Exercise Ameliorates Atherosclerosis via Up-Regulating Serum β-Hydroxybutyrate Levels
Abstract
:1. Introduction
2. Results
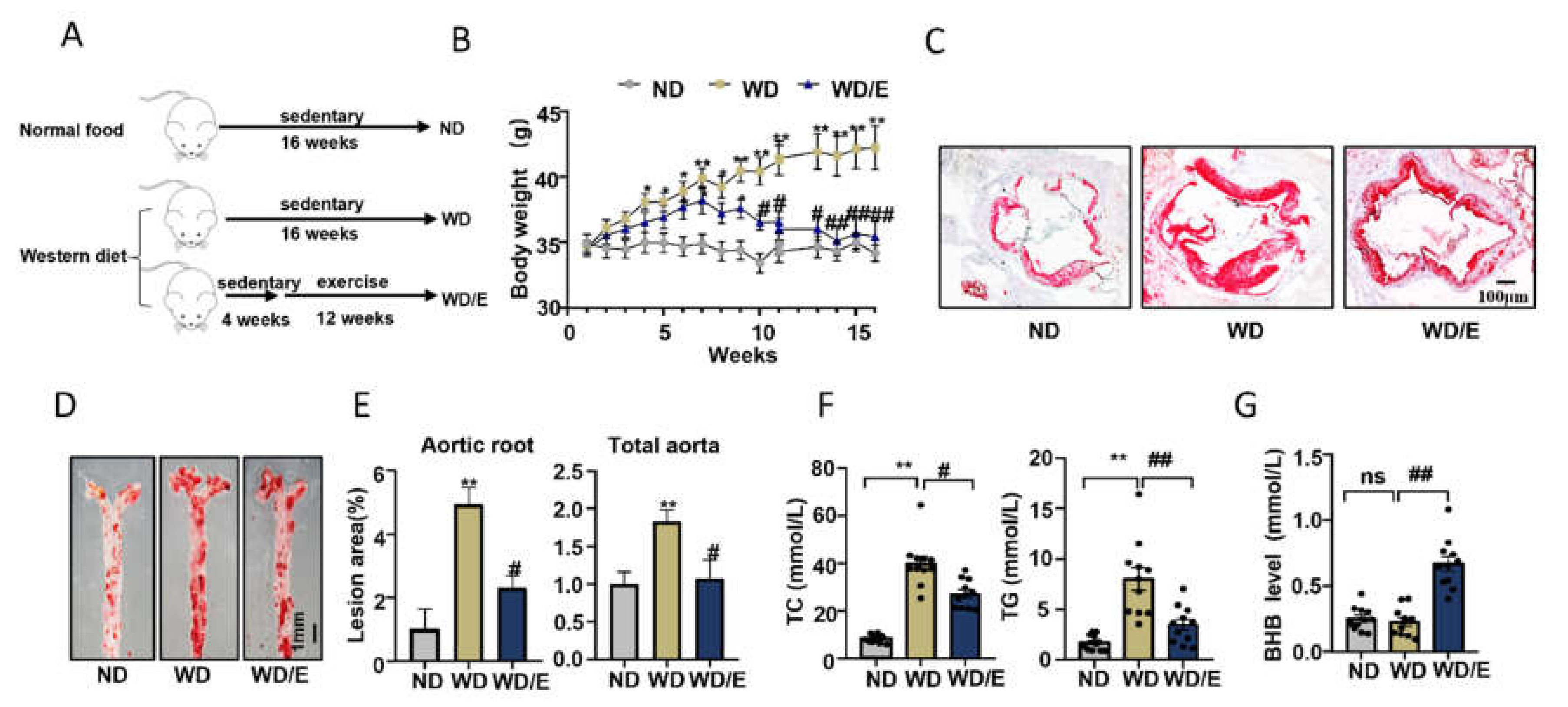
2.1. Regular Exercise Training Can Reduce Atherosclerotic Lesions and Increase Serum BHB Levels
2.2. Regular Exercise Training Increases the Expression of Lipid Transfer Proteins and Promotes Autophagy in Atherosclerotic Plaques
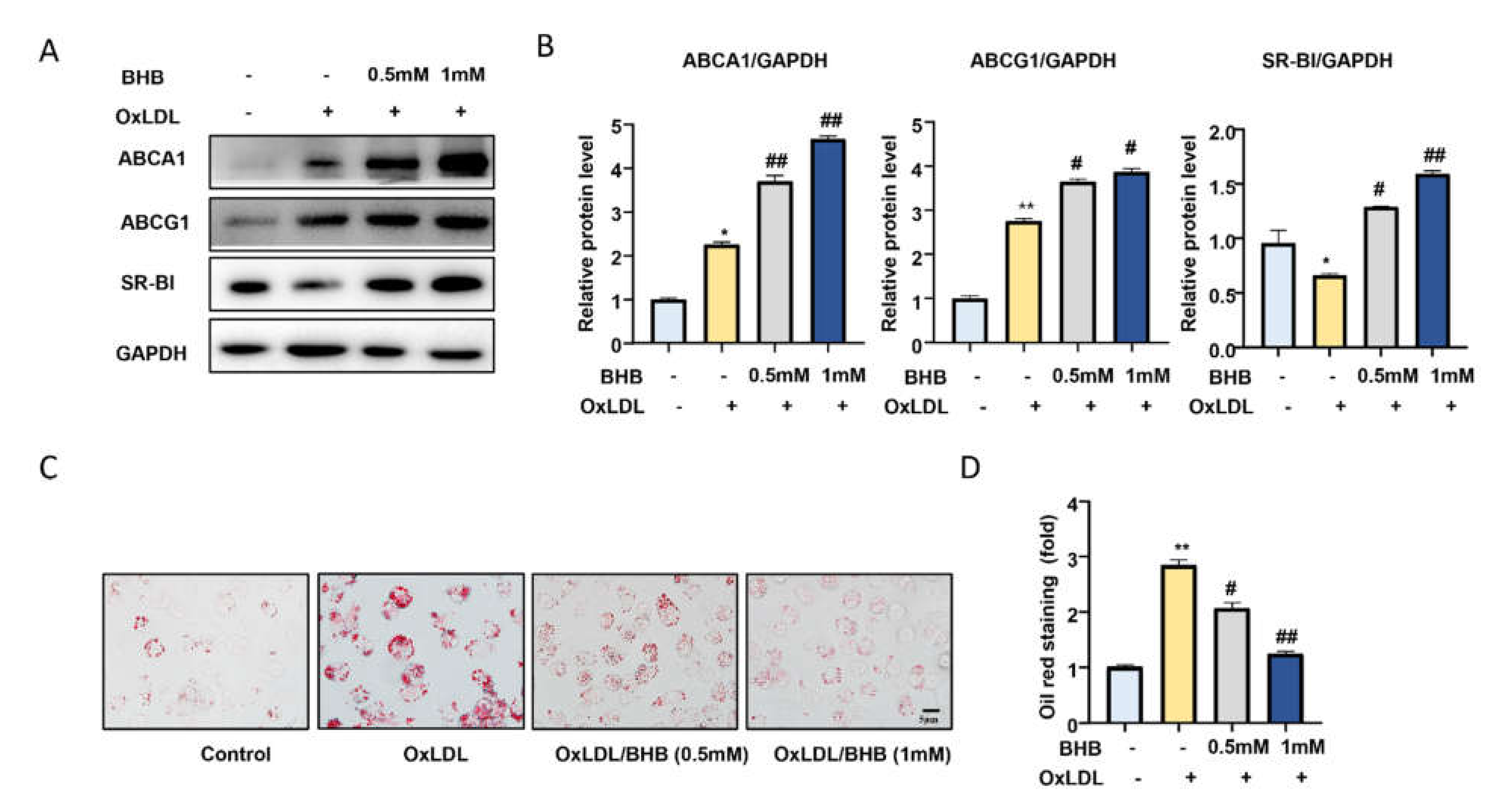
2.3. BHB Increases the Expression of Lipid Transfer Proteins and Inhibits Macrophage Foam Cell Formation In Vitro
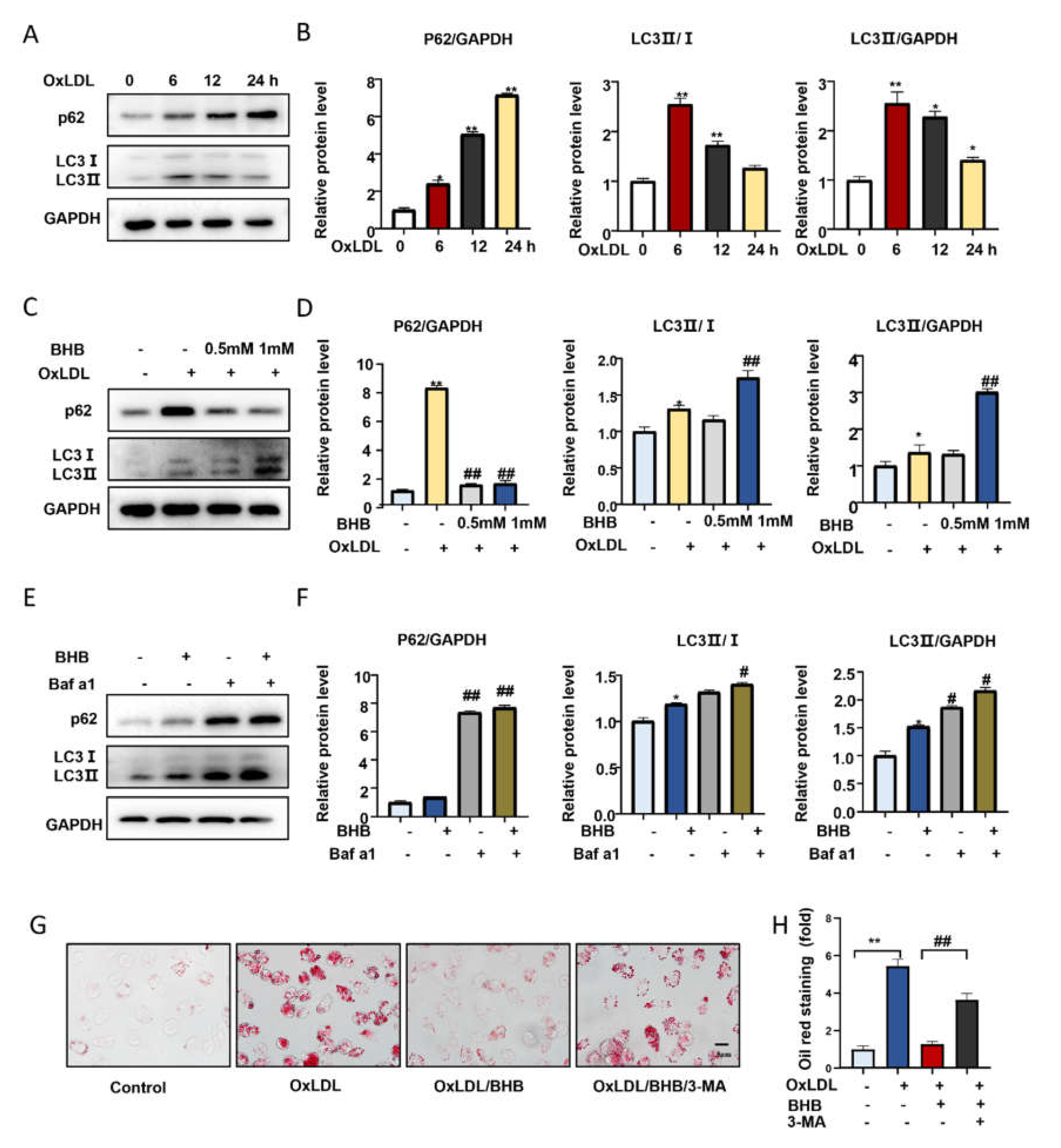
2.4. BHB Ameliorates Autophagy in Macrophages
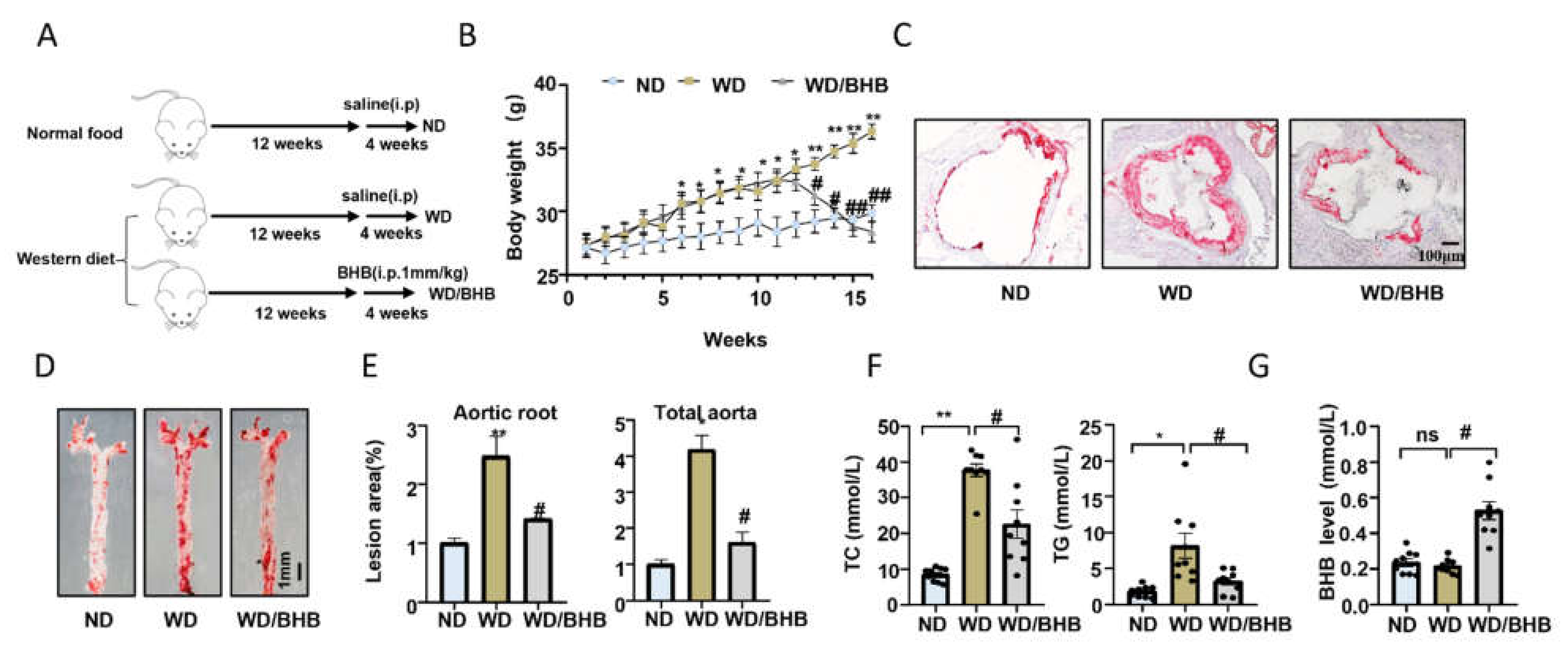
2.5. BHB Treatment Is Atheroprotective In Vivo
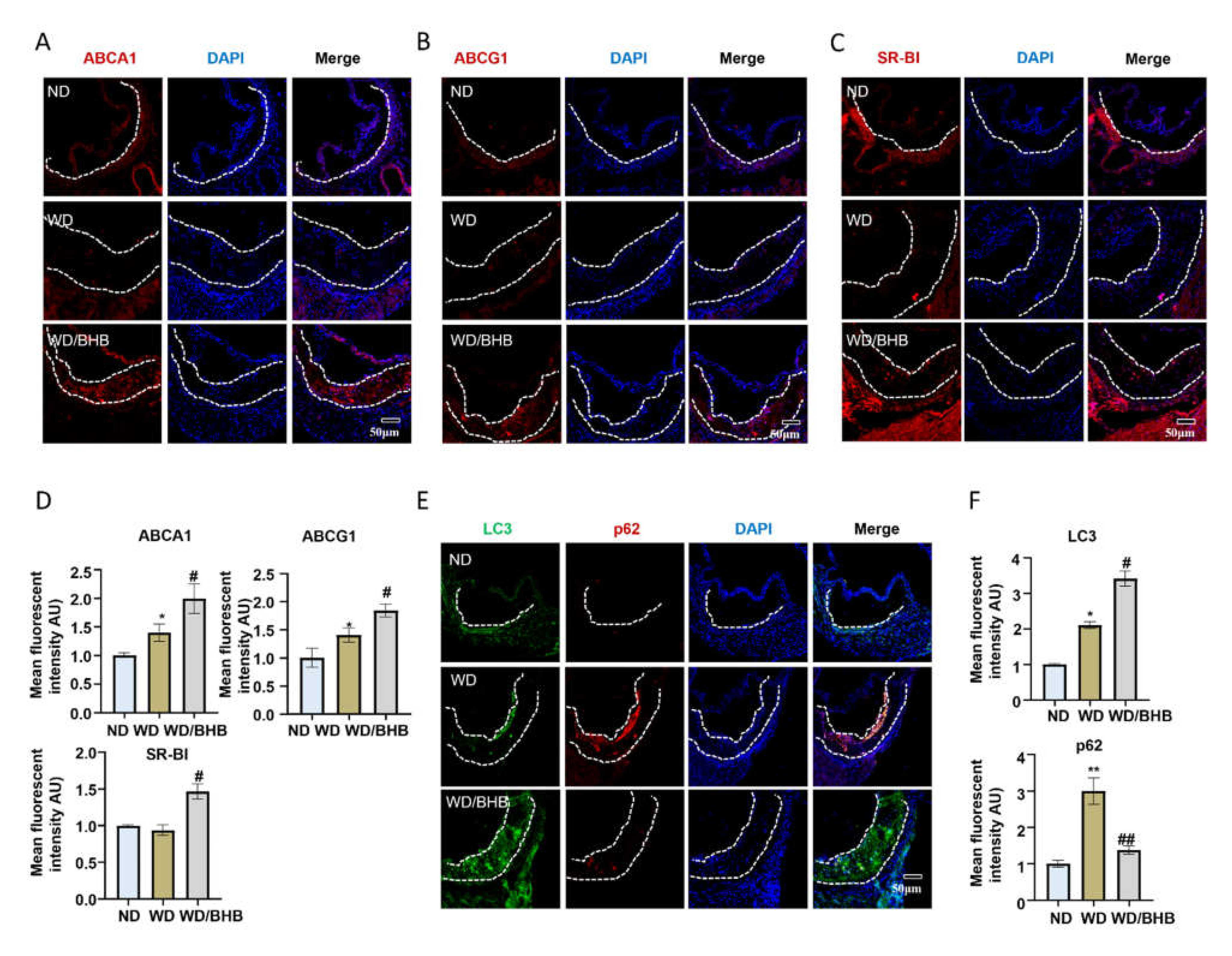
2.6. BHB Increases the Expression of Critical Cholesterol Transporters and Induces Autophagy in Atherosclerotic Plaques
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animal Studies
4.3. Exercise Method and BHB Injection
4.4. Atherosclerotic Lesion Analysis
4.5. Serum Lipid Measurement
4.6. Serum BHB Level Measurement
4.7. Serum Cytokine Analysis by Enzyme-Linked Immunoassay
4.8. Cell Culture
4.9. Foam Cell Formation Assays
4.10. Western Blotting Analysis
4.11. Immunofluorescence Staining
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHB | β-hydroxybutyric acid |
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG1 | ATP-binding cassette transporter G1 |
| SR-BI | Scavenger receptor class B type I |
| SRA | Scavenger receptor A |
| NRF2 | Nuclear factor E2-related factor 2 |
| MMPs | Matrix metalloproteinases |
| NLRP3 | NACHT, LRR, and PYD domains containing protein 3 |
| RCT | Reverse cholesterol transport |
| CE | Cholesteryl ester |
| ApoE−/− | ApoE deficiency |
| TC | Total cholesterol |
| TG | Triglyceride |
| ND | Normal food |
| WD | Western diet food |
| PMs | Peritoneal macrophages |
| 3-MA | 3-methyladenine |
| Baf a1 | Bafilomycin a1 |
| OxLDL | Oxidative low density lipoprotein |
| LDL | Low density lipoprotein |
| AMPK | Adenosine 5‘-monophosphate (AMP)-activated protein kinase |
| HDC3 | Histone deacetylase 3 |
| ER | Endoplasmic reticulum |
| OCT | Optimal cutting temperature compound |
References
- Ruiz-Leon, A.M.; Lapuente, M.; Estruch, R.; Casas, R. Clinical Advances in Immunonutrition and Atherosclerosis: A Review. Front. Immunol. 2019, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Bornfeldt, K.E.; Tall, A.R. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ. Res. 2016, 118, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Frodermann, V.; Rohde, D.; Courties, G.; Severe, N.; Schloss, M.J.; Amatullah, H.; McAlpine, C.S.; Cremer, S.; Hoyer, F.F.; Ji, F.; et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 2019, 25, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Zhang, D.W.; Zheng, X.L.; Tang, C.K. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res. 2019, 73, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, H.; Dai, X.; Wang, C.; Ding, Y.; Song, P.; Zou, M.H. Macrophage Liver Kinase B1 Inhibits Foam Cell Formation and Atherosclerosis. Circ. Res. 2017, 121, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, S.; Vanegas, D.; Kennedy, D.J.; Guy, E.; Nimako, G.; Morton, R.E.; Febbraio, M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 2008, 78, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Gui, Y.Z.; Yan, H.; Gao, F.; Xi, C.; Li, H.H.; Wang, Y.P. Betulin attenuates atherosclerosis in apoE(-/-) mice by up-regulating ABCA1 and ABCG1. Acta Pharm. Sin. 2016, 37, 1337–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tall, A.R.; Yvan-Charvet, L.; Terasaka, N.; Pagler, T.; Wang, N. HDL, ABC transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 2008, 7, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouimet, M.; Franklin, V.; Mak, E.; Liao, X.; Tabas, I.; Marcel, Y.L. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011, 13, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [Green Version]
- Ouimet, M.; Marcel, Y.L. Regulation of lipid droplet cholesterol efflux from macrophage foam cells. Arter. Thromb Vasc. Biol. 2012, 32, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Wu, W.Q.; Peng, S.; Wan, X.Q.; Lin, S.; Li, L.Y.; Song, Z.Y. Physical exercise inhibits atherosclerosis development by regulating the expression of neuropeptide Y in apolipoprotein E-deficient mice. Life Sci. 2019, 237, 116896. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Basse, A.L.; Schonke, M.; Chen, S.; Samad, M.; Altintas, A.; Laker, R.C.; Dalbram, E.; Barres, R.; Baldi, P.; et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab. 2019, 30, 92–110.e4. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, J.; Liu, H.; Chen, Y.; Ma, X.; Chen, S.; Chen, Y.; Bihl, J.I.; Yang, Y.I. Moderate Exercise Enhances Endothelial Progenitor Cell Exosomes Release and Function. Med. Sci. Sports Exerc. 2018, 50, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Polaki, V.; Chen, S.; Bihl, J.C. Exercise Improves Endothelial Function Associated with Alleviated Inflammation and Oxidative Stress of Perivascular Adipose Tissue in Type 2 Diabetic Mice. Oxid. Med. Cell Longev. 2020, 2020, 8830537. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Leaf, D.A. The effect of physical exercise on reverse cholesterol transport. Metabolism 2003, 52, 950–957. [Google Scholar] [CrossRef]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Goncalves, D.C. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2018, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.E.; Bae, J.H.; Lee, J.H.; Shin, H.E.; Zhang, D.; Cho, S.C.; Song, W. Effects of exercise-induced beta-hydroxybutyrate on muscle function and cognitive function. Physiol. Rep. 2021, 9, e14497. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.K.; Kim, D.H.; Bang, E.; Choi, Y.J.; Chung, H.Y. beta-Hydroxybutyrate Suppresses Lipid Accumulation in Aged Liver through GPR109A-mediated Signaling. Aging Dis. 2020, 11, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Uchida, Y.; Kadono, K.; Hirao, H.; Kawasoe, J.; Watanabe, T.; Ueda, S.; Okajima, H.; Terajima, H.; Uemoto, S. Up-regulation of FOXO1 and reduced inflammation by beta-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc. Natl. Acad. Sci. USA 2019, 116, 13533–13542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camberos-Luna, L.; Geronimo-Olvera, C.; Montiel, T.; Rincon-Heredia, R.; Massieu, L. The Ketone Body, beta-Hydroxybutyrate Stimulates the Autophagic Flux and Prevents Neuronal Death Induced by Glucose Deprivation in Cortical Cultured Neurons. Neurochem. Res. 2016, 41, 600–609. [Google Scholar] [CrossRef]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. beta-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflamm. 2020, 17, 280. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Cyranka, M.; Clarke, K.; de Wet, H. A Ketone Ester Drink Lowers Human Ghrelin and Appetite. Obesity 2018, 26, 269–273. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharm. 2020, 129, 110426. [Google Scholar] [CrossRef]
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A Multifunctional Receptor in Cholesterol Homeostasis and Atherosclerosis. Trends Endocrinol. Metab. 2017, 28, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Cretoiu, D.; Li, G.; Xiao, J. Exercise-mediated regulation of autophagy in the cardiovascular system. J. Sport Health Sci. 2020, 9, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Yang, R.; Wang, S.; Zhang, W.; Wang, X.; Li, H.; Dong, J.; Chen, W.; Yu, X.; Ji, F. Association of Serum β-Hydroxybutyrate and Coronary Artery Disease in an Urban Chinese Population. Front. Nutr. 2022, 9, 828824. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Yol, Y.; Turgay, F.; Yigitturk, O.; Asikovali, S.; Durmaz, B. The effects of regular aerobic exercise training on blood nitric oxide levels and oxidized LDL and the role of eNOS intron 4a/b polymorphism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165913. [Google Scholar] [CrossRef] [PubMed]
- Kojda, G.; Cheng, Y.C.; Burchfield, J.; Harrison, D.G. Dysfunctional Regulation of Endothelial Nitric Oxide Synthase (eNOS) Expression in Response to Exercise in Mice Lacking One eNOS Gene. Circulation 2001, 103, 2839–2844. [Google Scholar] [CrossRef] [Green Version]
- Moien-Afshari, F.; Ghosh, S.; Elmi, S.; Rahman, M.M.; Sallam, N.; Khazaei, M.; Kieffer, T.J.; Brownsey, R.W.; Laher, I. Exercise restores endothelial function independently of weight loss or hyperglycaemic status in db/db mice. Diabetologia 2008, 51, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Hambrecht, R.; Adams, V.; Erbs, S.; Linke, A.; Krankel, N.; Shu, Y.; Baither, Y.; Gielen, S.; Thiele, H.; Gummert, J.F.; et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 2003, 107, 3152–3158. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wu, Y.; Jiang, Y.; Liu, H. Aerobic exercise inhibits inflammatory response in atherosclerosis via Sestrin1 protein. Exp. Gerontol. 2021, 155, 111581. [Google Scholar] [CrossRef]
- Nascimento Dda, C.; Durigan Rde, C.; Tibana, R.A.; Durigan, J.L.; Navalta, J.W.; Prestes, J. The response of matrix metalloproteinase-9 and -2 to exercise. Sports Med. 2015, 45, 269–278. [Google Scholar] [CrossRef]
- Van Dijk, J.W.; Manders, R.J.; Tummers, K.; Bonomi, A.G.; Stehouwer, C.D.; Hartgens, F.; van Loon, L.J. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia 2012, 55, 1273–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borjesson, M.; Onerup, A.; Lundqvist, S.; Dahlof, B. Physical activity and exercise lower blood pressure in individuals with hypertension: Narrative review of 27 RCTs. Br. J. Sports Med. 2016, 50, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Stoner, L.; Rowlands, D.; Morrison, A.; Credeur, D.; Hamlin, M.; Gaffney, K.; Lambrick, D.; Matheson, A. Efficacy of Exercise Intervention for Weight Loss in Overweight and Obese Adolescents: Meta-Analysis and Implications. Sports Med. 2016, 46, 1737–1751. [Google Scholar] [CrossRef] [Green Version]
- Hoang, A.; Tefft, C.; Duffy, S.J.; Formosa, M.; Henstridge, D.C.; Kingwell, B.A.; Sviridov, D. ABCA1 expression in humans is associated with physical activity and alcohol consumption. Atherosclerosis 2008, 197, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Rahmati-Ahmadabad, S.; Broom, D.R.; Ghanbari-Niaki, A.; Shirvani, H. Effects of exercise on reverse cholesterol transport: A systemized narrative review of animal studies. Life Sci. 2019, 224, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21, e13024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brietzke, E.; Mansur, R.B.; Subramaniapillai, M.; Balanza-Martinez, V.; Vinberg, M.; Gonzalez-Pinto, A.; Rosenblat, J.D.; Ho, R.; McIntyre, R.S. Ketogenic diet as a metabolic therapy for mood disorders: Evidence and developments. Neurosci. Biobehav. Rev. 2018, 94, 11–16. [Google Scholar] [CrossRef]
- Wells, J.; Swaminathan, A.; Paseka, J.; Hanson, C. Efficacy and Safety of a Ketogenic Diet in Children and Adolescents with Refractory Epilepsy-A Review. Nutrients 2020, 12, 1809. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef]
- Li, B.; Yu, Y.; Liu, K.; Zhang, Y.; Geng, Q.; Zhang, F.; Li, Y.; Qi, J. beta-Hydroxybutyrate inhibits histone deacetylase 3 to promote claudin-5 generation and attenuate cardiac microvascular hyperpermeability in diabetes. Diabetologia 2021, 64, 226–239. [Google Scholar] [CrossRef]
- Gough, S.M.; Casella, A.; Ortega, K.J.; Hackam, A.S. Neuroprotection by the Ketogenic Diet: Evidence and Controversies. Front. Nutr. 2021, 8, 782657. [Google Scholar] [CrossRef] [PubMed]
- Simeone, T.A.; Simeone, K.A.; Rho, J.M. Ketone Bodies as Anti-Seizure Agents. Neurochem. Res. 2017, 42, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Whalen, C.A.; Gullette, S.; Mattie, F.J.; Florindo, C.; Heil, S.G.; Huang, N.K.; Neuberger, T.; Ross, A.C. A Hypomethylating Ketogenic Diet in Apolipoprotein E-Deficient Mice: A Pilot Study on Vascular Effects and Specific Epigenetic Changes. Nutrients 2021, 13, 3576. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Shalaurova, I.; Matyus, S.P.; Oskardmay, D.N.; Otvos, J.D.; Dullaart, R.P.F.; Connelly, M.A. Ketone Bodies Are Mildly Elevated in Subjects with Type 2 Diabetes Mellitus and Are Inversely Associated with Insulin Resistance as Measured by the Lipoprotein Insulin Resistance Index. J. Clin. Med. 2020, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, M.; Hwang, J.S.; Kim, M.; Kim, Y.J.; Seo, J.H.; Jung, J.; Ha, E. beta-hydroxybutyrate Impedes the Progression of Alzheimer’s Disease and Atherosclerosis in ApoE-Deficient Mice. Nutrients 2020, 12, 471. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.J.; Li, Z.H.; Zhang, Y.D.; Chen, J.; Li, Y.; Wu, F.Q.; Wang, W.; Cui, Z.J.; Chen, G.Q. Ketone Body 3-Hydroxybutyrate Ameliorates Atherosclerosis via Receptor Gpr109a-Mediated Calcium Influx. Adv. Sci. (Weinh) 2021, 8, 2003410. [Google Scholar] [CrossRef]
- Graff, E.C.; Fang, H.; Wanders, D.; Judd, R.L. Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism 2016, 65, 102–113. [Google Scholar] [CrossRef]
- Shao, B.Z.; Han, B.Z.; Zeng, Y.X.; Su, D.F.; Liu, C. The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol. Sin. 2016, 37, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Nanduri, R.; Bhagyaraj, E.; Kalra, R.; Ahuja, N.; Chacko, A.P.; Tiwari, D.; Sethi, K.; Saini, A.; Chandra, V.; et al. Vitamin D3-VDR-PTPN6 axis mediated autophagy contributes to the inhibition of macrophage foam cell formation. Autophagy 2021, 17, 2273–2289. [Google Scholar] [CrossRef]
- Liang, J.; Huang, J.; He, W.; Shi, G.; Chen, J.; Huang, H. beta-Hydroxybutyric Inhibits Vascular Calcification via Autophagy Enhancement in Models Induced by High Phosphate. Front. Cardiovasc. Med. 2021, 8, 685748. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Cao, W. Toll-Like Receptor Signaling Induces Nrf2 Pathway Activation through p62-Triggered Keap1 Degradation. Mol. Cell Biol. 2015, 35, 2673–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimura, J.; Itoh, K. Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic. Biol. Med. 2015, 88, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Pynn, M.; Schafer, K.; Konstantinides, S.; Halle, M. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein e-deficient mice. Circulation 2004, 109, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Wei, A.; Chen, F.; Chen, X.; Ding, W.; Ding, Z.; Wu, Z.; Du, R.; Cao, W. Enhancing PPARgamma by HDAC inhibition reduces foam cell formation and atherosclerosis in ApoE deficient mice. Pharm. Res. 2020, 160, 105059. [Google Scholar] [CrossRef]
- Cheng, C.W.; Biton, M.; Haber, A.L.; Gunduz, N.; Eng, G.; Gaynor, L.T.; Tripathi, S.; Calibasi-Kocal, G.; Rickelt, S.; Butty, V.L.; et al. Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell 2019, 178, 1115–1131.e1115. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.J.; Wang, L.; Tavakoli, S.; Nguyen, H.N.; Short, J.D.; Asmis, R. Glutaredoxin 1 controls monocyte reprogramming during nutrient stress and protects mice against obesity and atherosclerosis in a sex-specific manner. Nat. Commun. 2022, 13, 790. [Google Scholar] [CrossRef]
- Ding, W.; Ding, Z.; Wang, Y.; Zhu, Y.; Gao, Q.; Cao, W.; Du, R. Evodiamine Attenuates Experimental Colitis Injury Via Activating Autophagy and Inhibiting NLRP3 Inflammasome Assembly. Front. Pharm. 2020, 11, 573870. [Google Scholar] [CrossRef]
- Sergin, I.; Evans, T.D.; Zhang, X.; Bhattacharya, S.; Stokes, C.J.; Song, E.; Ali, S.; Dehestani, B.; Holloway, K.B.; Micevych, P.S.; et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun. 2017, 8, 15750. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhang, M.; Li, X.; Wang, Y.; Du, R. Exercise Ameliorates Atherosclerosis via Up-Regulating Serum β-Hydroxybutyrate Levels. Int. J. Mol. Sci. 2022, 23, 3788. https://doi.org/10.3390/ijms23073788
Xu Z, Zhang M, Li X, Wang Y, Du R. Exercise Ameliorates Atherosclerosis via Up-Regulating Serum β-Hydroxybutyrate Levels. International Journal of Molecular Sciences. 2022; 23(7):3788. https://doi.org/10.3390/ijms23073788
Chicago/Turabian StyleXu, Zhou, Mingyue Zhang, Xinran Li, Yong Wang, and Ronghui Du. 2022. "Exercise Ameliorates Atherosclerosis via Up-Regulating Serum β-Hydroxybutyrate Levels" International Journal of Molecular Sciences 23, no. 7: 3788. https://doi.org/10.3390/ijms23073788
APA StyleXu, Z., Zhang, M., Li, X., Wang, Y., & Du, R. (2022). Exercise Ameliorates Atherosclerosis via Up-Regulating Serum β-Hydroxybutyrate Levels. International Journal of Molecular Sciences, 23(7), 3788. https://doi.org/10.3390/ijms23073788





