In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors
Abstract
1. Introduction
2. Results
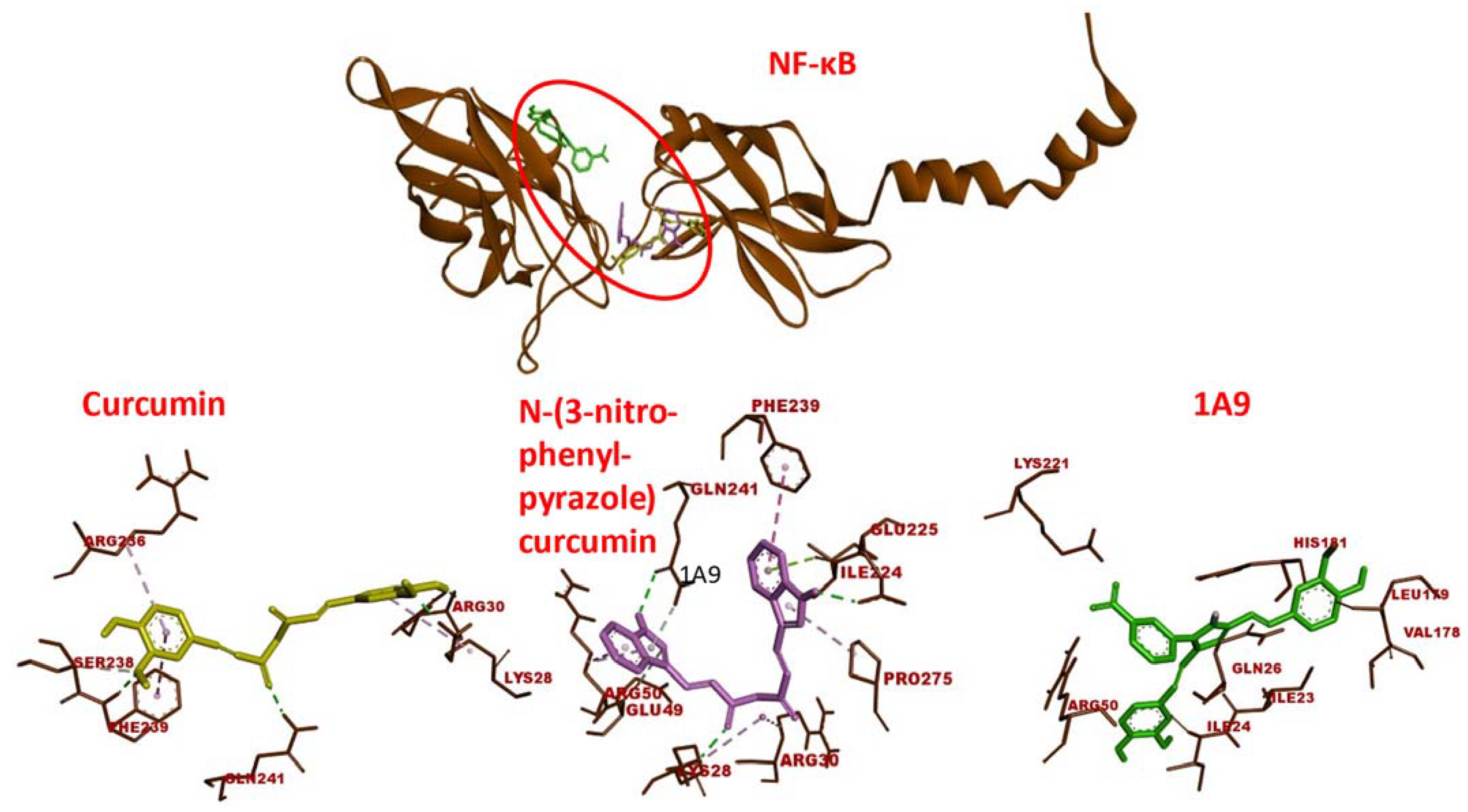
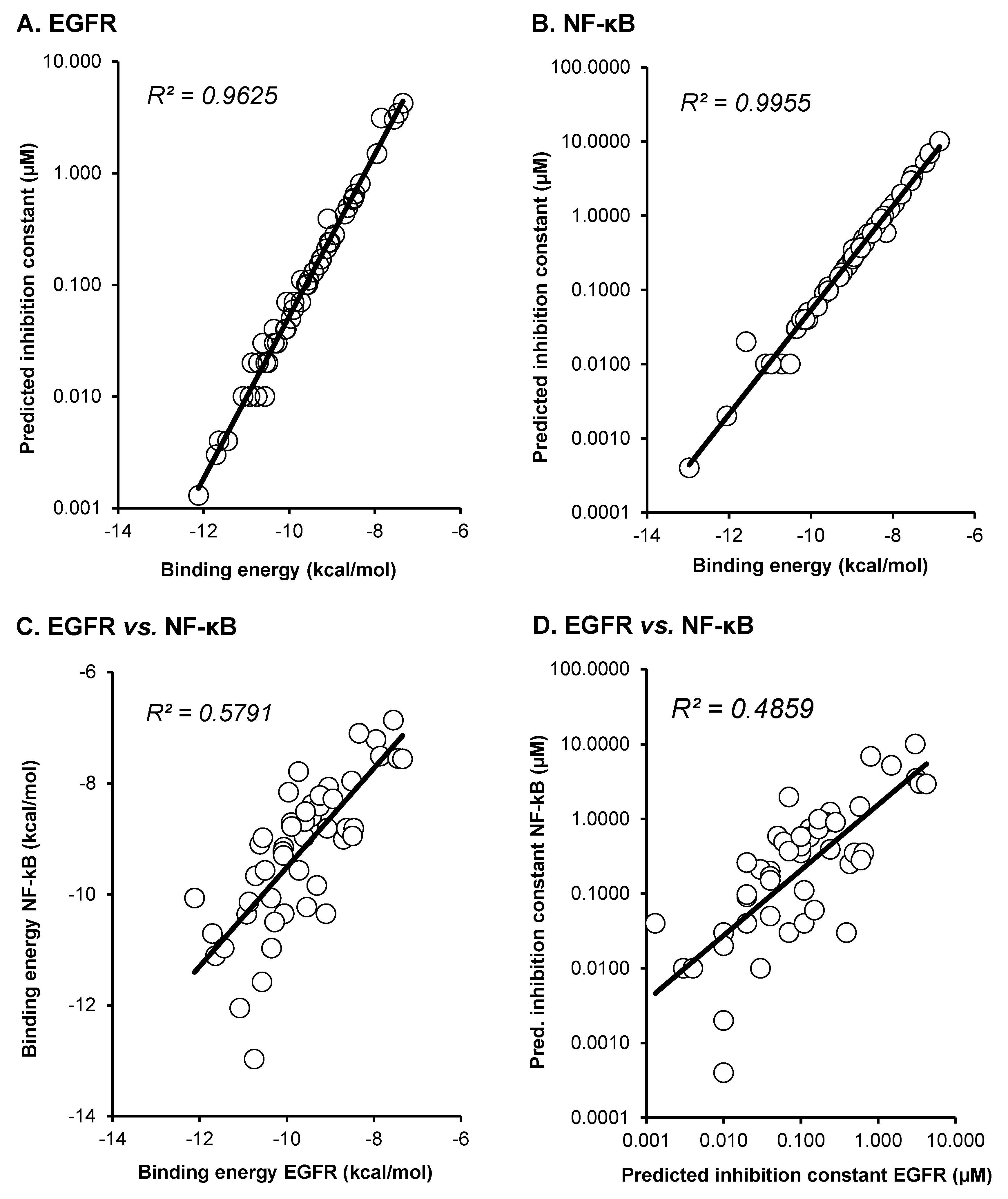
2.1. Molecular Docking
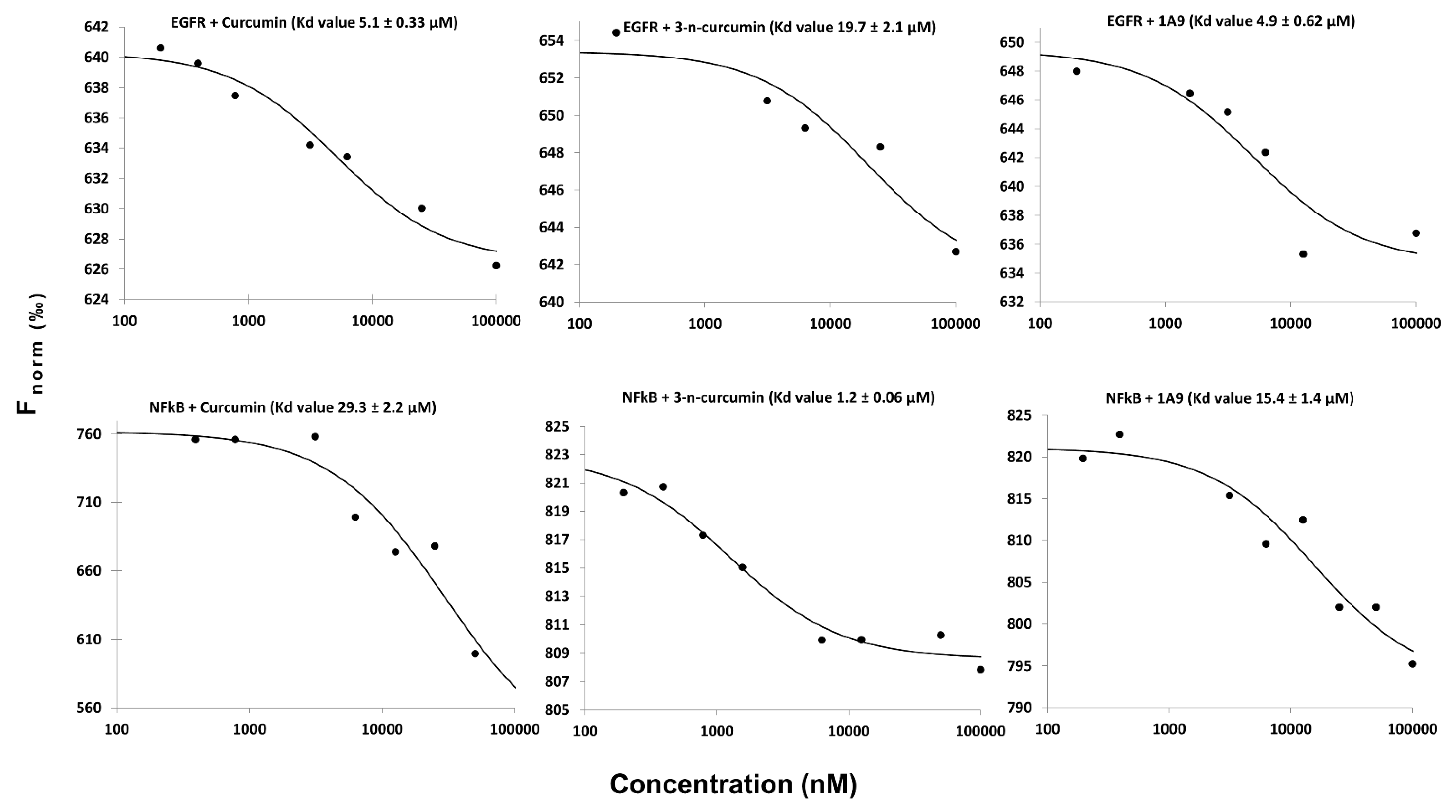
2.2. Microscale Thermophoresis
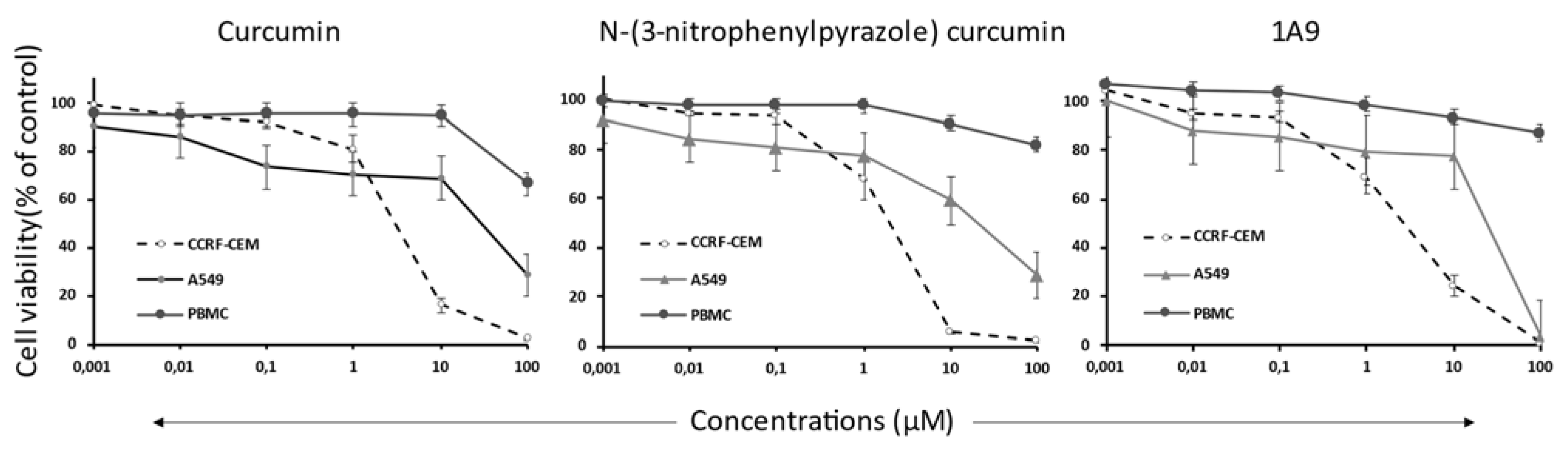
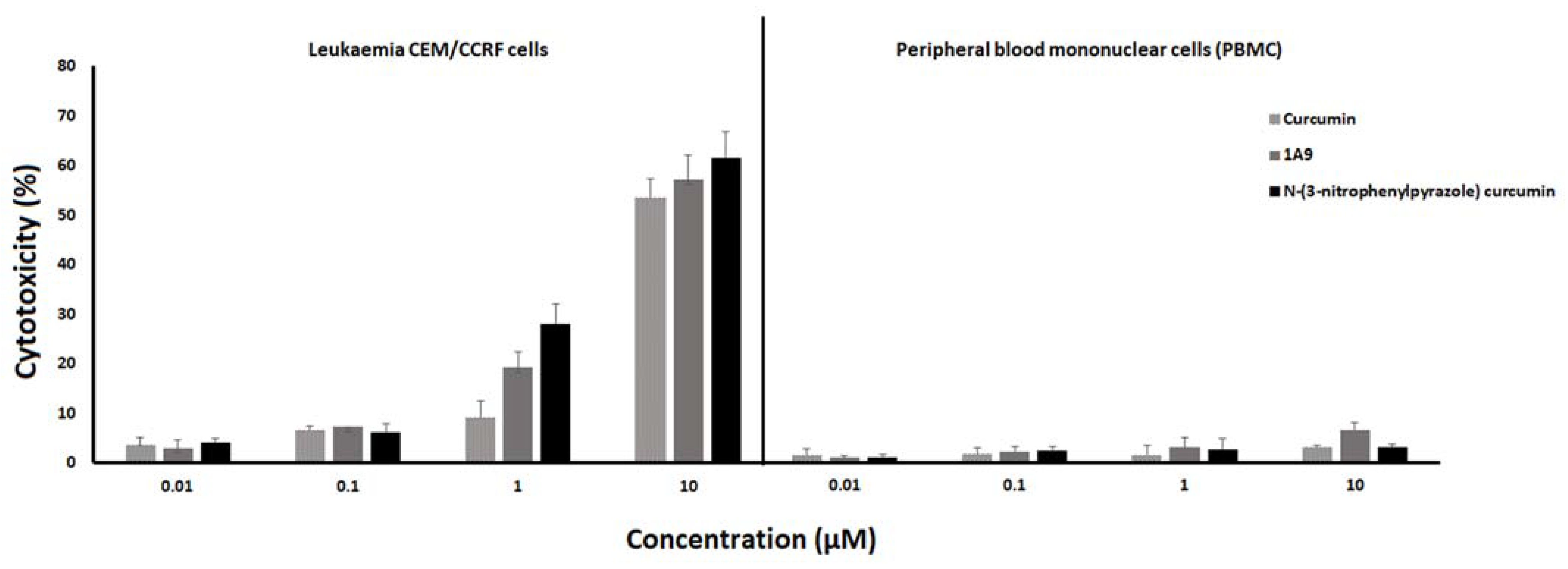
2.3. Resazurin Assay
2.4. LDH Assay
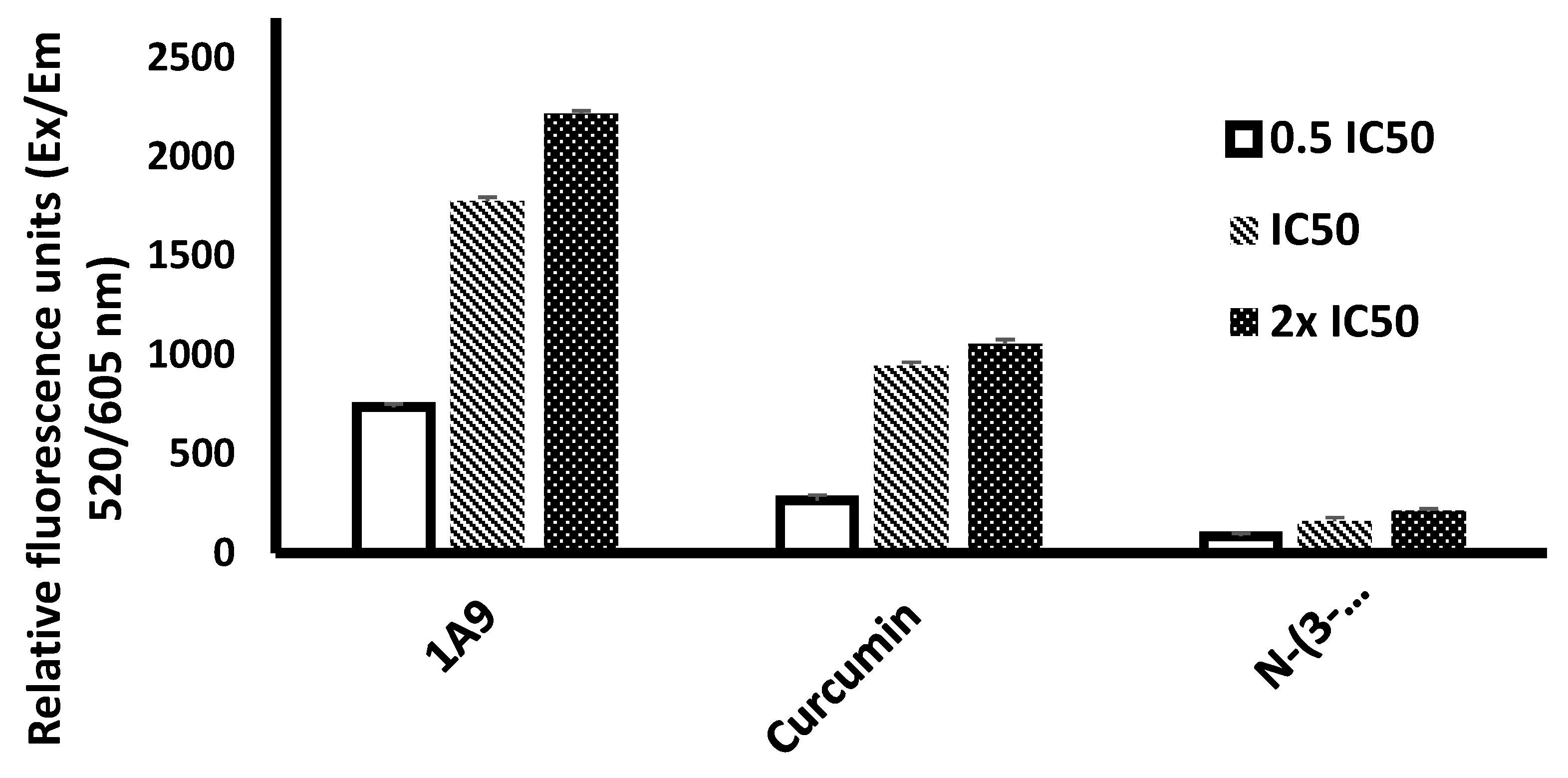
2.5. ROS Assay
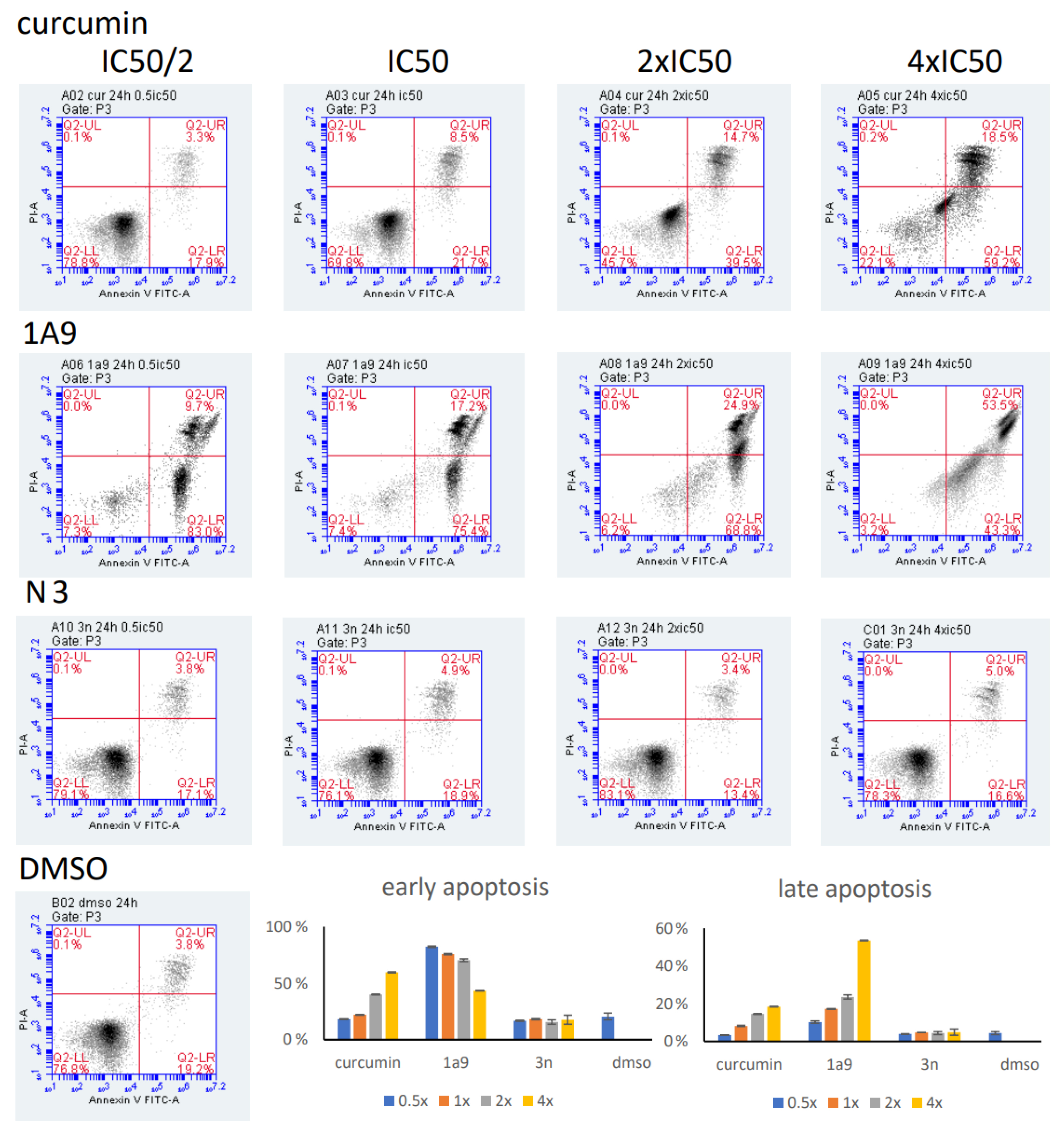
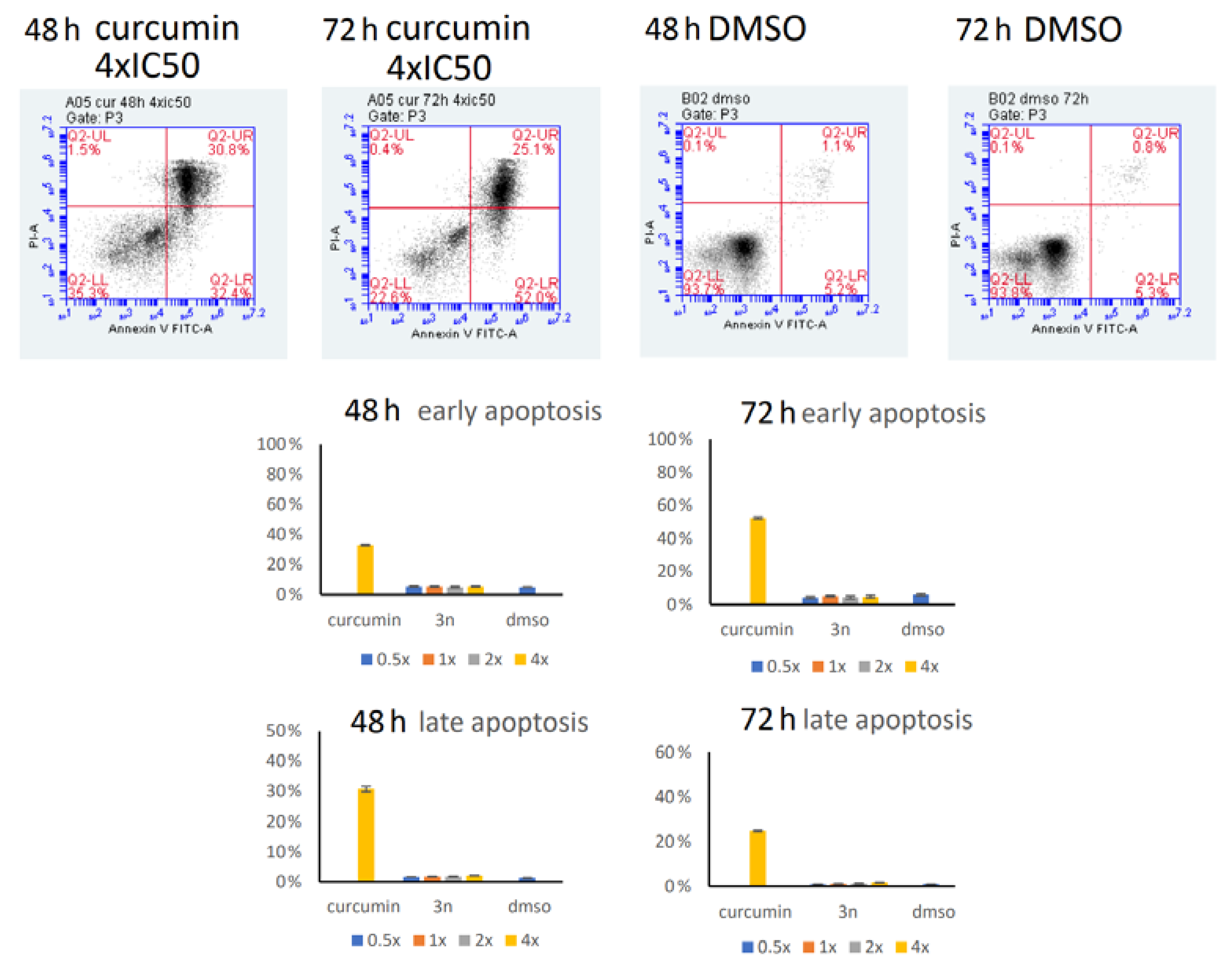
2.6. Annexin/PI Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Docking
4.3. Microscale Thermophoresis
4.4. Cell Culture
4.5. Resazurin Cell Viability Assay
4.6. Lactate Dehydrogenase (LDH) Assay
4.7. Reactive Oxygen Species (ROS) Detection Assay
4.8. Annexin V/Propidium Iodide (PI) Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Tabuchi, T.; Nakayama, T.; Hojo, S.; Yoshioka, S.; Maeura, Y. Past trends and future estimation of annual breast cancer incidence in Osaka, Japan. Asian Pac. J. Cancer Prev. 2016, 17, 52. [Google Scholar]
- Mattiuzzi, C.; Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Efferth, T. African flora has the potential to fight multidrug resistance of cancer. BioMed Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef]
- Efferth, T.; Saeed, M.E.M.; Kadioglu, O.; Seo, E.J.; Shirooie, S.; Mbaveng, A.T.; Nabavi, S.M.; Kuete, V. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol. Adv. 2020, 38, 107342. [Google Scholar] [CrossRef]
- Efferth, T.; Kadioglu, O.; Saeed, M.E.M.; Seo, E.-J.; Mbaveng, A.T.; Kuete, V. Medicinal plants and phytochemicals against multidrug-resistant tumor cells expressing ABCB1, ABCG2, or ABCB5: A synopsis of 2 decades. Phytochem. Rev. 2021, 20, 7–53. [Google Scholar] [CrossRef]
- Sertel, S.; Plinkert, P.K.; Efferth, T. Natural products derived from traditional Chinese medicine as novel inhibitors of the epidermal growth factor receptor. Comb. Chem. High Throughput Screen. 2010, 13, 849–854. [Google Scholar] [CrossRef]
- Zhao, Q.; Kretschmer, N.; Bauer, R.; Efferth, T. Shikonin and its derivatives inhibit the epidermal growth factor receptor signaling and synergistically kill glioblastoma cells in combination with erlotinib. Int. J. Cancer 2015, 137, 1446–1456. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Rahama, M.; Kuete, V.; Dawood, M.; Elbadawi, M.; Sugimoto, Y.; Efferth, T. Collateral sensitivity of drug-resistant ABCB5- and mutation-activated EGFR overexpressing cells towards resveratrol due to modulation of SIRT1 expression. Phytomedicine 2019, 59, 152890. [Google Scholar] [CrossRef]
- Lee, H.Y.J.; Meng, M.; Liu, Y.; Su, T.; Kwan, H.Y. Medicinal herbs and bioactive compounds overcome the drug resistance to epidermal growth factor receptor inhibitors in non-small cell lung cancer. Oncol. Lett. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Hamdoun, S.; Efferth, T. Ginkgolic acids inhibit migration in breast cancer cells by inhibition of NEMO sumoylation and NF-κB activity. Oncotarget 2017, 8, 35103–35115. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, R.H. Potential mechanisms of action of dietary phytochemicals for cancer prevention by targeting cellular signaling transduction pathways. J. Agric. Food. Chem. 2018, 66, 3260–3276. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.; Ooko, E.; Efferth, T. Collateral sensitivity of parthenolide via NF-κB and HIF-α inhibition and epigenetic changes in drug-resistant cancer cell lines. Front. Pharmacol. 2019, 10, 542. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar]
- Ooko, E.; Alsalim, T.; Saeed, B.; Saeed, M.E.; Kadioglu, O.; Abbo, H.S.; Titinchi, S.J.; Efferth, T. Modulation of P-glycoprotein activity by novel synthetic curcumin derivatives in sensitive and multidrug-resistant T-cell acute lymphoblastic leukemia cell lines. Tox. Appl. Pharmacol. 2016, 305, 216–233. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Curcumin inhibits proteasome activity in triple-negative breast cancer cells through regulating p300/miR-142-3p/PSMB5 axis. Phytomedicine 2020, 78, 153312. [Google Scholar] [CrossRef]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine 2021, 80, 153395. [Google Scholar] [CrossRef]
- Seo, E.J.; Efferth, T.; Panossian, A. Curcumin downregulates expression of opioid-related nociceptin receptor gene (OPRL1) in isolated neuroglia cells. Phytomedicine 2018, 50, 285–299. [Google Scholar] [CrossRef]
- Hsiao, A.F.; Lien, Y.C.; Tzeng, I.S.; Liu, C.T.; Chou, S.H.; Horng, Y.S. The efficacy of high- and low-dose curcumin in knee osteoarthritis: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 63, 102775. [Google Scholar] [CrossRef] [PubMed]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, Q.; Liu, Y.; Chen, Z.; Hu, H. Efficacy and safety of curcumin supplement on improvement of insulin resistance in people with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Evid.-Based Complement. Altern. Med. 2021, 2021, 4471944. [Google Scholar] [CrossRef]
- Sertel, S.; Eichhorn, T.; Bauer, J.; Hock, K.; Plinkert, P.K.; Efferth, T. Pharmacogenomic determination of genes associated with sensitivity or resistance of tumor cells to curcumin and curcumin derivatives. J. Nutr. Biochem. 2012, 23, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.X.; Li, Y.; Yin, H.; Zhang, J. Curcumin: Updated molecular mechanisms and intervention targets in human lung cancer. Int. J. Mol. Sci. 2012, 13, 3959–3978. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ma, W.; Liu, X.; Zu, Y.; Fu, Y.; Wu, N.; Liang, L.; Yao, L.; Efferth, T. Cytotoxic activity of curcumin towards CCRF-CEM leukemia cells and its effect on DNA damage. Molecules 2009, 14, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Wu, X.; Liu, Y.; Liu, Y.; Zhou, J.; Jiang, X.; Zhang, L.; He, X.; Ma, L. Curcumin-induced DNA demethylation in human gastric cancer cells is mediated by the DNA-damage response pathway. Oxid. Med. Cell. Longev. 2020, 2020, 2543504. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.; Nerlich, A.G.; Iancu, C.M.; Cilli, M.; Schleicher, E.; Vené, R.; Dell’Eva, R.; Jochum, M.; Albini, A.; Pfeffer, U. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol. Biochem. 2007, 19, 137–152. [Google Scholar] [CrossRef]
- Killian, P.H.; Kronski, E.; Michalik, K.M.; Barbieri, O.; Astigiano, S.; Sommerhoff, C.P.; Pfeffer, U.; Nerlich, A.G.; Bachmeier, B.E. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis 2012, 33, 2507–2519. [Google Scholar] [CrossRef]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL-1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef]
- Ruiz de Porras, V.; Bystrup, S.; Martínez-Cardús, A.; Pluvinet, R.; Sumoy, L.; Howells, L.; James, M.I.; Iwuji, C.; Manzano, J.L.; Layos, L.; et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci. Rep. 2016, 6, 24675. [Google Scholar] [CrossRef] [PubMed]
- Norooznezhad, F.; Rodriguez-Merchan, E.C.; Asadi, S.; Norooznezhad, A.H. Curcumin: Hopeful treatment of hemophilic arthropathy via inhibition of inflammation and angiogenesis. Expert Rev. Hematol. 2020, 13, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Bhandarkar, S.S.; Arbiser, J.L. Curcumin as an inhibitor of angiogenesis. Adv. Exp. Med. Biol. 2007, 595, 185–195. [Google Scholar] [PubMed]
- Shafiee, M.; Mohamadzade, E.; Shahid Sales, S.; Khakpouri, S.; Maftouh, M.; Parizadeh, S.A.; Hasanian, S.M.; Avan, A. Current status and perspectives regarding the therapeutic potential of targeting EGFR pathway by curcumin in lung cancer. Curr. Pharm. Des. 2017, 23, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Wan Mohd Tajuddin, W.N.B.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanistic understanding of curcumin’s therapeutic effects in lung cancer. Nutrients 2019, 11, 2989. [Google Scholar] [CrossRef]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141. [Google Scholar] [CrossRef]
- Ruiz de Porras, V.; Layos, L.; Martínez-Balibrea, E. Curcumin: A therapeutic strategy for colorectal cancer? Semin. Cancer Biol. 2021, 73, 321–330. [Google Scholar] [CrossRef]
- Thomas, R.K.; Sos, M.L.; Zander, T.; Mani, O.; Popov, A.; Berenbrinker, D.; Smola-Hess, S.; Schultze, J.L.; Wolf, J. Inhibition of nuclear translocation of nuclear factor-kappaB despite lack of functional IkappaBalpha protein overcomes multiple defects in apoptosis signaling in human B-cell malignancies. Clin. Cancer Res. 2005, 11, 8186–8194. [Google Scholar] [CrossRef]
- Zambre, A.P.; Kulkarni, V.M.; Padhye, S.; Sandur, S.K.; Aggarwal, B.B. Novel curcumin analogs targeting TNF-induced NF-kappaB activation and proliferation in human leukemic KBM-5 cells. Bioorg. Med. Chem. 2006, 14, 7196–7204. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Queisser, N.; Wolfson, M.L.; Fraga, C.G.; Adamo, A.M.; Oteiza, P.I. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin’s lymphoma cells. Int. J. Cancer 2008, 123, 56–65. [Google Scholar] [CrossRef]
- Shehzad, A.; Lee, J.; Lee, Y.S. Curcumin in various cancers. Biofactors 2013, 39, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Shen, Q.; Jiang, H.; Ji, O.; Zhu, L.; Zhang, L. Curcumin inhibited the growth and invasion of human monocytic leukaemia SHI-1 cells in vivo by altering MAPK and MMP signalling. Pharm. Biol. 2020, 58, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, M.; Dai, E.; Luo, Y. Molecular targets of curcumin in breast cancer (Review). Mol. Med. Rep. 2019, 19, 23–29. [Google Scholar] [CrossRef]
- Akbari, S.; Kariznavi, E.; Jannati, M.; Elyasi, S.; Tayarani-Najaran, Z. Curcumin as a preventive or therapeutic measure for chemotherapy and radiotherapy induced adverse reaction: A comprehensive review. Food Chem. Toxicol. 2020, 145, 111699. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Moghadam, E.R.; Hashemi, F.; Entezari, M.; Hushmandi, K.; Mohammadinejad, R.; Najafi, M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020, 256, 117984. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Vyas, A.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr. Pharm. Des. 2013, 19, 2047–2069. [Google Scholar]
- Ooko, E.; Kadioglu, O.; Greten, H.J.; Efferth, T. Pharmacogenomic characterization and isobologram analysis of the combination of ascorbic acid and curcumin-two main metabolites of Curcuma longa-in cancer cells. Front. Pharmacol. 2017, 8, 38. [Google Scholar] [CrossRef]
- Karimpour, M.; Feizi, M.A.H.; Mahdavi, M.; Krammer, B.; Verwanger, T.; Najafi, F.; Babaei, E. Development of curcumin-loaded gemini surfactant nanoparticles: Synthesis, characterization and evaluation of anticancer activity against human breast cancer cell lines. Phytomedicine 2019, 57, 183–190. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K. Molecular targets of curcumin. Adv. Exp. Med. Biol. 2007, 595, 227–243. [Google Scholar] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.E.; Efferth, T. Broad-spectrum cross-resistance to anticancer drugs mediated by epidermal growth factor receptor. Anticancer Res. 2019, 39, 3585–3593. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Attri, B.K.; Gill, R.K.; Bariwal, J. Review on EGFR inhibitors: Critical updates. Mini Rev. Med. Chem. 2016, 16, 1134–1166. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Saeed, M.E.M.; Foersch, S.; Schneider, J.; Roth, W.; Efferth, T. Relationship between EGFR expression and subcellular localization with cancer development and clinical outcome. Oncotarget 2019, 10, 1918–1931. [Google Scholar] [CrossRef]
- Kadioglu, O.; Saeed, M.E.M.; Mahmoud, N.; Azawi, S.; Mrasek, K.; Liehr, T.; Efferth, T. Identification of novel drug resistance mechanisms by genomic and transcriptomic profiling of glioblastoma cells with mutation-activated EGFR. Life Sci. 2021, 284, 119601. [Google Scholar] [CrossRef]
- Efferth, T. Signal transduction pathways of the epidermal growth factor receptor in colorectal cancer and their inhibition by small molecules. Curr. Med. Chem. 2012, 19, 5735–5744. [Google Scholar] [CrossRef]
- Kadioglu, O.; Cao, J.; Saeed, M.E.; Greten, H.J.; Efferth, T. Targeting epidermal growth factor receptors and downstream signaling pathways in cancer by phytochemicals. Target. Oncol. 2015, 10, 337–353. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D.; Herscovitch, M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 2006, 25, 6887–6899. [Google Scholar] [CrossRef] [PubMed]
- Gaptulbarova, K.A.; Tsyganov, M.M.; Pevzner, A.M.; Ibragimova, M.K.; Litviakov, N.V. NF-kB as a potential prognostic marker and a candidate for targeted therapy of cancer. Exp. Oncol. 2020, 42, 263–269. [Google Scholar]
- Ríos, J.L.; Recio, M.C.; Escandell, J.M.; Andújar, I. Inhibition of transcription factors by plant-derived compounds and their implications in inflammation and cancer. Curr. Pharm. Des. 2009, 15, 1212–1237. [Google Scholar] [CrossRef]
- Luqman, S.; Pezzuto, J.M. NFkappaB: A promising target for natural products in cancer chemoprevention. Phytother. Res. 2010, 24, 949–963. [Google Scholar] [CrossRef]
- Soukhtanloo, M.; Mohtashami, E.; Maghrouni, A.; Mollazadeh, H.; Mousavi, S.H.; Roshan, M.K.; Tabatabaeizadeh, S.A.; Hosseini, A.; Vahedi, M.M.; Jalili-Nik, M.; et al. Natural products as promising targets in glioblastoma multiforme: A focus on NF-κB signaling pathway. Pharmacol. Rep. 2020, 72, 285–295. [Google Scholar] [CrossRef]
- Shostak, K.; Chariot, A. EGFR and NF-κB: Partners in cancer. Trends Mol. Med. 2015, 21, 385–393. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Starok, M.; Preira, P.; Vayssade, M.; Haupt, K.; Salome, L.; Rossi, C. EGFR Inhibition by curcumin in cancer cells: A dual mode of action. Biomacromolecules 2015, 16, 1634–1642. [Google Scholar] [CrossRef]
- Wada, K.; Lee, J.Y.; Hung, H.Y.; Shi, Q.; Lin, L.; Zhao, Y.; Goto, M.; Yang, P.C.; Kuo, S.C.; Chen, H.W.; et al. Novel curcumin analogs to overcome EGFR-TKI lung adenocarcinoma drug resistance and reduce EGFR-TKI-induced GI adverse effects. Bioorg. Med. Chem. 2015, 23, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, A.A.; Sahebkar, A. Difluorinated curcumin: A promising curcumin analogue with improved anti-tumor activity and pharmacokinetic profile. Curr. Pharm. Des. 2016, 22, 4386–4397. [Google Scholar] [CrossRef] [PubMed]
- Hackler, L., Jr.; Ozsvari, B.; Gyuris, M.; Sipos, P.; Fabian, G.; Molnar, E.; Marton, A.; Farago, N.; Mihaly, J.; Nagy, L.I.; et al. The curcumin analog C-150, influencing NF-kappaB, UPR and Akt/Notch pathways has potent anticancer activity in vitro and in vivo. PLoS ONE 2016, 11, e0149832. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Reed, J.C. Bcl-2: Prevention of apoptosis as a mechanism of drug resistance. Hematol. Oncol. Clin. N. Am. 1995, 9, 451–473. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Kadioglu, O.; Saeed, M.E.; Valoti, M.; Frosini, M.; Sgaragli, G.; Efferth, T. Interactions of human P-glycoprotein transport substrates and inhibitors at the drug binding domain: Functional and molecular docking analyses. Biochem. Pharmacol. 2016, 104, 42–51. [Google Scholar] [CrossRef]
- Abdelfatah, S.; Böckers, M.; Asensio, M.; Kadioglu, O.; Klinger, A.; Fleischer, E.; Efferth, T. Isopetasin and S-isopetasin as novel P-glycoprotein inhibitors against multidrug-resistant cancer cells. Phytomedicine 2021, 86, 153196. [Google Scholar] [CrossRef]
- Lu, X.; Yan, G.; Dawood, M.; Klauck, S.M.; Sugimoto, Y.; Klinger, A.; Fleischer, E.; Shan, L.; Efferth, T. A novel moniliformin derivative as pan-inhibitor of histone deacetylases triggering apoptosis of leukemia cells. Biochem. Pharmacol. 2021, 194, 114677. [Google Scholar] [CrossRef]
- Naß, J.; Abdelfatah, S.; Efferth, T. The triterpenoid ursolic acid ameliorates stress in Caenorhabditis elegans by affecting the depression-associated genes skn-1 and prdx2. Phytomedicine 2021, 88, 153598. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ahuja, V.; Sankar, M.J.; Kumar, A.; Moss, A.C. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2012, 10, CD008424. [Google Scholar] [PubMed]
- Bukhari, S.N.; Franzblau, S.G.; Jantan, I.; Jasamai, M. Current prospects of synthetic curcumin analogs and chalcone derivatives against Mycobacterium tuberculosis. Med. Chem. 2013, 9, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and neurodegenerative diseases. BioFactors 2013, 39, 122–132. [Google Scholar] [CrossRef]
- Cicero, A.F.; Colletti, A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine 2016, 23, 1134–1144. [Google Scholar] [CrossRef]
- Hernandez, M.; Wicz, S.; Corral, R.S. Cardioprotective actions of curcumin on the pathogenic NFAT/COX-2/prostaglandin E2 pathway induced during Trypanosoma cruzi infection. Phytomedicine 2016, 23, 1392–1400. [Google Scholar] [CrossRef]


| Compounds | EGFR (Mean Binding Energy, kcal/mol) | EGFR (Mean pKi, µM) | NF-κB (Mean Binding Energy, kcal/mol) | NF-κB (Mean pKi, µM) | |
|---|---|---|---|---|---|
| 1 | Curcumin | −9.42 ± 0.09 | 0.13 ± 0.02 | −8.38 ± 0.16 | 0.73 ± 0.21 |
| 2 | Bisdemethoxycurcumin | −8.51 ± 0.02 | 0.58 ± 0.02 | −7.96 ± 0.05 | 1.46 ± 0.11 |
| 3 | Diacetylcurcumin | −10.72 ± 0.49 | 0.02 ± 0.02 | −9.67 ± 0.26 | 0.09 ± 0.04 |
| 4 | [18FP]-curcumin | −8.70 ± 0.21 | 0.43 ± 0.14 | −9.01 ± 0.07 | 0.25 ± 0.03 |
| 5 | Monodemethoxycurcumin | −9.04 ± 0.06 | 0.24 ± 0.02 | −8.07 ± 0.10 | 1.23 ± 0.20 |
| 6 | (E,E)-Bis(2-hydroxybenzylidene)acetone | −7.95 ± 0.01 | 1.49 ± 0.04 | −7.21 ± 0.06 | 5.19 ± 0.50 |
| 7 | Curcumin monoglucoside | −9.72 ± 0.76 | 0.11 ± 0.11 | −9.57 ± 0.34 | 0.11 ± 0.06 |
| 8 | Di-O-(2-thienoyl) curcumin | −11.71 ± 0.26 | 0.003 ± 0.002 | −10.71 ± 0.17 | 0.01 ± <0.01 |
| 9 | Cis-curcumin | −9.07 ± 0.30 | 0.24 ± 0.10 | −8.81 ± 0.33 | 0.39 ± 0.21 |
| 10 | Curcumin diglucoside | −9.10 ± 0.99 | 0.39 ± 0.35 | −10.35 ± 0.35 | 0.03 ± 0.02 |
| 11 | Tetrahydrocurcumin | −9.13 ± 0.17 | 0.21 ± 0.06 | −8.28 ± 0.21 | 0.90 ± 0.34 |
| 12 | Allyl curcumin | −10.07 ± 0.07 | 0.04 ± 0.01 | −9.15 ± 0.04 | 0.20 ± 0.01 |
| 13 | Monodemethylcurcumin | −9.96 ± 0.10 | 0.05 ± 0.01 | −8.16 ± 0.56 | 0.59 ± 0.12 |
| 14 | Didemethylcurcumin | −9.90 ± 0.07 | 0.06 ± 0.01 | −8.71 ± 0.25 | 0.49 ± 0.18 |
| 15 | Curcumin-4′-O-β-d-gentiotrioside | −7.85 ± 0,59 | 3.11 ± 0.23 | −7.51 ± 0.37 | 3.47 ± 2.05 |
| 16 | 4-Benzylidene curcumin | −10.62 ± 0.72 | 0.03 ± 0.03 | −9.10 ± 0.10 | 0.21 ± 0.04 |
| 17 | Monoglycinoyl curcumin | −12.12 ± 0.21 | 0.0013 ± 0.0006 | −10.07 ± 0.08 | 0.04 ± 0.01 |
| 18 | Ethyl curcumin | −10.09 ± 0.11 | 0.04 ± 0.01 | −9.23 ± 0.05 | 0.17 ± 0.01 |
| 19 | Curcumin dimer 1 | −11.64 ± 0.56 | 0.004 ± 0.003 | −11.11 ± 0.65 | 0.01 ± 0.01 |
| 20 | N-phenylpyrazole curcumin | −8.63 ± 0.19 | 0.49 ± 0.14 | −8.81 ± 0.01 | 0.35 ± 0.01 |
| 21 | Curcumin β-d-glucuronide | −10.36 ± 0.19 | 0.04 ± 0.02 | −10.07 ± 0.36 | 0.05 ± 0.03 |
| 22 | 3,4-Difluorobenzylidene curcumin | −10.08 ± 0.13 | 0.04 ± 0.01 | −9.30 ± 0.07 | 0.15 ± 0.02 |
| 23 | Di-O-(2-hydroxyethyl) curcumin | −9.60 ± 0.27 | 0.10 ± 0.05 | −8.97 ± 0.55 | 0.35 ± 0.27 |
| 24 | 4-(4-hydroxybenzylidene) curcumin | −10.55 ± 0.12 | 0.02 ± <0.01 | −8.98 ± 0.04 | 0.26 ± 0.02 |
| 25 | N-(3-nitrophenylpyrazole) curcumin | −10.50 ± 0.02 | 0.02 ± <0.01 | −9.57 ± 0.01 | 0.097 ± <0.01 |
| 26 | N-(4-flurophenylpyrazole) curcumin | −8.46 ± 0.19 | 0.65 ± 0.19 | −8.81 ± 0.09 | 0.35 ± 0.06 |
| 27 | N-(4-methoxyphenylpyrazole) curcumin | −8.49 ± 0.04 | 0.60 ± 0.03 | −8.95 ± 0.11 | 0.28 ± 0.05 |
| 28 | Di-O-chloropropionylethyl curcumin | −10.92 ± 0.25 | 0.01 ± <0.01 | −10.36 ± 0.59 | 0.03 ± 0.02 |
| 29 | Curcumin tri-adamantylaniniethylcarbonate | −10.75 ± 0.23 | 0.01 ± 0.01 | −12.97 ± 0.47 | 0.0004 ± 0.0003 |
| 30 | Curcumin dimer 2 | −11.08 ± 0.24 | 0.01 ± <0.01 | −12.05 ± 0.46 | 0.002 ± 0.001 |
| 31 | Curcumin tri-trithiadiazolaminoethylcarbonate | −10.35 ± 0.55 | 0.03 ± 0.02 | −10.97 ± 0.53 | 0.01 ± 0.01 |
| 32 | 4-(4-hydroxy-3-methoxybenzylidene) curcumin | −11.44 ± 0.16 | 0.004 ± 0.002 | −10.97 ± 0.53 | 0.01 ± 0.01 |
| 33 | Curcumin-β-d-glucuronide triacetate methyl ester | −10.06 ± 0.79 | 0.07 ± 0.06 | −10.35 ± 0.35 | 0.03 ± 0.02 |
| 34 | Curcumin 4′-O-β-d-gentiobiosyl 4″-O-β-d-glucoside | −9.54 ± 0.25 | 0.11 ± 0.05 | −10.23 ± 0.59 | 0.04 ± 0.04 |
| 35 | Tetrahydrocurcumin isoxazole | −9.43 ± 0.20 | 0.13 ± 0.04 | −8.60 ± 0.41 | 0.57 ± 0.32 |
| 36 | Hexahydrocurcumin | −9.25 ± 0.11 | 0.17 ± 0.03 | −8.41 ± 0.27 | 0.73 ± 0.35 |
| 37 | Bisdemethoxycurcumin isoxazole | −7.45 ± 0.01 | 3.45 ± 0.10 | −7.55 ± 0.10 | 2.96 ± 0.47 |
| 38 | Curcumin dimer 3 | −10.28 ± 0.22 | 0.03 ± 0.01 | −10.50 ± 0.77 | 0.01 ± 0.01 |
| 39 | Curcumin ED | −9.59 ± 0.08 | 0.10 ± 0.02 | −8.69 ± 0.14 | 0.43 ± 0.10 |
| 40 | Curcumin PE | −9.24 ± 0.12 | 0.17 ± 0.03 | −8.22 ± 0.22 | 0.99 ± 0.33 |
| 41 | Curcumin sulfate | −9.31 ± 0.16 | 0.15 ± 0.04 | −9.84 ± 0.08 | 0.06 ± 0.01 |
| 42 | Ferrocenyl curcumin | −8.34 ± 0.22 | 0.80 ± 0.29 | −7.10 ± 0.33 | 6.87 ± 3.92 |
| 43 | Di-O-deconyl curcumin | −10.57 ± 0.17 | 0.01 ± <0.01 | −11.58 ± 0.85 | 0.02 ± 0.01 |
| 44 | GNF-pf-2695 ((2E,5E)-2,5-bis[(3,4,5-trimethoxyphenyl) methylidene]cyclopentan-1-one) | −7.34 ± 0.07 | 4.22 ± 0.51 | −7.56 ± 0.15 | 2.93 ± 0.78 |
| 45 | Perfluoro curcumin | −7.55 ± 0.19 | 3.03 ± 0.86 | −6.86 ± 0.28 | 10.05 ± 4.03 |
| 46 | Keto-curcumin | −9.89 ± 0.41 | 0.07 ± 0.04 | −8.78 ± 0.15 | 0.37 ± 0.09 |
| 47 | Disalicyloyl curcumin | −7.67 ± 0.58 | 0.02 ± 0.02 | −6.24 ± 0.06 | 26.77 ± 3.36 |
| 48 | HO-3867 (3E,5E)-3,5-bis[(4-fluorophenyl)methylidene]-1-[(1-hydroxy-2,2,5,5-tetramethylpyrrol-3-yl)methyl]piperidin-4-one) | −9.73 ± 0.03 | 0.07 ± <0.01 | −7.79 ± 0.10 | 1.96 ± 0.33 |
| 49 | 1A6 ((1E,6E)-4-chloro-1,7-bis(3,4-dimethoxyphenyl)hepta-1,6-diene-3,5-dione) | −8.94 ± 0.06 | 0.28 ± 0.03 | −8.28 ± 0.21 | 0.90 ± 0.34 |
| 50 | 1A9 ((1E,6E)-4-chloro-1,7-di(1H-indol-3-yl)hepta-1,6-diene-3,5-dione) | −9.57 ± 0.02 | 0.10 ± 0.03 | −8.51 ± 0.11 | 0.58 ± <0.01 |
| Cells | Compounds | ||
|---|---|---|---|
| Curcumin | N-(3-Nitrophenylpyrazole) Curcumin | 1A9 | |
| CCRF-CEM | 3.0 ± 0.4 | 1.9 ± 0.4 | 2.6 ± 0.3 |
| A549 | 29.4 ± 1.9 | 18.9 ± 1.4 | 23.3 ± 1.2 |
| PBMC | >100 | >100 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.E.M.; Yücer, R.; Dawood, M.; Hegazy, M.-E.F.; Drif, A.; Ooko, E.; Kadioglu, O.; Seo, E.-J.; Kamounah, F.S.; Titinchi, S.J.; et al. In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors. Int. J. Mol. Sci. 2022, 23, 3966. https://doi.org/10.3390/ijms23073966
Saeed MEM, Yücer R, Dawood M, Hegazy M-EF, Drif A, Ooko E, Kadioglu O, Seo E-J, Kamounah FS, Titinchi SJ, et al. In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors. International Journal of Molecular Sciences. 2022; 23(7):3966. https://doi.org/10.3390/ijms23073966
Chicago/Turabian StyleSaeed, Mohamed E. M., Rümeysa Yücer, Mona Dawood, Mohamed-Elamir F. Hegazy, Assia Drif, Edna Ooko, Onat Kadioglu, Ean-Jeong Seo, Fadhil S. Kamounah, Salam J. Titinchi, and et al. 2022. "In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors" International Journal of Molecular Sciences 23, no. 7: 3966. https://doi.org/10.3390/ijms23073966
APA StyleSaeed, M. E. M., Yücer, R., Dawood, M., Hegazy, M.-E. F., Drif, A., Ooko, E., Kadioglu, O., Seo, E.-J., Kamounah, F. S., Titinchi, S. J., Bachmeier, B., & Efferth, T. (2022). In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors. International Journal of Molecular Sciences, 23(7), 3966. https://doi.org/10.3390/ijms23073966









