Targeting of Proteins for Translocation at the Endoplasmic Reticulum
Abstract
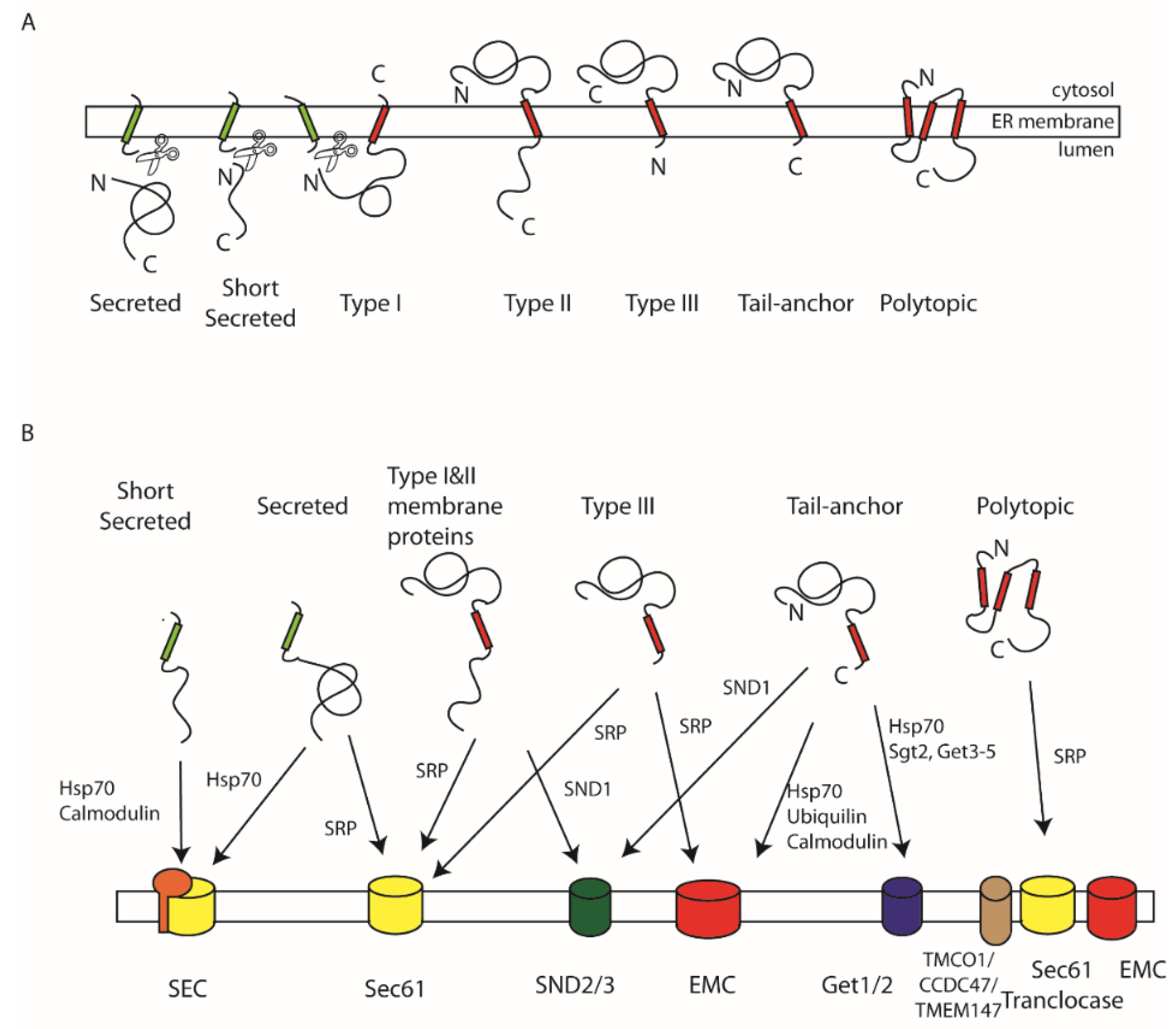
1. Targeting Signals
2. ER Protein Translocases and Insertases
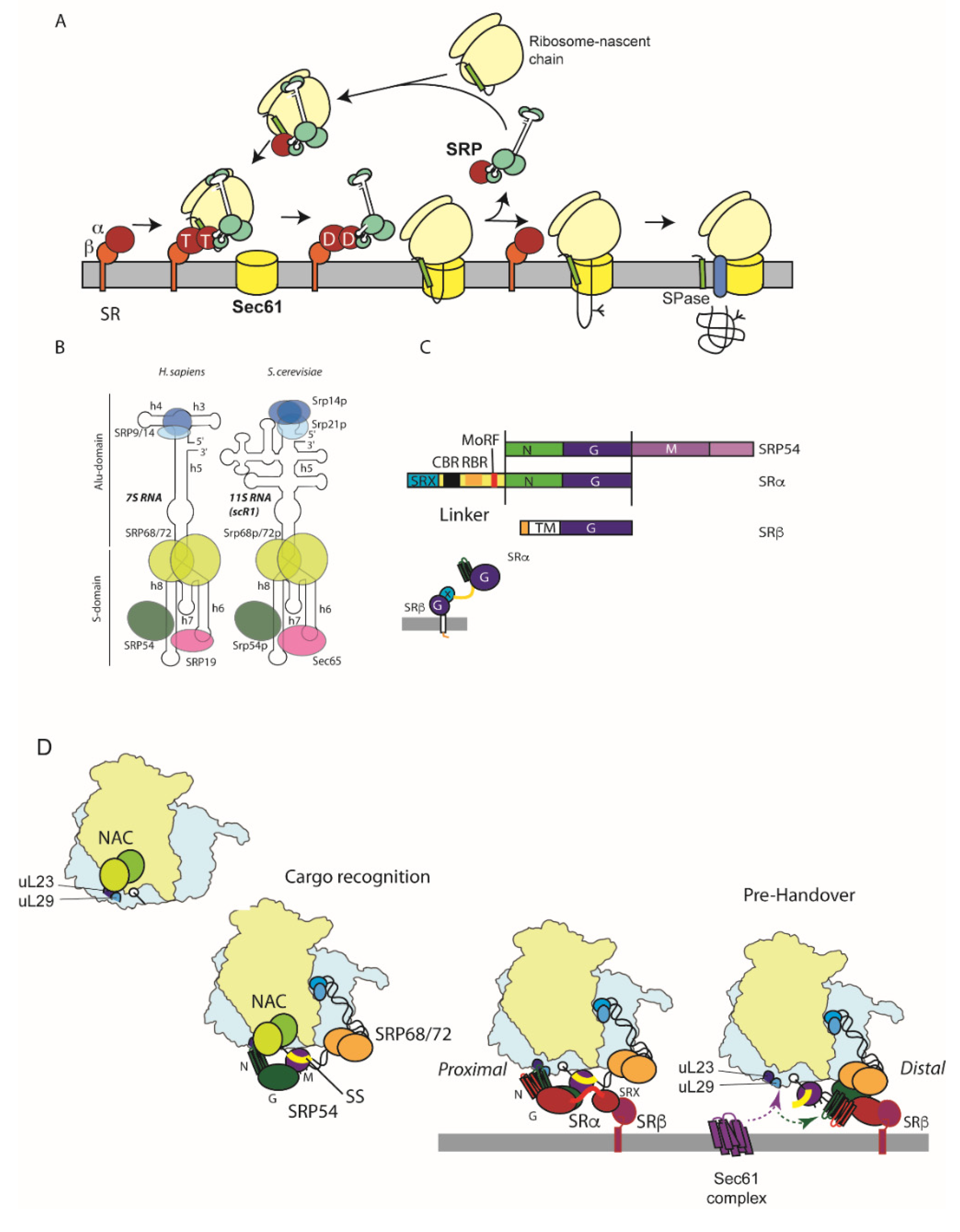
3. SRP-Dependent Targeting
4. SND-Targeting Pathway
5. Sec62-Dependent Targeting
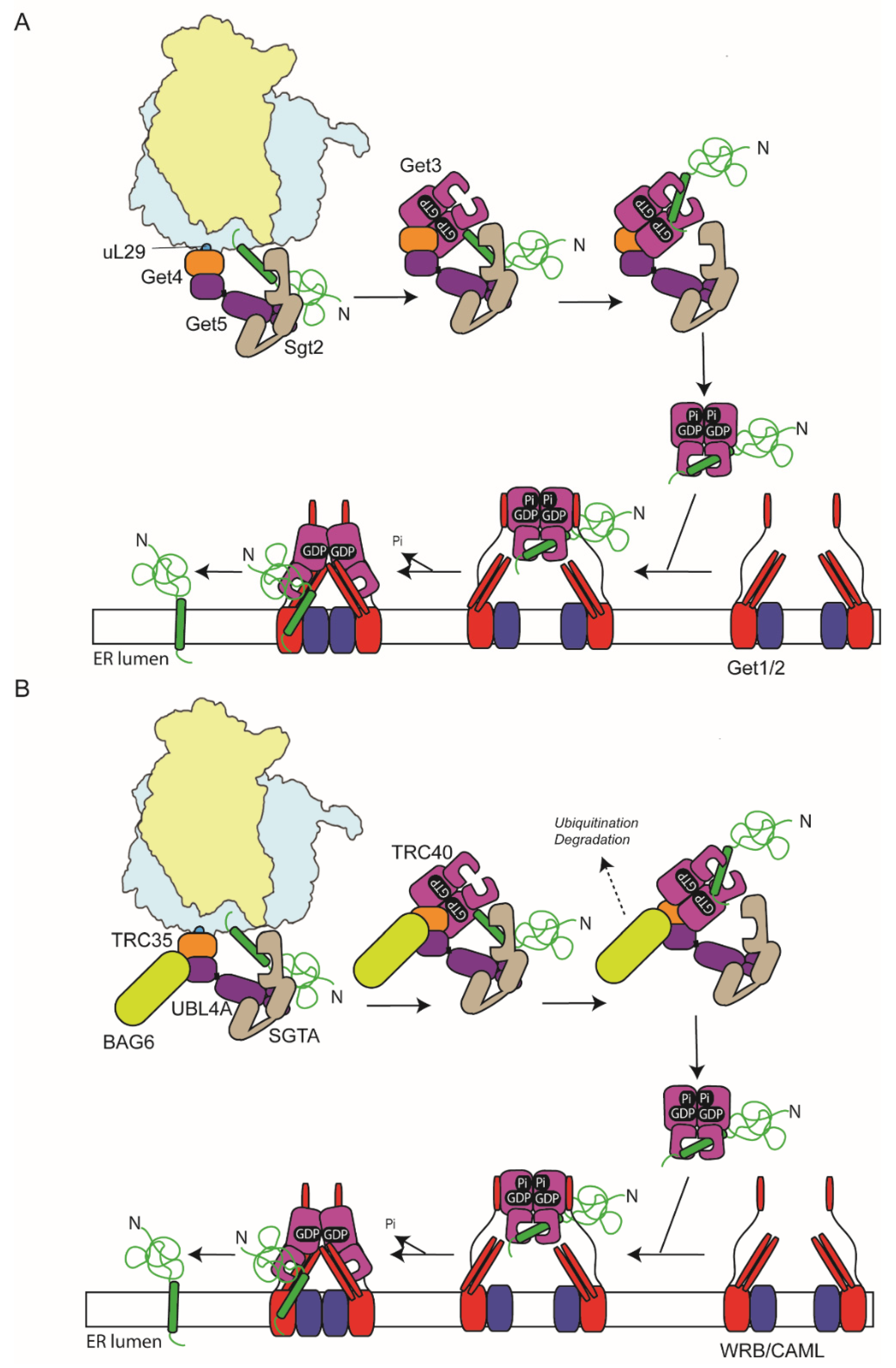
6. GET-Targeting Pathway
7. EMC Translocase
8. Defective ER Targeting and Human Disease
Future Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blobel, G.; Dobberstein, B. Transfer of proteins across membranes I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 1975, 67, 835–851. [Google Scholar] [CrossRef]
- Martoglio, B.; Dobberstein, B. Signal sequences: More than just greasy peptides. Trends Cell Biol. 1998, 8, 410–415. [Google Scholar] [CrossRef]
- Von Heijne, G. Signal sequences. The limits of variation. J. Mol. Biol. 1985, 184, 99–105. [Google Scholar] [CrossRef]
- Hegde, R.S.; Keenan, R.J. The mechanisms of integral membrane protein biogenesis. Nat. Rev. Mol. Cell Biol. 2021, 23, 107–124. [Google Scholar] [CrossRef]
- O’Keefe, S.; Pool, M.R.; High, S. Membrane protein biogenesis at the ER: The highways and byways. FEBS J. 2021. [Google Scholar] [CrossRef]
- Hegde, R.S.; Keenan, R.J. Tail-anchored membrane protein insertion into the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2011, 12, 787–798. [Google Scholar] [CrossRef]
- McGilvray, P.T.; Anghel, S.A.; Sundaram, A.; Zhong, F.; Trnka, M.J.; Fuller, J.R.; Hu, H.; Burlingame, A.L.; Keenan, R.J. An ER translocon for multi-pass membrane protein biogenesis. eLife 2020, 9, e56889. [Google Scholar] [CrossRef]
- Mateja, A.; Keenan, R.J. A structural perspective on tail-anchored protein biogenesis by the GET pathway. Curr. Opin. Struct. Biol. 2018, 51, 195–202. [Google Scholar] [CrossRef]
- Chitwood, P.J.; Hegde, R.S. The Role of EMC during Membrane Protein Biogenesis. Trends Cell Biol. 2019, 29, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Pool, M.R. Signal recognition particles in chloroplasts, bacteria, yeast and mammals (review). Mol. Membr. Biol. 2005, 22, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, R.M.; Hegde, R.S. Structures of the scanning and engaged states of the mammalian SRP-ribosome complex. eLife 2015, 4, e07975. [Google Scholar] [CrossRef]
- Walter, P.; Blobel, G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1980, 77, 7112–7116. [Google Scholar] [CrossRef]
- Meyer, D.I.; Dobberstein, B. Identification and characterization of a membrane component essential for the translocation of nascent secretory proteins across the membrane of the endoplasmic reticulum. J. Cell Biol. 1980, 87, 503–508. [Google Scholar] [CrossRef]
- Song, W.; Raden, D.; Mandon, E.C.; Gilmore, R. Role of Sec61a in the regulated transfer of the ribosome-nascent chain complex from the signal recognition particle to the translocation channel. Cell 2000, 100, 333–343. [Google Scholar] [CrossRef]
- Siegel, V.; Walter, P. Elongation arrest is not a prerequisite for secretory protein translocation across the microsomal membrane. J. Cell Biol. 1985, 100, 1913–1921. [Google Scholar] [CrossRef]
- Gundelfinger, E.D.; Krause, E.; Melli, M.; Dobberstein, B. The organization of the 7SL RNA in the signal recognition particle. Nucleic Acids Res. 1983, 11, 7363–7374. [Google Scholar] [CrossRef][Green Version]
- Brown, J.D.; Hann, B.C.; Medzihradszky, K.F.; Niwa, M.; Burlingame, A.L.; Walter, P. Subunits of the Saccharomyces cerevisiae signal recognition particle required for its functional expression. EMBO J. 1994, 13, 4390–4400. [Google Scholar] [CrossRef]
- Siegel, V.; Walter, P. Each of the activities of signal recognition particle (SRP) is contained within a distinct domain: Analysis of biochemical mutants of SRP. Cell 1988, 52, 39–49. [Google Scholar] [CrossRef]
- Ogg, S.C.; Barz, W.P.; Walter, P. A functional GTPase domain, but not its transmembrane domain, is required for function of the SRP receptor beta-subunit. J. Cell Biol. 1998, 142, 341–354. [Google Scholar] [CrossRef]
- Halic, M.; Becker, T.; Pool, M.R.; Spahn, C.M.; Grassucci, R.A.; Frank, J.; Beckmann, R. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature 2004, 427, 808–814. [Google Scholar] [CrossRef]
- Wild, K.; Becker, M.M.M.; Kempf, G.; Sinning, I. Structure, dynamics and interactions of large SRP variants. Biol. Chem. 2019, 401, 63–80. [Google Scholar] [CrossRef]
- Hainzl, T.; Sauer-Eriksson, A.E. Signal-sequence induced conformational changes in the signal recognition particle. Nat. Commun. 2015, 6, 7163. [Google Scholar] [CrossRef]
- Freymann, D.M.; Keenan, R.J.; Stroud, R.N.; Walter, P. Structure of the conserved GTPase domain of the signal recognition particle. Nature 1997, 385, 361–364. [Google Scholar] [CrossRef]
- Janda, C.Y.; Li, J.; Oubridge, C.; Hernandez, H.; Robinson, C.V.; Nagai, K. Recognition of a signal peptide by the signal recognition particle. Nature 2010, 465, 507–510. [Google Scholar] [CrossRef]
- Keenan, R.J.; Freymann, D.M.; Walter, P.; Stroud, R.M. Crystal structure of the signal sequence binding protein of the signal recognition particle. Cell 1998, 94, 181–191. [Google Scholar] [CrossRef]
- Hainzl, T.; Huang, S.; Sauer-Eriksson, A.E. Structure of the SRP19 RNA complex and implications for signal recognition particle assembly. Nature 2002, 417, 767–771. [Google Scholar] [CrossRef]
- Ogg, S.C.; Walter, P. SRP samples nascent chains for the presence of signal sequences by interacting with the ribosome at a discrete step during translation elongation. Cell 1995, 81, 1075–1084. [Google Scholar] [CrossRef]
- Flanagan, J.J.; Chen, J.C.; Miao, Y.; Shao, Y.; Lin, J.; Bock, P.E.; Johnson, A.E. SRP binds to ribosome-bound signal sequences with fluorescence-detected subnanomolar affinity that does not diminish as the nascent chain lengthens. J. Biol. Chem. 2003, 278, 18628–18637. [Google Scholar] [CrossRef]
- Chartron, J.W.; Hunt, K.C.; Frydman, J. Cotranslational signal-independent SRP preloading during membrane targeting. Nature 2016, 536, 224–228. [Google Scholar] [CrossRef]
- Pool, M.R.; Stumm, J.; Fulga, T.A.; Sinning, I.; Dobberstein, B. Distinct Modes of Signal Recognition Particle Interaction with the Ribosome. Science 2002, 297, 1345–1348. [Google Scholar] [CrossRef]
- Hauser, S.; Bacher, G.; Dobberstein, B.; Lütcke, H. A complex comprising the signal sequence binding protein and the SRP RNA promotes translocation of nascent proteins. EMBO J. 1995, 14, 5485–5493. [Google Scholar] [CrossRef]
- Dalley, J.A.; Selkirk, A.; Pool, M.R. Access to Ribosomal Protein Rpl25p by the Signal Recognition Particle Is Required for Efficient Cotranslational Translocation. Mol. Biol. Cell 2008, 19, 2876–2884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mason, N.; Ciufo, L.F.; Brown, J.D. Elongation arrest is a physiologically important function of the signal recognition particle. EMBO J. 2000, 19, 4164–4174. [Google Scholar] [CrossRef]
- Lakkaraju, A.K.; Mary, C.; Scherrer, A.; Johnson, A.E.; Strub, K. SRP keeps polypeptides translocation-competent by slowing translation to match limiting ER-targeting sites. Cell 2008, 133, 440–451. [Google Scholar] [CrossRef]
- Pechmann, S.; Chartron, J.W.; Frydman, J. Local slowdown of translation by nonoptimal codons promotes nascent-chain recognition by SRP in vivo. Nat. Struct. Mol. Biol. 2014, 21, 1100–1105. [Google Scholar] [CrossRef]
- Bange, G.; Sinning, I. SIMIBI twins in protein targeting and localization. Nat. Struct. Mol. Biol. 2013, 20, 776–780. [Google Scholar] [CrossRef]
- Montoya, G.; Svensson, C.; Luirink, J.; Sinning, I. Crytsal structure of the NG domain from the signal recognition particle receptor FtsY. Nature 1997, 385, 365–368. [Google Scholar] [CrossRef]
- Young, J.C.; Ursini, J.; Legate, K.R.; Miller, J.D.; Walter, P.; Andrews, D.W. An amino-terminal domain containing hydrophobic and hydrophilic sequences binds the signal recognition particle receptor a subunit to the b subunit on the endoplasmic reticulum membrane. J. Biol. Chem. 1995, 270, 15650–15657. [Google Scholar] [CrossRef]
- Schwartz, T.; Blobel, G. Structural basis for the function of the beta subunit of the eukaryotic signal recognition particle receptor. Cell 2003, 112, 793–803. [Google Scholar] [CrossRef]
- Schlenker, O.; Hendricks, A.; Sinning, I.; Wild, K. The structure of the mammalian signal recognition particle (SRP) receptor as prototype for the interaction of small GTPases with Longin domains. J. Biol. Chem. 2006, 281, 8898–8906. [Google Scholar] [CrossRef]
- Egea, P.F.; Shan, S.O.; Napetschnig, J.; Savage, D.F.; Walter, P.; Stroud, R.M. Substrate twinning activates the signal recognition particle and its receptor. Nature 2004, 427, 215–221. [Google Scholar] [CrossRef]
- Focia, P.J.; Shepotinovskaya, I.V.; Seidler, J.A.; Freymann, D.M. Heterodimeric GTPase core of the SRP targeting complex. Science 2004, 303, 373–377. [Google Scholar] [CrossRef]
- Kobayashi, K.; Jomaa, A.; Lee, J.H.; Chandrasekar, S.; Boehringer, D.; Shan, S.O.; Ban, N. Structure of a prehandover mammalian ribosomal SRP.SRP receptor targeting complex. Science 2018, 360, 323–327. [Google Scholar] [CrossRef]
- Jomaa, A.; Eitzinger, S.; Zhu, Z.; Chandrasekar, S.; Kobayashi, K.; Shan, S.O.; Ban, N. Molecular mechanism of cargo recognition and handover by the mammalian signal recognition particle. Cell Rep. 2021, 36, 109350. [Google Scholar] [CrossRef]
- Hwang Fu, Y.H.; Chandrasekar, S.; Lee, J.H.; Shan, S.O. A molecular recognition feature mediates ribosome-induced SRP-receptor assembly during protein targeting. J. Cell Biol. 2019, 218, 3307–3319. [Google Scholar] [CrossRef]
- Peluso, P.; Herschlag, D.; Nock, S.; Freymann, D.; Johnson, A.E.; Walter, P. Role of the 4.5S RNA in Assembly of the Bacterial Signal Recognition Particle with its Receptor. Science 2000, 288, 1640–1643. [Google Scholar] [CrossRef]
- Bradshaw, N.; Neher, S.B.; Booth, D.S.; Walter, P. Signal sequences activate the catalytic switch of SRP RNA. Science 2009, 323, 127–130. [Google Scholar] [CrossRef]
- Neher, S.B.; Bradshaw, N.; Floor, S.N.; Gross, J.D.; Walter, P. SRP RNA controls a conformational switch regulating the SRP-SRP receptor interaction. Nat. Struct. Mol. Biol. 2008, 15, 916–923. [Google Scholar] [CrossRef]
- Lee, J.H.; Jomaa, A.; Chung, S.; Hwang Fu, Y.H.; Qian, R.; Sun, X.; Hsieh, H.H.; Chandrasekar, S.; Bi, X.; Mattei, S.; et al. Receptor compaction and GTPase rearrangement drive SRP-mediated cotranslational protein translocation into the ER. Sci. Adv. 2021, 7, eabg0942. [Google Scholar] [CrossRef]
- Halic, M.; Gartmann, M.; Schlenker, O.; Mielke, T.; Pool, M.R.; Sinning, I.; Beckmann, R. Signal Recognition Particle Receptor Exposes the Ribosomal Translocon Binding Site. Science 2006, 312, 745–747. [Google Scholar] [CrossRef]
- Lee, J.H.; Chandrasekar, S.; Chung, S.; Hwang Fu, Y.H.; Liu, D.; Weiss, S.; Shan, S.O. Sequential activation of human signal recognition particle by the ribosome and signal sequence drives efficient protein targeting. Proc. Natl. Acad. Sci. USA 2018, 115, E5487–E5496. [Google Scholar] [CrossRef]
- Jomaa, A.; Fu, Y.H.; Boehringer, D.; Leibundgut, M.; Shan, S.O.; Ban, N. Structure of the quaternary complex between SRP, SR, and translocon bound to the translating ribosome. Nat. Commun. 2017, 8, 15470. [Google Scholar] [CrossRef] [PubMed]
- Connolly, T.; Rapiejko, P.J.; Gilmore, R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science 1991, 252, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.H.; Lee, J.H.; Chandrasekar, S.; Shan, S.O. A ribosome-associated chaperone enables substrate triage in a cotranslational protein targeting complex. Nat. Commun. 2020, 11, 5840. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, B.; Sakai, H.; Davis, T.A.; Wiedmann, M. A protein complex required for signal-sequence-specific sorting and translocation. Nature 1994, 370, 434–440. [Google Scholar] [CrossRef]
- Nyathi, Y.; Pool, M.R. Analysis of the interplay of protein biogenesis factors at the ribosome exit site reveals new role for NAC. J. Cell Biol. 2015, 210, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Gamerdinger, M.; Kobayashi, K.; Wallisch, A.; Kreft, S.G.; Sailer, C.; Schlomer, R.; Sachs, N.; Jomaa, A.; Stengel, F.; Ban, N.; et al. Early Scanning of Nascent Polypeptides inside the Ribosomal Tunnel by NAC. Mol. Cell 2019, 75, 996–1006. [Google Scholar] [CrossRef]
- Del Alamo, M.; Hogan, D.J.; Pechmann, S.; Albanese, V.; Brown, P.O.; Frydman, J. Defining the specificity of cotranslationally acting chaperones by systematic analysis of mRNAs associated with ribosome-nascent chain complexes. PLoS Biol. 2011, 9, e1001100. [Google Scholar] [CrossRef]
- Schibich, D.; Gloge, F.; Pohner, I.; Bjorkholm, P.; Wade, R.C.; von Heijne, G.; Bukau, B.; Kramer, G. Global profiling of SRP interaction with nascent polypeptides. Nature 2016, 536, 219–223. [Google Scholar] [CrossRef]
- Costa, E.A.; Subramanian, K.; Nunnari, J.; Weissman, J.S. Defining the physiological role of SRP in protein-targeting efficiency and specificity. Science 2018, 359, 689–692. [Google Scholar] [CrossRef]
- Matsuo, Y.; Inada, T. The ribosome collision sensor Hel2 functions as preventive quality control in the secretory pathway. Cell Rep. 2021, 34, 108877. [Google Scholar] [CrossRef] [PubMed]
- Hann, B.C.; Walter, P. The Signal Recognition Particle in S. cerevisiae. Cell 1991, 67, 131–144. [Google Scholar] [CrossRef]
- Aviram, N.; Ast, T.; Costa, E.A.; Arakel, E.C.; Chuartzman, S.G.; Jan, C.H.; Hassdenteufel, S.; Dudek, J.; Jung, M.; Schorr, S.; et al. The SND proteins constitute an alternative targeting route to the endoplasmic reticulum. Nature 2016, 540, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Hassdenteufel, S.; Sicking, M.; Schorr, S.; Aviram, N.; Fecher-Trost, C.; Schuldiner, M.; Jung, M.; Zimmermann, R.; Lang, S. hSnd2 protein represents an alternative targeting factor to the endoplasmic reticulum in human cells. FEBS Lett. 2017, 591, 3211–3224. [Google Scholar] [CrossRef]
- Yang, J.; Hirata, T.; Liu, Y.S.; Guo, X.Y.; Gao, X.D.; Kinoshita, T.; Fujita, M. Human SND2 mediates ER targeting of GPI-anchored proteins with low hydrophobic GPI attachment signals. FEBS Lett. 2021, 595, 1542–1558. [Google Scholar] [CrossRef]
- Deshaies, R.J.; Schekman, R. SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J. Cell Biol. 1989, 109, 2644–2653. [Google Scholar] [CrossRef]
- Deshaies, R.J.; Sanders, S.L.; Feldheim, D.A.; Schekman, R. Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature 1991, 349, 806–808. [Google Scholar] [CrossRef]
- Stirling, C.J.; Rothblatt, J.; Hosobuchi, M.; Deshaies, R.; Schekman, R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol. Biol. Cell 1992, 3, 129–142. [Google Scholar] [CrossRef]
- Ng, D.T.; Brown, J.D.; Walter, P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J. Cell Biol. 1996, 134, 269–278. [Google Scholar] [CrossRef]
- Waters, M.G.; Blobel, G. Secretory protein translocation in a yeast cell-free system can occur post-translationally and requires ATP-hydrolysis. J. Cell Biol. 1986, 102, 1543–1550. [Google Scholar] [CrossRef]
- Prinz, A.; Behrens, C.; Rapoport, T.A.; Hartmann, E.; Kalies, K.U. Evolutionarily conserved binding of ribosomes to the translocation channel via the large ribosomal RNA. EMBO J. 2000, 19, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.A.; Grau, H.; Kraft, R.; Kostka, S.; Prehn, S.; Kalies, K.U.; Hartmann, E. Mammalian Sec61 is associated with Sec62 and Sec63. J. Biol. Chem. 2000, 275, 14550–14557. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.H.; Steinchen, W.; Beatrix, B.; Berninghausen, O.; Becker, T.; Bange, G.; Cheng, J.; Beckmann, R. Architecture of the active post-translational Sec translocon. EMBO J. 2021, 40, e105643. [Google Scholar] [CrossRef]
- Chirico, W.J.; Waters, M.G.; Blobel, G. 70 K heat shock related proteins stimulate protein translocation into microsomes. Nature 1988, 332, 805–810. [Google Scholar] [CrossRef]
- Ngosuwan, J.; Wang, N.M.; Fung, K.L.; Chirico, W.J. Roles of cytosolic Hsp70 and Hsp40 molecular chaperones in post-translational translocation of presecretory proteins into the endoplasmic reticulum. J. Biol. Chem. 2003, 278, 7034–7042. [Google Scholar] [CrossRef]
- Tripathi, A.; Mandon, E.C.; Gilmore, R.; Rapoport, T.T.A. Two alternative binding mechanisms connect the protein translocation Sec71-Sec72 complex with heat shock proteins. J. Biol. Chem. 2017, 292, 8007–8018. [Google Scholar] [CrossRef] [PubMed]
- Jan, C.H.; Williams, C.C.; Weissman, J.S. Principles of ER cotranslational translocation revealed by proximity-specific ribosome profiling. Science 2014, 346, 1257521. [Google Scholar] [CrossRef] [PubMed]
- Tyedmers, J.; Lerner, M.; Bies, C.; Dudek, J.; Skowronek, M.H.; Haas, I.G.; Heim, N.; Nastainczyk, W.; Volkmer, J.; Zimmermann, R. Homologs of the yeast Sec complex subunits Sec62p and Sec63p are abundant proteins in dog pancreas microsomes. Proc. Natl. Acad. Sci. USA 2000, 97, 7214–7219. [Google Scholar] [CrossRef]
- Muller, L.; de Escauriaza, M.D.; Lajoie, P.; Theis, M.; Jung, M.; Muller, A.; Burgard, C.; Greiner, M.; Snapp, E.L.; Dudek, J.; et al. Evolutionary gain of function for the ER membrane protein Sec62 from yeast to humans. Mol. Biol. Cell 2010, 21, 691–703. [Google Scholar] [CrossRef]
- Müller, G.; Zimmermann, R. Import of honeybee prepromelittin into the endoplasmic reticulum: Structural basis for independence of SRP and docking protein. EMBO J. 1987, 6, 2099–2107. [Google Scholar] [CrossRef]
- Lakkaraju, A.K.; Thankappan, R.; Mary, C.; Garrison, J.L.; Taunton, J.; Strub, K. Efficient secretion of small proteins in mammalian cells relies on Sec62-dependent posttranslational translocation. Mol. Biol. Cell 2012, 23, 2712–2722. [Google Scholar] [CrossRef]
- Lang, S.; Benedix, J.; Fedeles, S.V.; Schorr, S.; Schirra, C.; Schauble, N.; Jalal, C.; Greiner, M.; Hassdenteufel, S.; Tatzelt, J.; et al. Different effects of Sec61alpha, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J. Cell Sci. 2012, 125, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Hassdenteufel, S.; Theis, M.; Paton, A.W.; Paton, J.C.; Zimmermann, R.; High, S. The signal sequence influences post-translational ER translocation at distinct stages. PLoS ONE 2013, 8, e75394. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Vilardi, F.; Lang, S.; Leznicki, P.; Zimmermann, R.; High, S. TRC40 can deliver short secretory proteins to the Sec61 translocon. J. Cell Sci. 2012, 125, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Hegde, R.S. A calmodulin-dependent translocation pathway for small secretory proteins. Cell 2011, 147, 1576–1588. [Google Scholar] [CrossRef]
- Schorr, S.; Nguyen, D.; Hassdenteufel, S.; Nagaraj, N.; Cavalie, A.; Greiner, M.; Weissgerber, P.; Loi, M.; Paton, A.W.; Paton, J.C.; et al. Identification of signal peptide features for substrate specificity in human Sec62/Sec63-dependent ER protein import. FEBS J. 2020, 287, 4612–4640. [Google Scholar] [CrossRef]
- Reithinger, J.H.; Kim, J.E.; Kim, H. Sec62 protein mediates membrane insertion and orientation of moderately hydrophobic signal anchor proteins in the endoplasmic reticulum (ER). J. Biol. Chem. 2013, 288, 18058–18067. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, J.E.; Reithinger, J.H.; Kim, H. The Sec62-Sec63 translocon facilitates translocation of the C-terminus of membrane proteins. J. Cell Sci. 2014, 127, 4270–4278. [Google Scholar] [CrossRef]
- Kutay, U.; Ahnert-Hilger, G.; Hartmann, E.; Wiedenmann, B.; Rapoport, T.A. Transport route for synaptobrevin via a novel transport pathway of insertion into the endoplasmic reticulum membrane. EMBO J. 1995, 14, 217–223. [Google Scholar] [CrossRef]
- Shan, S.O. Guiding tail-anchored membrane proteins to the endoplasmic reticulum in a chaperone cascade. J. Biol. Chem. 2019, 294, 16577–16586. [Google Scholar] [CrossRef]
- Borgese, N.; Coy-Vergara, J.; Colombo, S.F.; Schwappach, B. The Ways of Tails: The GET Pathway and more. Protein J. 2019, 38, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Jonikas, M.C.; Collins, S.R.; Denic, V.; Oh, E.; Quan, E.M.; Schmid, V.; Weibezahn, J.; Schwappach, B.; Walter, P.; Weissman, J.S.; et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 2009, 323, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Brown, E.C.; Mak, G.; Zhuang, J.; Denic, V. A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol. Cell 2010, 40, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, M.; Metz, J.; Schmid, V.; Denic, V.; Rakwalska, M.; Schmitt, H.D.; Schwappach, B.; Weissman, J.S. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 2008, 134, 634–645. [Google Scholar] [CrossRef]
- Stefanovic, S.; Hegde, R.S. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell 2007, 128, 1147–1159. [Google Scholar] [CrossRef]
- Favaloro, V.; Spasic, M.; Schwappach, B.; Dobberstein, B. Distinct targeting pathways for the membrane insertion of tail-anchored (TA) proteins. J. Cell Sci. 2008, 121, 1832–1840. [Google Scholar] [CrossRef]
- Mariappan, M.; Li, X.; Stefanovic, S.; Sharma, A.; Mateja, A.; Keenan, R.J.; Hegde, R.S. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature 2010, 466, 1120–1124. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sakisaka, T. Molecular machinery for insertion of tail-anchored membrane proteins into the endoplasmic reticulum membrane in mammalian cells. Mol. Cell 2012, 48, 387–397. [Google Scholar] [CrossRef]
- Shao, S.; Rodrigo-Brenni, M.C.; Kivlen, M.H.; Hegde, R.S. Mechanistic basis for a molecular triage reaction. Science 2017, 355, 298–302. [Google Scholar] [CrossRef]
- Leznicki, P.; High, S. SGTA antagonizes BAG6-mediated protein triage. Proc. Natl. Acad. Sci. USA 2012, 109, 19214–19219. [Google Scholar] [CrossRef]
- Leznicki, P.; High, S. SGTA associates with nascent membrane protein precursors. EMBO Rep. 2020, 21, e48835. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Shichino, Y.; Schneider-Poetsch, T.; Mito, M.; Hashimoto, S.; Udagawa, T.; Kohno, K.; Yoshida, M.; Mishima, Y.; Inada, T.; et al. Genome-wide Survey of Ribosome Collision. Cell Rep. 2020, 31, 107610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; De Laurentiis, E.; Bohnsack, K.E.; Wahlig, M.; Ranjan, N.; Gruseck, S.; Hackert, P.; Wolfle, T.; Rodnina, M.V.; Schwappach, B.; et al. Ribosome-bound Get4/5 facilitates the capture of tail-anchored proteins by Sgt2 in yeast. Nat. Commun. 2021, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Berndt, U.; Golz, H.; Tais, A.; Oellerer, S.; Wolfle, T.; Fitzke, E.; Rospert, S. NAC functions as a modulator of SRP during the early steps of protein targeting to the endoplasmic reticulum. Mol. Biol. Cell 2012, 23, 3027–3040. [Google Scholar] [CrossRef]
- Berndt, U.; Oellerer, S.; Zhang, Y.; Johnson, A.E.; Rospert, S. A signal-anchor sequence stimulates signal recognition particle binding to ribosomes from inside the exit tunnel. Proc. Natl. Acad. Sci. USA 2009, 106, 1398–1403. [Google Scholar] [CrossRef]
- Cho, H.; Shan, S.O. Substrate relay in an Hsp70-cochaperone cascade safeguards tail-anchored membrane protein targeting. EMBO J. 2018, 37, e99264. [Google Scholar] [CrossRef]
- Mateja, A.; Szlachcic, A.; Downing, M.E.; Dobosz, M.; Mariappan, M.; Hegde, R.S.; Keenan, R.J. The structural basis of tail-anchored membrane protein recognition by Get3. Nature 2009, 461, 361–366. [Google Scholar] [CrossRef]
- Bozkurt, G.; Stjepanovic, G.; Vilardi, F.; Amlacher, S.; Wild, K.; Bange, G.; Favaloro, V.; Rippe, K.; Hurt, E.; Dobberstein, B.; et al. Structural insights into tail-anchored protein binding and membrane insertion by Get3. Proc. Natl. Acad. Sci. USA 2009, 106, 21131–21136. [Google Scholar] [CrossRef]
- Chartron, J.W.; Suloway, C.J.; Zaslaver, M.; Clemons, W.M., Jr. Structural characterization of the Get4/Get5 complex and its interaction with Get3. Proc. Natl. Acad. Sci. USA 2010, 107, 12127–12132. [Google Scholar] [CrossRef]
- Rome, M.E.; Rao, M.; Clemons, W.M.; Shan, S.O. Precise timing of ATPase activation drives targeting of tail-anchored proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 7666–7671. [Google Scholar] [CrossRef]
- Chio, U.S.; Chung, S.; Weiss, S.; Shan, S.O. A Chaperone Lid Ensures Efficient and Privileged Client Transfer during Tail-Anchored Protein Targeting. Cell Rep. 2019, 26, 37–44. [Google Scholar] [CrossRef]
- Zalisko, B.E.; Chan, C.; Denic, V.; Rock, R.S.; Keenan, R.J. Tail-Anchored Protein Insertion by a Single Get1/2 Heterodimer. Cell Rep. 2017, 20, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Stefer, S.; Reitz, S.; Wang, F.; Wild, K.; Pang, Y.Y.; Schwarz, D.; Bomke, J.; Hein, C.; Lohr, F.; Bernhard, F.; et al. Structural basis for tail-anchored membrane protein biogenesis by the Get3-receptor complex. Science 2011, 333, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Whynot, A.; Tung, M.; Denic, V. The mechanism of tail-anchored protein insertion into the ER membrane. Mol. Cell 2011, 43, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, M.; Mateja, A.; Dobosz, M.; Bove, E.; Hegde, R.S.; Keenan, R.J. The mechanism of membrane-associated steps in tail-anchored protein insertion. Nature 2011, 477, 61–66. [Google Scholar] [CrossRef]
- McDowell, M.A.; Heimes, M.; Fiorentino, F.; Mehmood, S.; Farkas, A.; Coy-Vergara, J.; Wu, D.; Bolla, J.R.; Schmid, V.; Heinze, R.; et al. Structural Basis of Tail-Anchored Membrane Protein Biogenesis by the GET Insertase Complex. Mol. Cell 2020, 80, 72–86. [Google Scholar] [CrossRef]
- Guna, A.; Volkmar, N.; Christianson, J.C.; Hegde, R.S. The ER membrane protein complex is a transmembrane domain insertase. Science 2018, 359, 470–473. [Google Scholar] [CrossRef]
- Volkmar, N.; Thezenas, M.L.; Louie, S.M.; Juszkiewicz, S.; Nomura, D.K.; Hegde, R.S.; Kessler, B.M.; Christianson, J.C. The ER membrane protein complex promotes biogenesis of sterol-related enzymes maintaining cholesterol homeostasis. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef]
- Abell, B.M.; Pool, M.R.; Schlenker, O.; Sinning, I.; High, S. Signal recognition particle mediates post-translational targeting in eukaryotes. EMBO J. 2004, 23, 2755–2764. [Google Scholar] [CrossRef]
- Shurtleff, M.J.; Itzhak, D.N.; Hussmann, J.A.; Schirle Oakdale, N.T.; Costa, E.A.; Jonikas, M.; Weibezahn, J.; Popova, K.D.; Jan, C.H.; Sinitcyn, P.; et al. The ER membrane protein complex interacts cotranslationally to enable biogenesis of multipass membrane proteins. eLife 2018, 7, e37018. [Google Scholar] [CrossRef]
- O’Keefe, S.; Zong, G.; Duah, K.B.; Andrews, L.E.; Shi, W.Q.; High, S. An alternative pathway for membrane protein biogenesis at the endoplasmic reticulum. Commun. Biol. 2021, 4, 828. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, P.J.; Juszkiewicz, S.; Guna, A.; Shao, S.; Hegde, R.S. EMC Is Required to Initiate Accurate Membrane Protein Topogenesis. Cell 2018, 175, 1507–1519.e16. [Google Scholar] [CrossRef] [PubMed]
- Miller-Vedam, L.E.; Brauning, B.; Popova, K.D.; Schirle Oakdale, N.T.; Bonnar, J.L.; Prabu, J.R.; Boydston, E.A.; Sevillano, N.; Shurtleff, M.J.; Stroud, R.M.; et al. Structural and mechanistic basis of the EMC-dependent biogenesis of distinct transmembrane clients. eLife 2020, 9, e62611. [Google Scholar] [CrossRef] [PubMed]
- Spiller, M.P.; Stirling, C.J. Preferential targeting of a signal recognition particle-dependent precursor to the Ssh1p translocon in yeast. J. Biol. Chem. 2011, 286, 21953–21960. [Google Scholar] [CrossRef] [PubMed]
- Carapito, R.; Konantz, M.; Paillard, C.; Miao, Z.; Pichot, A.; Leduc, M.S.; Yang, Y.; Bergstrom, K.L.; Mahoney, D.H.; Shardy, D.L.; et al. Mutations in signal recognition particle SRP54 cause syndromic neutropenia with Shwachman-Diamond-like features. J. Clin. Investig. 2017, 127, 4090–4103. [Google Scholar] [CrossRef]
- Bellanne-Chantelot, C.; Schmaltz-Panneau, B.; Marty, C.; Fenneteau, O.; Callebaut, I.; Clauin, S.; Docet, A.; Damaj, G.L.; Leblanc, T.; Pellier, I.; et al. Mutations in the SRP54 gene cause severe congenital neutropenia as well as Shwachman-Diamond-like syndrome. Blood 2018, 132, 1318–1331. [Google Scholar] [CrossRef]
- Juaire, K.D.; Lapouge, K.; Becker, M.M.M.; Kotova, I.; Michelhans, M.; Carapito, R.; Wild, K.; Bahram, S.; Sinning, I. Structural and Functional Impact of SRP54 Mutations Causing Severe Congenital Neutropenia. Structure 2021, 29, 15–28.e7. [Google Scholar] [CrossRef]
- Schurch, C.; Schaefer, T.; Muller, J.S.; Hanns, P.; Arnone, M.; Dumlin, A.; Scharer, J.; Sinning, I.; Wild, K.; Skokowa, J.; et al. SRP54 mutations induce congenital neutropenia via dominant-negative effects on XBP1 splicing. Blood 2021, 137, 1340–1352. [Google Scholar] [CrossRef]
- Kanda, S.; Yanagitani, K.; Yokota, Y.; Esaki, Y.; Kohno, K. Autonomous translational pausing is required for XBP1u mRNA recruitment to the ER via the SRP pathway. Proc. Natl. Acad. Sci. USA 2016, 113, E5886–E5895. [Google Scholar] [CrossRef]
- Schmaltz-Panneau, B.; Pagnier, A.; Clauin, S.; Buratti, J.; Marty, C.; Fenneteau, O.; Dieterich, K.; Beaupain, B.; Donadieu, J.; Plo, I.; et al. Identification of biallelic germline variants of SRP68 in a sporadic case with severe congenital neutropenia. Haematologica 2021, 106, 1216–1219. [Google Scholar] [CrossRef]
- Kirwan, M.; Walne, A.J.; Plagnol, V.; Velangi, M.; Ho, A.; Hossain, U.; Vulliamy, T.; Dokal, I. Exome sequencing identifies autosomal-dominant SRP72 mutations associated with familial aplasia and myelodysplasia. Am. J. Hum. Genet. 2012, 90, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Karamyshev, A.L.; Tikhonova, E.B.; Karamysheva, Z.N. Translational Control of Secretory Proteins in Health and Disease. Int. J. Mol. Sci. 2020, 21, 2538. [Google Scholar] [CrossRef] [PubMed]
- Karamyshev, A.L.; Patrick, A.E.; Karamysheva, Z.N.; Griesemer, D.S.; Hudson, H.; Tjon-Kon-Sang, S.; Nilsson, I.; Otto, H.; Liu, Q.; Rospert, S.; et al. Inefficient SRP interaction with a nascent chain triggers a mRNA quality control pathway. Cell 2014, 156, 146–157. [Google Scholar] [CrossRef]
- Tikhonova, E.B.; Karamysheva, Z.N.; von Heijne, G.; Karamyshev, A.L. Silencing of Aberrant Secretory Protein Expression by Disease-Associated Mutations. J. Mol. Biol. 2019, 431, 2567–2580. [Google Scholar] [CrossRef]
- Pinarbasi, E.S.; Karamyshev, A.L.; Tikhonova, E.B.; Wu, I.H.; Hudson, H.; Thomas, P.J. Pathogenic Signal Sequence Mutations in Progranulin Disrupt SRP Interactions Required for mRNA Stability. Cell Rep. 2018, 23, 2844–2851. [Google Scholar] [CrossRef]
- Saarela, J.; von Schantz, C.; Peltonen, L.; Jalanko, A. A novel aspartylglucosaminuria mutation affects translocation of aspartylglucosaminidase. Hum. Mutat. 2004, 24, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Seppen, J.; Steenken, E.; Lindhout, D.; Bosma, P.J.; Elferink, R.P. A mutation which disrupts the hydrophobic core of the signal peptide of bilirubin UDP-glucuronosyltransferase, an endoplasmic reticulum membrane protein, causes Crigler-Najjar type II. FEBS Lett. 1996, 390, 294–298. [Google Scholar] [CrossRef]
- Donnarumma, M.; Regis, S.; Tappino, B.; Rosano, C.; Assereto, S.; Corsolini, F.; Di Rocco, M.; Filocamo, M. Molecular analysis and characterization of nine novel CTSK mutations in twelve patients affected by pycnodysostosis. Mutation in brief #961. Online. Hum. Mutat. 2007, 28, 524. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pool, M.R. Targeting of Proteins for Translocation at the Endoplasmic Reticulum. Int. J. Mol. Sci. 2022, 23, 3773. https://doi.org/10.3390/ijms23073773
Pool MR. Targeting of Proteins for Translocation at the Endoplasmic Reticulum. International Journal of Molecular Sciences. 2022; 23(7):3773. https://doi.org/10.3390/ijms23073773
Chicago/Turabian StylePool, Martin R. 2022. "Targeting of Proteins for Translocation at the Endoplasmic Reticulum" International Journal of Molecular Sciences 23, no. 7: 3773. https://doi.org/10.3390/ijms23073773
APA StylePool, M. R. (2022). Targeting of Proteins for Translocation at the Endoplasmic Reticulum. International Journal of Molecular Sciences, 23(7), 3773. https://doi.org/10.3390/ijms23073773




