Store-Operated Ca2+ Entry in Skeletal Muscle Contributes to the Increase in Body Temperature during Exertional Stress
Abstract
1. Introduction
2. Results
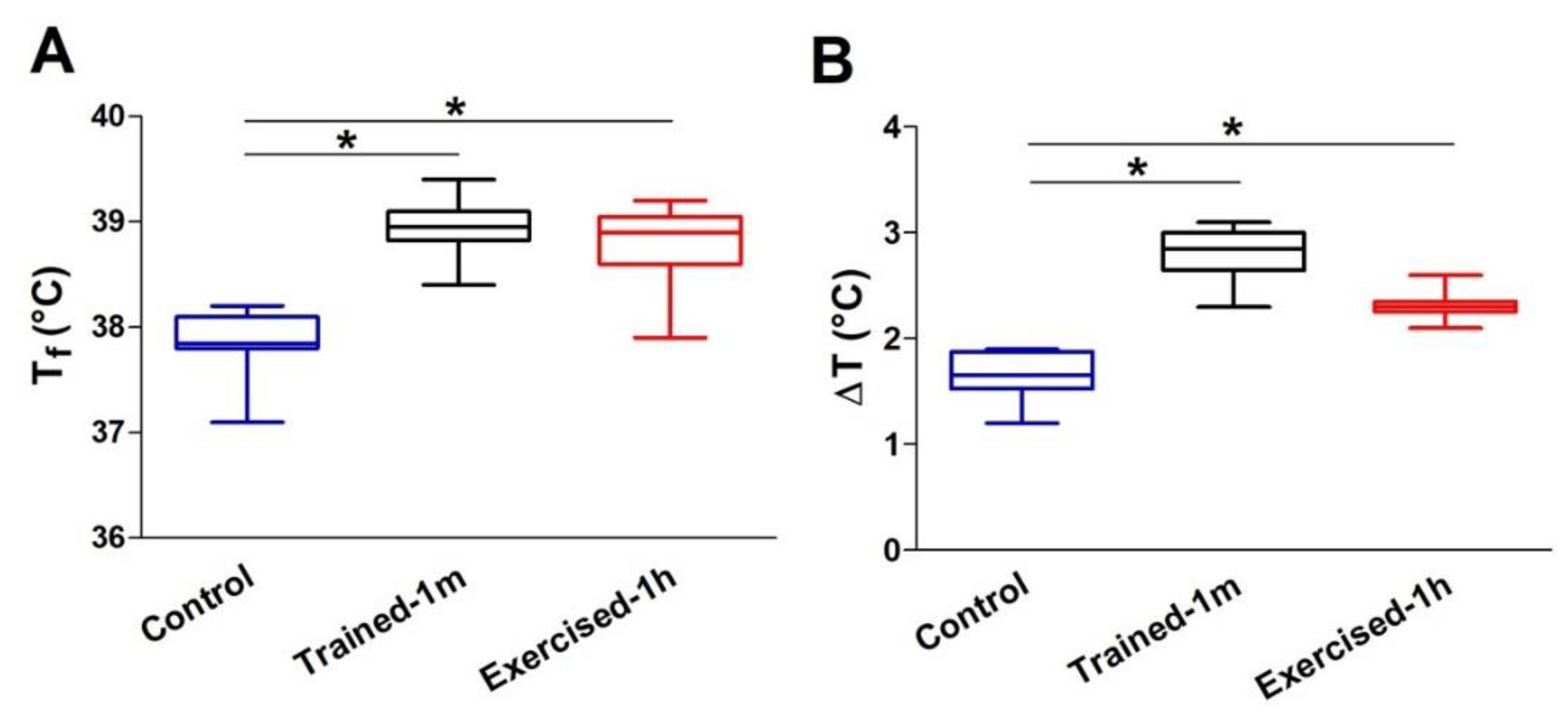
- The increase in body temperature was greater in the trained-1 m and exercised-1 h mice than in the controls. The difference with the control mice was statistically significant, both as an absolute temperature (Figure 1A: 38.9 ± 0.2 in trained-1 m and 38.8 ± 0.5 in exercised-1 h vs. 37.9 ± 0.2 in control) and as ΔT, i.e., the difference between the temperatures measured at the beginning (T0) and at the end (Tf) of the experiment (Figure 1B: 2.7 ± 0.2 in trained-1 m and 2.3 ± 0.1 in exercised-1 h vs. 1.7 ± 0.1 in control). See also Supplementary Table S1. Results collected using the infrared thermometer display a similar trend, both as an absolute temperature (Supplementary Figure S2A: 36.5 ± 0.4 in trained-1 m and 36.3 ± 0.5 in exercised-1 h vs. 35.6 ± 0.3 in control) and as ΔT (Supplementary Figure S2B: 2.7 ± 0.3 in trained-1 m and 2.3 ± 0.1 in exercised-1 h vs. 1.6 ± 0.3 in control).
- Note that all graphs (Figure 1 and Supplementary Figure S2) indicate an increase in body temperature that is slightly greater in the trained-1 m mice than in the exercised-1 h mice; this difference, though, was not statistically significant.
3. Discussion
- (a)
- the results collected in Supplementary Figure S2 indicate that measuring the temperature of animals using a simple infrared thermometer for cutaneous temperature (Supplementary Figures S1 and S2) can be a valid and less invasive alternative method for avoiding the use of the rectal thermometer in future studies.
- (b)
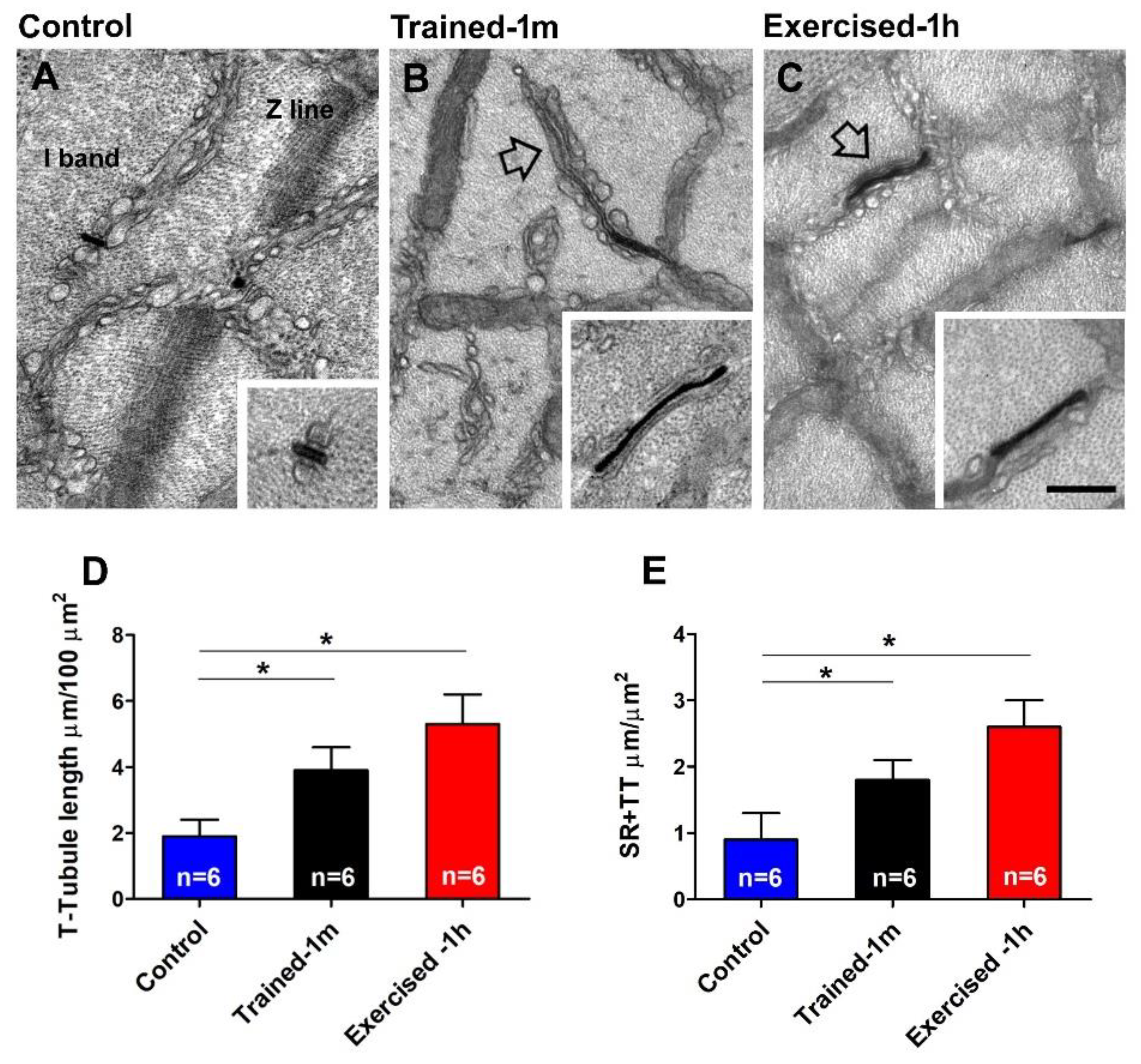
- The assembly of CEUs is also promoted by voluntary training in wheel cages; this result is novel, i.e., it was not previously reported (Figure 3 and Figure 4). The following remain to be determined: (i) whether CEUs assembled by training are more stable that those formed during acute exercise, and (ii) whether training promotes an increased expression of STIM1 and Orai1. Hence, the effect of prolonged training will need to be investigated more in depth. Though, the fact that, during heat stress (Supplementary Figure S3A), EDL from trained-1 m mice did not behave differently from those of the controls suggests that TTs do retract quickly following exercise in the trained-1 m mice as well (see next section for additional detail).
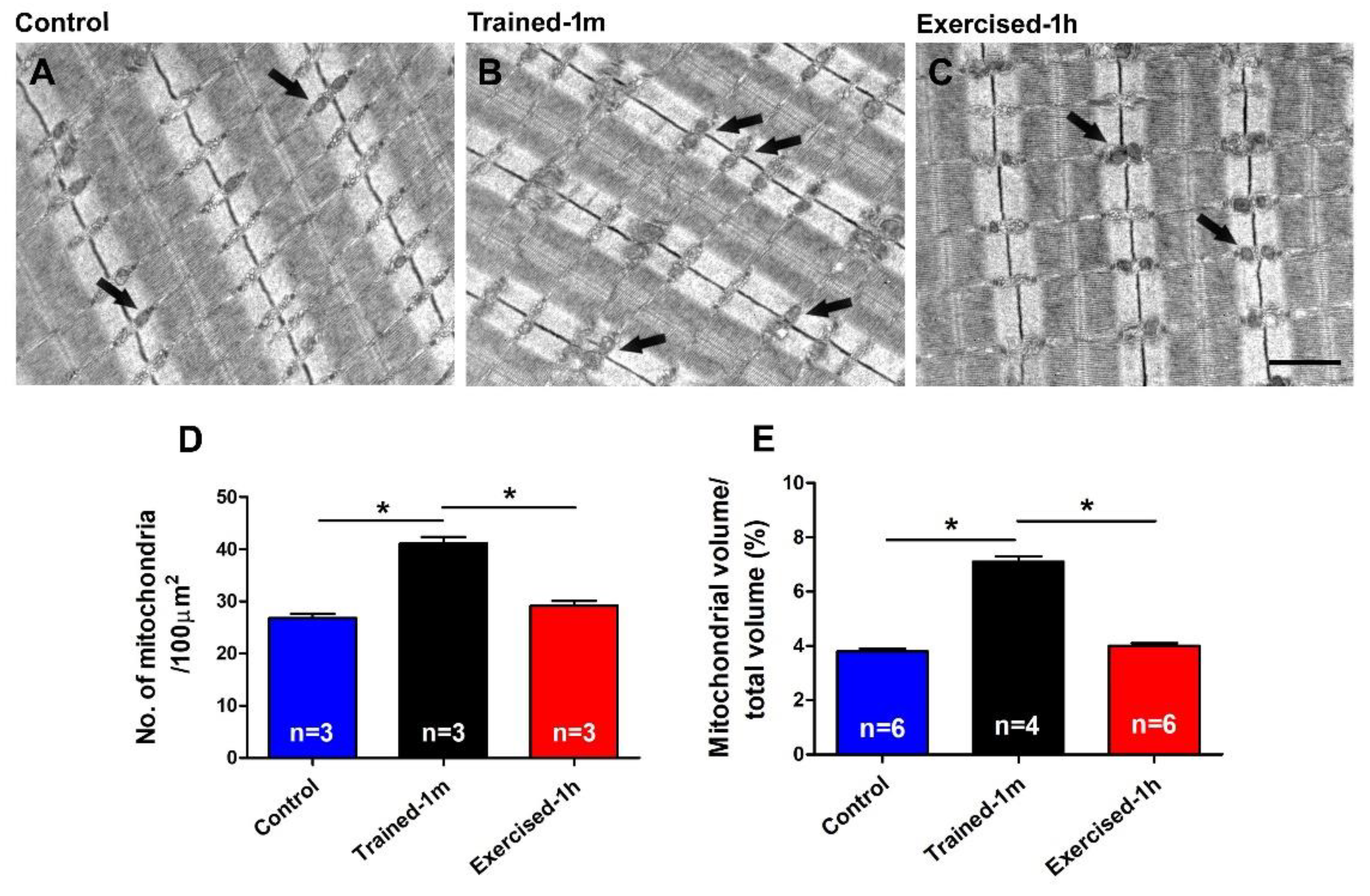
- the mice trained-1 m in wheel cages, when exposed to the ES protocol, reached a final temperature that is slightly higher than that of the mice exercised-1 h (Figure 1). This result is consistent both when measuring the internal temperature with a rectal thermometer and the cutaneous temperature with the infrared thermometer (compare Figure 1 and Supplementary Figure S2). To determine a possible explanation for this outcome, additional investigation is needed. However, one of the possible explanations is the fact that the muscle in the mice trained-1 m has a higher number/volume of mitochondria than in the other two groups (Figure 5). Indeed, it is well known that training increases mitochondrial volume and number [60,61,62,63,64] and that the augmented mitochondrial activity during aerobic respiration could generate additional heat [55,56].
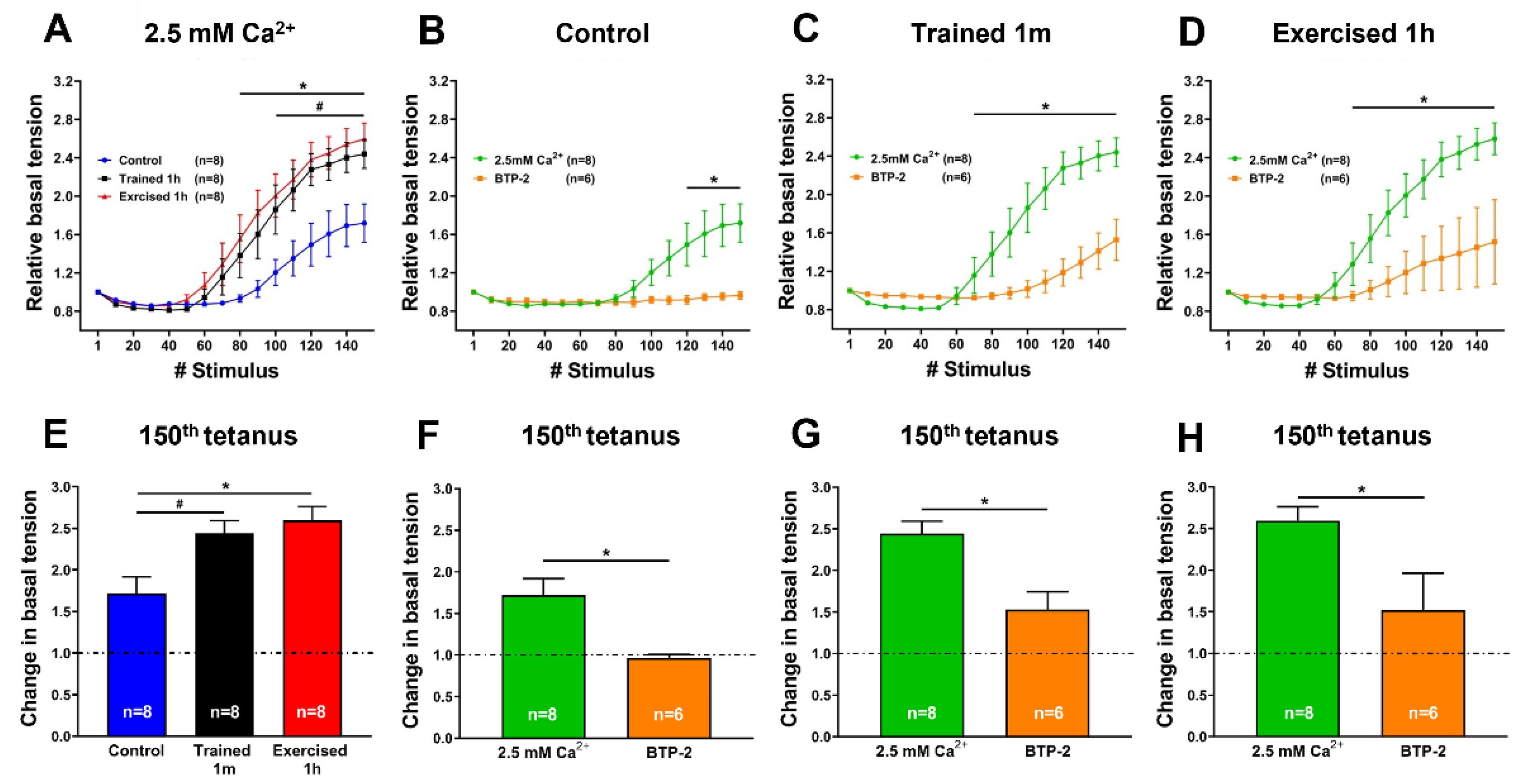
- In the ex-vivo heat-stress protocol (Supplementary Figure S3), we exposed EDL muscles to increasing steps of temperature to assess whether the temperature alone was a relevant factor in determining hyper-contracture of muscle. The results indicate that the EDL muscles dissected from mice exercised-1 h (by a treadmill run protocol which promotes the assembly of the CEUs; [44]) developed a greater basal tension compared to that of the controls, while the EDL muscles from trained-1 m mice did not behave differently from those of the controls. The explanation for this unexpected result could reside in the following observation: the extension of TTs at the I band is more pronounced in the mice exercised-1 h than in the mice trained-1 m (Figure 4). The reason for this could be the plasticity of TTs, which allows their retraction from the I band following exercise [49]. This considered, (A) EDL muscles from exercised-1 h mice were dissected for functional studies and EM immediately after the protocol of incremental running designed to induce fatigue and promote assembly of CEUs (as in [44,49]); in this case, the TTs did not have much time to retract before being dissected for experiments. (B) Alternatively, the muscles of mice trained-1 m were dissected from mice kept in cages equipped with running wheels and likely did not exercise intensively immediately before the collection of muscle samples for functional studies and EM. In the latter case, the TTs had enough time to retract, as shown by quantitative analysis in Figure 4. As the assembly of functional CEUs requires the presence of TTs at the I band and of their contact with SR-stacks [49], the EDL muscles dissected from mice trained-1 m are less predisposed (than muscles from mice exercised-1 h) to develop contractures solely from being exposed to heat (Supplementary Figure S3).
4. Materials and Methods
4.1. Animals
4.2. In Vivo-Experiments
4.3. In-Vitro Contracture Test (IVCT)
4.4. Preparation of Samples for Light (LM) and Electron Microscopy (EM)
5. Quantitative Analyses by Electron Microscopy (EM)
6. Quantitative Analysis by Histology
7. Statistical Analyses
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bouchama, A.; Knochel, J.P. Heat stroke. N. Engl. J. Med. 2002, 346, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.M.; Ellis, F.R.; Halsall, P.J. Evidence for related myopathies in exertional heat stroke and malignant hyperthermia. Lancet 1991, 338, 1491–1492. [Google Scholar] [CrossRef]
- Lehmann-Horn, F.; Klingler, W.; Jurkat-Rott, K. Nonanesthetic malignant hyperthermia. Anesthesiology 2011, 115, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, L.; Canini, F. On the nature of the link between malignant hyperthermia and exertional heatstroke. Med. Hypotheses 1995, 45, 268–270. [Google Scholar] [CrossRef]
- Denborough, M.; Hopkinson, K.C. Propofol and malignant hyperpyrexia. Lancet 1988, 1, 191. [Google Scholar] [CrossRef]
- MacLennan, D.H. The genetic basis of malignant hyperthermia. Trends Pharmacol. Sci. 1992, 13, 330–334. [Google Scholar] [CrossRef]
- Finsterer, J. Current concepts in malignant hyperthermia. J. Clin. Neuromuscul. Dis. 2002, 4, 64–74. [Google Scholar] [CrossRef]
- Glahn, K.P.; Ellis, F.R.; Halsall, P.J.; Muller, C.R.; Snoeck, M.M.; Urwyler, A.; Wappler, F. Recognizing and managing a malignant hyperthermia crisis: Guidelines from the European Malignant Hyperthermia Group. Br. J. Anaesth. 2010, 105, 417–420. [Google Scholar] [CrossRef]
- Iaizzo, P.A.; Wedel, D.J.; Gallagher, W.J. In vitro contracture testing for determination of susceptibility to malignant hyperthermia: A methodologic update. Mayo Clin. Proc. 1991, 66, 998–1004. [Google Scholar] [CrossRef]
- Wappler, F.; Roewer, N.; Kochling, A.; Scholz, J.; Steinfath, M.; Schulte am Esch, J. In vitro diagnosis of malignant hyperthermia susceptibility with ryanodine-induced contractures in human skeletal muscles. Anesth. Analg. 1996, 82, 1230–1236. [Google Scholar]
- Nelson, T.E. Abnormality in calcium release from skeletal sarcoplasmic reticulum of pigs susceptible to malignant hyperthermia. J. Clin. Investig. 1983, 72, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.E. SR function in malignant hyperthermia. Cell Calcium 1988, 9, 257–265. [Google Scholar] [CrossRef]
- Fill, M.; Stefani, E.; Nelson, T.E. Abnormal human sarcoplasmic reticulum Ca2+ release channels in malignant hyperthermic skeletal muscle. Biophys. J. 1991, 59, 1085–1090. [Google Scholar] [CrossRef]
- Mickelson, J.R.; Louis, C.F. Malignant hyperthermia: Excitation-contraction coupling, Ca2+ release channel, and cell Ca2+ regulation defects. Physiol. Rev. 1996, 76, 537–592. [Google Scholar] [CrossRef]
- Wu, S.; Ibarra, M.C.; Malicdan, M.C.; Murayama, K.; Ichihara, Y.; Kikuchi, H.; Nonaka, I.; Noguchi, S.; Hayashi, Y.K.; Nishino, I. Central core disease is due to RYR1 mutations in more than 90% of patients. Brain 2006, 129, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Galli, L.; Orrico, A.; Lorenzini, S.; Censini, S.; Falciani, M.; Covacci, A.; Tegazzin, V.; Sorrentino, V. Frequency and localization of mutations in the 106 exons of the RYR1 gene in 50 individuals with malignant hyperthermia. Hum. Mutat. 2006, 27, 830. [Google Scholar] [CrossRef]
- Rios, E.; Ma, J.J.; Gonzalez, A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J. Muscle Res. Cell Motil. 1991, 12, 127–135. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Protasi, F. Ryanodine receptors of striated muscles: A complex channel capable of multiple interactions. Physiol. Rev. 1997, 77, 699–729. [Google Scholar] [CrossRef]
- Nelson, T.E.; Bedell, D.M.; Jones, E.W. Porcine malignant hyperthermia: Effects of temperature and extracellular calcium concentration on halothane-induced contracture of susceptible skeletal muscle. Anesthesiology 1975, 42, 301–306. [Google Scholar] [CrossRef]
- Jones, E.W.; Nelson, T.E.; Anderson, I.L.; Kerr, D.D.; Burnap, T.K. Malignant hyperthermia of swine. Anesthesiology 1972, 36, 42–51. [Google Scholar] [CrossRef]
- Paolini, C.; Quarta, M.; Nori, A.; Boncompagni, S.; Canato, M.; Volpe, P.; Allen, P.D.; Reggiani, C.; Protasi, F. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J. Physiol. 2007, 583, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.G.; Goonasekera, S.A.; Durham, W.J.; Tang, W.; Lueck, J.D.; Riehl, J.; Pessah, I.N.; Zhang, P.; Bhattacharjee, M.B.; Dirksen, R.T.; et al. Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. FASEB J. 2006, 20, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Riehl, J.; Esteve, E.; Matthaei, K.I.; Goth, S.; Allen, P.D.; Pessah, I.N.; Lopez, J.R. Pharmacologic and functional characterization of malignant hyperthermia in the R163C RyR1 knock-in mouse. Anesthesiology 2006, 105, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Aracena-Parks, P.; Long, C.; Rossi, A.E.; Goonasekera, S.A.; Boncompagni, S.; Galvan, D.L.; Gilman, C.P.; Baker, M.R.; Shirokova, N.; et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 2008, 133, 53–65. [Google Scholar] [CrossRef]
- Dainese, M.; Quarta, M.; Lyfenko, A.D.; Paolini, C.; Canato, M.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Anesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice. FASEB J. 2009, 23, 1710–1720. [Google Scholar] [CrossRef]
- Protasi, F.; Paolini, C.; Dainese, M. Calsequestrin-1: A new candidate gene for malignant hyperthermia and exertional/environmental heat stroke. J. Physiol. 2009, 587, 3095–3100. [Google Scholar] [CrossRef]
- Michelucci, A.; Paolini, C.; Boncompagni, S.; Canato, M.; Reggiani, C.; Protasi, F. Strenuous exercise triggers a life-threatening response in mice susceptible to malignant hyperthermia. FASEB J. 2017, 31, 3649–3662. [Google Scholar] [CrossRef]
- Duke, A.M.; Hopkins, P.M.; Calaghan, S.C.; Halsall, J.P.; Steele, D.S. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J. Biol. Chem 2010, 285, 25645–25653. [Google Scholar] [CrossRef]
- Yarotskyy, V.; Protasi, F.; Dirksen, R.T. Accelerated activation of SOCE current in myotubes from two mouse models of anesthetic- and heat-induced sudden death. PLoS ONE 2013, 8, e77633. [Google Scholar] [CrossRef]
- Fletcher, J.E.; Huggins, F.J.; Rosenberg, H. The importance of calcium ions for in vitro malignant hyperthermia testing. Can. J. Anaesth. 1990, 37, 695–698. [Google Scholar] [CrossRef]
- Putney, J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Parekh, A.B.; Penner, R. Store depletion and calcium influx. Physiol. Rev. 1997, 77, 901–930. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, N.; Ogawa, Y. Calcium dynamics in skeletal muscle. Clin. Calcium 2001, 11, 1417–1423. [Google Scholar] [PubMed]
- Launikonis, B.S.; Barnes, M.; Stephenson, D.G. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2941–2944. [Google Scholar] [CrossRef] [PubMed]
- Launikonis, B.S.; Rios, E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J. Physiol. 2007, 583, 81–97. [Google Scholar] [CrossRef]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef]
- Lyfenko, A.D.; Dirksen, R.T. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J. Physiol. 2008, 586, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, R.T. Checking your SOCCs and feet: The molecular mechanisms of Ca2+ entry in skeletal muscle. J. Physiol. 2009, 587, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Wei-Lapierre, L.; Carrell, E.M.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 2013, 4, 2805. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Michelucci, A.; Pietrangelo, L.; Dirksen, R.T.; Protasi, F. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci. Rep. 2017, 7, 14286. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Yang, D.; Nagaraj, R.Y.; Nosek, T.A.; Nishi, M.; Takeshima, H.; Cheng, H.; Ma, J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat. Cell Biol. 2002, 4, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Carrell, E.M.; Coppola, A.R.; McBride, H.J.; Dirksen, R.T. Orai1 enhances muscle endurance by promoting fatigue-resistant type I fiber content but not through acute store-operated Ca2+ entry. FASEB J. 2016, 30, 4109–4119. [Google Scholar] [CrossRef]
- Boncompagni, S.; Michelucci, A.; Pietrangelo, L.; Dirksen, R.T.; Protasi, F. Addendum: Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci. Rep. 2018, 8, 17463. [Google Scholar] [CrossRef]
- Boncompagni, S.; Pecorai, C.; Michelucci, A.; Pietrangelo, L.; Protasi, F. Long-Term Exercise Reduces Formation of Tubular Aggregates and Promotes Maintenance of Ca(2+) Entry Units in Aged Muscle. Front. Physiol. 2021, 11, 601057. [Google Scholar] [CrossRef]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Garcia-Castaneda, M.; Takano, T.; Malik, S.; Dirksen, R.T.; Protasi, F. Transverse tubule remodeling enhances Orai1-dependent Ca(2+) entry in skeletal muscle. eLife 2019, 8, e47576. [Google Scholar] [CrossRef]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Takano, T.; Protasi, F.; Dirksen, R.T. Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice. J. Gen. Physiol. 2020, 152, e202012617. [Google Scholar] [CrossRef]
- Melzer, W. ECC meets CEU-New focus on the backdoor for calcium ions in skeletal muscle cells. J. Gen. Physiol. 2020, 152, e202012679. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Pietrangelo, L.; Boncompagni, S. Calcium entry units (CEUs): Perspectives in skeletal muscle function and disease. J. Muscle Res. Cell Motil. 2021, 42, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Canato, M.; Scorzeto, M.; Giacomello, M.; Protasi, F.; Reggiani, C.; Stienen, G.J. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc. Natl. Acad. Sci. USA 2010, 107, 22326–22331. [Google Scholar] [CrossRef] [PubMed]
- Zitt, C.; Strauss, B.; Schwarz, E.C.; Spaeth, N.; Rast, G.; Hatzelmann, A.; Hoth, M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 2004, 279, 12427–12437. [Google Scholar] [CrossRef]
- Krustrup, P.; Ferguson, R.A.; Kjaer, M.; Bangsbo, J. ATP and heat production in human skeletal muscle during dynamic exercise: Higher efficiency of anaerobic than aerobic ATP resynthesis. J. Physiol. 2003, 549, 255–269. [Google Scholar] [CrossRef]
- Willis, W.T.; Jackman, M.R. Mitochondrial function during heavy exercise. Med. Sci. Sports Exerc. 1994, 26, 1347–1353. [Google Scholar] [CrossRef]
- Pietrangelo, L.; D’Incecco, A.; Ainbinder, A.; Michelucci, A.; Kern, H.; Dirksen, R.T.; Boncompagni, S.; Protasi, F. Age-dependent uncoupling of mitochondria from Ca(2+) release units in skeletal muscle. Oncotarget 2015, 6, 35358–35371. [Google Scholar] [CrossRef]
- Pietrangelo, L.; Michelucci, A.; Ambrogini, P.; Sartini, S.; Guarnier, F.A.; Fusella, A.; Zamparo, I.; Mammucari, C.; Protasi, F.; Boncompagni, S. Muscle activity prevents the uncoupling of mitochondria from Ca(2+) Release Units induced by ageing and disuse. Arch. Biochem. Biophys. 2019, 663, 22–33. [Google Scholar] [CrossRef]
- Zampieri, S.; Pietrangelo, L.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Pond, A.; Grim-Stieger, M.; Cvecka, J.; Sedliak, M.; et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 163–173. [Google Scholar] [CrossRef]
- Vina, J.; Gomez-Cabrera, M.C.; Borras, C.; Froio, T.; Sanchis-Gomar, F.; Martinez-Bello, V.E.; Pallardo, F.V. Mitochondrial biogenesis in exercise and in ageing. Adv. Drug Deliv. Rev. 2009, 61, 1369–1374. [Google Scholar] [CrossRef]
- Derbre, F.; Gomez-Cabrera, M.C.; Nascimento, A.L.; Sanchis-Gomar, F.; Martinez-Bello, V.E.; Tresguerres, J.A.; Fuentes, T.; Gratas-Delamarche, A.; Monsalve, M.; Vina, J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1alpha to exercise training. Age 2012, 34, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2021, 599, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Lundby, C.; Jacobs, R.A. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Paolini, C.; Canato, M.; Wei-Lapierre, L.; Pietrangelo, L.; De Marco, A.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Antioxidants protect calsequestrin-1 knockout mice from halothane- and heat-induced sudden death. Anesthesiology 2015, 123, 603–617. [Google Scholar] [CrossRef]
- Adnet, P.J.; Krivosic-Horber, R.M.; Adamantidis, M.M.; Reyford, H.; Cordonnier, C.; Haudecoeur, G. Effects of calcium-free solution, calcium antagonists, and the calcium agonist BAY K 8644 on mechanical responses of skeletal muscle from patients susceptible to malignant hyperthermia. Anesthesiology 1991, 75, 413–419. [Google Scholar] [CrossRef]
- Loud, A.V.; Barany, W.C.; Pack, B.A. Quantitative Evaluation of Cytoplasmic Structures in Electron Micrographs. Lab. Investig. 1965, 14, 996–1008. [Google Scholar]
- Mobley, B.A.; Eisenberg, B.R. Sizes of components in frog skeletal muscle measured by methods of stereology. J. Gen. Physiol. 1975, 66, 31–45. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolami, B.; Serano, M.; Michelucci, A.; Pietrangelo, L.; Protasi, F. Store-Operated Ca2+ Entry in Skeletal Muscle Contributes to the Increase in Body Temperature during Exertional Stress. Int. J. Mol. Sci. 2022, 23, 3772. https://doi.org/10.3390/ijms23073772
Girolami B, Serano M, Michelucci A, Pietrangelo L, Protasi F. Store-Operated Ca2+ Entry in Skeletal Muscle Contributes to the Increase in Body Temperature during Exertional Stress. International Journal of Molecular Sciences. 2022; 23(7):3772. https://doi.org/10.3390/ijms23073772
Chicago/Turabian StyleGirolami, Barbara, Matteo Serano, Antonio Michelucci, Laura Pietrangelo, and Feliciano Protasi. 2022. "Store-Operated Ca2+ Entry in Skeletal Muscle Contributes to the Increase in Body Temperature during Exertional Stress" International Journal of Molecular Sciences 23, no. 7: 3772. https://doi.org/10.3390/ijms23073772
APA StyleGirolami, B., Serano, M., Michelucci, A., Pietrangelo, L., & Protasi, F. (2022). Store-Operated Ca2+ Entry in Skeletal Muscle Contributes to the Increase in Body Temperature during Exertional Stress. International Journal of Molecular Sciences, 23(7), 3772. https://doi.org/10.3390/ijms23073772





