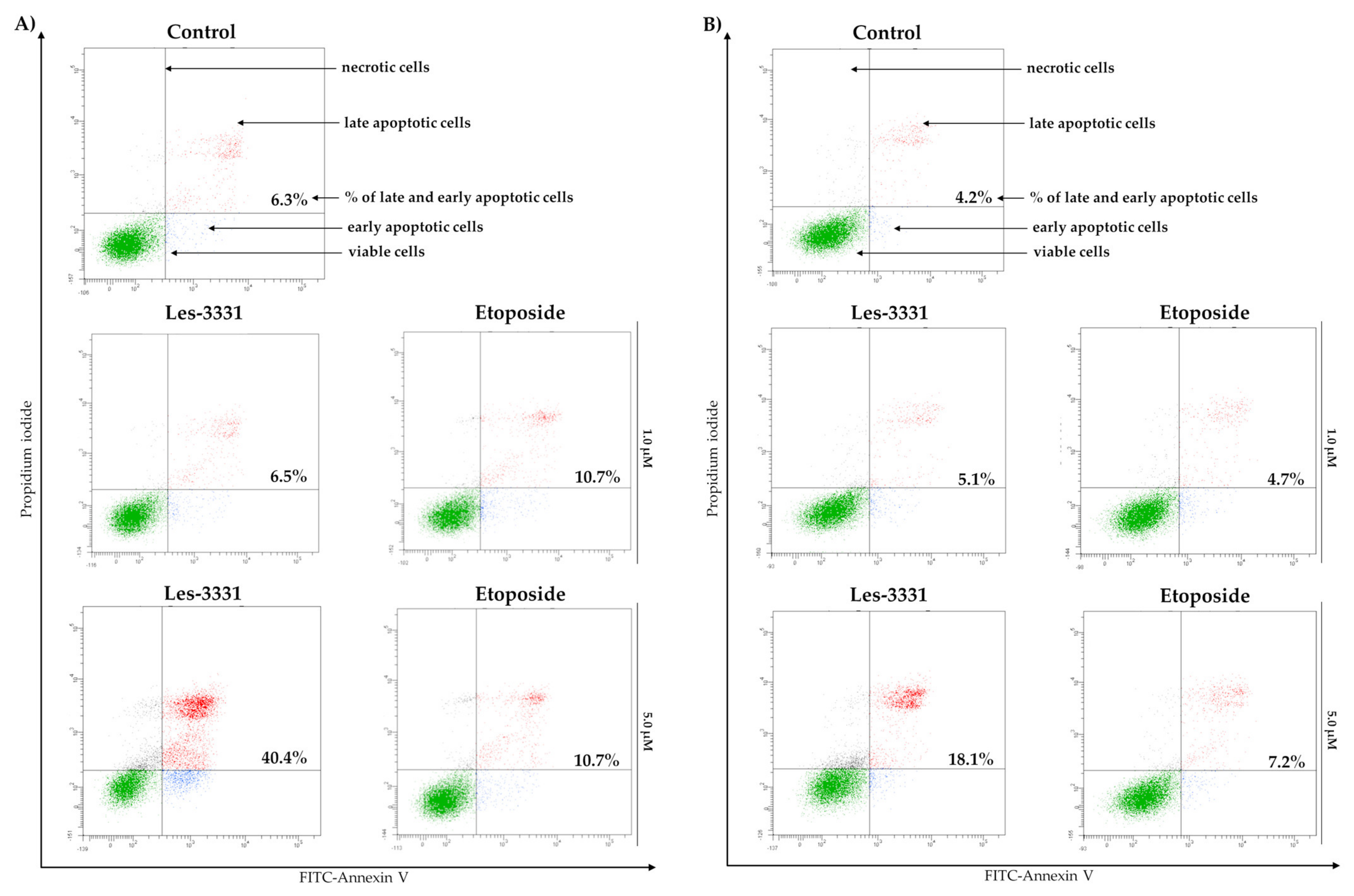
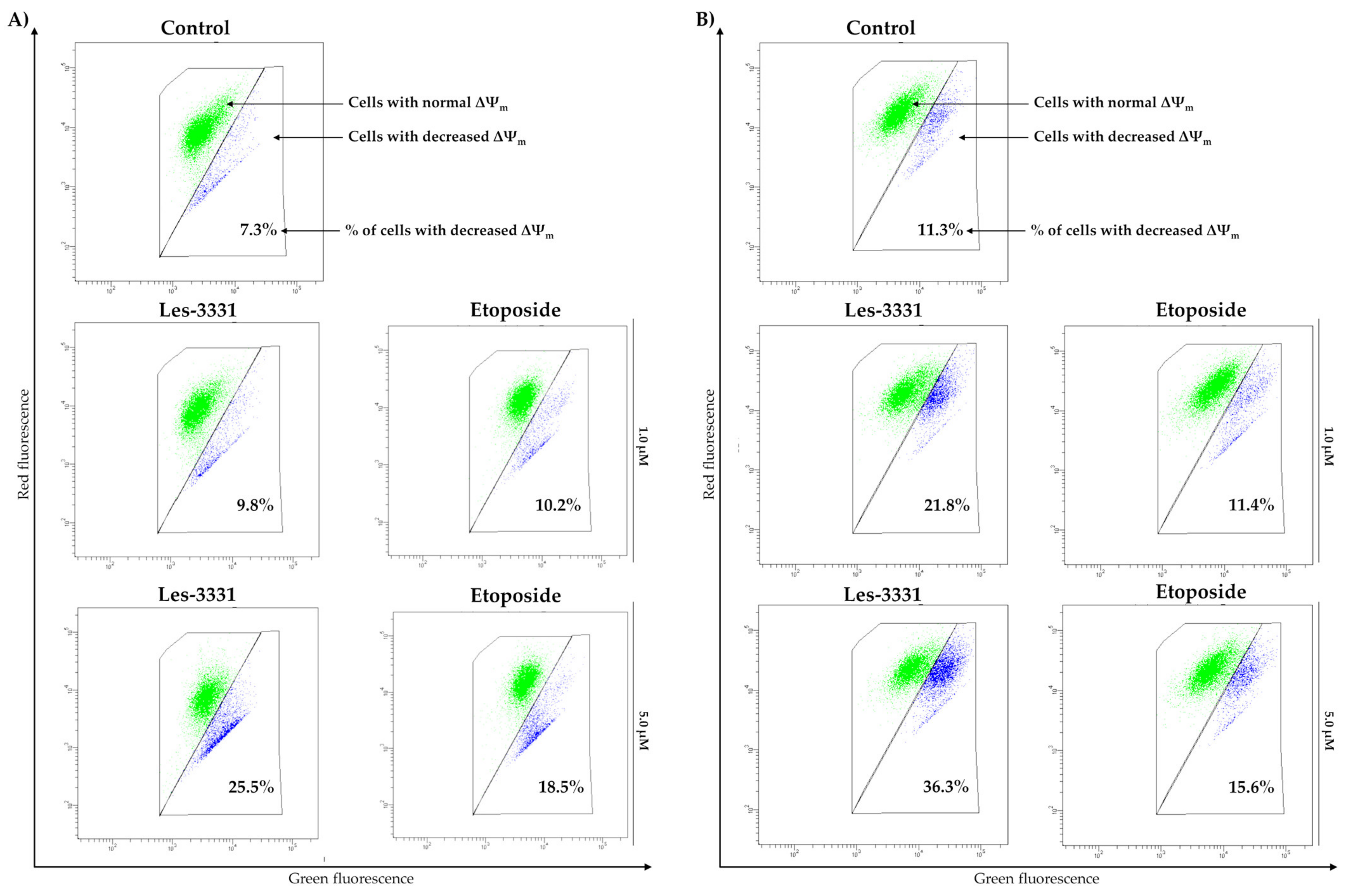
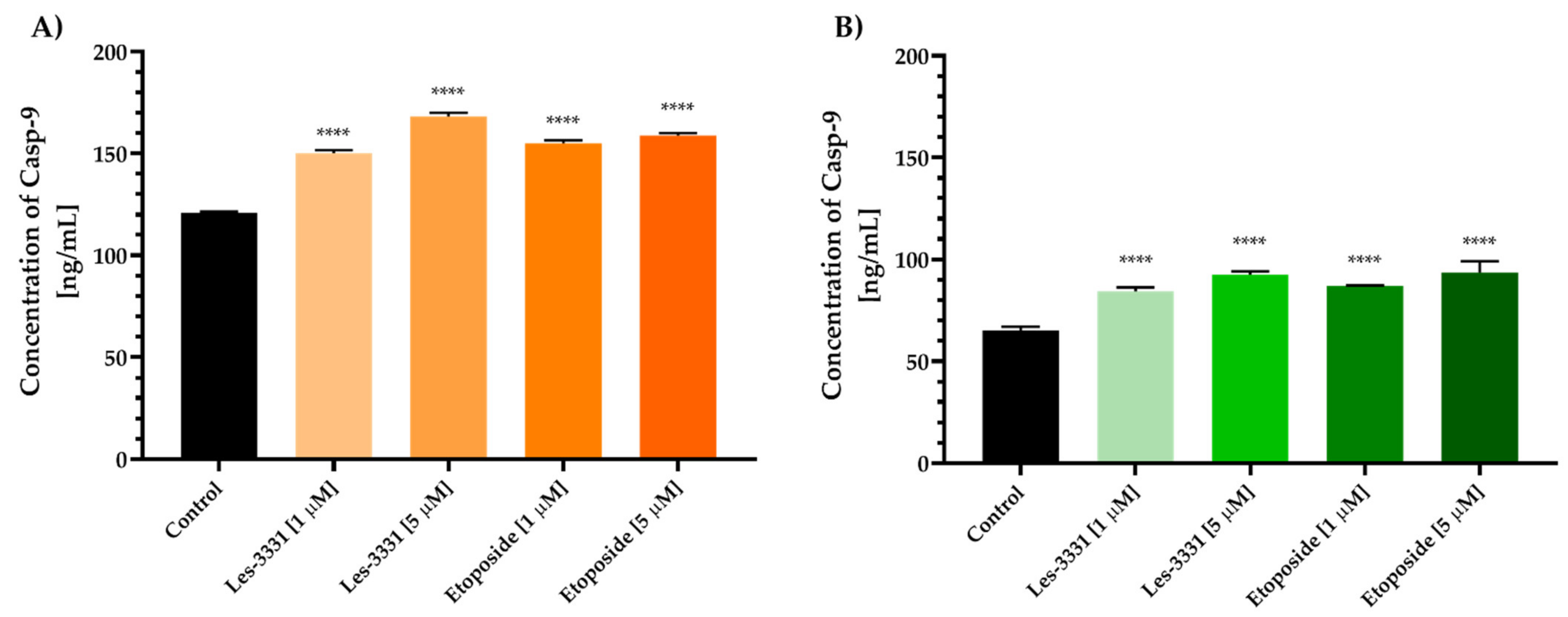
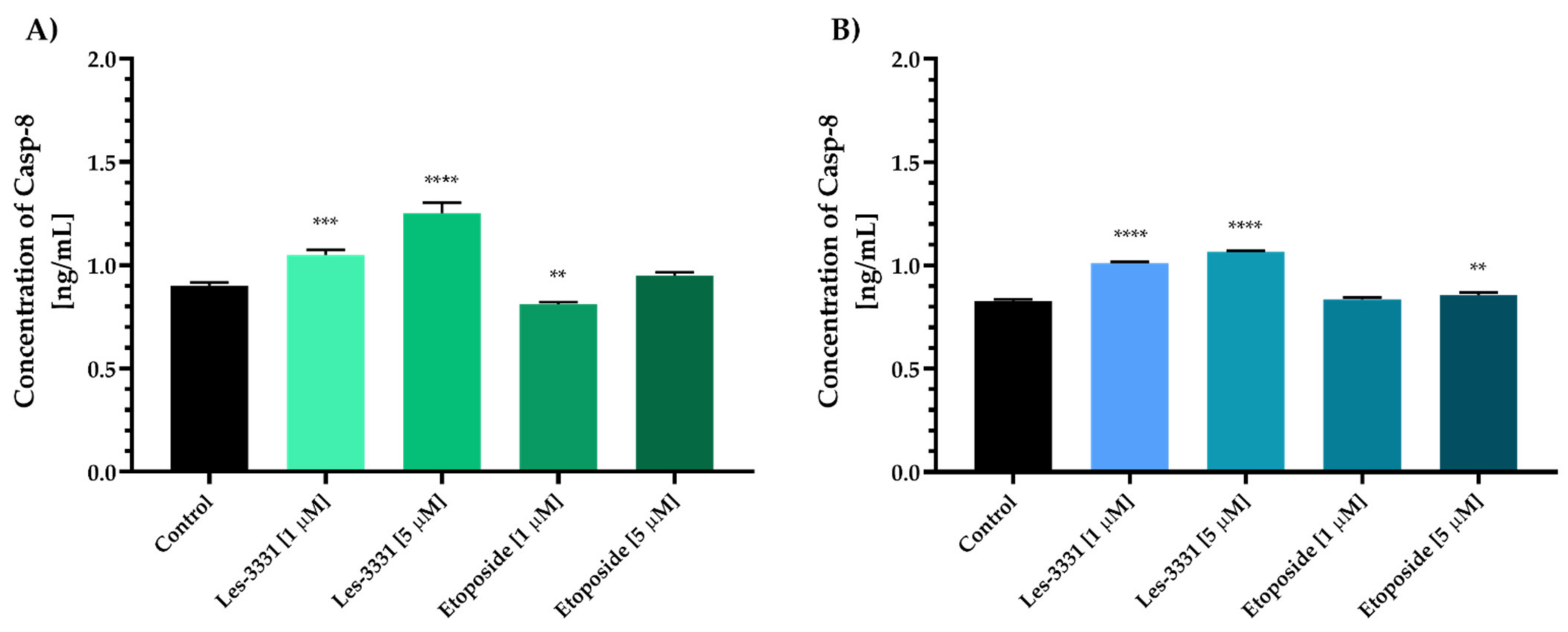
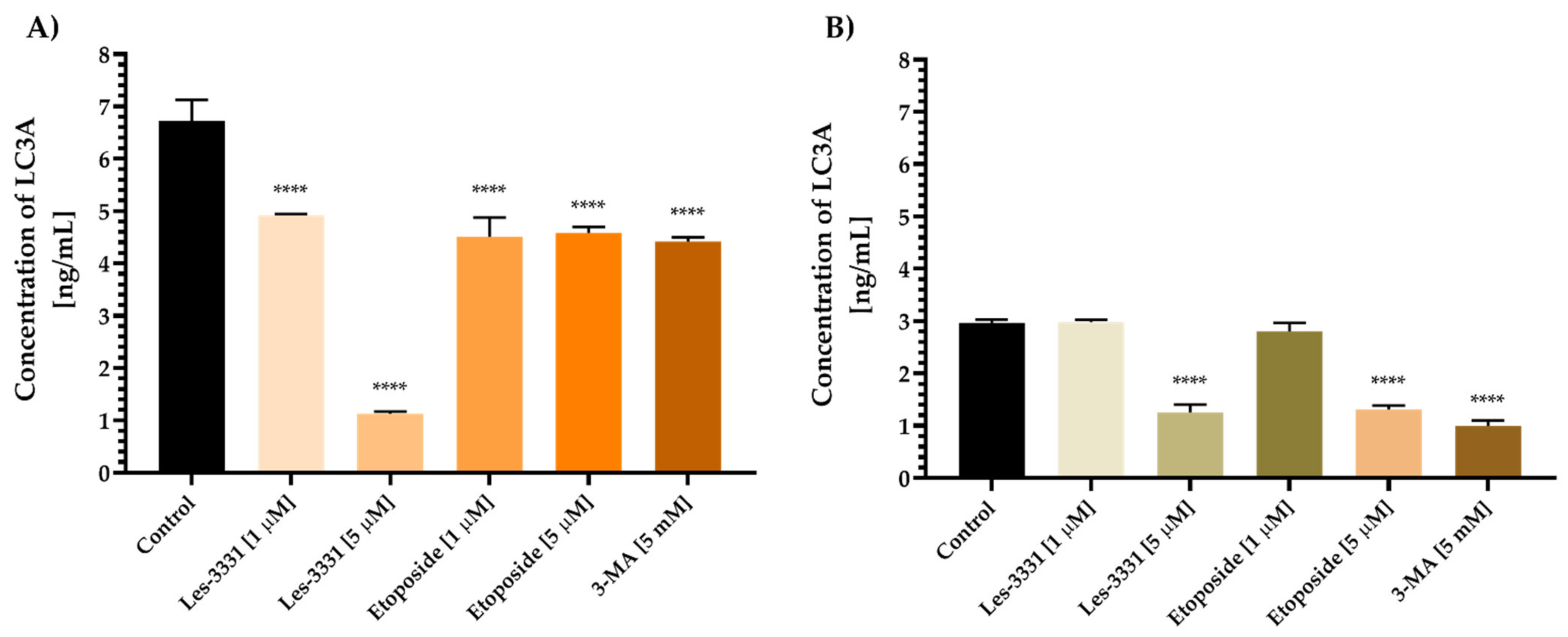
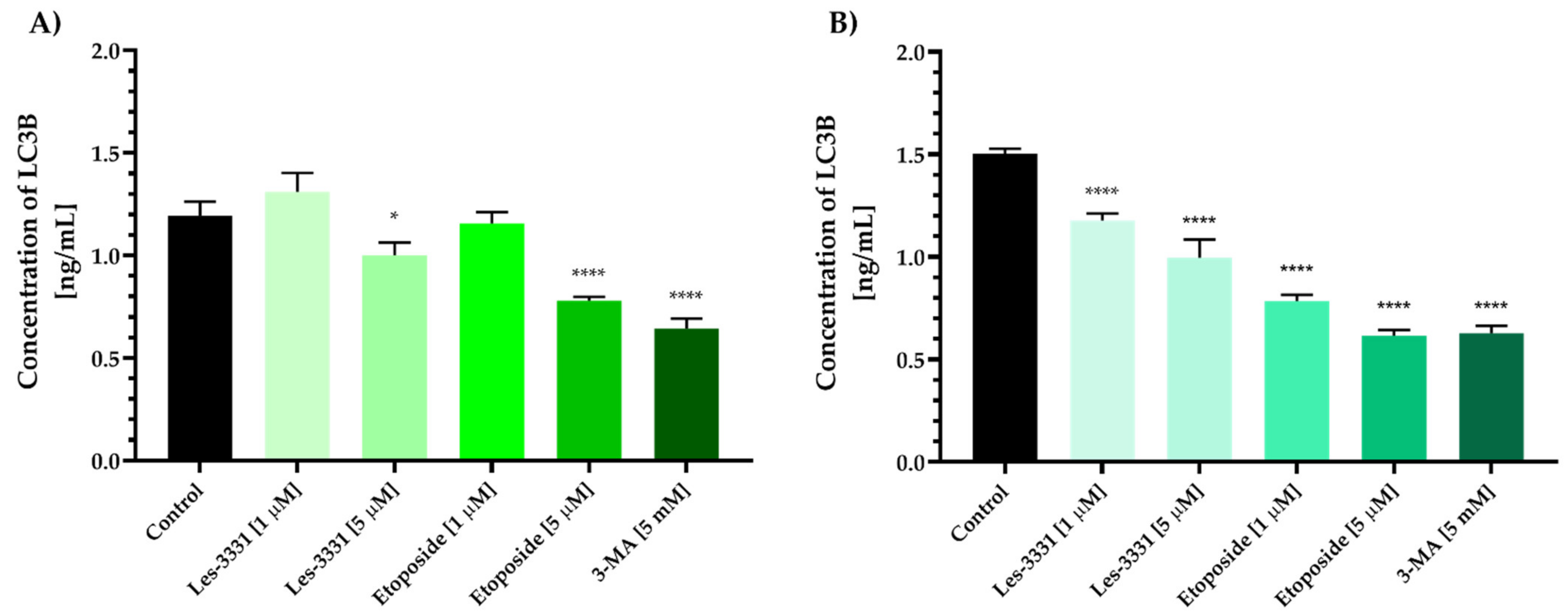
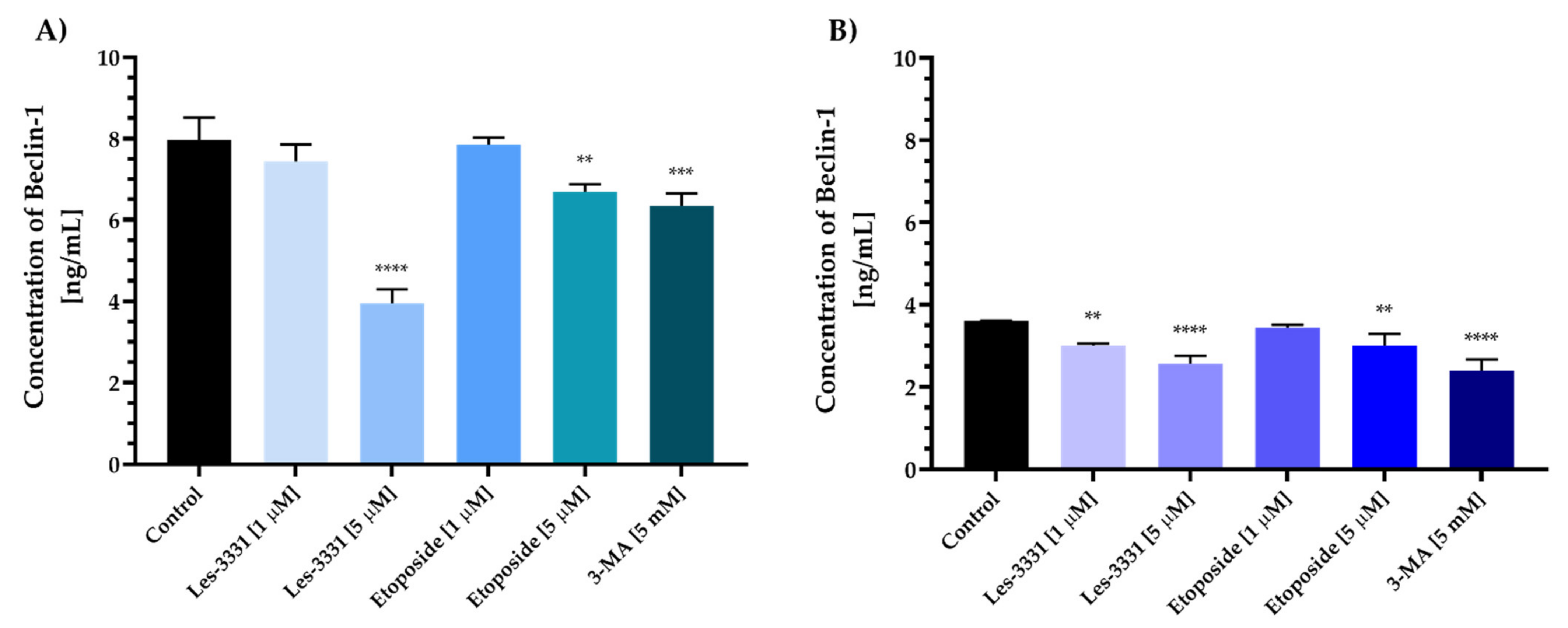
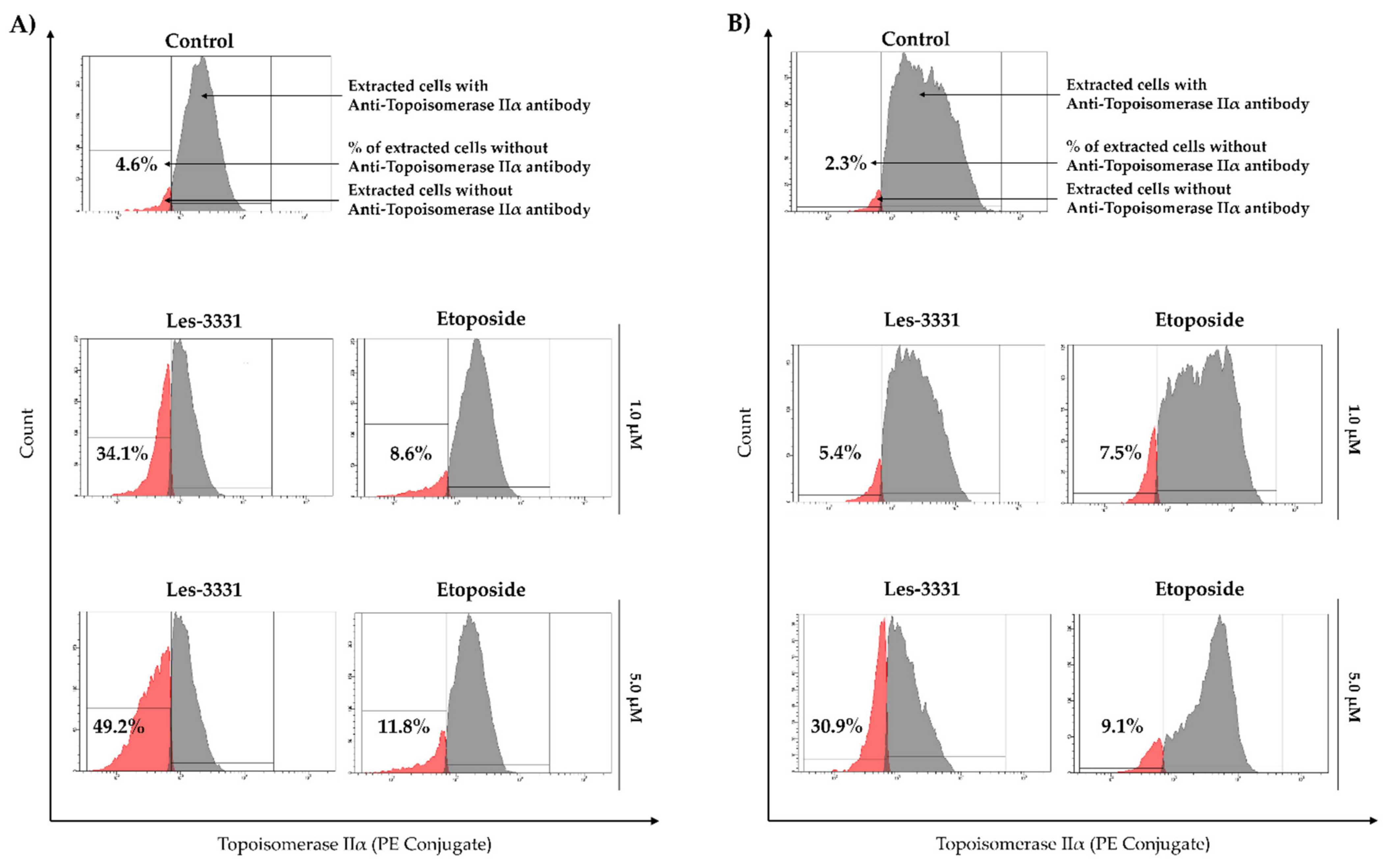
2.2. Biological Studies
2.3. Molecular Docking Simulations
2.4. ADME-Tox In Vitro
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Synthesis of 2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid (Les-3331)
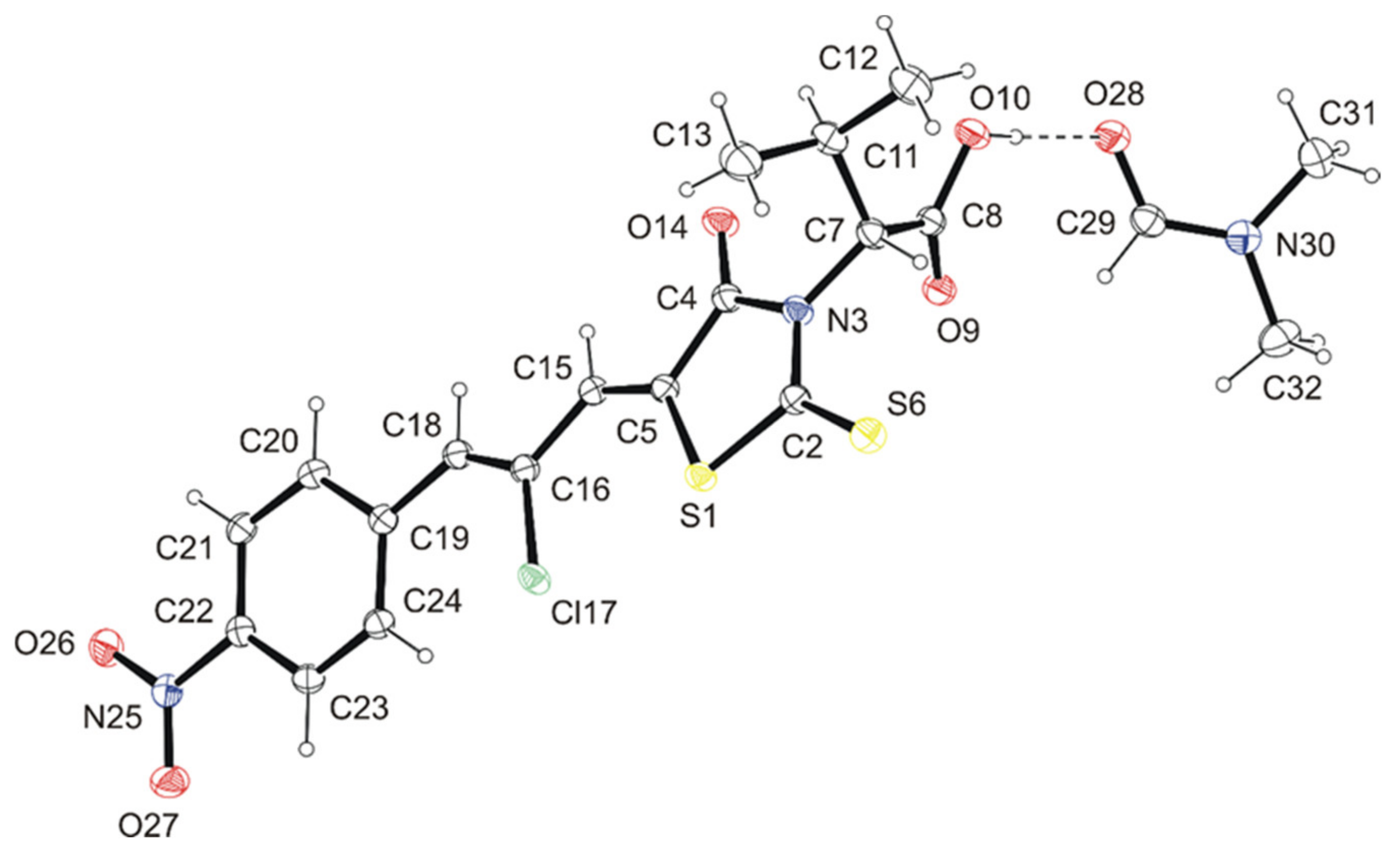
4.3. Crystal Structure Determination of Les-3331*DMF
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. [3H]-Thymidine Incorporation Assay
4.7. Flow Cytometry Assessment of Annexin V Binding
4.8. Mitochondrial Membrane Potential (ΔΨm) Analysis
4.9. Determination of Caspase-8 and Caspase-9, LC3A, LC3B, Beclin-1, and Topoisomerase II Concentration
4.10. Molecular Docking Studies
4.11. Flow Cytometric Analysis of Anti-Topoisomerase IIα antibody
4.12. ADME-Tox In Vitro
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 3-MA | 3-Methyladenine |
| ANOVA | analysis of variance |
| BC | breast cancer |
| Casp-8 | caspase-8 |
| Casp-9 | caspase-9 |
| DDIs | drug-drug interactions |
| ELISA | enzyme-linked immunosorbent assay |
| ER | estrogen receptor |
| FBS | fetal bovine serum |
| LC3A | microtubule-associated protein 1A/1B light chain 3A |
| LC3B | microtubule-associated protein 1A/1B light chain 3B |
| MCB | medium control baseline |
| MPF | microplate fluctuation protocol |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide |
| PAMPA | parallel artificial membrane permeability assay |
| PBS | phosphate-buffered saline |
| PE | phycoerythrin |
| PR | progesterone receptor |
| RLMs | rat liver microsomes |
| RT | room temperature |
| TCA | trichloroacetic acid |
| TMS | tetramethylsilane |
| TNBC | triple-negative breast cancer |
| Top II | topoisomerase II |
| UPLC | ultra-performance liquid chromatography |
| WHO | World Health Organization |
| ΔΨm | mitochondrial membrane potential |
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization–Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 19 January 2022).
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Brown, F.C. 4-Thiazolidinones. Chem. Rev. 1961, 61, 463–521. [Google Scholar] [CrossRef]
- Newkome, G.R.; Nayak, A. 4-Thiazolidinones. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: Cambridge, MA, USA, 1980; Volume 25, pp. 83–112. [Google Scholar]
- Singh, S.P.; Parmar, S.S.; Raman, K.; Stenberg, V.I. Chemistry and biological activity of thiazolidinones. Chem. Rev. 1981, 81, 175–203. [Google Scholar] [CrossRef]
- Tomašić, T.; Peterlin Mašič, L. Rhodanine as a scaffold in drug discovery: A critical review of its biological activities and mechanisms of target modulation. Expert Opin. Drug Discov. 2012, 7, 549–560. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef]
- Lesyk, R.B.; Zimenkovsky, B.S. 4-Thiazolidones: Centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004, 8, 1547–1577. [Google Scholar] [CrossRef]
- Lesyk, R.B.; Zimenkovsky, B.S.; Kaminskyy, D.V.; Kryshchyshyn, A.P.; Havryluk, D.Y.; Atamanyuk, D.V.; Subtel’na, I.Y.; Khyluk, D.V. Thiazolidinone motif in anticancer drug discovery. Experience of DH LNMU medicinal chemistry scientific group. Biopolym. Cell 2011, 27, 107–117. [Google Scholar] [CrossRef]
- Tomasic, T.; Masic, P.L. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009, 16, 1596–1629. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones–An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Tahmasvand, R.; Bayat, P.; Vahdaniparast, S.M.; Dehghani, S.; Kooshafar, Z.; Khaleghi, S.; Almasirad, A.; Salimi, M. Design and synthesis of novel 4-thiazolidinone derivatives with promising anti-breast cancer activity: Synthesis, characterization, in vitro and in vivo results. Bioorg. Chem. 2020, 104, 104276. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K.; Agrawal, R.K. Recent developments and biological activities of thiazolidinone derivatives: A review. Bioorg. Med. Chem. 2012, 20, 3378–3395. [Google Scholar] [CrossRef]
- Sohda, T.; Momose, Y.; Meguro, K.; Kawamatsu, Y.; Sugiyama, Y.; Ikeda, H. Studies on antidiabetic agents. Synthesis and hypoglycemic activity of 5-[4-(pyridylalkoxy)benzyl]-2,4-thiazolidinediones. Arzneimittelforschung 1990, 40, 37–42. [Google Scholar] [CrossRef]
- Charlier, C.; Michaux, C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur. J. Med. Chem. 2003, 38, 645–659. [Google Scholar] [CrossRef]
- Ramirez, M.A.; Borja, N.L. Epalrestat: An aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy 2008, 28, 646–655. [Google Scholar] [CrossRef]
- Elkaeed, E.B.; Salam, H.A.A.E.; Sabt, A.; Al-Ansary, G.H.; Eldehna, W.M. Recent advancements in the development of anti-breast cancer synthetic small molecules. Molecules 2021, 26, 7611. [Google Scholar] [CrossRef]
- Lesyk, R. Drug design: 4-thiazolidinones applications. Part 1. Synthetic routes to the drug-like molecules. J. Med. Sci. 2020, 89, 33–49. [Google Scholar] [CrossRef]
- Lesyk, R. Drug design: 4-thiazolidinones applications. Part 2. Pharmacological profiles. J. Med. Sci. 2020, 89, 132–141. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent developments with rhodanine as a scaffold for drug discovery. Expert Opin. Drug Discov. 2017, 12, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Buzun, K.; Kryshchyshyn-Dylevych, A.; Senkiv, J.; Roman, O.; Gzella, A.; Bielawski, K.; Bielawska, A.; Lesyk, R. Synthesis and anticancer activity evaluation of 5-[2-chloro-3-(4-nitrophenyl)-2-propenylidene]-4-thiazolidinones. Molecules 2021, 26, 3057. [Google Scholar] [CrossRef] [PubMed]
- Subtel’na, I.; Atamanyuk, D.; Szymańska, E.; Kieć-Kononowicz, K.; Zimenkovsky, B.; Vasylenko, O.; Gzella, A.; Lesyk, R. Synthesis of 5-arylidene-2-amino-4-azolones and evaluation of their anticancer activity. Bioorg. Med. Chem. 2010, 18, 5090–5102. [Google Scholar] [CrossRef] [PubMed]
- Panchuk, R.; Chumak, V.; Fil, M.R.; Havrylyuk, D.; Zimenkovsky, B.S.; Lesyk, R.; Stoika, R.S. Study of molecular mechanisms of proapoptotic action of novel heterocyclic 4-thiazolidone derivatives. Biopolym. Cell 2012, 28, 121–128. [Google Scholar] [CrossRef]
- Slepikas, L.; Chiriano, G.; Perozzo, R.; Tardy, S.; Kranjc, A.; Patthey-Vuadens, O.; Ouertatani-Sakouhi, H.; Kicka, S.; Harrison, C.F.; Scrignari, T.; et al. In silico driven design and synthesis of Rhodanine derivatives as novel antibacterials targeting the enoyl reductase InhA. J. Med. Chem. 2016, 59, 10917–10928. [Google Scholar] [CrossRef] [PubMed]
- Jagiello-Gruszfeld, A.I.; Meluch, M.; Kunkiel, M.; Gorniak, A.; Majstrak-Hulewska, A.; Gorska, K.; Konieczna, A.; Nowecki, Z. Oral etoposide in heavily pre-treated metastatic breast cancer: A retrospective study. J. Clin. Oncol. 2021, 39, e13070. [Google Scholar] [CrossRef]
- Hu, N.; Zhu, A.; Si, Y.; Yue, J.; Wang, X.; Wang, J.; Ma, F.; Xu, B.; Yuan, P. A Phase II, Single-Arm Study of Apatinib and Oral Etoposide in Heavily Pre-Treated Metastatic Breast Cancer. Front. Oncol. 2021, 10, 3246. [Google Scholar] [CrossRef]
- Giannone, G.; Milani, A.; Ghisoni, E.; Genta, S.; Mittica, G.; Montemurro, F.; Valabrega, G. Oral etoposide in heavily pre-treated metastatic breast cancer: A retrospective series. Breast 2018, 38, 160–164. [Google Scholar] [CrossRef]
- Gornowicz, A.; Kałuża, Z.; Bielawska, A.; Gabryel-Porowska, H.; Czarnomysy, R.; Bielawski, K. Cytotoxic efficacy of a novel dinuclear platinum(II) complex used with anti-MUC1 in human breast cancer cells. Mol. Cell. Biochem. 2014, 392, 161–174. [Google Scholar] [CrossRef][Green Version]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Bielawski, K.; Bielawska, A. Autophagy modulators in cancer therapy. Int. J. Mol. Sci. 2021, 22, 5804. [Google Scholar] [CrossRef]
- Buzun, K.; Bielawska, A.; Bielawski, K.; Gornowicz, A. DNA topoisomerases as molecular targets for anticancer drugs. J. Enzym. Inhib. Med. 2020, 35, 1781–1799. [Google Scholar] [CrossRef] [PubMed]
- Sniecikowska, J.; Gluch-Lutwin, M.; Bucki, A.; Więckowska, A.; Siwek, A.; Jastrzebska-Wiesek, M.; Partyka, A.; Wilczyńska, D.; Pytka, K.; Latacz, G.; et al. Discovery of novel pERK1/2- or β-arrestin-preferring 5-HT1A receptor-biased agonists: Diversified therapeutic-like versus side effect profile. J. Med. Chem. 2020, 63, 10946–10971. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-G.; Wang, C.; Li, Y.; Wang, L.; Zhou, B.; Xu, B.; Jiang, X.; Zhou, Z.-G.; Sun, X.-F. Downregulation of caspase-9 is a frequent event in patients with stage II colorectal cancer and correlates with poor clinical outcome. Colorectal Dis. 2010, 12, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, E.; Sahin, A.A.; Chen, J.S.; Krishnamurthy, R.R.; Aggarwal, N.; Brun, A.-M.; Sapino, A.; Zhang, F.; Sharma, D.; Yang, X.-H.; et al. Down-regulation of caspase 3 in breast cancer: A possible mechanism for chemoresistance. Oncogene 2002, 21, 8843–8851. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting autophagy in cancer: Recent advances and future directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, M.; Zhao, J.; Wang, J.; Zhang, Y.; Zhang, Q. High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Med. Oncol. 2013, 30, 475. [Google Scholar] [CrossRef]
- Gornowicz, A.; Szymanowska, A.; Mojzych, M.; Bielawski, K.; Bielawska, A. The effect of novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine sulfonamide derivatives on apoptosis and autophagy in DLD-1 and HT-29 colon cancer cells. Int. J. Mol. Sci. 2020, 21, 5221. [Google Scholar] [CrossRef]
- CrysAlis. PRO, Version 1.171.41.110a; Rigaku Oxford Diffraction: Yarnton, UK, 2021. [Google Scholar]
- Sheldrick, G. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, N.; Gornowicz, A.; Bielawska, A.; Surażyński, A.; Szymanowska, A.; Czarnomysy, R.; Bielawski, K. The molecular mechanism of anticancer action of novel octahydropyrazino[2,1-a:5,4-a′]diisoquinoline derivatives in human gastric cancer cells. Investig. New Drugs 2018, 36, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Mullick, P.; Khan, S.A.; Begum, T.; Verma, S.; Kaushik, D.; Alam, O. Synthesis of 1,2,4-triazine derivatives as potential anti-anxiety and anti-inflammatory agents. Acta Pol. Pharm. 2009, 66, 379–385. [Google Scholar] [PubMed]
- Gornowicz, A.; Bielawska, A.; Czarnomysy, R.; Gabryel-Porowska, H.; Muszyńska, A.; Bielawski, K. The combined treatment with novel platinum(II) complex and anti-MUC1 increases apoptotic response in MDA-MB-231 breast cancer cells. Mol. Cell. Biochem. 2015, 408, 103–113. [Google Scholar] [CrossRef]
- Czarnomysy, R.; Surażyński, A.; Muszynska, A.; Gornowicz, A.; Bielawska, A.; Bielawski, K. A novel series of pyrazole-platinum(II) complexes as potential anti-cancer agents that induce cell cycle arrest and apoptosis in breast cancer cells. J. Enzyme Inhib. Med. 2018, 33, 1006–1023. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Latacz, G.; Lubelska, A.; Jastrzębska-Więsek, M.; Partyka, A.; Marć, M.A.; Satała, G.; Wilczyńska, D.; Kotańska, M.; Więcek, M.; Kamińska, K.; et al. The 1,3,5-triazine derivatives as innovative chemical family of 5-HT6 serotonin receptor agents with therapeutic perspectives for cognitive impairment. Int. J. Mol. Sci. 2019, 20, 3420. [Google Scholar] [CrossRef]



| Compounds | Topoisomerase II | |
|---|---|---|
| Binding Energy, kcal/mol | Inhibition Constant, Ki, nM | |
| Les-3331 | −8.79 | 360.70 |
| Etoposide | −11.97 | 1.69 |
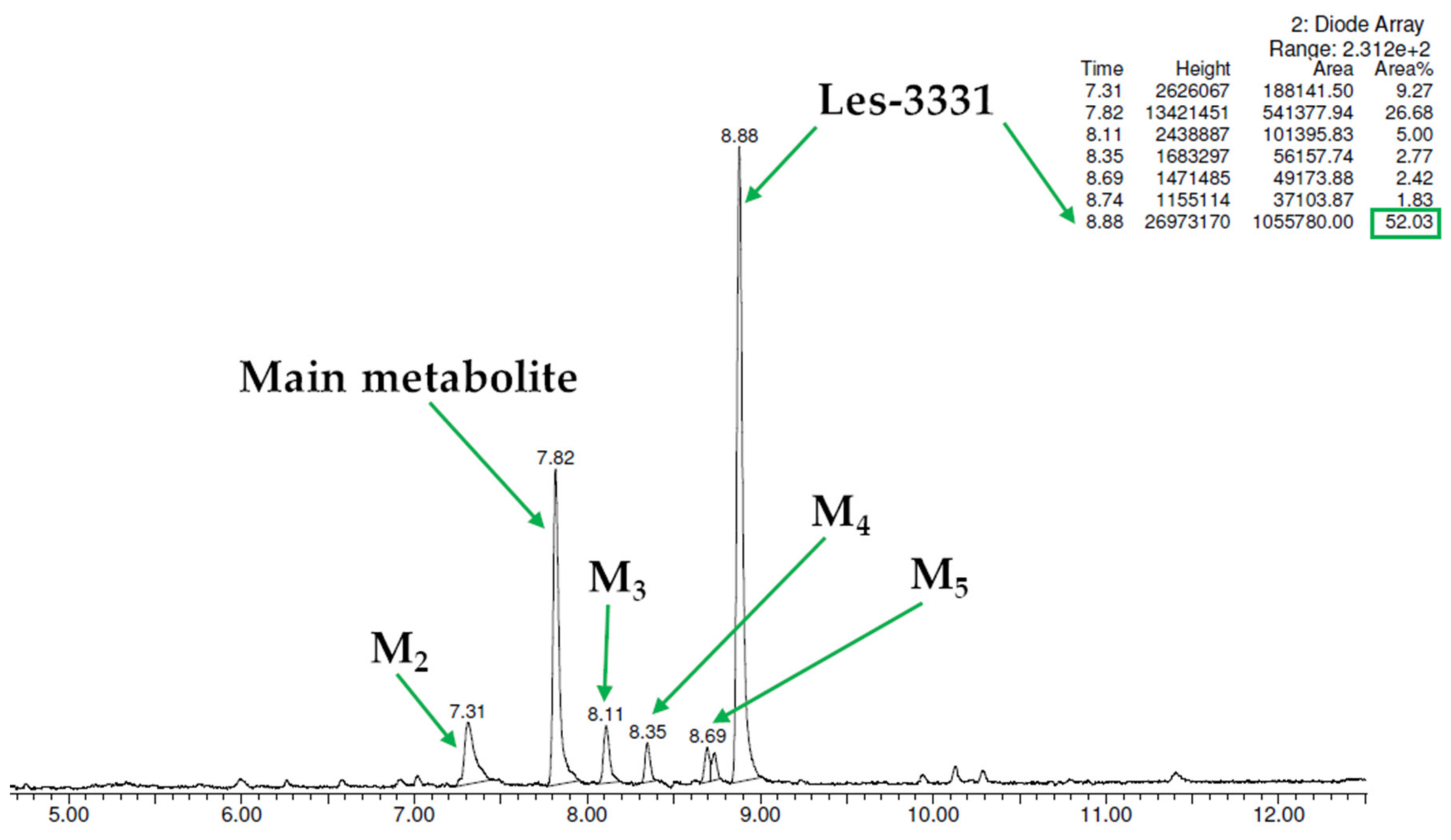
| Substrate | Molecular Mass (m/z) | % of the Remaining Substrate | The Retention Time of Metabolite (min.) | The Molecular Mass of the Metabolite (m/z) | Proposed Metabolic Pathway |
|---|---|---|---|---|---|
| Les-3331 | 427.13 | 52.03 | Main metabolite M1 7.82 | 397.16. | decomposition |
| M2 7.31 | 413.17 | decomposition and hydroxylation | |||
| M3 8.11 | 393.17 | decomposition and dehydrogenation | |||
| M4 8.35 | 397.16 | decomposition | |||
| M5 8.69 | not estimated | not estimated | |||
| Verapamil * | 454.60 | 37.28 | M1 4.94 | 441.42 | demethylation (norverapamil) |
| M2 3.99 | 291.35 | defragmentation | |||
| M3 4.67 | 441.42 | demethylation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzun, K.; Gornowicz, A.; Lesyk, R.; Kryshchyshyn-Dylevych, A.; Gzella, A.; Czarnomysy, R.; Latacz, G.; Olejarz-Maciej, A.; Handzlik, J.; Bielawski, K.; et al. 2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule. Int. J. Mol. Sci. 2022, 23, 4091. https://doi.org/10.3390/ijms23084091
Buzun K, Gornowicz A, Lesyk R, Kryshchyshyn-Dylevych A, Gzella A, Czarnomysy R, Latacz G, Olejarz-Maciej A, Handzlik J, Bielawski K, et al. 2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule. International Journal of Molecular Sciences. 2022; 23(8):4091. https://doi.org/10.3390/ijms23084091
Chicago/Turabian StyleBuzun, Kamila, Agnieszka Gornowicz, Roman Lesyk, Anna Kryshchyshyn-Dylevych, Andrzej Gzella, Robert Czarnomysy, Gniewomir Latacz, Agnieszka Olejarz-Maciej, Jadwiga Handzlik, Krzysztof Bielawski, and et al. 2022. "2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule" International Journal of Molecular Sciences 23, no. 8: 4091. https://doi.org/10.3390/ijms23084091
APA StyleBuzun, K., Gornowicz, A., Lesyk, R., Kryshchyshyn-Dylevych, A., Gzella, A., Czarnomysy, R., Latacz, G., Olejarz-Maciej, A., Handzlik, J., Bielawski, K., & Bielawska, A. (2022). 2-{5-[(Z,2Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule. International Journal of Molecular Sciences, 23(8), 4091. https://doi.org/10.3390/ijms23084091







