Synthesis and Biological Evaluation of Novel Bufalin Derivatives
Abstract
:1. Introduction
2. Results
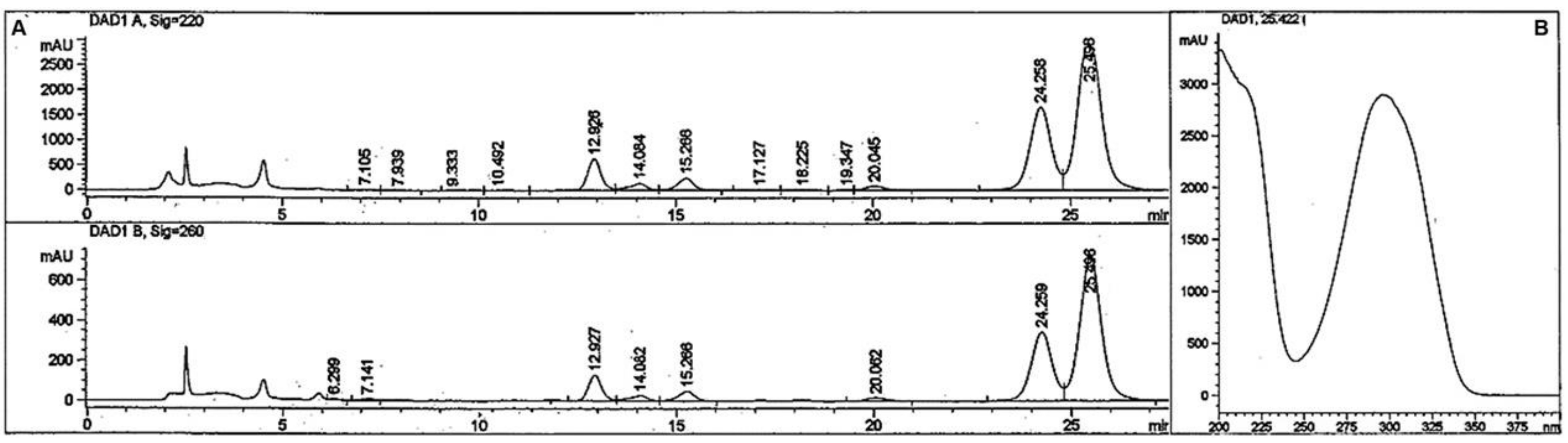
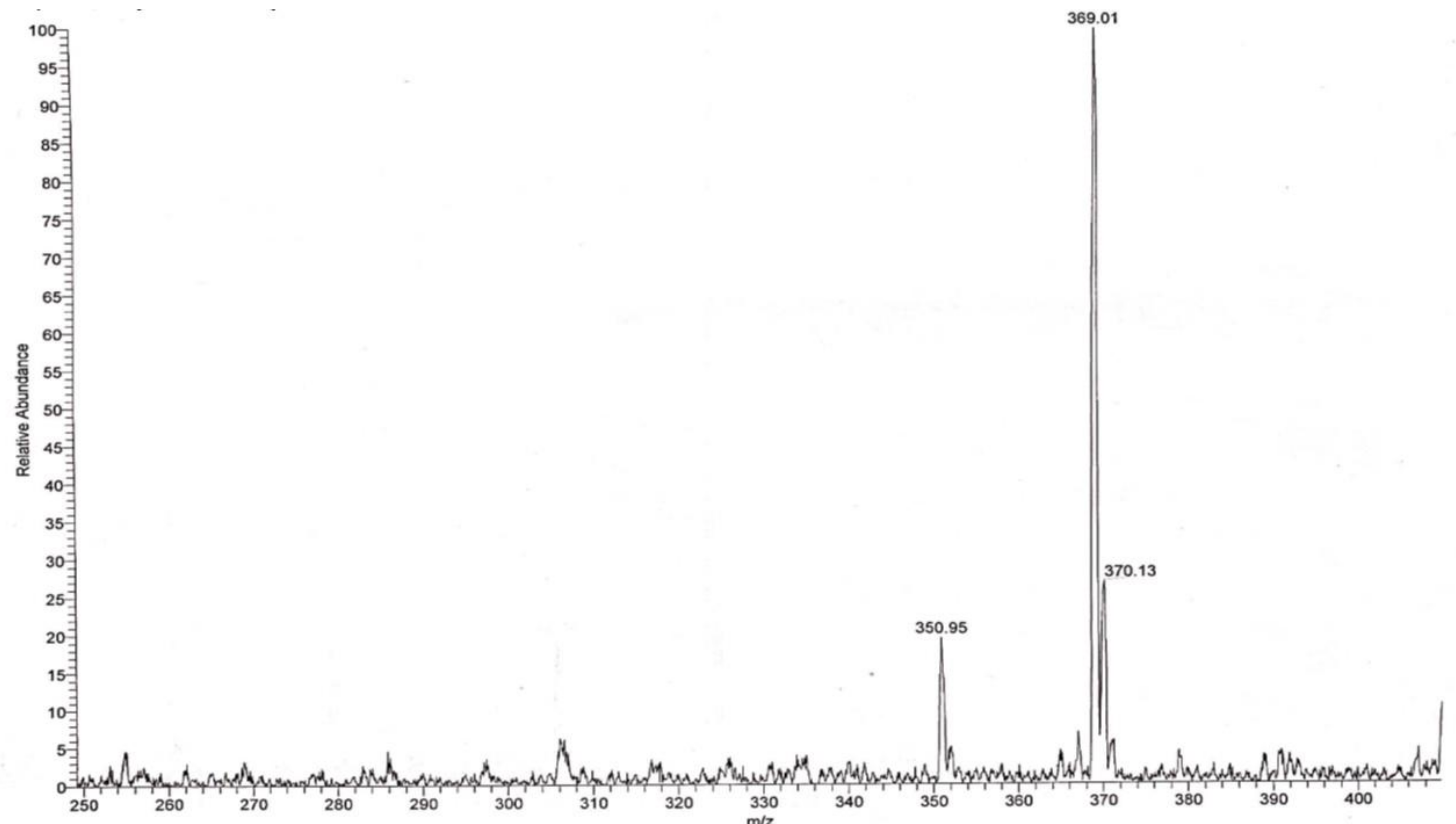
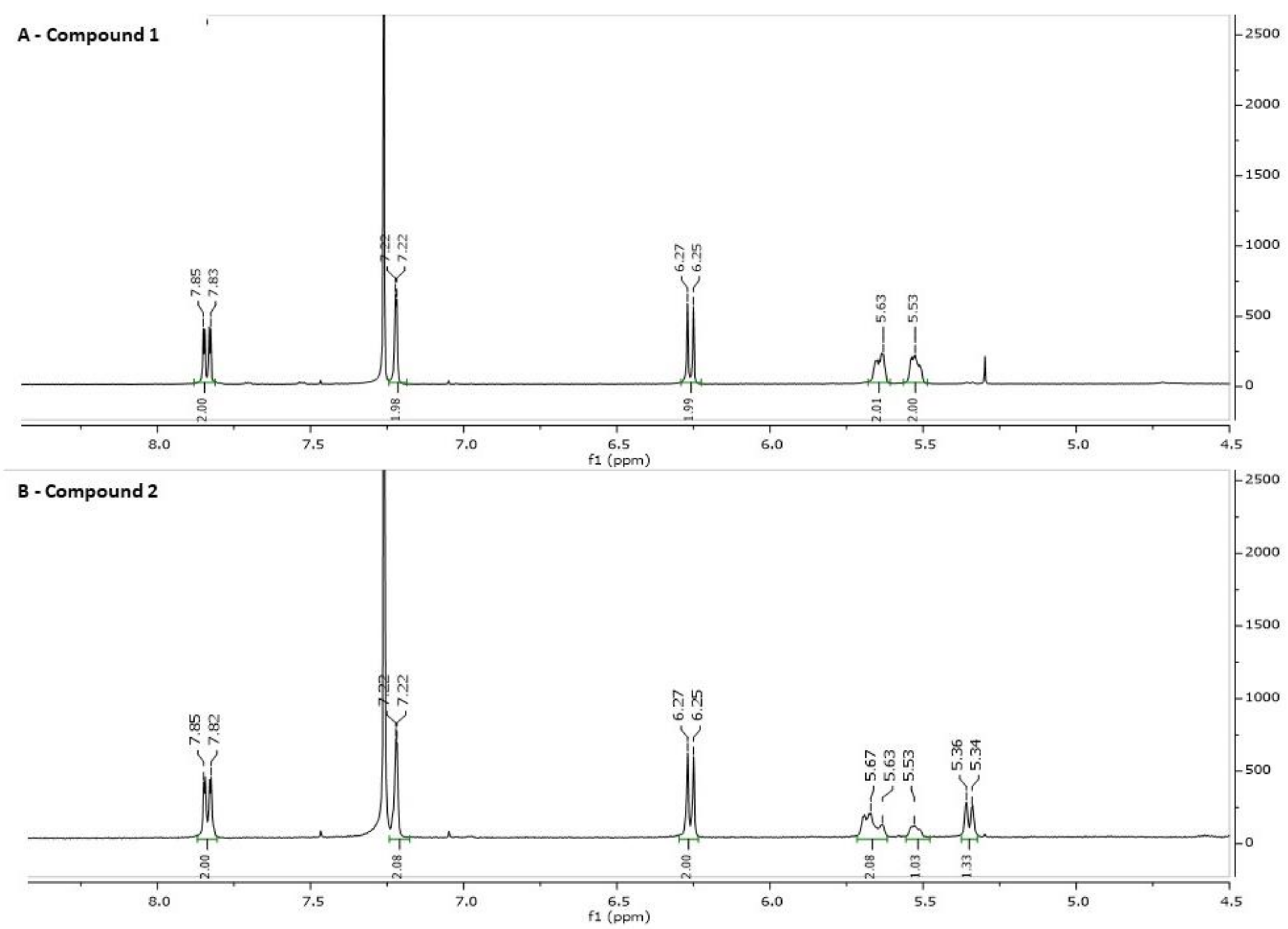
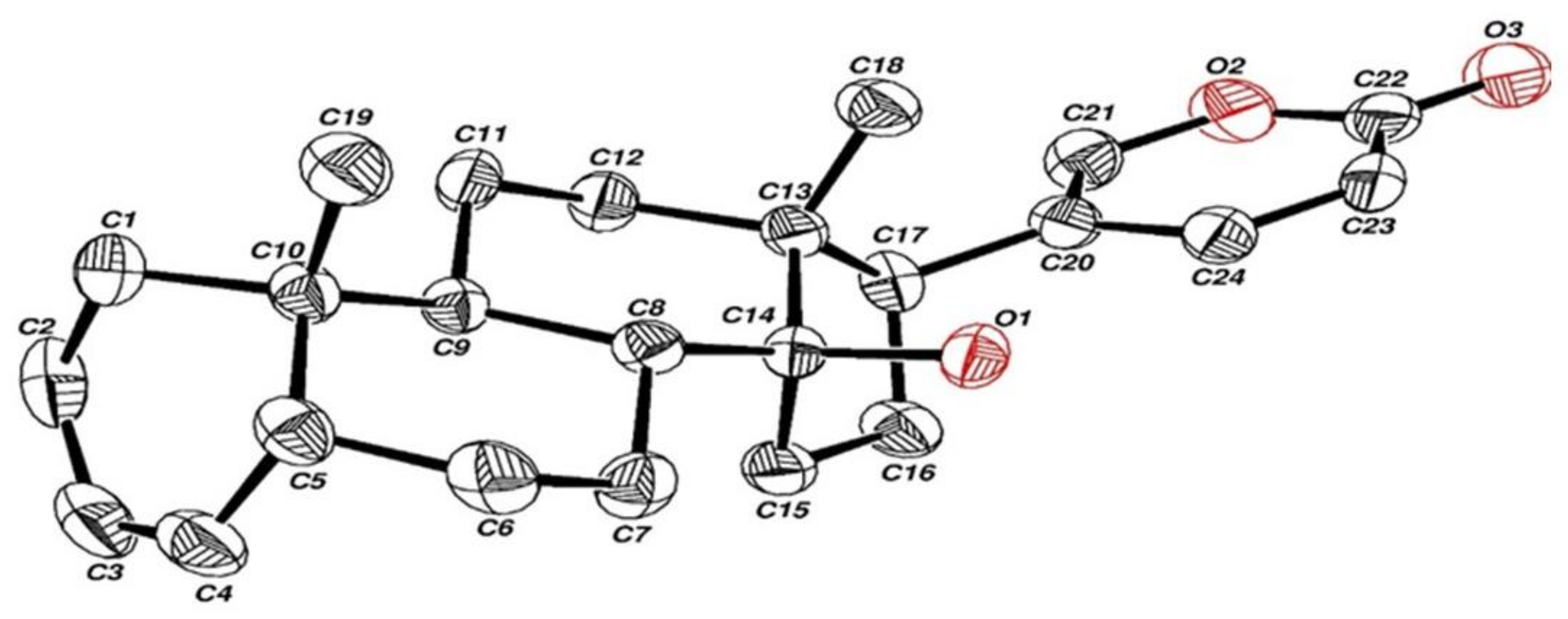
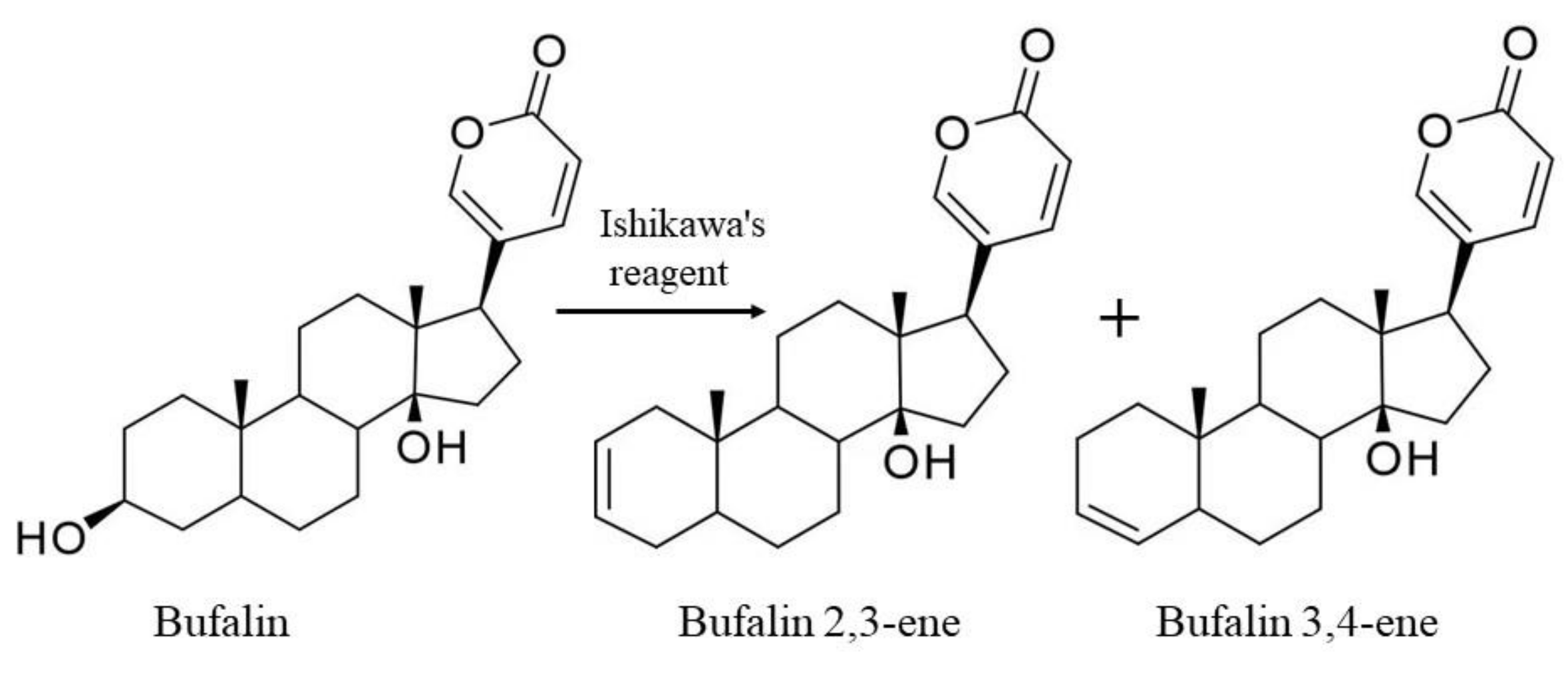
2.1. Synthesis and Structure Validation of Bufalin Derivatives
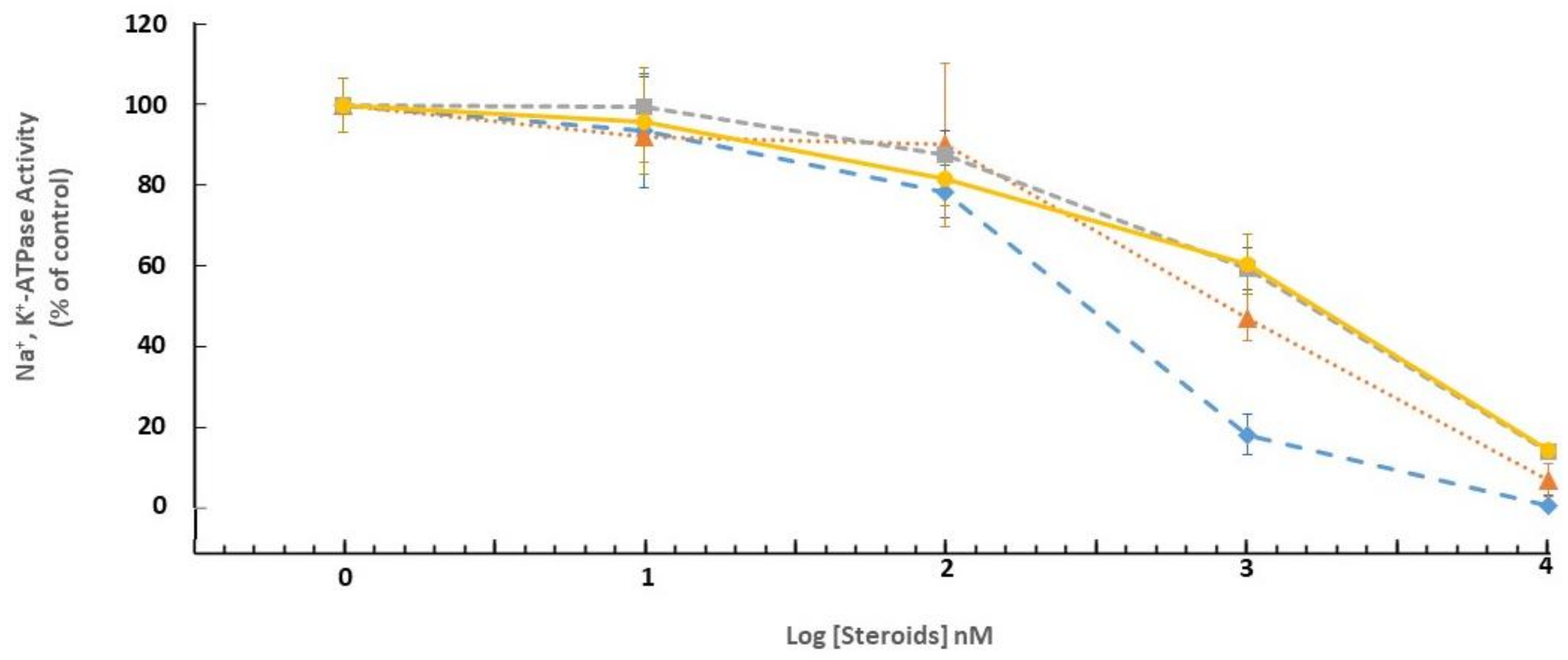
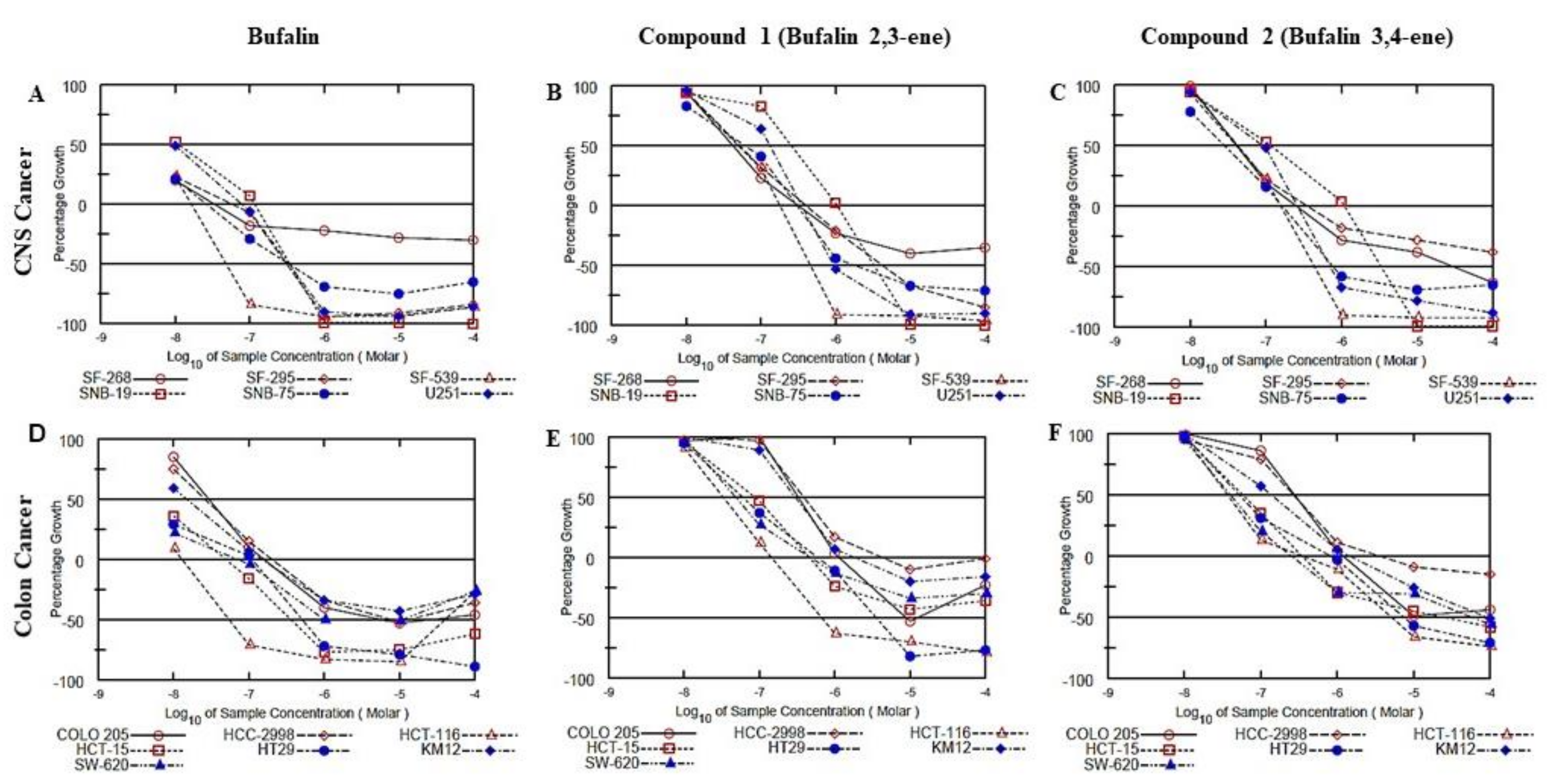
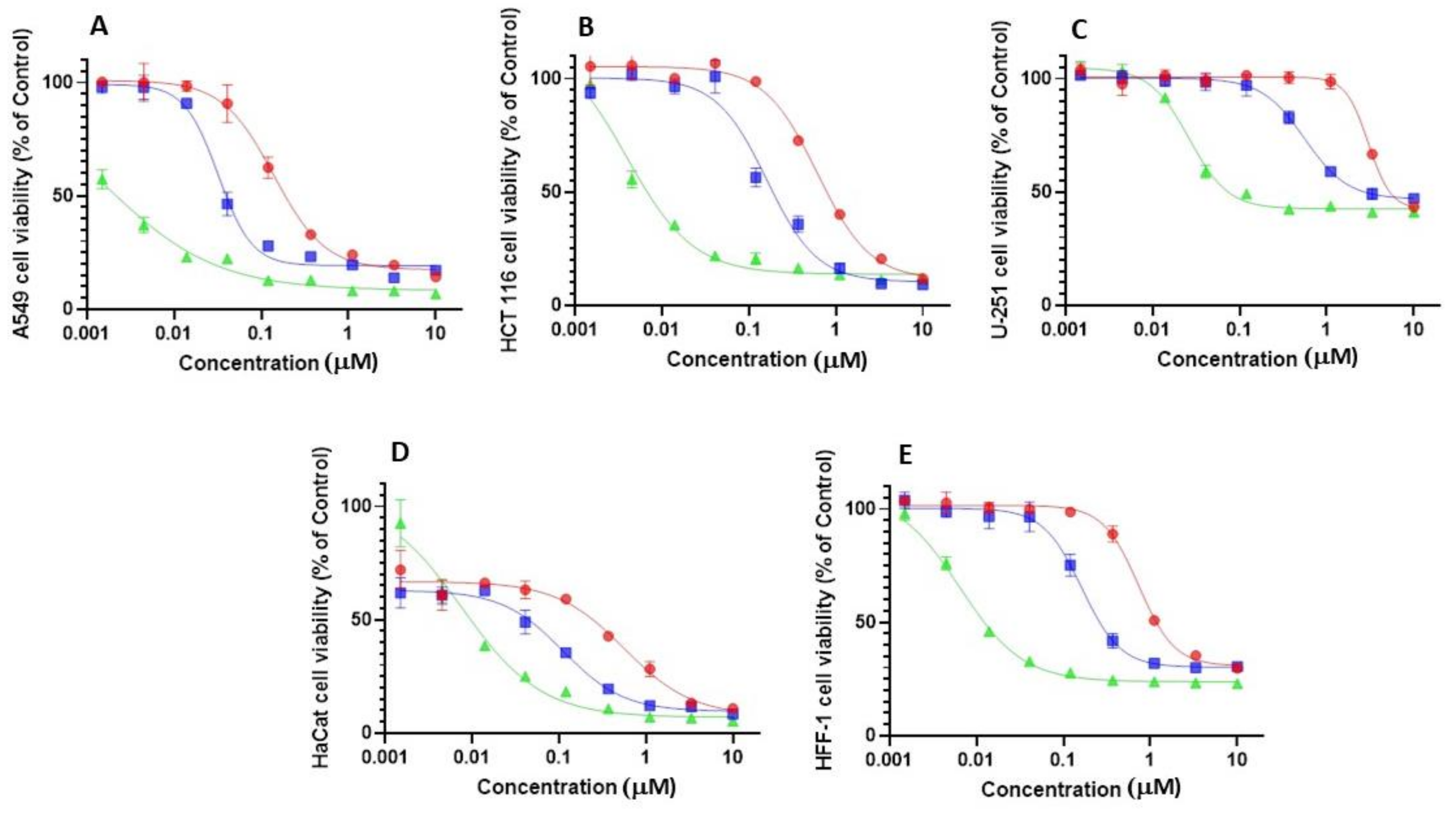
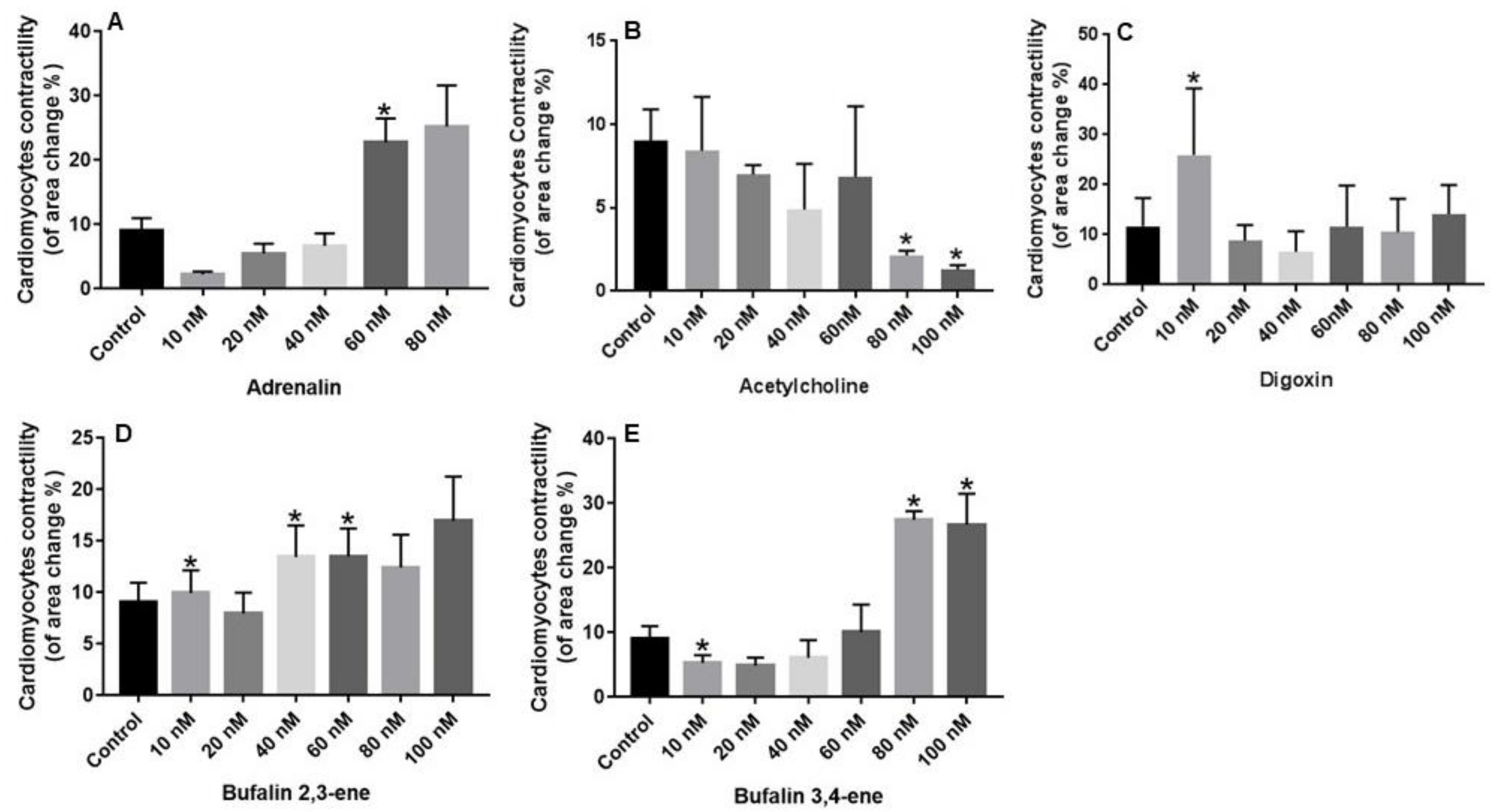
2.2. Biological Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. High Performance Liquid Chromatography
4.3. NMR and Mass Spectroscopy
4.4. Crystallographic Structure Analysis
4.5. Chemical Experimental Procedures
4.6. ATPase Activity
4.7. Cytotoxicity against Cancer Cells
4.8. Quail Cardiac Muscle Cell Contractility
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krenn, L.; Kopp, B. Bufadienolides from animal and plant sources. Phytochemistry 1998, 48, 1–29. [Google Scholar] [CrossRef]
- Li, F.J.; Hu, J.H.; Ren, X.; Zhou, C.M.; Liu, Q.; Zhang, Y.Q. Toad venom: A comprehensive review of chemical constituents, anticancer activities, and mechanisms. Arch. Pharm. 2021, 354, e2100060. [Google Scholar] [CrossRef] [PubMed]
- Nesher, M.; Shpolansky, U.; Viola, N.; Dvela, M.; Buzaglo, N.; Ben-Ami, H.C.; Rosen, H.; Lichtstein, D. Ouabain attenuates cardiotoxicity induced by other cardiac steroids. Br. J. Pharmacol. 2010, 160, 346–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prassas, I.; Diamandis, E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yuan, Y.; Han, L.; Qiu, Y.; Huang, X.; Gao, F.; Fan, G.; Zhang, Y.; Tang, X.; He, X.; et al. Bufalin-loaded vitamin E succinate-grafted-chitosan oligosaccharide/RGD conjugated TPGS mixed micelles demonstrated improved antitumor activity against drug-resistant colon cancer. Int. J. Nanomed. 2018, 13, 7533–7548. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zeng, C.; Cao, W.; Zhou, X.; Pan, Y.; Xie, Y.; Zhang, Y.; Yang, Q.; Wang, S. Bufalin-loaded PEGylated liposomes: Antitumor efficacy, acute toxicity, and tissue distribution. Nanoscale Res. Lett. 2019, 14, 223. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, J. Transferrin and folic acid co-modified bufalin-loaded nanoliposomes: Preparation, characterization, and application in anticancer activity. Int. J. Nanomed. 2018, 13, 6009–6018. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-S.; Wang, J.; Chen, J.; Kuo, K.T.; Tang, J.; Gao, H.; Chen, L.; Chen, Z.; Meng, Z. New therapeutic aspects of steroidal cardiac glycosides: The anticancer properties of Huachansu and its main active constituent Bufalin. Cancer Cell Int. 2019, 19, 92. [Google Scholar] [CrossRef]
- Zhakeer, Z.; Hadeer, M.; Tuerxun, Z.; Tuerxun, K. Bufalin inhibits the inflammatory effects in asthmatic mice through the suppression of nuclear factor-kappa B activity. Pharmacology 2017, 99, 179–187. [Google Scholar] [CrossRef]
- Pollard, B.S.; Blanco, J.C.; Pollard, J.R. Classical drug digitoxin inhibits influenza cytokine storm, with implications for COVID-19 therapy. In Vivo 2020, 34, 3723–3730. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Madan, N.; Xu, Y.; Duan, Q.; Banerjee, M.; Larre, I.; Pierre, S.V.; Xie, Z. Src-independent ERK signaling through the rat α3 isoform of Na/K-ATPase. Am. J. Physiol. Cell Physiol. 2017, 312, C222–C232. [Google Scholar] [CrossRef]
- Cui, X.; Xie, Z. Protein interaction and Na/K-ATPase-mediated signal transduction. Molecules 2017, 22, 990. [Google Scholar] [CrossRef] [Green Version]
- Patocka, J.; Nepovimova, E.; Wu, W.; Kuca, K. Digoxin: Pharmacology and toxicology—A review. Environ. Toxicol. Pharmacol. 2020, 79, 103400. [Google Scholar] [CrossRef]
- Buzaglo, N.; Rosen, H.; Ben Ami, H.C.; Inbal, A.; Lichtstein, D. Essential opposite roles of ERK and Akt signaling in cardiac steroid-induced increase in heart contractility. J. Pharmacol. Exp. Ther. 2016, 357, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Su, H.; Wang, G.; Li, B.; Shen, G.; Gao, Q. Anti-tumor activity of bufalin by inhibiting c-MET mediated MEK/ERK and PI3K/AKT signaling pathways in gallbladder cancer. J. Cancer 2020, 11, 3114–3123. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.W.; Chang, H.Y.; Lee, Y.Z.; Hsu, H.Y.; Lee, S.J. The cardenolide ouabain suppresses coronaviral replication via augmenting a Na(+)/K(+)-ATPase-dependent PI3K_PDK1 axis signaling. Toxicol. Appl. Pharmacol. 2018, 356, 90–97. [Google Scholar] [CrossRef]
- Cornelius, F.; Kanai, R.; Toyoshima, C. A structural view on the functional importance of the sugar moiety and steroid hydroxyls of cardiotonic steroids in binding to Na,K-ATPase*. J. Biol. Chem. 2013, 288, 6602–6616. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, J.; Jang, H.G.; Mansur, N.; Ilovich, O.; Shpolansky, U.; Galili, D.; Feldman, T.; Rosen, H.; Lichstein, D. 4-(3′alpha15′beta-dihydroxy-5′beta-estran-17′beta-yl)furan-2-methyl alcohol: An anti-digoxin agent with a novel mechanism of action. J. Med. Chem. 2006, 49, 600–606. [Google Scholar] [CrossRef]
- Takaoka, A.; Iwakiri, H.; Ishikawa, N. F-propene-dialkylamine reaction products as fluorinating agents. Bull. Chem. Soc. Jpn. 1979, 52, 3337. [Google Scholar] [CrossRef] [Green Version]
- Ando, K.; Nakajima, Y.; Yamagishi, T.; Yamamoto, S.; Nakamura, H. Development of proximal coronary arteries in quail embryonic heart: Multiple capillaries penetrating the aortic sinus fuse to form main coronary trunk. Circ. Res. 2004, 94, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Cakstina, I.; Riekstina, U.; Boroduskis, M.; Nakurte, I.; Ancans, J.; Zile, M.H.; Muiznieks, I. Primary culture of avian embryonic heart forming region cells to study the regulation of vertebrate early heart morphogenesis by vitamin A. BMC Dev. Biol. 2014, 14, 10. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.; Yu, F.; Wu, W.; Liu, J.; Li, J.; Guo, F.; Xu, L.; Wang, F.; Cui, X. Bufalin enhances the killing efficacy of NK cells against hepatocellular carcinoma by inhibiting MICA shedding. Int. Immunopharmacol. 2021, 101, 108195. [Google Scholar] [CrossRef]
- Brownlee, A.A.; Johnson, P.; Mills, I.H. Actions of bufalin and cinobufotalin, two bufadienolides respectively more active and less active than ouabain, on ouabain binding and 86Rb uptake by human erythrocytes. Clin. Sci. 1990, 78, 169–174. [Google Scholar] [CrossRef]
- Laursen, M.; Gregersen, J.L.; Yatime, L.; Nissen, P.; Fedosova, N.U. Structures and characterization of digoxin- and bufalin-bound Na+,K+-ATPase compared with the ouabain-bound complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1755–1760. [Google Scholar] [CrossRef] [Green Version]
- Pamnani, M.B.; Chen, S.; Bryant, H.J.; Schooley, J.F.; Eliades, D.C.; Yuan, C.M.; Haddy, F.J. Effects of three sodium-potassium adenosine triphosphatase inhibitors. Hypertension 1991, 18, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Pamnani, M.B.; Chen, S.; Yuan, C.M.; Haddy, F.J. Chronic blood pressure effects of bufalin, a sodium-potassium ATPase inhibitor, in rats. Hypertension 1994, 23, I106–I109. [Google Scholar] [CrossRef] [Green Version]
- Karaś, K.; Sałkowska, A.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. Cardiac glycosides with target at direct and indirect interactions with nuclear receptors. Biomed. Pharmacother. 2020, 127, 110106. [Google Scholar] [CrossRef]
- Wang, Y.; Lonard, D.M.; Yu, Y.; Chow, D.-C.; Palzkill, T.G.; Wang, J.; Qi, R.; Matzuk, A.J.; Song, X.; Madoux, F.; et al. Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res. 2014, 74, 1506–1517. [Google Scholar] [CrossRef] [Green Version]
- Stenkvist, B.; Bengtsson, E.; Dahlqvist, B.; Eriksson, O.; Jarkrans, T.; Nordin, B. Cardiac glycosides and breast cancer, revisited. N. Engl. J. Med. 1982, 306, 484. [Google Scholar]
- Jing, Y.; Ohizumi, H.; Kawazoe, N.; Hashimoto, S.; Masuda, Y.; Nakajo, S.; Yoshida, T.; Kuroiwa, Y.; Nakaya, K. Selective inhibitory effect of bufalin on growth of human tumor cells in vitro: Association with the induction of apoptosis in leukemia HL-60 cells. Jpn. J. Cancer Res. 1994, 85, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, W.; Schonfeld, R.; Menke, K.H.; Weiland, J.; Repke, K.R. Origin of differences of inhibitory potency of cardiac glycosides in Na+/K+-transporting ATPase from human cardiac muscle, human brain cortex and guinea-pig cardiac muscle. Biochem. Pharmacol. 1986, 35, 3221–3231. [Google Scholar] [CrossRef]
- Huss, D.; Poynter, G.; Lansford, R. Japanese quail (Coturnix japonica) as a laboratory animal model. Lab. Anim. 2008, 37, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ershad, M.; Meredith, A.; Shah, N.; Khalid, M.M. Cardioactive Steroid Toxicity; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Buzaglo, N.; Golomb, M.; Rosen, H.; Beeri, R.; Ami, H.C.-B.; Langane, F.; Pierre, S.; Lichtstein, D. Augmentation of ouabain-induced increase in heart muscle contractility by akt inhibitor MK-2206. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 78–89. [Google Scholar] [CrossRef]
- Lichtstein, D.; Samuelov, S.; Bourrit, A. Characterization of the stimulation of neuronal Na(+), K(+)-ATPase activity by low concentrations of ouabain. Neurochem. Int. 1985, 7, 709–715. [Google Scholar] [CrossRef]
- Bagniski, E.; Zak, B. Micro-determination of serum phosphate and phospholipids. Clin. Chim. Acta 1960, 5, 834–838. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampath, V.; Horesh, N.; Sasi, B.; Zannadeh, H.; Pogodin, I.; Singh, S.V.; Deutsch, J.; Lichtstein, D. Synthesis and Biological Evaluation of Novel Bufalin Derivatives. Int. J. Mol. Sci. 2022, 23, 4007. https://doi.org/10.3390/ijms23074007
Sampath V, Horesh N, Sasi B, Zannadeh H, Pogodin I, Singh SV, Deutsch J, Lichtstein D. Synthesis and Biological Evaluation of Novel Bufalin Derivatives. International Journal of Molecular Sciences. 2022; 23(7):4007. https://doi.org/10.3390/ijms23074007
Chicago/Turabian StyleSampath, VishnuPriya, Noa Horesh, Ben Sasi, Hiba Zannadeh, Ilana Pogodin, Shiv Vardan Singh, Joseph Deutsch, and David Lichtstein. 2022. "Synthesis and Biological Evaluation of Novel Bufalin Derivatives" International Journal of Molecular Sciences 23, no. 7: 4007. https://doi.org/10.3390/ijms23074007
APA StyleSampath, V., Horesh, N., Sasi, B., Zannadeh, H., Pogodin, I., Singh, S. V., Deutsch, J., & Lichtstein, D. (2022). Synthesis and Biological Evaluation of Novel Bufalin Derivatives. International Journal of Molecular Sciences, 23(7), 4007. https://doi.org/10.3390/ijms23074007




