Sunitinib and Pterostilbene Combination Treatment Exerts Antitumor Effects in Gastric Cancer via Suppression of PDZD8
Abstract
:1. Introduction
2. Results
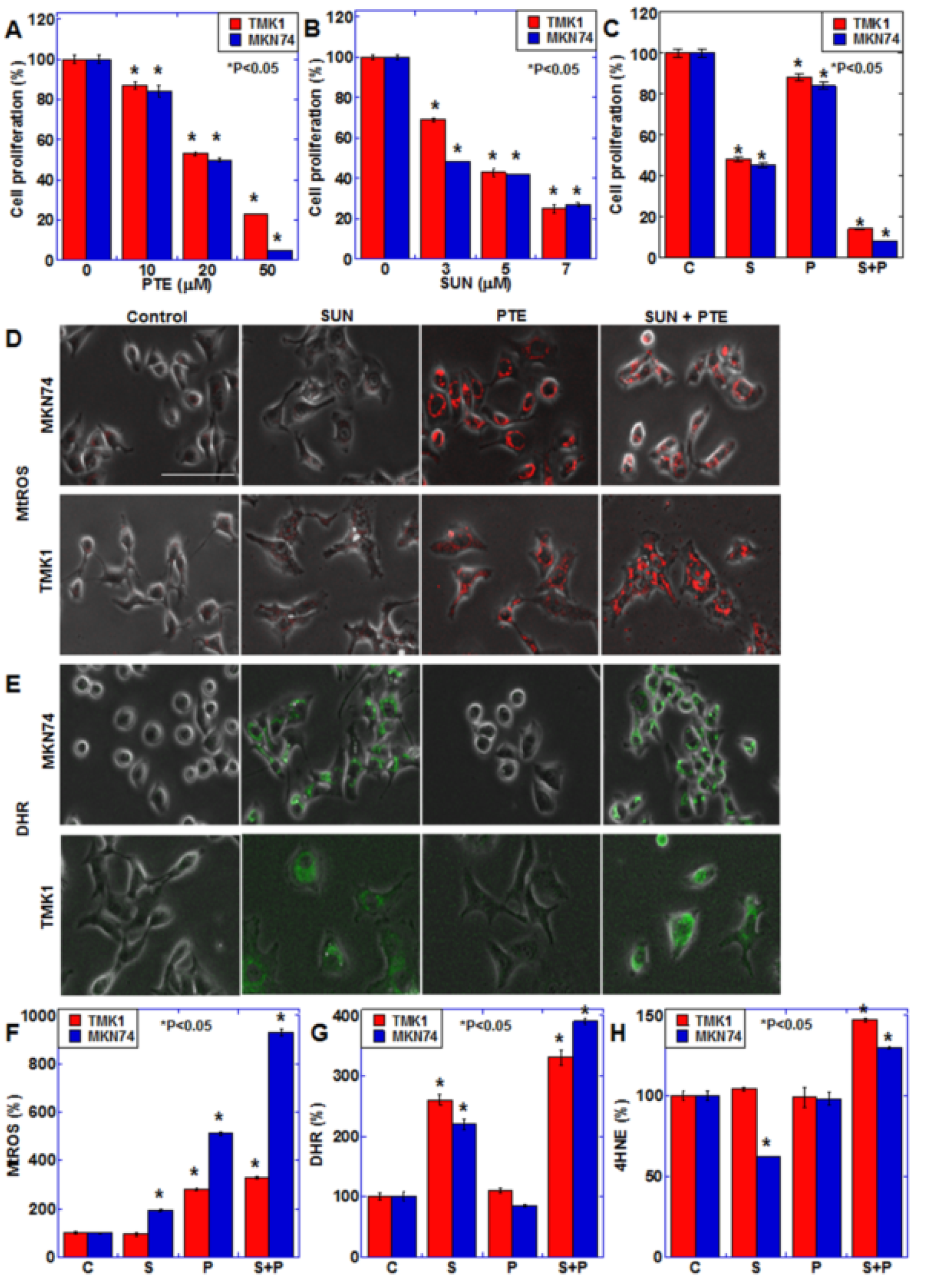
2.1. Effect of SUN and PTE on Gastric Cancer Cells
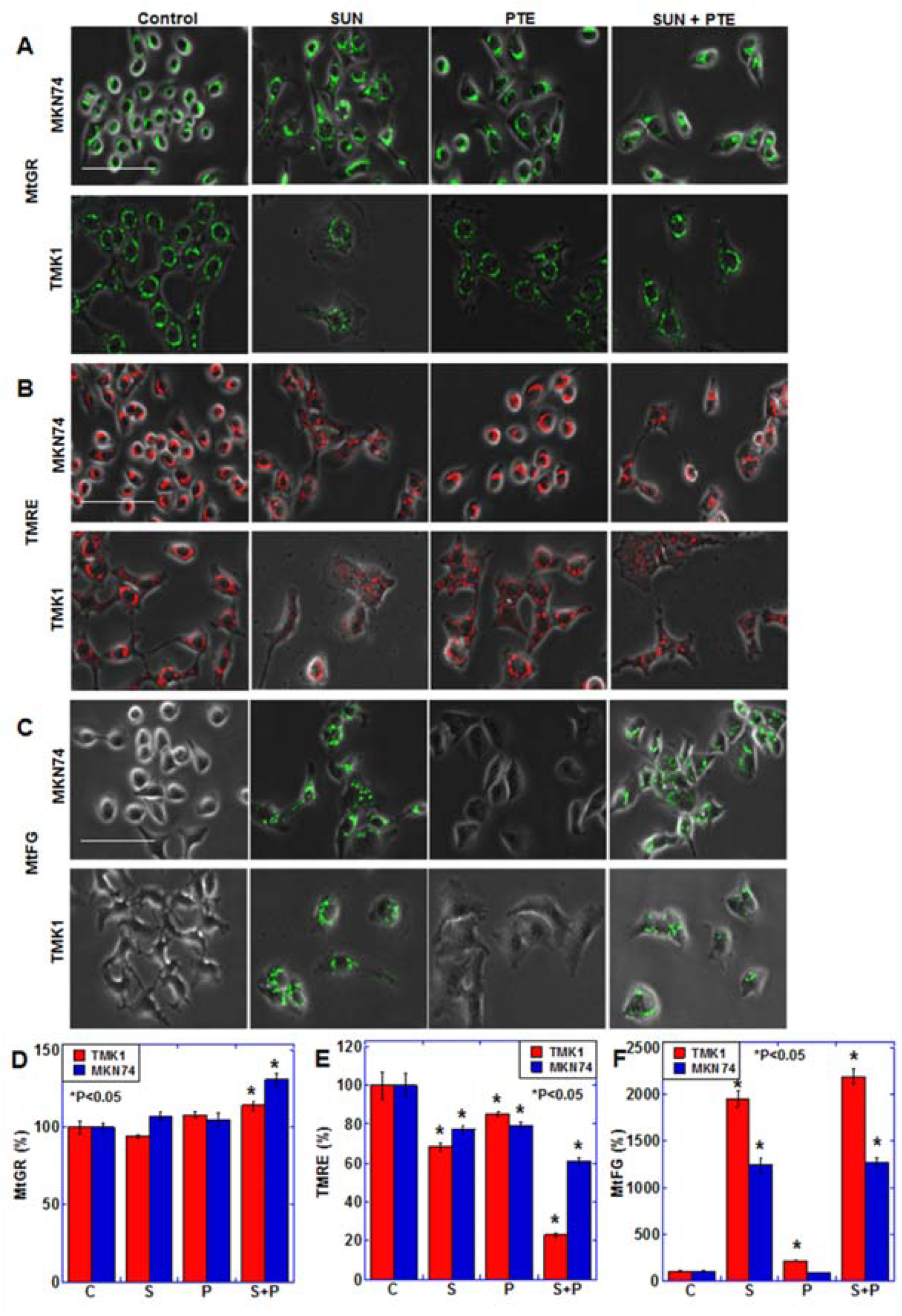
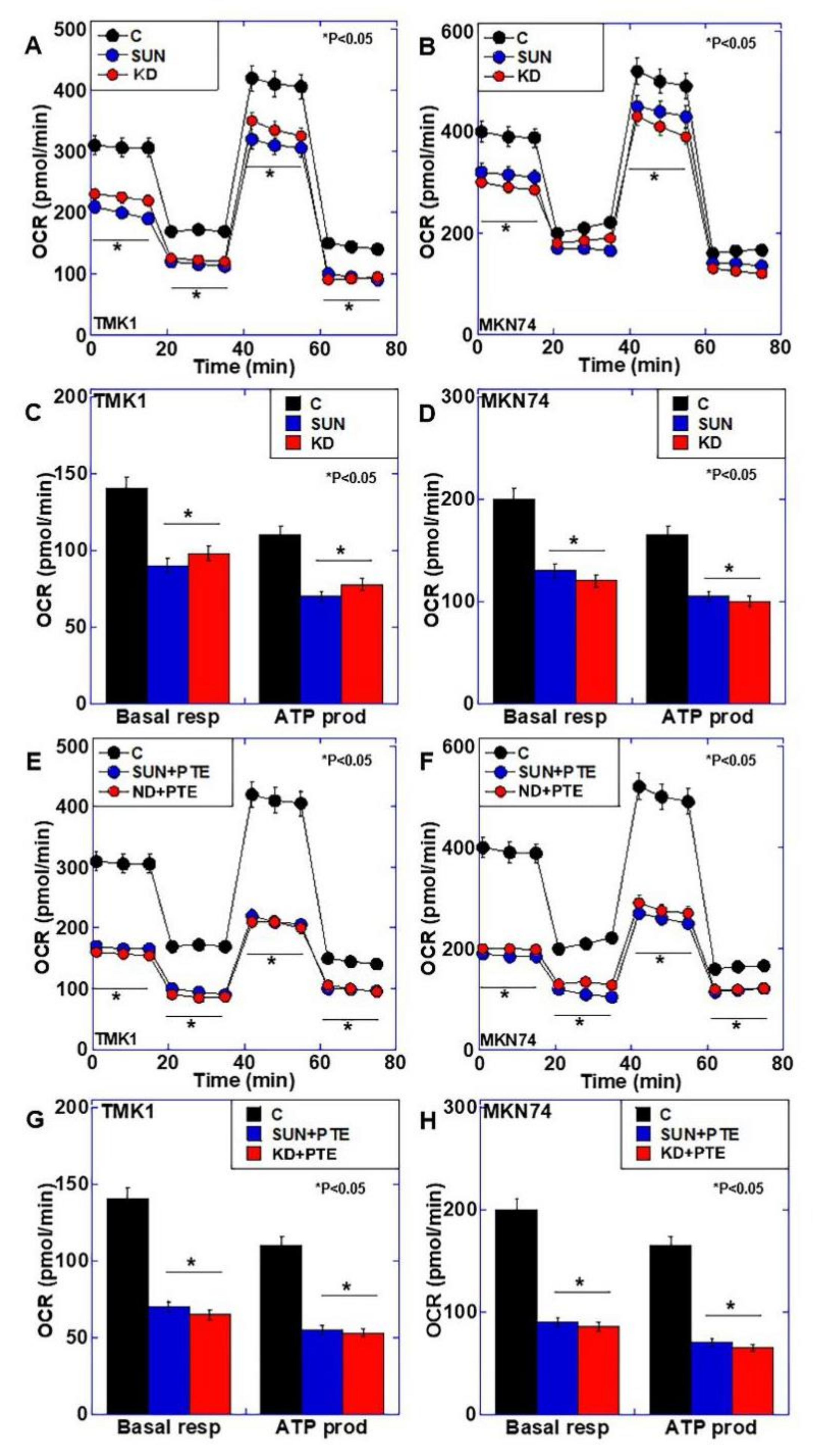
2.2. Effect of SUN on Mitochondrial Function
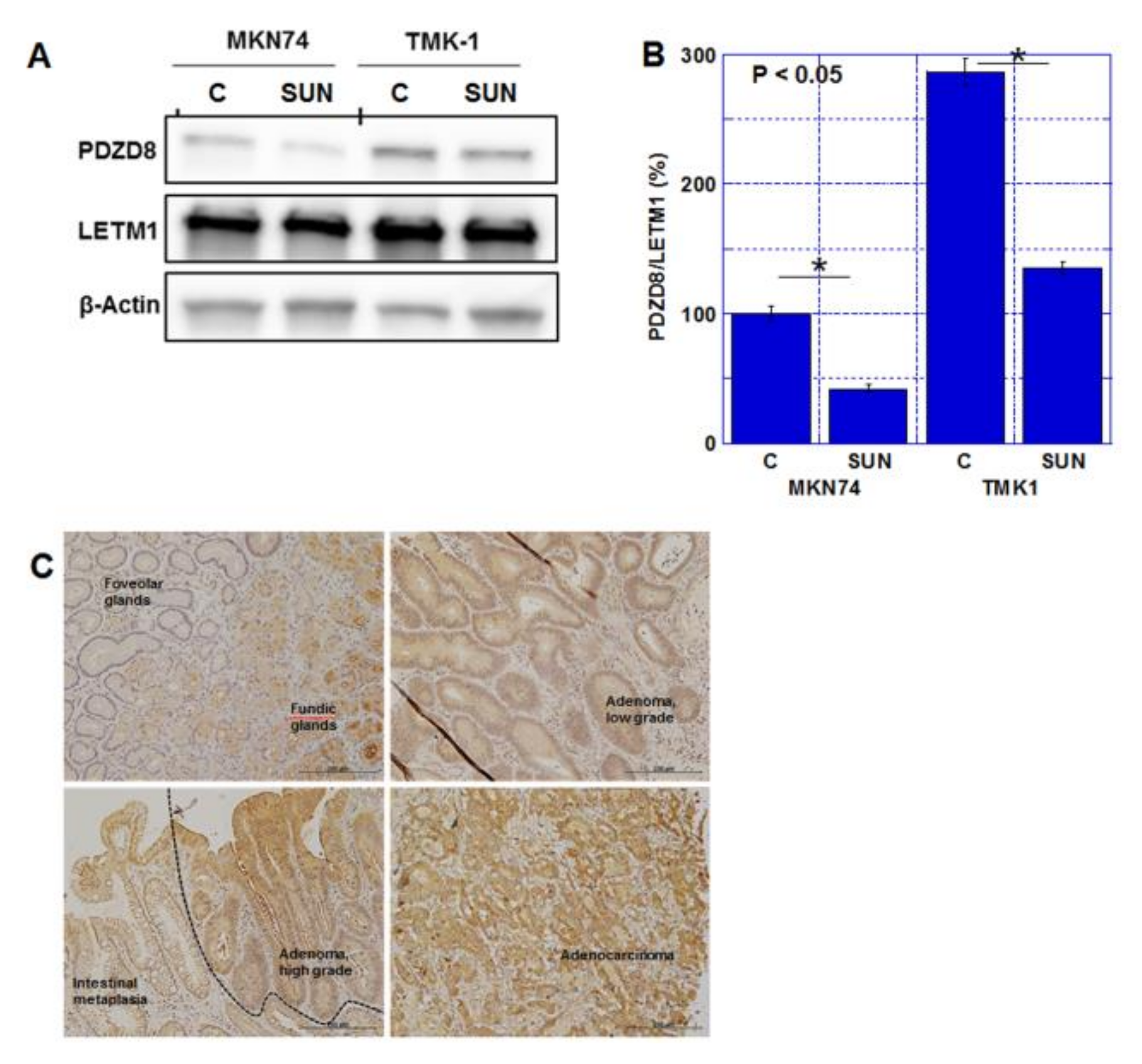
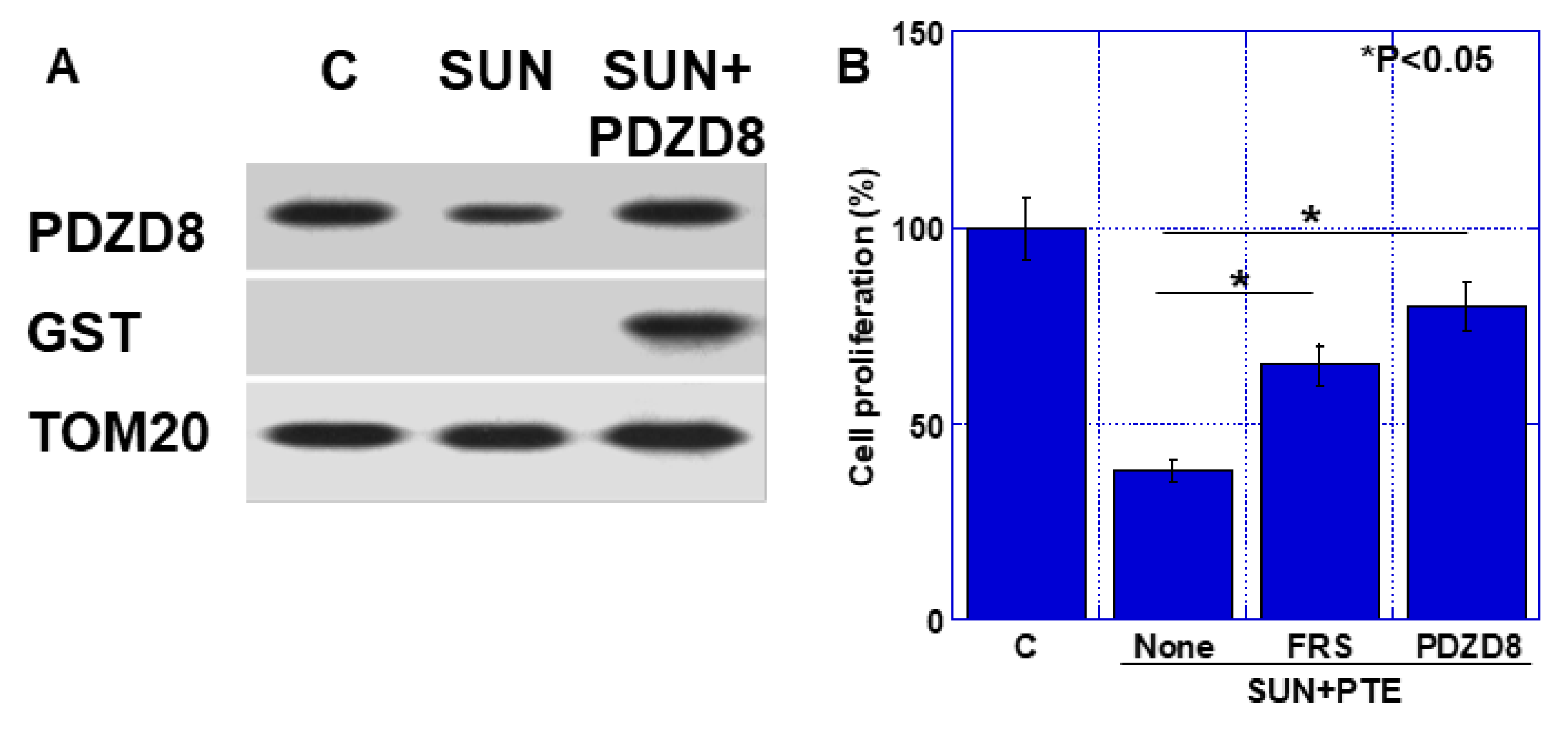
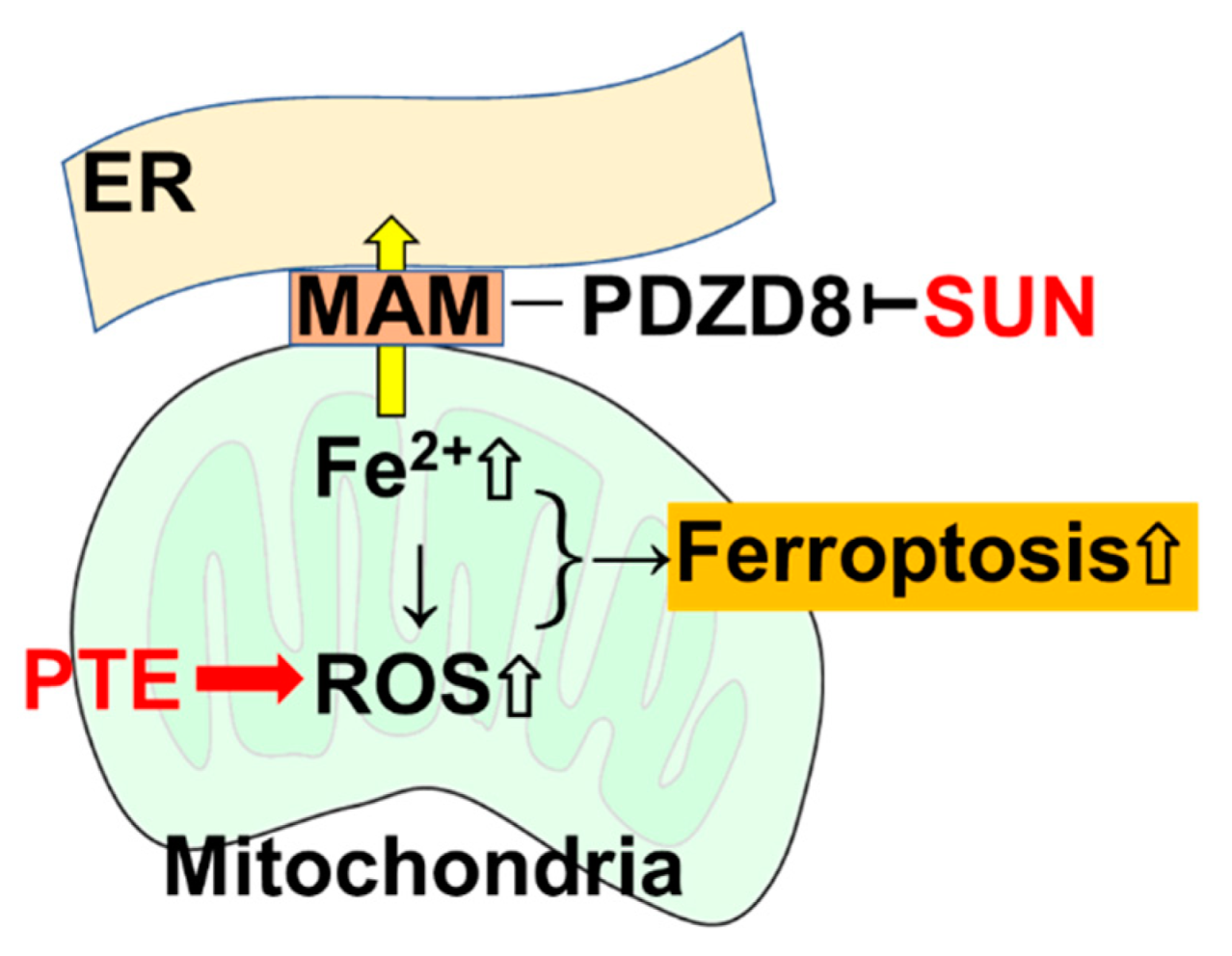
2.3. Role of PDZD8 in Gastric Cancer Cells
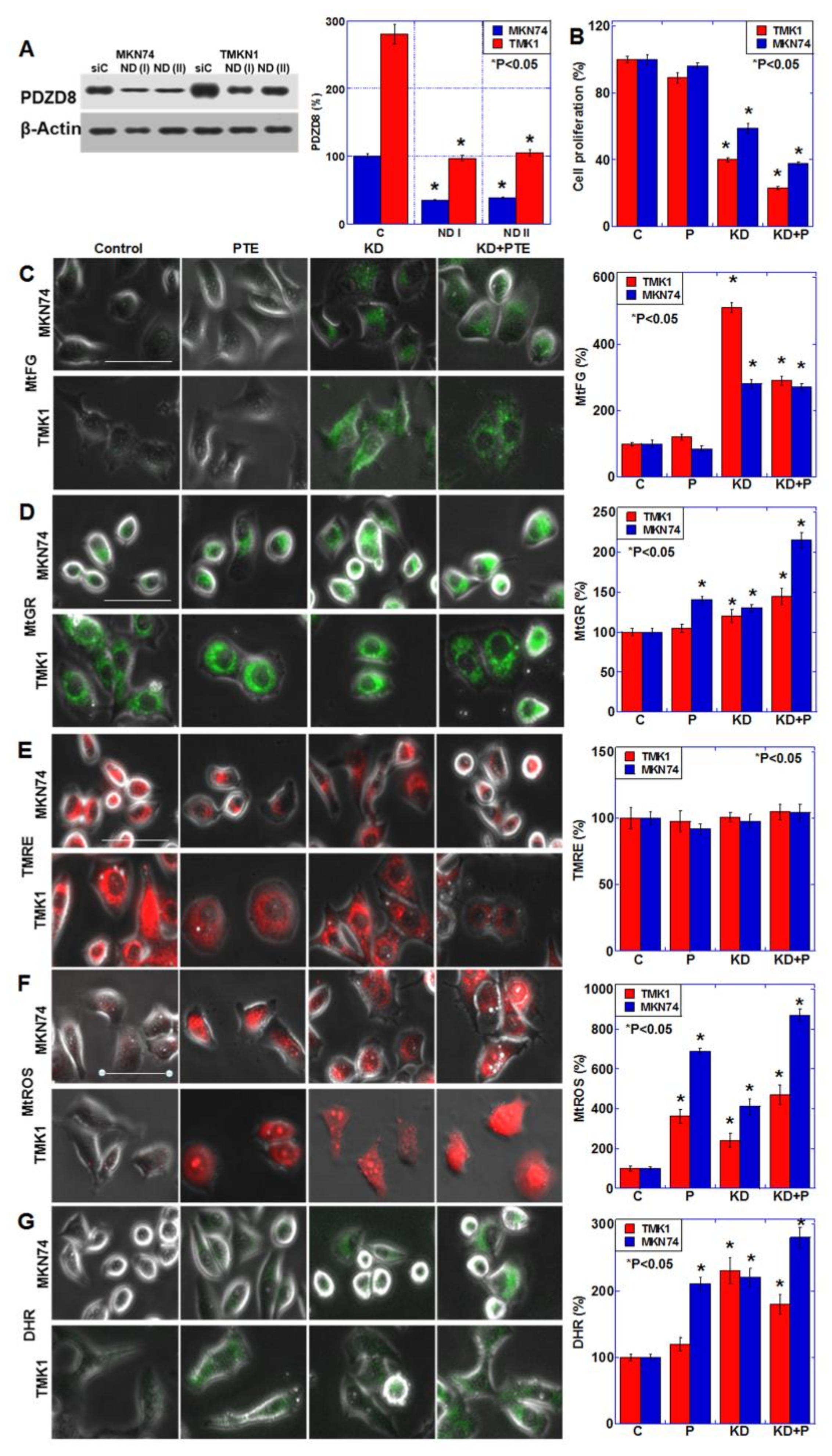
2.4. Effects of SUN Treatment or PDZD8 Knockdown on Mitochondrial Respiration
2.5. Effect of Extrinsic PDZD Protein on SUN and PTE-Induced Cell Growth Inhibition in TMK1 Cells
2.6. Effect of SUN and PTE on Mouse Tumors
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Cell Growth and Apoptosis
4.3. Mitochondrial Imaging
4.4. mRNA Profiling
4.5. Extracellular Flux Analysis
4.6. Protein Extraction
4.7. Immunoblot Analysis
4.8. Immunoprecipitation
4.9. Tissue Microarray and Surgical Specimens
4.10. Small Interfering RNA
4.11. Enzyme-Linked Immunosorbent Assay (ELISA)
4.12. Extrinsic PDZD8 Protein Administration
4.13. RT-PCR
4.14. Animals
4.15. Animal Tumor Models
4.16. Immunohistochemistry
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SUN | sunitinib |
| PTE | pterostilbene |
| TKI | tyrosine kinase inhibitor |
| ER | endoplasmic reticulum |
| MAM | Mitochondria-associated endoplasmic reticulum membrane |
| PDZD8 | PDZ domain-containing protein 8 |
| MMP | mitochondrial membrane potential |
| OCR | oxygen consumption rate |
| LETM1 | leucine zipper and EF-hand containing transmembrane protein 1 |
| QFM | quadriceps femoris muscle |
| VDAC1 | voltage-dependent anion channel 1 |
References
- Statistics Bureau Ministry of Internal Affairs and Communications Japan. Statistical Handbook of Japan 2017; Statistics Bureau Ministry of Internal Affairs and Communications: Tokyo, Japan, 2017. [Google Scholar]
- Mikami, H.; Nagase, H. Cancer survival rates at Japanese Association of Clinical Cancer Centers. Available online: https://kapweb.chiba-cancer-registry.org/ (accessed on 1 June 2021).
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Khan, U.; Shah, M.A. Ramucirumab for the treatment of gastric or gastro-esophageal junction cancer. Exp. Opin. Biol. Ther. 2019, 19, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Haap, M.; Kopp, H.G.; Lipp, H.P. Tyrosine kinase inhibitors—A review on pharmacology, metabolism and side effects. Curr. Drug Metab. 2009, 10, 470–481. [Google Scholar] [CrossRef]
- Orphanos, G.S.; Ioannidis, G.N.; Ardavanis, A.G. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009, 48, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Sadek, I. Sunitinib: The antiangiogenic effects and beyond. Onco. Targets Ther. 2016, 9, 5495–5505. [Google Scholar] [CrossRef] [Green Version]
- Papaetis, G.S.; Syrigos, K.N. Sunitinib: A multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs 2009, 23, 377–389. [Google Scholar] [CrossRef]
- Morishita, A.; Gong, J.; Masaki, T. Targeting receptor tyrosine kinases in gastric cancer. World J. Gastroenterol. 2014, 20, 4536–4545. [Google Scholar] [CrossRef]
- Daniel, M.; Tollefsbol, T.O. Pterostilbene down-regulates hTERT at physiological concentrations in breast cancer cells: Potentially through the inhibition of cMyc. J. Cell Biochem. 2018, 119, 3326–3337. [Google Scholar] [CrossRef]
- Moon, D.; McCormack, D.; McDonald, D.; McFadden, D. Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro. J. Surg. Res. 2013, 180, 208–215. [Google Scholar] [CrossRef]
- Mori, S.; Kishi, S.; Honoki, K.; Fujiwara-Tani, R.; Moriguchi, T.; Sasaki, T.; Fujii, K.; Tsukamoto, S.; Fujii, H.; Kido, A.; et al. Anti-Stem Cell Property of Pterostilbene in Gastrointestinal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9437. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef] [Green Version]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid. Med. Cell Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef] [Green Version]
- Filadi, R.; Greotti, E.; Pizzo, P. Highlighting the endoplasmic reticulum-mitochondria connection: Focus on Mitofusin 2. Pharm. Res. 2018, 128, 42–51. [Google Scholar] [CrossRef]
- Raturi, A.; Simmen, T. Where the endoplasmic reticulum and the mitochondrion tie the knot: The mitochondria-associated membrane (MAM). Biochim. Biophys. Acta 2013, 1833, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Simmen, T.; Herrera-Cruz, M.S. Plastic mitochondria-endoplasmic reticulum (ER) contacts use chaperones and tethers to mould their structure and signaling. Curr. Opin. Cell Biol. 2018, 53, 61–69. [Google Scholar] [CrossRef]
- Jeyasimman, D.; Saheki, Y. SMP domain proteins in membrane lipid dynamics. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2020, 1865, 158447. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, J.; Chu, Q.Z.; Ji, W.K. PDZD8-mediated lipid transfer at contacts between the ER and late endosomes/lysosomes is required for neurite outgrowth. J. Cell Sci. 2022, 135, jcs255026. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Kwon, S.K.; Paek, H.; Pernice, W.M.; Paul, M.A.; Lee, J.; Erfani, P.; Raczkowski, A.; Petrey, D.S.; Pon, L.A.; et al. ER-mitochondria tethering by PDZD8 regulates Ca(2+) dynamics in mammalian neurons. Science 2017, 358, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Schmollinger, S.; Attar, N.; Campos, O.A.; Vogelauer, M.; Carey, M.F.; Merchant, S.S.; Kurdistani, S.K. Endoplasmic reticulum-mitochondria junction is required for iron homeostasis. J. Biol. Chem. 2017, 292, 13197–13204. [Google Scholar] [CrossRef] [Green Version]
- Nechushtai, R.; Karmi, O.; Zuo, K.; Marjault, H.B.; Darash-Yahana, M.; Sohn, Y.S.; King, S.D.; Zandalinas, S.I.; Carloni, P.; Mittler, R. The balancing act of NEET proteins: Iron, ROS, calcium and metabolism. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118805. [Google Scholar] [CrossRef]
- Lipper, C.H.; Stofleth, J.T.; Bai, F.; Sohn, Y.S.; Roy, S.; Mittler, R.; Nechushtai, R.; Onuchic, J.N.; Jennings, P.A. Redox-dependent gating of VDAC by mitoNEET. Proc. Natl. Acad. Sci. USA 2019, 116, 19924–19929. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Darash-Yahana, M.; Sohn, Y.S.; Bai, F.; Song, L.; Cabantchik, I.Z.; Jennings, P.A.; Onuchic, J.N.; Nechushtai, R. NEET Proteins: A New Link Between Iron Metabolism, Reactive Oxygen Species, and Cancer. Antioxid. Redox Signal. 2019, 30, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur. J. Pharm. 2016, 789, 229–243. [Google Scholar] [CrossRef]
- Dong, J.; Guo, H.; Chen, Y. Pterostilbene induces apoptosis through caspase activation in ovarian cancer cells. Eur. J. Gynaecol. Oncol. 2016, 37, 342–347. [Google Scholar]
- Kong, Y.; Chen, G.; Xu, Z.; Yang, G.; Li, B.; Wu, X.; Xiao, W.; Xie, B.; Hu, L.; Sun, X.; et al. Pterostilbene induces apoptosis and cell cycle arrest in diffuse large B-cell lymphoma cells. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alosi, J.A.; McDonald, D.E.; Schneider, J.S.; Privette, A.R.; McFadden, D.W. Pterostilbene inhibits breast cancer in vitro through mitochondrial depolarization and induction of caspase-dependent apoptosis. J. Surg. Res. 2010, 161, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeHart, D.N.; Fang, D.; Heslop, K.; Li, L.; Lemasters, J.J.; Maldonado, E.N. Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells. Biochem. Pharm. 2018, 148, 155–162. [Google Scholar] [CrossRef]
- Hsiao, P.C.; Chou, Y.E.; Tan, P.; Lee, W.J.; Yang, S.F.; Chow, J.M.; Chen, H.Y.; Lin, C.H.; Lee, L.M.; Chien, M.H. Pterostilbene simultaneously induced G0/G1-phase arrest and MAPK-mediated mitochondrial-derived apoptosis in human acute myeloid leukemia cell lines. PLoS ONE 2014, 9, e105342. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Ferrous ion-EDTA-stimulated phospholipid peroxidation. A reaction changing from alkoxyl-radical- to hydroxyl-radical-dependent initiation. Biochem. J. 1984, 224, 697–701. [Google Scholar] [CrossRef] [Green Version]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef] [Green Version]
- Kuniyasu, H.; Oue, N.; Wakikawa, A.; Shigeishi, H.; Matsutani, N.; Kuraoka, K.; Ito, R.; Yokozaki, H.; Yasui, W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J. Pathol. 2002, 196, 163–170. [Google Scholar] [CrossRef]
- Matsushima-Otsuka, S.; Fujiwara-Tani, R.; Sasaki, T.; Ohmori, H.; Nakashima, C.; Kishi, S.; Nishiguchi, Y.; Fujii, K.; Luo, Y.; Kuniyasu, H. Significance of intranuclear angiotensin-II type 2 receptor in oral squamous cell carcinoma. Oncotarget 2018, 9, 36561–36574. [Google Scholar] [CrossRef] [Green Version]
- Kuniyasu, H.; Yasui, W.; Pettaway, C.A.; Yano, S.; Oue, N.; Tahara, E.; Fidler, I.J. Interferon-alpha prevents selection of doxorubicin-resistant undifferentiated-androgen-insensitive metastatic human prostate cancer cells. Prostate 2001, 49, 19–29. [Google Scholar] [CrossRef]

| Gene Symbol | MKN74 | TMK1 | Gene Symbol | MKN74 | TMK1 |
|---|---|---|---|---|---|
| PCNA | −2.91 | −5.16 | GPT | 1.18 | 1.15 |
| SLC38A5 | −1.53 | −11.7 | LDHD | 1.21 | 1.11 |
| TGM2 | −1.38 | −58.84 | SLC16A13 | 1.24 | 1.11 |
| ME2 | −2.57 | −2.33 | ACO1 | 1.29 | 1.24 |
| CKLF | −1.64 | −4.35 | GAD1 | 1.12 | 1.43 |
| L2HGDH | −2.59 | −1.93 | ME3 | 1.88 | 1.2 |
| PDHA1 | −2.58 | −1.57 | ACO2 | 1.18 | 1.91 |
| PFKP | −2.34 | −1.78 | SLC16A5 | 1.53 | 1.58 |
| CKB | −1.39 | −2.81 | SLC38A10 | 1.55 | 1.59 |
| PDK1 | −1.12 | −2.82 | PDK2 | 1.72 | 1.82 |
| SLC38A4 | −2.18 | −1.21 | SLC38A9 | 2.19 | 1.49 |
| GAD2 | −1.13 | −2.11 | SLC38A2 | 2.34 | 1.46 |
| TGM1 | −1.65 | −1.63 | PDP1 | 2.88 | 1.12 |
| LDHB | −1.66 | −1.26 | GLS | 4.35 | 1.11 |
| SLC16A3 | −1.15 | −1.66 | ME1 | 2.17 | 1.69 |
| GPT2 | −1.38 | −1.59 | SLC17A5 | 1.65 | 3.09 |
| GOT2 | −1.56 | −1.1 | SLC16A4 | 3.63 | 2.17 |
| OGFOD1 | −1.18 | −1.52 | PDK4 | 1.74 | 5.81 |
| PKM | −1.31 | −1.5 | |||
| PDHX | −1.48 | −1.21 | |||
| OGDHL | −1.17 | −1.31 | |||
| CKM | −1.29 | −1.15 | |||
| PFKL | −1.11 | −1.22 |
| Gene Symbol | MKN74 | TMK1 | Gene Symbol | MKN74 | TMK1 |
|---|---|---|---|---|---|
| IRF2BPL | −3.27 | −2.66 | ZNF474 | 2.13 | 1.03 |
| IRF2BP2 | −2.59 | −2 | MSS51 | 2.07 | 1.51 |
| PLEKHM3 | −2.47 | −2.77 | ZNF608 | 1.91 | 1.43 |
| PLEKHM1 | −2.25 | −2.33 | ENPP4 | 1.86 | −1.13 |
| TIMM10B | −2.01 | −2.05 | SNRNP48 | 1.84 | 1.74 |
| ZNF428 | −1.98 | 1.05 | ITGAE | 1.84 | 1.73 |
| LENG9 | −1.79 | −1.29 | FREM3 | 1.65 | 1.28 |
| ASPHD2 | −1.79 | −1.09 | PDE6A | 1.65 | 1.16 |
| AMDHD1 | −1.64 | −1.55 | TOPAZ1 | 1.61 | 1.18 |
| ZNF609 | −1.56 | −1.22 | SLC39A5 | 1.61 | 1.18 |
| PDZD8 | −1.45 | −1.38 | SLC39A8 | 1.57 | 1.63 |
| MOB2 | −1.44 | −1.56 | FRAS1 | 1.51 | −1.27 |
| Parameter | n | PDZD8 Expression | High | p Value (1) | ||
|---|---|---|---|---|---|---|
| Low | Moderate | |||||
| Total | 85 | 8 | 39 | 38 | ||
| Age | 32–62 yrs (4) | 42 | 4 | 18 | 20 | NS (2) |
| 63–90 yrs | 43 | 4 | 21 | 18 | ||
| Sex | Male | 68 | 5 | 31 | 32 | NS |
| Female | 17 | 3 | 8 | 6 | ||
| pT (3) | 1 | 10 | 3 | 5 | 2 | 0.031 |
| 2 | 25 | 4 | 12 | 9 | ||
| 3 | 44 | 1 | 21 | 22 | ||
| 4 | 6 | 0 | 1 | 5 | ||
| pN (3) | 0 | 65 | 8 | 31 | 26 | NS |
| 1–2 | 20 | 0 | 8 | 12 | ||
| pStage (3) | 1 | 34 | 7 | 16 | 11 | 0.0106 |
| 2 | 44 | 1 | 22 | 21 | ||
| 3–4 | 7 | 0 | 1 | 6 | ||
| Grade (3) | 1 | 14 | 3 | 5 | 6 | NS |
| 2 | 24 | 1 | 11 | 12 | ||
| 3 | 47 | 4 | 23 | 20 | ||
| Tissue | n | PDZD8 Expression | Moderate | High | p Value (1) | |
|---|---|---|---|---|---|---|
| Negative | Low | |||||
| Foveolar epithelium | 35 | 35 | 0 | 0 | 0 | |
| Pyloric gland | 24 | 24 | 0 | 0 | 0 | |
| Fundic gland | 11 | 11 | 0 | 0 | 0 | |
| Intestinal metaplasia | 12 | 12 | 0 | 0 | 0 | |
| Adenoma | 11 | 11 | 0 | 0 | 0 | |
| Adenocarcinoma | 85 | 0 | 8 | 39 | 38 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojo, Y.; Kishi, S.; Mori, S.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Nishiguchi, Y.; Nakashima, C.; Luo, Y.; Shinohara, H.; et al. Sunitinib and Pterostilbene Combination Treatment Exerts Antitumor Effects in Gastric Cancer via Suppression of PDZD8. Int. J. Mol. Sci. 2022, 23, 4002. https://doi.org/10.3390/ijms23074002
Hojo Y, Kishi S, Mori S, Fujiwara-Tani R, Sasaki T, Fujii K, Nishiguchi Y, Nakashima C, Luo Y, Shinohara H, et al. Sunitinib and Pterostilbene Combination Treatment Exerts Antitumor Effects in Gastric Cancer via Suppression of PDZD8. International Journal of Molecular Sciences. 2022; 23(7):4002. https://doi.org/10.3390/ijms23074002
Chicago/Turabian StyleHojo, Yudai, Shingo Kishi, Shiori Mori, Rina Fujiwara-Tani, Takamitsu Sasaki, Kiyomu Fujii, Yukiko Nishiguchi, Chie Nakashima, Yi Luo, Hisashi Shinohara, and et al. 2022. "Sunitinib and Pterostilbene Combination Treatment Exerts Antitumor Effects in Gastric Cancer via Suppression of PDZD8" International Journal of Molecular Sciences 23, no. 7: 4002. https://doi.org/10.3390/ijms23074002
APA StyleHojo, Y., Kishi, S., Mori, S., Fujiwara-Tani, R., Sasaki, T., Fujii, K., Nishiguchi, Y., Nakashima, C., Luo, Y., Shinohara, H., & Kuniyasu, H. (2022). Sunitinib and Pterostilbene Combination Treatment Exerts Antitumor Effects in Gastric Cancer via Suppression of PDZD8. International Journal of Molecular Sciences, 23(7), 4002. https://doi.org/10.3390/ijms23074002






