Psychic Life-Biological Molecule Bidirectional Relationship: Pathways, Mechanisms, and Consequences for Medical and Psychological Sciences—A Narrative Review
Abstract
:1. Introduction
2. Psyche and Biological Systems: The Bidirectional Pathways
2.1. What Is the Psyche?
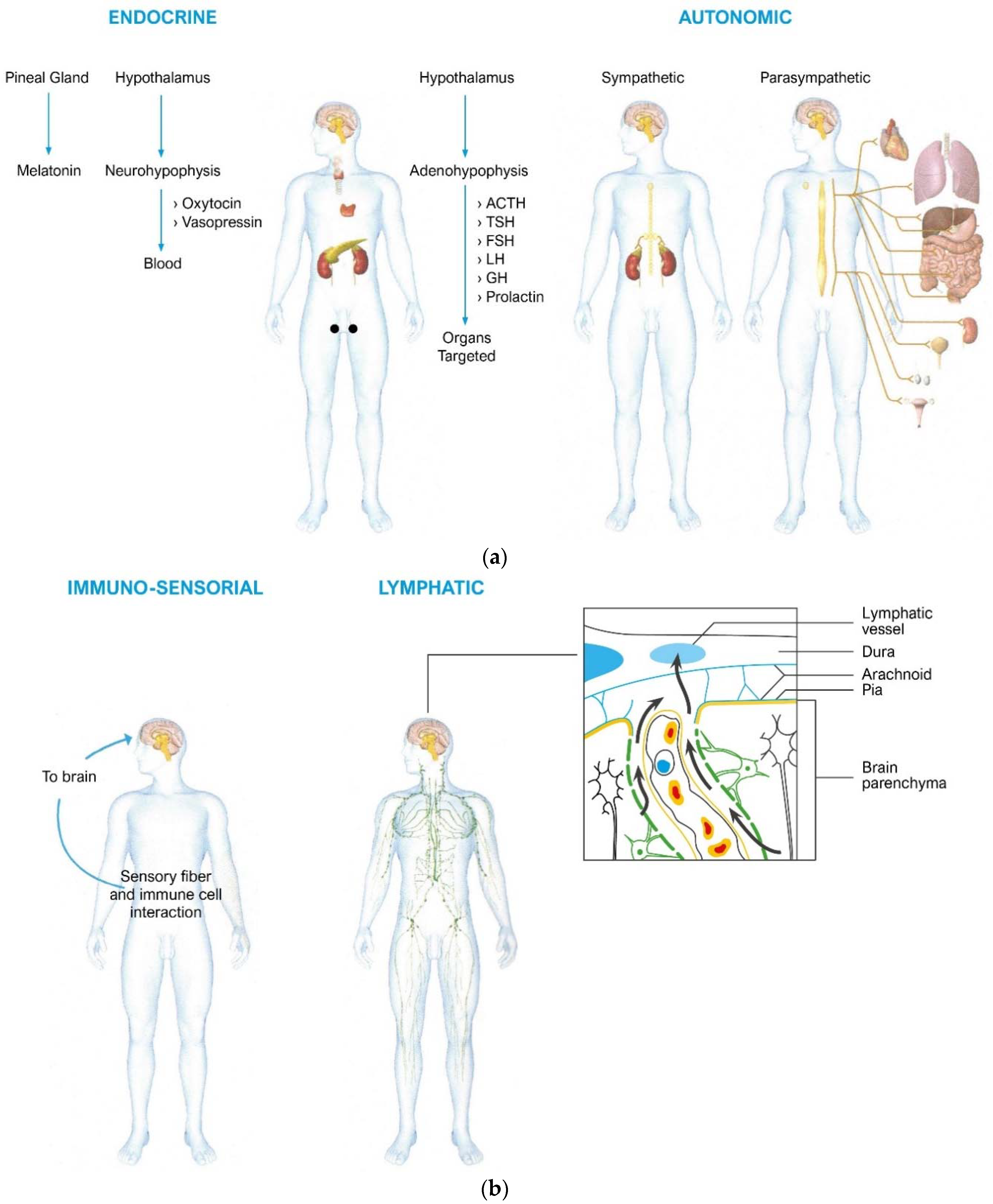
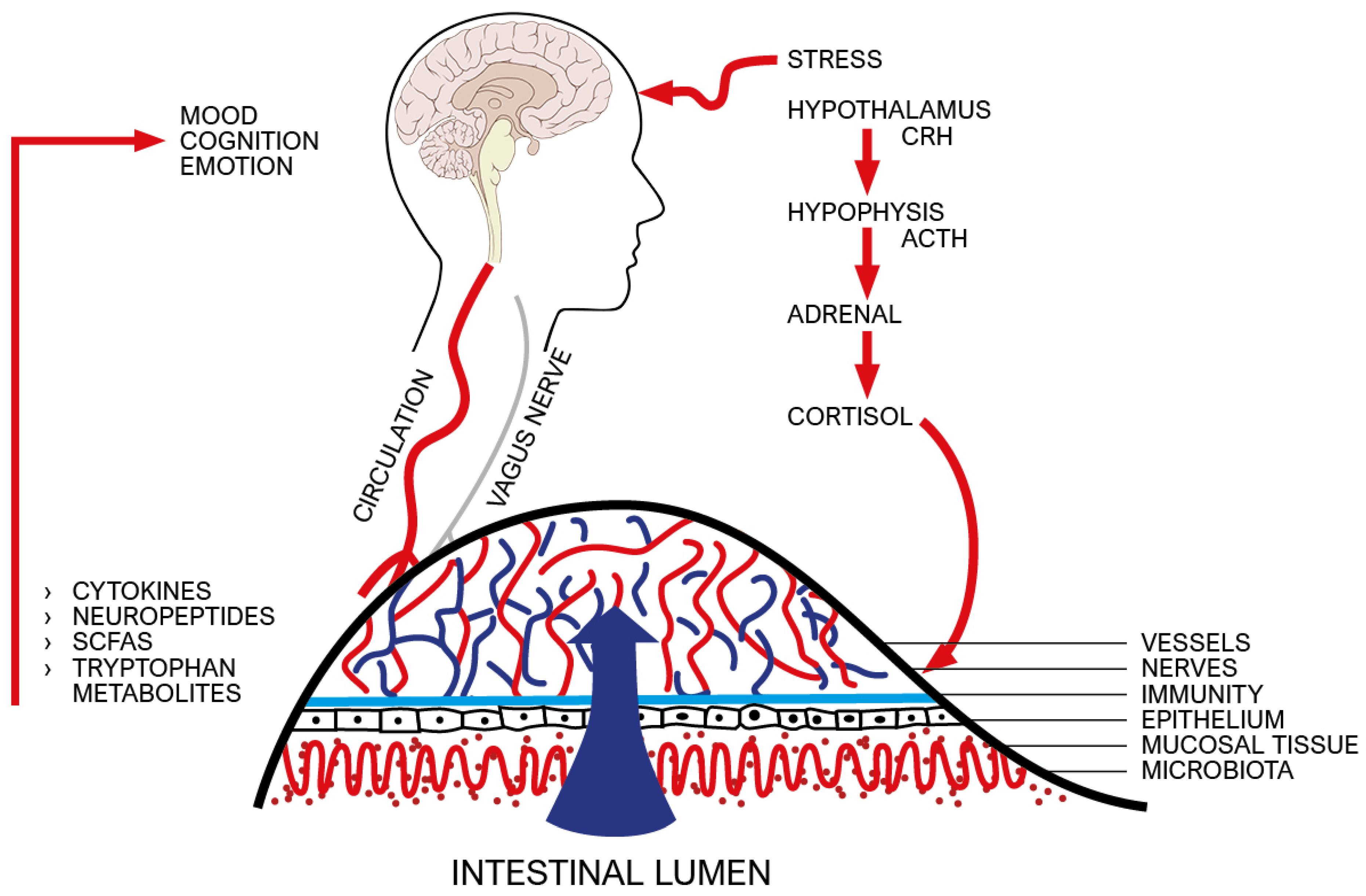
2.2. Pathways
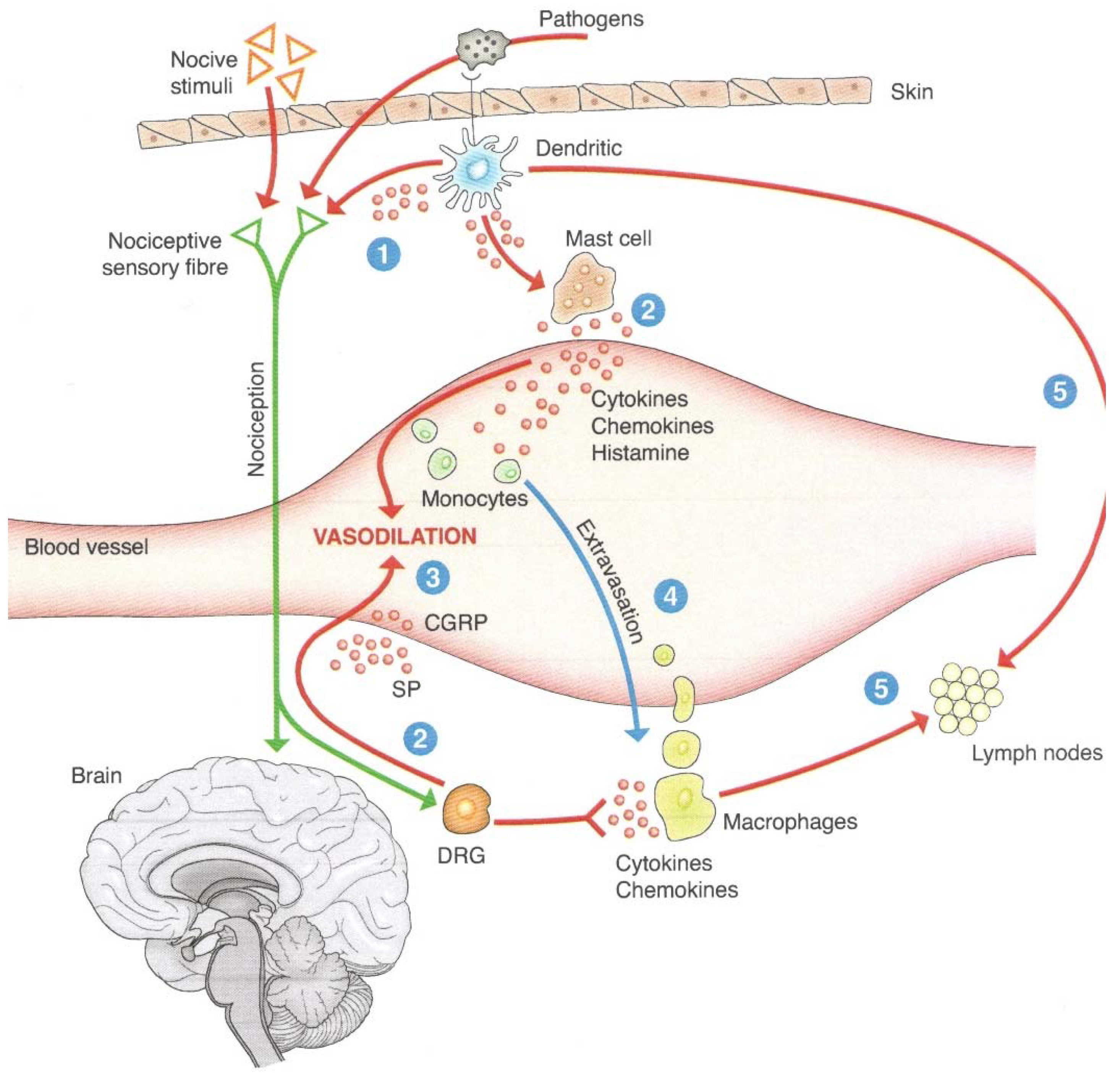
2.2.1. From the Nervous System to the Immune System
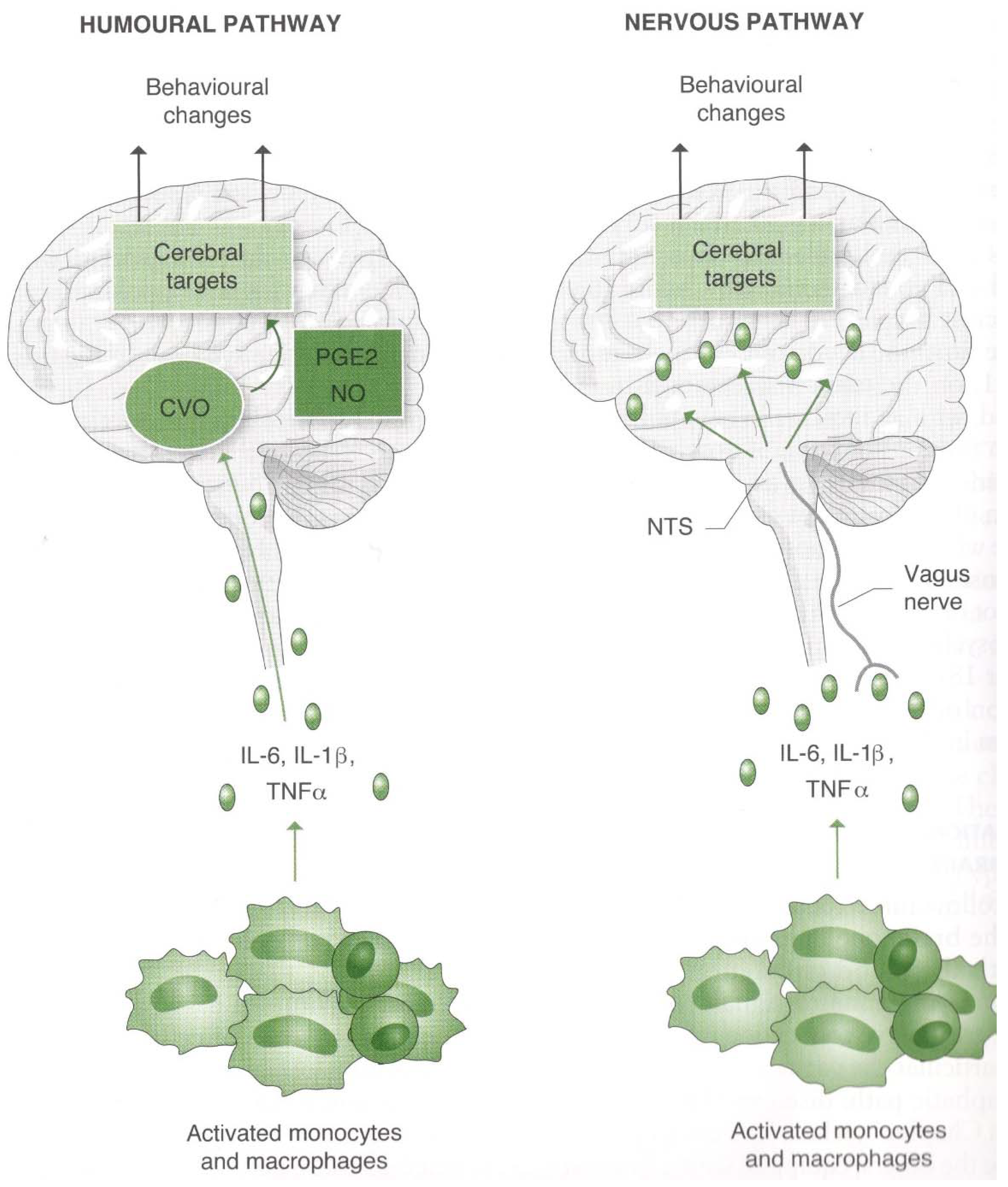
2.2.2. From the Immune System to the Psyche–Brain System
2.2.3. The Brain
2.2.4. The Immune System
3. Mental States and Molecular Biology
3.1. Epigenetics as a Main Pathway
3.2. Early Life Adversities Molecular Markers
3.3. Loneliness
3.4. Social Adversity and Social Inequality
3.5. Depression, and Other Psychiatric Diseases
3.6. Stress, Mental Condition and Vaccine Effectiveness
3.7. Epigenetic Signature Reversion and Inflammatory Mediator Regulation by Psychological and Body–Mind Interventions
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, W. The Principles of Psychology; Dover: New York, NY, USA, 1950; Volume 1, p. 5. [Google Scholar]
- Bottaccioli, F.; Bottaccioli, A.G. PsychoNeuroEndocrineimmunology and the Science of Integrated Care. The Manual; Edra: Milano, Italy, 2020. [Google Scholar]
- Kemenade, B.L.V.-V.; Cohen, N.; Chadzinska, M. Neuroendocrine-immune interaction: Evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Dev. Comp. Immunol. 2017, 66, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Godinho-Silva, C.; Cardoso, F.; Veiga-Fernandes, H. Neuro–Immune Cell Units: A New Paradigm in Physiology. Annu. Rev. Immunol. 2019, 37, 19–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberger, N.I.; Moieni, M.; Inagaki, T.K.; Muscatell, K.A.; Irwin, M.R. In Sickness and in Health: The Co-Regulation of Inflammation and Social Behavior. Neuropsychopharmacology 2017, 42, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Muscatell, K.A.; Inagaki, T.K. Beyond social withdrawal: New perspectives on the effects of inflammation on social behavior. Brain Behav. Immun.-Health 2021, 16, 100302. [Google Scholar] [CrossRef]
- Bottaccioli, A.G.; Bottaccioli, F.; Minelli, A. Stress and the psyche-brain-immune network in psychiatric diseases based on psychoneuroendocrineimmunology: A concise review. Ann. N. Y. Acad. Sci. 2018, 1437, 31–42. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Bauer, M.E. (Eds.) Immunopsychiatry: A Clinician’s Introduction to the Immune Basis of Mental Disorders; Oxford University Press: Oxford, MS, USA, 2019. [Google Scholar]
- Koch, C.; Massimini, M.; Boly, M.; Tononi, G. Neural correlates of consciousness: Progress and problems. Nat. Rev. Neurosci. 2016, 17, 307–321. [Google Scholar] [CrossRef]
- Ginsburg, S.; Jablonka, E. The Evolution of the Sensitive Soul: Learning and the Origins of Consciousness; MIT Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Dennett, D.C. Facing up to the hard question of consciousness. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170342. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.T. Cognitive Therapy of the Emotional Disorders; New American Library: New York, NY, USA, 1976. [Google Scholar]
- Simon, H.A. Models of Man; John Wiley: New York, NY, USA, 1957. [Google Scholar]
- Tversky, A.; Kahneman, D. Judgment under Uncertainty: Heuristics and Biases. Science 1974, 185, 1124–1131. [Google Scholar] [CrossRef]
- Kahneman, D. Thinking, Fast and Slow; Farrar Straus and Giroux: New York, NY, USA, 2011. [Google Scholar]
- Freud, S. The Complete Psychological Works of Sigmund Freud, Volume 22: New Introductory Lectures on Psycho-Analysis and Other Works (1932–1936); The Vintage Classis Penguin Books: London, UK, 2001. [Google Scholar]
- Damasio, A. Feeling & knowing: Making minds conscious. Cogn. Neurosci. 2020, 12, 65–66. [Google Scholar] [CrossRef]
- Parisi, G. In Un Volo Di Storni; Rizzoli: Milano, Italy, 2021; p. 95. [Google Scholar]
- Voosholz, J.; Gabriel, M. (Eds.) Top-Down Causation and Emergence; MIT Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Gardner, C.; Kleinman, A. Medicine and the Mind—The Consequences of Psychiatry’s Identity Crisis. N. Engl. J. Med. 2019, 381, 1697–1699. [Google Scholar] [CrossRef]
- LeDoux, J. Anxious. Using the Brain to Understand and Treat Fear and Anxiety; Penguin Books: New York, NY, USA, 2015; pp. 329–336. [Google Scholar]
- Jung, C.G. The Relations between the Ego and the Unconscious; Routledge: London, UK, 1953; Volume 7. [Google Scholar]
- Antonovsky, A. The sense of coherence: An historical and future perspective. Isr. J. Med. Sci. 1996, 32, 170–178. [Google Scholar]
- Gadamer, H.-G. The Enigma of Health; Stanford University Press: Stanford, CA, USA, 1996. [Google Scholar]
- Lieberman, P. Hominid evolution, supralaryngeal vocal tract physiology, and the fossil evidence for reconstructions. Brain Lang. 1979, 7, 101–126. [Google Scholar] [CrossRef]
- Lieberman, P. The evolution of language and thought. J. Anthr. Sci 2016, 94, 127–146. [Google Scholar] [CrossRef]
- Street, S.E.; Navarrete, A.F.; Reader, S.M.; Laland, K.N. Coevolution of cultural intelligence, extended life history, sociality, and brain size in primates. Proc. Natl. Acad. Sci. USA 2017, 114, 7908–7914. [Google Scholar] [CrossRef] [Green Version]
- Markov, A.V.; Markov, M.A. Coevolution of Brain, Culture, and Lifespan: Insights from Computer Simulations. Biochemistry 2021, 86, 1503–1525. [Google Scholar] [CrossRef]
- Blalock, J.E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol. Rev. 1989, 69, 1–32. [Google Scholar] [CrossRef]
- Ader, R. (Ed.) Psychoneuroimmunology, 4th ed.; Academic Press: Rochester, NY, USA, 2007. [Google Scholar]
- Del Rey, A.; Besedovsky, H. The immune-neuroendocrine network in health and disease. In The Wiley-Blackwell Handbook of Psychoneuroimmunology; Kusnekov, A.W., Anisman, H., Eds.; Wiley-Blackwell: Chichester, UK, 2014; pp. 99–119. [Google Scholar]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef]
- Huh, J.R.; Veiga-Fernandes, H. Neuroimmune circuits in inter-organ communication. Nat. Rev. Immunol. 2019, 20, 217–228. [Google Scholar] [CrossRef]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 2020, 21, 20–36. [Google Scholar] [CrossRef]
- Liu, J.; Yu, J.; Cheung, C. Immune Actions on the Peripheral Nervous System in Pain. Int. J. Mol. Sci. 2021, 22, 1448. [Google Scholar] [CrossRef]
- Felten, D.L.; Felten, S.Y.; Carlson, S.L.; Olschowka, J.A.; Livnat, S. Noradrenergic and peptidergic innervation of lymphoid tissue. J. Immunol. 1985, 135, 755–765. [Google Scholar]
- Silver, R.; Silverman, A.-J.; Vitković, L.; Lederhendler, I.I. Mast cells in the brain: Evidence and functional significance. Trends Neurosci. 1996, 19, 25–31. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Kulka, M. Decoding Mast Cell-Microglia Communication in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 1093. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and Functional Neuroscience in Immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef] [PubMed]
- Pintér, E.; Helyes, Z.; Szolcsányi, J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol. Ther. 2006, 112, 440–456. [Google Scholar] [CrossRef]
- Brunner, S.M.; Reichmann, F.; Leitner, J.; Wölfl, S.; Bereswill, S.; Farzi, A.; Schneider, A.-M.; Klieser, E.; Neureiter, D.; Emberger, M.; et al. Galanin receptor 3 attenuates inflammation and influences the gut microbiota in an experimental murine colitis model. Sci. Rep. 2021, 11, 564. [Google Scholar] [CrossRef]
- Tarnawski, L.; Olofsson, P.S. Inflammation neuroscience: Neuro-immune crosstalk and interfaces. Clin. Transl. Immunol. 2021, 10, e1352. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Teratani, T.; Mikami, Y.; Nakamoto, N.; Suzuki, T.; Harada, Y.; Okabayashi, K.; Hagihara, Y.; Taniki, N.; Kohno, K.; Shibata, S.; et al. The liver–brain–gut neural arc maintains the Treg cell niche in the gut. Nature 2020, 585, 591–596. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural. Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.-H.; Kam, T.-I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.E.; Wade-Martins, R.; Burnet, P.W.J. What Is Our Understanding of the Influence of Gut Microbiota on the Pathophysiology of Parkinson’s Disease? Front. Neurosci. 2021, 15, 708587. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.; del Rey, A.; Sorkin, E.; Dinarello, C.A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 1986, 233, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef]
- Gehrlach, D.A.; Dolensek, N.; Klein, A.; Chowdhury, R.R.; Matthys, A.; Junghänel, M.; Gaitanos, T.N.; Podgornik, A.; Black, T.D.; Vaka, N.R.; et al. Aversive state processing in the posterior insular cortex. Nat. Neurosci. 2019, 22, 1424–1437. [Google Scholar] [CrossRef]
- Jung, J.; Choi, S.; Han, K.-M.; Kim, A.; Kang, W.; Paik, J.-W.; Lee, H.-W.; Ham, B.-J. Alterations in functional brain networks in depressed patients with a suicide attempt history. Neuropsychopharmacology 2019, 45, 964–974. [Google Scholar] [CrossRef]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 6211. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Sekirov, I.; Finlay, B.B. Human and microbe: United we stand. Nat. Med. 2006, 12, 736–737. [Google Scholar] [CrossRef]
- Tetel, M.J.; De Vries, G.J.; Melcangi, R.C.; Panzica, G.; O’Mahony, S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocr. 2017, 30, e12548. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Arshad, M.; Sameen, A.; Oh, D.-H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Nawreen, N.; Smail, M.A.; Cotella, E.M. Brain mechanisms of HPA axis regulation: Neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress 2020, 23, 617–632. [Google Scholar] [CrossRef]
- Antoni, F.A. Hypothalamic Control of Adrenocorticotropin Secretion: Advances since the Discovery of 41-Residue Corticotropin-Releasing Factor. Endocr. Rev. 1986, 7, 351–378. [Google Scholar] [CrossRef]
- Sterling, P.; Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition, and Health; Fisher, S., Reason, J., Eds.; Wiley: Chichester, UK, 1988; pp. 629–649. [Google Scholar]
- McEwen, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Selye, H. The Stress of Life; McGraw Hill: New York, NY, USA, 1956. [Google Scholar]
- McEwen, B.S. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef] [Green Version]
- Slavich, G.M. Psychoneuroimmunology of Stress and Mental Health. In The Oxford Handbook of Stress and Mental Health; Oxford Handbooks Online; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Berens, A.E.; Jensen, S.K.G.; Nelson, C.A. Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Med. 2017, 15, 135. [Google Scholar] [CrossRef]
- Ibrahim, P.; Almeida, D.; Nagy, C.; Turecki, G. Molecular impacts of childhood abuse on the human brain. Neurobiol. Stress 2021, 15, 100343. [Google Scholar] [CrossRef]
- Chen, M.A.; LeRoy, A.S.; Majd, M.; Chen, J.Y.; Brown, R.L.; Christian, L.M.; Fagundes, C.P. Immune and Epigenetic Pathways Linking Childhood Adversity and Health Across the Lifespan. Front. Psychol. 2021, 12, 788351. [Google Scholar] [CrossRef] [PubMed]
- Brew, B.K.; Lundholm, C.; Osvald, E.C.; Chambers, G.; Öberg, S.; Fang, F.; Almqvist, C. Early-Life Adversity Due to Bereavement and Inflammatory Diseases in the Next Generation: A Population Study in Transgenerational Stress Exposure. Am. J. Epidemiol. 2021, 191, 38–48. [Google Scholar] [CrossRef]
- Szabo, Y.Z.; Slavish, D.C.; Graham-Engeland, J.E. The effect of acute stress on salivary markers of inflammation: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Slavish, D.C.; Szabo, Y.Z. What moderates salivary markers of inflammation reactivity to stress? A descriptive report and meta-regression. Stress 2021, 24, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.D.; Godbout, J.P.; Sheridan, J.F. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology 2016, 42, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.R.; Cole, S.W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011, 11, 625–632. [Google Scholar] [CrossRef]
- Cole, S.W. Human Social Genomics. PLoS Genet. 2014, 10, e1004601. [Google Scholar] [CrossRef]
- Cole, S.W.; Capitanio, J.P.; Chun, K.; Arevalo, J.M.G.; Ma, J.; Cacioppo, J.T. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc. Natl. Acad. Sci. USA 2015, 112, 15142–15147. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.W.; Cacioppo, J.T.; Cacioppo, S.; Bone, K.; Del Rosso, L.A.; Spinner, A.; Arevalo, J.M.G.; Dizon, T.P.; Capitanio, J.P. The Type I interferon antiviral gene program is impaired by lockdown and preserved by caregiving. Proc. Natl. Acad. Sci. USA 2021, 118, e2105803118. [Google Scholar] [CrossRef]
- Hamilton, O.S.; Cadar, D.; Steptoe, A. Systemic inflammation and emotional responses during the COVID-19 pandemic. Transl. Psychiatry 2021, 11, 626. [Google Scholar] [CrossRef]
- Perlmutter, A. Immunological Interfaces: The COVID-19 Pandemic and Depression. Front. Neurol. 2021, 12, 657004. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Y.; Zheng, Y.; You, C.; Tan, J.; Hu, L.; Zhang, Z.; Ding, L. Factors related to mental health of inpatients with COVID-19 in Wuhan, China. Brain Behav. Immun. 2020, 89, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, R.; Volkow, N.D. Increased risk of COVID-19 infection and mortality in people with mental disorders: Analysis from electronic health records in the United States. World Psychiatry 2020, 20, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Alexander, F. Psychological Aspects of Medicine. Psychosom. Med. 1939, 1, 7–18. [Google Scholar] [CrossRef]
- Engel, G.L. The Need for a New Medical Model: A Challenge for Biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef]
- Monod, J. Le Hasard et la Nécessité. Essai sur la Philosophie Naturelle de la Biologie Moderne; Editions du Seuil: Paris, France, 1970. [Google Scholar]
- Crick, F. Central Dogma of Molecular Biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef]
- Waddington, C.H. The Nature of Life; Atheneum: New York, NY, USA, 1961. [Google Scholar]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Szyf, M. The epigenetics of perinatal stress. Dialog-Clin. Neurosci. 2019, 21, 369–378. [Google Scholar] [CrossRef]
- Nur, S.M.; Rath, S.; Ahmad, V.; Ahmad, A.; Ateeq, B.; Khan, M.I. Nutritive vitamins as epidrugs. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–13. [Google Scholar] [CrossRef]
- Rodriguez, N.; Martinez-Pinteño, A.; Blázquez, A.; Ortiz, A.E.; Moreno, E.; Gassó, P.; Lafuente, A.; Lazaro, L.; Mas, S. Integrative DNA Methylation and Gene Expression Analysis of Cognitive Behavioral Therapy Response in Children and Adolescents with Obsessive-Compulsive Disorder; a Pilot Study. Pharmacogenom. Pers. Med. 2021, 14, 757–766. [Google Scholar] [CrossRef]
- Buric, I.; Farias, M.; Jong, J.; Mee, C.; Brazil, I. What Is the Molecular Signature of Mind–Body Interventions? A Systematic Review of Gene Expression Changes Induced by Meditation and Related Practices. Front. Immunol. 2017, 8, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda Furtado, C.L.; Dos Santos Luciano, M.C.; da Silva Santos, R.; Furtado, G.P.; Moraes, M.O.; Pessoa, C. Epidrugs: Targeting epigenetic marks in cancer treatment. Epigenetics 2019, 14, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Bošković, A.; Rando, O.J. Transgenerational Epigenetic Inheritance. Annu. Rev. Genet. 2018, 52, 21–41. [Google Scholar] [CrossRef]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 1–17. [Google Scholar] [CrossRef]
- Stein, Z.; Susser, M. The Dutch famine, 1944–1945, and the reproductive process. I. Effects on six indices at birth. Pediatr. Res. 1975, 9, 70–76. [Google Scholar]
- Schulz, L.C. The Dutch Hunger Winter and the development origins of health and disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16757–16758. [Google Scholar] [CrossRef] [Green Version]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [Green Version]
- Tobi, E.W.; Slieker, R.C.; Stein, A.D.; Suchiman, H.E.D.; Slagboom, P.E.; Van Zwet, E.W.; Heijmans, B.T.; Lumey, L.H. Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. Int. J. Epidemiol. 2015, 44, 1211–1223. [Google Scholar] [CrossRef] [Green Version]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epi-genetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Gudiel, H.P.; Córdova-Palomera, A.; Eixarch, E.; Deuschle, M.; Fañanás, L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: A meta-analysis. Epigenetics 2015, 10, 893–902. [Google Scholar] [CrossRef] [Green Version]
- Coussons-Read, M.E.; Okun, M.L.; Nettles, C. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav. Immun. 2007, 21, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Koliada, A.K. Early-life adversity and long-term neurobehavioral outcomes: Epigenome as a bridge? Hum. Genom. 2017, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szyf, M. Perinatal stress and epigenetics. Handb. Clin. Neurol. 2021, 180, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Manning, K.Y.; Long, X.; Watts, D.; Madsen, L.T.; Giesbrecht, G.F.; Lebel, C. Prenatal maternal distress during the COVID-19 pandemic and its effects on the infant brain. medRxiv 2021. [Google Scholar] [CrossRef]
- Lafortune, S.; Laplante, D.P.; Elgbeili, G.; Li, X.; Lebel, S.; Dagenais, C.; King, S. Effect of Natural Disaster-Related Prenatal Maternal Stress on Child Development and Health: A Meta-Analytic Review. Int. J. Environ. Res. Public Health 2021, 18, 8332. [Google Scholar] [CrossRef]
- Veru, F.; Dancause, K.; Laplante, D.P.; King, S.; Luheshi, G. Prenatal maternal stress predicts reductions in CD4+ lymphocytes, increases in innate-derived cytokines, and a Th2 shift in adolescents: Project Ice Storm. Physiol. Behav. 2015, 144, 137–145. [Google Scholar] [CrossRef]
- Turcotte-Tremblay, A.-M.; Lim, R.; Laplante, D.P.; Kobzik, L.; Brunet, A.; King, S. Prenatal Maternal Stress Predicts Childhood Asthma in Girls: Project Ice Storm. BioMed Res. Int. 2014, 2014, 201717. [Google Scholar] [CrossRef] [Green Version]
- Bermick, J.; Schaller, M. Epigenetic regulation of pediatric and neonatal immune responses. Pediatr. Res. 2022, 91, 297–327. [Google Scholar] [CrossRef]
- Dos Santos, R.M. Isolation, social stress, low socioeconomic status and its relationship to immune response in Covid-19 pandemic context. Brain Behav. Immun. Health 2020, 7, 100103, Erratum in Brain Behav. Immun. Health 2021, 19, 100408. [Google Scholar] [CrossRef]
- Bower, J.E.; Shiao, S.L.; Sullivan, P.; Lamkin, D.M.; Atienza, R.; Mercado, F.; Arevalo, J.; Asher, A.; Ganz, P.A.; Cole, S.W. Prometastatic Molecular Profiles in Breast Tumors From Socially Isolated Women. JNCI Cancer Spectr. 2018, 2, pky029. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Penedo, F.; Goodheart, M.J.; Dahmoush, L.; Bs, J.M.G.A.; Thaker, P.H.; Slavich, G.M.; Sood, A.K.; Cole, S.W. Epithelial-mesenchymal transition polarization in ovarian carcinomas from patients with high social isolation. Cancer 2020, 126, 4407–4413. [Google Scholar] [CrossRef]
- Stahn, A.C.; Gunga, H.-C.; Kohlberg, E.; Gallinat, J.; Dinges, D.F.; Kühn, S. Brain Changes in Response to Long Antarctic Expeditions. N. Engl. J. Med. 2019, 381, 2273–2275. [Google Scholar] [CrossRef]
- Lam, J.A.; Murray, E.R.; Yu, K.E.; Ramsey, M.; Nguyen, T.T.; Mishra, J.; Martis, B.; Thomas, M.L.; Lee, E.E. Neurobiology of loneliness: A systematic review. Neuropsychopharmacology 2021, 46, 1873–1887. [Google Scholar] [CrossRef]
- Pourriyahi, H.; Yazdanpanah, N.; Saghazadeh, A.; Rezaei, N. Loneliness: An Immunometabolic Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 12162. [Google Scholar] [CrossRef]
- Engelmann, J.M.; Herrmann, E.; Tomasello, M. Five-Year Olds, but Not Chimpanzees, Attempt to Manage Their Reputations. PLoS ONE 2012, 7, e48433. [Google Scholar] [CrossRef] [PubMed]
- Klafka, M.; Liszkowski, U. The Emergence of Lying for Reputational Concerns in 5-Year-Olds. Front. Psychol. 2021, 12, 700695. [Google Scholar] [CrossRef]
- Christopoulos, K.A.; Neilands, T.B.; Dilworth, S.; Lisha, N.; Sauceda, J.; Mugavero, M.J.; Crane, H.M.; Fredericksen, R.J.; Mathews, W.C.; Moore, R.D.; et al. Internalized HIV stigma predicts subsequent viremia in US HIV patients through depressive symptoms and antiretroviral therapy adherence. AIDS 2020, 34, 1665–1671. [Google Scholar] [CrossRef]
- Li, M.J.; Takada, S.; Okafor, C.N.; Gorbach, P.M.; Shoptaw, S.J.; Cole, S.W. Experienced homophobia and gene expression alterations in Black and Latino men who have sex with men in Los Angeles County. Brain Behav. Immun. 2019, 83, 120–125. [Google Scholar] [CrossRef]
- Flentje, A.; Heck, N.C.; Brennan, J.M.; Meyer, I.H. The relationship between minority stress and biological outcomes: A systematic review. J. Behav. Med. 2019, 43, 673–694. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E.; Aziz, N.; Kim, K.H.; Fahey, J.L. Immunological effects of induced shame and guilt. Psychosom. Med. 2004, 66, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Smyth, A.P.J.; Gammage, K.L.; Lamarche, L.; Muir, C. Examining University Men’s Psychobiological and Behavioral Response-Recovery Profile From a Social-Evaluative Body Image Threat. Am. J. Mens Health 2020, 14, 1557988320910831. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K. Marriage, divorce, and the immune system. Am. Psychol. 2018, 73, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Sbarra, D.A.; Hasselmo, K.; Bourassa, K.J. Divorce and health: Beyond individual differences. Curr. Dir. Psychol. Sci. 2015, 24, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Whisman, M.A.; Robustelli, B.L.; Sbarra, D.A. Marital disruption is associated with shorter salivary telomere length in a probability sample of older adults. Soc. Sci. Med. 2016, 157, 60–67. [Google Scholar] [CrossRef]
- Berger, E.; Castagné, R.; Chadeau-Hyam, M.; Bochud, M.; D’Errico, A.; Gandini, M.; Karimi, M.; Kivimäki, M.; Krogh, V.; Marmot, M.; et al. Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat. Commun. 2019, 10, 773. [Google Scholar] [CrossRef] [Green Version]
- Vineis, P.; Avendano-Pabon, M.; Barros, H.; Bartley, M.; Carmeli, C.; Carra, L.; Chadeau-Hyam, M.; Costa, G.; Delpierre, C.; D’Errico, A.; et al. Special Report: The Biology of Inequalities in Health: The Lifepath Consortium. Front. Public Health 2020, 8, 118. [Google Scholar] [CrossRef]
- Pariante, C.M. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur. Neuropsychopharmacol. 2017, 27, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Remes, O.; Mendes, J.F.; Templeton, P. Biological, Psychological, and Social Determinants of Depression: A Review of Recent Literature. Brain Sci. 2021, 11, 1633. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Peacock, B.N.; Scheiderer, D.J.; Kellermann, G.H. Biomolecular aspects of depression: A retrospective analysis. Compr. Psychiatry 2017, 73, 168–180. [Google Scholar] [CrossRef]
- Lynall, M.-E.; Turner, L.; Bhatti, J.; Cavanagh, J.; de Boer, P.; Mondelli, V.; Jones, D.; Drevets, W.C.; Cowen, P.; Harrison, N.A.; et al. Peripheral Blood Cell–Stratified Subgroups of Inflamed Depression. Biol. Psychiatry 2019, 88, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and Inflammation in Coronary Artery Disease: A Review Psychoneuroendocrineimmunology-Based. Front. Immunol. 2018, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Tonhajzerova, I.; Sekaninova, N.; Olexova, L.B.; Visnovcova, Z. Novel Insight into Neuroimmune Regulatory Mechanisms and Biomarkers Linking Major Depression and Vascular Diseases: The Dilemma Continues. Int. J. Mol. Sci. 2020, 21, 2317. [Google Scholar] [CrossRef] [Green Version]
- Rao, N.P.; Venkatasubramanian, G.; Ravi, V. Plasma cytokine abnormalities in drug naive, comorbidity-free obsessive-compulsive disorder. Psychiatry Res. 2015, 229, 949–952. [Google Scholar] [CrossRef]
- de Oliveira, K.C.; Camilo, C.; Gastaldi, V.D.; Feltrin, A.S.; Lisboa, B.C.G.; Paula, V.D.J.R.D.; Moretto, A.C.; Lafer, B.; Hoexter, M.Q.; Miguel, E.C.; et al. Brain areas involved with obsessive-compulsive disorder present different DNA methylation modulation. BMC Genom. Data 2021, 22, 45. [Google Scholar] [CrossRef]
- Chang, K.; Koplewicz, H.S.; Steingard, R. Special Issue on Pediatric Acute-Onset Neuropsychiatric Syndrome. J. Child Adolesc. Psychopharmacol. 2015, 25, 1–2. [Google Scholar] [CrossRef] [Green Version]
- van der Wal, J.M.; van Borkulo, C.D.; Deserno, M.K.; Breedvelt, J.J.F.; Lees, M.; Lokman, J.C.; Borsboom, D.; Denys, D.; van Holst, R.J.; Smidt, M.P.; et al. Advancing urban mental health research: From complexity science to actionable targets for intervention. Lancet Psychiatry 2021, 8, 991–1000. [Google Scholar] [CrossRef]
- Howes, O.D.; Murray, R.M. Schizophrenia: An integrated sociodevelopmental-cognitive model. Lancet 2013, 383, 1677–1687. [Google Scholar] [CrossRef] [Green Version]
- Davies, C.; Appiah-Kusi, E.; Wilson, R.; Blest-Hopley, G.; Bossong, M.G.; Valmaggia, L.; Brammer, M.; Perez, J.; Allen, P.; Murray, R.M.; et al. Altered relationship between cortisol response to social stress and mediotemporal function during fear processing in people at clinical high risk for psychosis: A preliminary report. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 272, 461–475. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Teixeira, A.L.; Salgado, J.V. Evidence for an Immune Role on Cognition in Schizophrenia: A Systematic Review. Curr. Neuropharmacol. 2014, 12, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Klaus, F.; Mitchell, K.; Liou, S.C.; Eyler, L.T.; Nguyen, T.T. Chemokine MCP1 is associated with cognitive flexibility in schizophrenia: A preliminary analysis. J. Psychiatr. Res. 2021, 138, 139–145. [Google Scholar] [CrossRef]
- Madison, A.A.; Shrout, M.R.; Renna, M.E.; Kiecolt-Glaser, J.K. Psychological and Behavioral Predictors of Vaccine Efficacy: Considerations for COVID-19. Perspect. Psychol. Sci. 2021, 16, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Bottaccioli, A.G.; Lazzari, D.; Bottaccioli, F. Promoting the Resilience of the Italian Population Against SARS-CoV-2. Front. Psychiatry 2021, 11, 560017. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Desarnaud, F.; Makotkine, I.; Lehrner, A.L.; Koch, E.; Flory, J.D.; Buxbaum, J.D.; Meaney, M.J.; Bierer, L.M. Epigenetic Biomarkers as Predictors and Correlates of Symptom Improvement Following Psychotherapy in Combat Veterans with PTSD. Front. Psychiatry 2013, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Vinkers, C.H.; Geuze, E.; van Rooij, S.J.H.; Kennis, M.; Schür, R.R.; Nispeling, D.M.; Smith, A.K.; Nievergelt, C.M.; Uddin, M.; Rutten, B.P.F.; et al. Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Mol. Psychiatry 2019, 26, 1264–1271. [Google Scholar] [CrossRef]
- Lopresti, A.L. Cognitive behaviour therapy and inflammation: A systematic review of its relationship and the potential implications for the treatment of depression. Aust. N. Z. J. Psychiatry 2017, 51, 565–582. [Google Scholar] [CrossRef]
- Romero-Sanchiz, P.; Nogueira-Arjona, R.; Araos, P.; Serrano, A.; Barrios, V.; Argente, J.; Garcia-Marchena, N.; Lopez-Tellez, A.; Rodriguez-Moreno, S.; Mayoral-Cleries, F.; et al. Variation in chemokines plasma concentrations in primary care depressed patients associated with Internet-based cognitive-behavioral therapy. Sci. Rep. 2020, 10, 1078. [Google Scholar] [CrossRef] [Green Version]
- Shields, G.S.; Spahr, C.M.; Slavich, G.M. Psychosocial Interventions and Immune System Function: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2020, 77, 1031–1043. [Google Scholar] [CrossRef]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef]
- Krylova, M.; Skouras, S.; Razi, A.; Nicholson, A.A.; Karner, A.; Steyrl, D.; Boukrina, O.; Rees, G.; Scharnowski, F.; Koush, Y. Progressive modulation of resting-state brain activity during neurofeedback of positive-social emotion regulation networks. Sci. Rep. 2021, 11, 23363. [Google Scholar] [CrossRef]
- Bottaccioli, F.; Carosella, A.; Cardone, R.; Mambelli, M.; Cemin, M.; D’Errico, M.M.; Ponzio, E.; Bottaccioli, A.G.; Minelli, A. Brief Training of Psychoneuroendocrinoimmunology-Based Meditation (PNEIMED) Reduces Stress Symptom Ratings and Improves Control on Salivary Cortisol Secretion Under Basal and Stimulated Conditions. Explore 2014, 10, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottaccioli, A.G.; Bottaccioli, F.; Carosella, A.; Cofini, V.; Muzi, P.; Bologna, M. Psychoneuroendocrinoimmunology-based meditation (PNEIMED) training reduces salivary cortisol under basal and stressful conditions in healthy university students: Results of a randomized controlled study. Explore 2019, 16, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Irwin, M.R. Mind–body therapies and control of inflammatory biology: A descriptive review. Brain Behav. Immun. 2016, 51, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemirovsky, A.; Ilan, K.; Lerner, L.; Cohen-Lavi, L.; Schwartz, D.; Goren, G.; Sergienko, R.; Greenberg, D.; Slonim-Nevo, V.; Sarid, O.; et al. Brain-immune axis regulation is responsive to cognitive behavioral therapy and mindfulness intervention: Observations from a randomized controlled trial in patients with Crohn’s disease. Brain Behav. Immun.-Health 2021, 19, 100407. [Google Scholar] [CrossRef]
- Feng, F.; Tuchman, S.; Denninger, J.W.; Fricchione, G.L.; Yeung, A. Qigong for the Prevention, Treatment, and Rehabilitation of COVID-19 Infection in Older Adults. Am. J. Geriatr. Psychiatry 2020, 28, 812–819. [Google Scholar] [CrossRef]
- Vancampfort, D.; Stubbs, B.; Van Damme, T.; Smith, L.; Hallgren, M.; Schuch, F.; Deenik, J.; Rosenbaum, S.; Ashdown-Franks, G.; Mugisha, J.; et al. The efficacy of meditation-based mind-body interventions for mental disorders: A meta-review of 17 meta-analyses of randomized controlled trials. J. Psychiatr. Res. 2020, 134, 181–191. [Google Scholar] [CrossRef]
- Fernández-Alvarez, J.; Grassi, M.; Colombo, D.; Botella, C.; Cipresso, P.; Perna, G.; Riva, G. Efficacy of bio- and neurofeedback for depression: A meta-analysis. Psychol. Med. 2021, 52, 201–216. [Google Scholar] [CrossRef]
- Lambez, B.; Harwood-Gross, A.; Golumbic, E.Z.; Rassovsky, Y. Non-pharmacological interventions for cognitive difficulties in ADHD: A systematic review and meta-analysis. J. Psychiatr. Res. 2019, 120, 40–55. [Google Scholar] [CrossRef]
- Rahmani, E.; Mahvelati, A.; Alizadeh, A.; Mokhayeri, Y.; Rahmani, M.; Zarabi, H.; Hassanvandi, S. Is neurofeedback effective in children with ADHD? A systematic review and meta-analysis. Neurocase 2022, 1–12. [Google Scholar] [CrossRef]
- Girardeau, G.; Lopes-Dos-Santos, V. Brain neural patterns and the memory function of sleep. Science 2021, 374, 560–564. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Bottaccioli, F. Filosofia per la Medicina. Medicina per la Filosofia. Oriente e Occidente a Confronto, 2nd ed.; Tecniche Nuove: Milano, Italy, 2020. [Google Scholar]
- McEwen, C.A. Connecting the biology of stress, allostatic load and epigenetics to social structures and processes. Neurobiol. Stress 2022, 17, 100426. [Google Scholar] [CrossRef]
- Janet, P. La Médecine Psychologique; Republié chez l’Harmattan: Paris, France, 2005. [Google Scholar]
- Kuhn, T. The Structure of Scientific Revolutions, 2nd ed.; University of Chicago: Chicago, IL, USA, 1986. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottaccioli, A.G.; Bologna, M.; Bottaccioli, F. Psychic Life-Biological Molecule Bidirectional Relationship: Pathways, Mechanisms, and Consequences for Medical and Psychological Sciences—A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3932. https://doi.org/10.3390/ijms23073932
Bottaccioli AG, Bologna M, Bottaccioli F. Psychic Life-Biological Molecule Bidirectional Relationship: Pathways, Mechanisms, and Consequences for Medical and Psychological Sciences—A Narrative Review. International Journal of Molecular Sciences. 2022; 23(7):3932. https://doi.org/10.3390/ijms23073932
Chicago/Turabian StyleBottaccioli, Anna Giulia, Mauro Bologna, and Francesco Bottaccioli. 2022. "Psychic Life-Biological Molecule Bidirectional Relationship: Pathways, Mechanisms, and Consequences for Medical and Psychological Sciences—A Narrative Review" International Journal of Molecular Sciences 23, no. 7: 3932. https://doi.org/10.3390/ijms23073932





