Differential Oxygen Exposure Modulates Mesenchymal Stem Cell Metabolism and Proliferation through mTOR Signaling
Abstract
:1. Introduction
2. Results
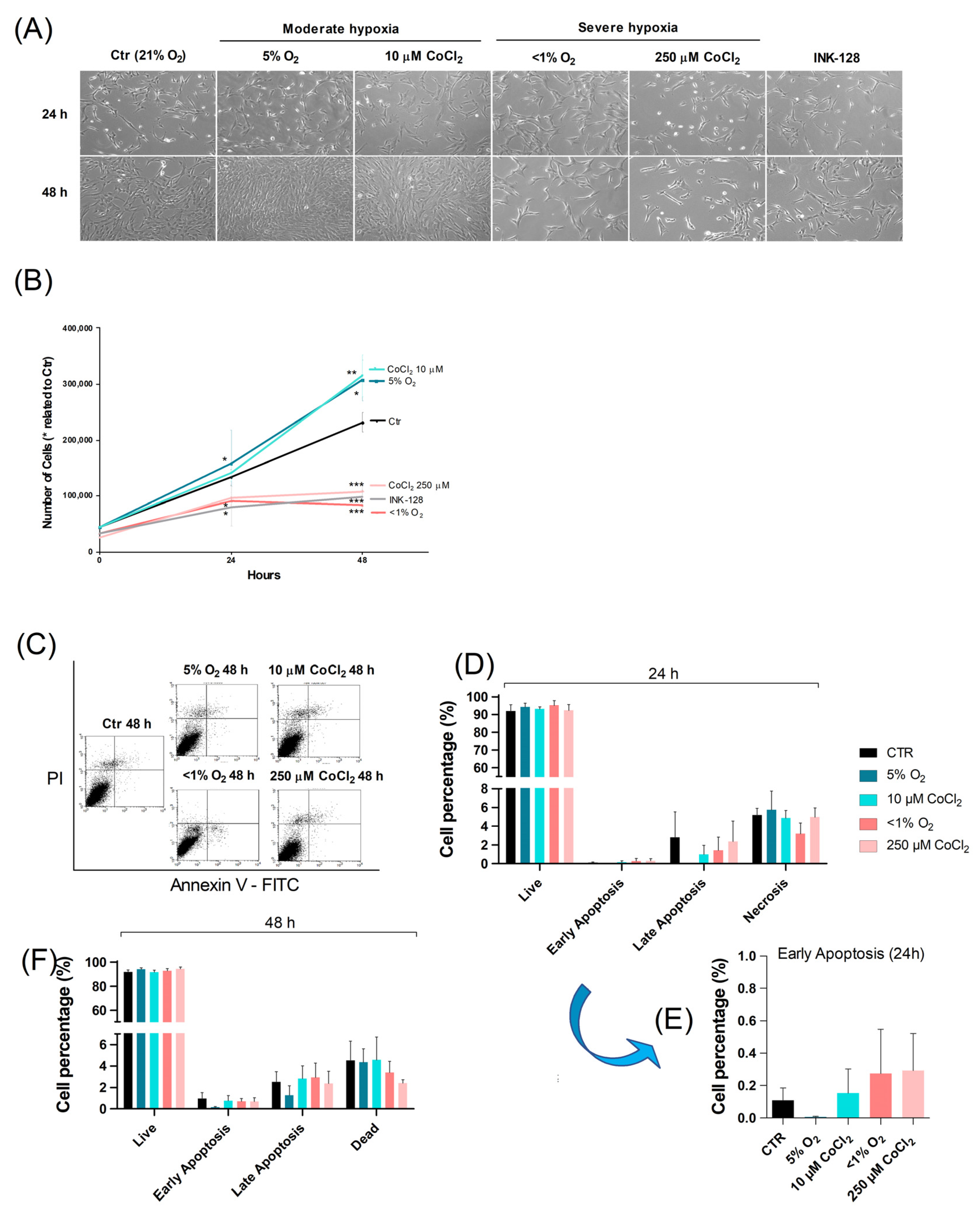
2.1. Severe Hypoxia Promotes a Quiescence-Like State
2.2. Oxidative Phosphorylation Shifts toward Glycolysis in Severe Hypoxia
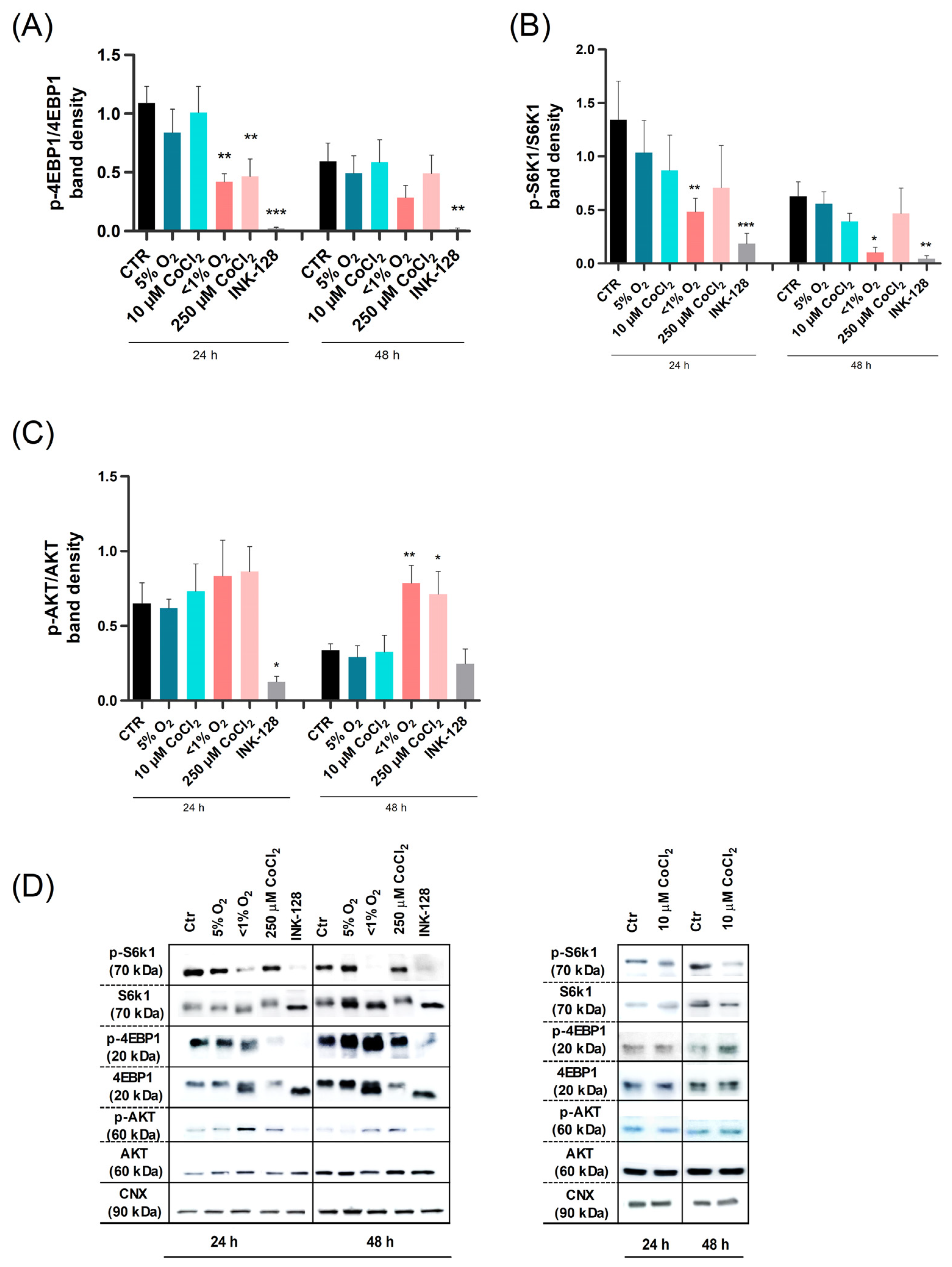
2.3. Severe Hypoxia Influences mTOR Signaling
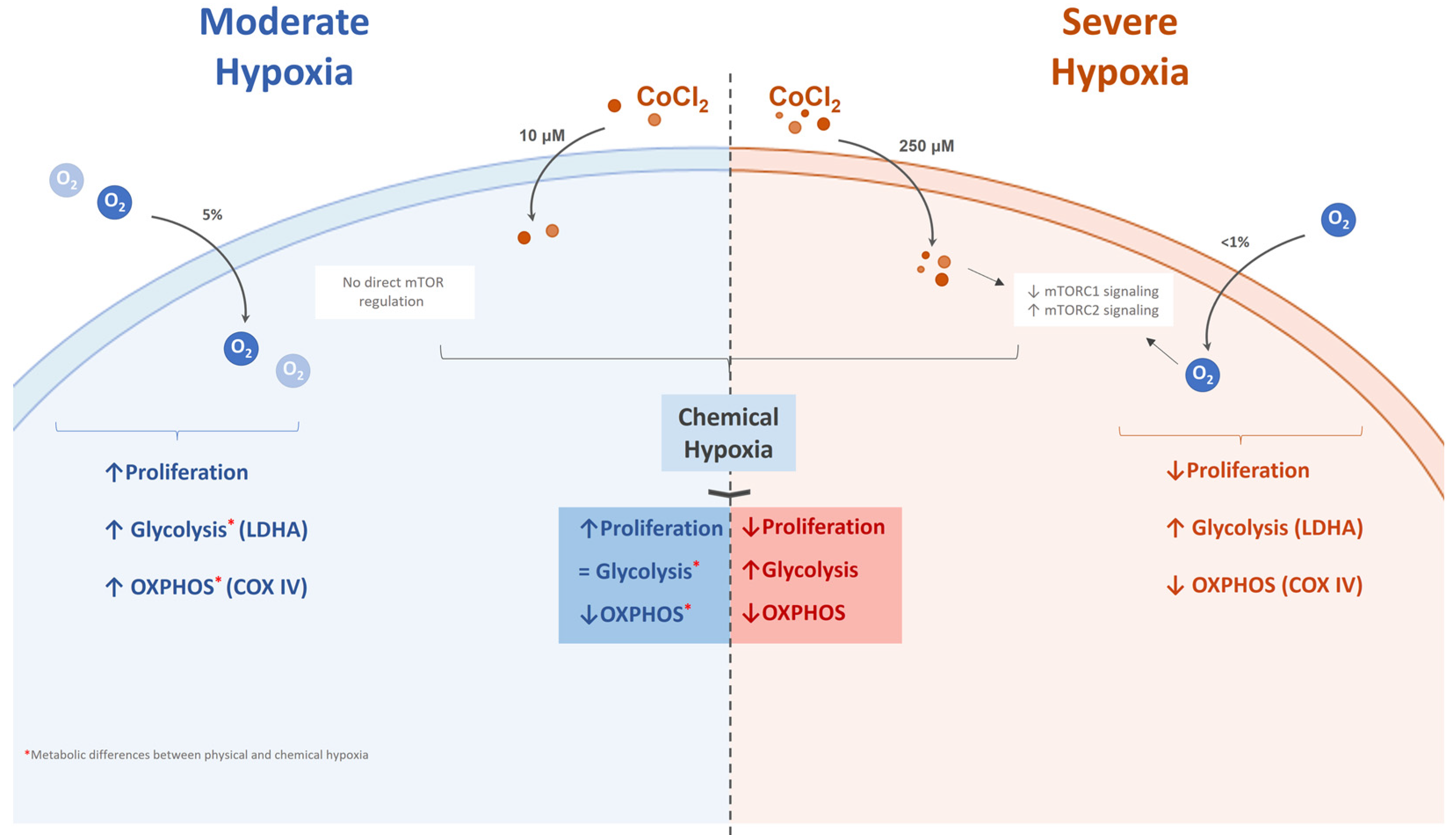
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Hypoxic Treatment and Experimental Design
4.3. Live Cell Imaging, Cell Growth and Cell Viability
4.4. Flow Cytometry
4.5. Western Blotting
4.6. Live Cell Metabolic Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, L.; Castaldi, M.A.; Rosamilio, R.; Ragni, E.; Vitolo, R.; Fulgione, C.; Castaldi, S.G.; Serio, B.; Bianco, R.; Guida, M.; et al. Mesenchymal Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential. Int. J. Stem Cells 2019, 12, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why Is the Partial Oxygen Pressure of Human Tissues a Crucial Parameter? Small Molecules and Hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Golinska, M.; Griffiths, J.R. HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells. Cells 2021, 10, 2371. [Google Scholar] [CrossRef]
- Drela, K.; Sarnowska, A.; Siedlecka, P.; Szablowska-Gadomska, I.; Wielgos, M.; Jurga, M.; Lukomska, B.; Domanska-Janik, K. Low Oxygen Atmosphere Facilitates Proliferation and Maintains Undifferentiated State of Umbilical Cord Mesenchymal Stem Cells in an Hypoxia Inducible Factor-Dependent Manner. Cytotherapy 2014, 16, 881–892. [Google Scholar] [CrossRef]
- Obradovic, H.; Krstic, J.; Trivanovic, D.; Mojsilovic, S.; Okic, I.; Kukolj, T.; Ilic, V.; Jaukovic, A.; Terzic, M.; Bugarski, D. Improving Stemness and Functional Features of Mesenchymal Stem Cells from Wharton’s Jelly of a Human Umbilical Cord by Mimicking the Native, Low Oxygen Stem Cell Niche. Placenta 2019, 82, 25–34. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int. J. Mol. Sci. 2017, 18, 1320. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, H.; Kwon, S.; Choi, S.; Oh, S.; Ryu, G.H.; Jeon, H.B.; Chang, J.W. Pressure Stimuli Improve the Proliferation of Wharton’s Jelly-Derived Mesenchymal Stem Cells under Hypoxic Culture Conditions. Int. J. Mol. Sci. 2020, 21, 7092. [Google Scholar] [CrossRef]
- Nekanti, U.; Dastidar, S.; Venugopal, P.; Totey, S.; Ta, M. Increased Proliferation and Analysis of Differential Gene Expression in Human Wharton’s Jelly-Derived Mesenchymal Stromal Cells under Hypoxia. Int. J. Biol. Sci. 2010, 6, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Ye, H.; Yang, A. Mathematical Modelling of Interacting Mechanisms for Hypoxia Mediated Cell Cycle Commitment for Mesenchymal Stromal Cells. BMC Syst. Biol. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.M.; Wang, Y.; Mathieu, J.; Margaretha, L.; Song, C.; Jones, D.C.; Cavanaugh, C.; Miklas, J.W.; Mahen, E.; Showalter, M.R.; et al. Metabolic Control over MTOR-Dependent Diapause-like State. Dev. Cell 2020, 52, 236–250.e7. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, J.; Lefèvre, P.; Banerjee, A.; Murphy, B. Regulation of Diapause in Carnivores. Reprod. Domest. Anim. 2017, 52, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tümpel, S.; Rudolph, K.L. Quiescence: Good and Bad of Stem Cell Aging. Trends Cell Biol. 2019, 29, 672–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laplante, M.; Sabatini, D.M. MTOR Signaling at a Glance. J. Cell Sci. 2009, 122, 3589–3594. Available online: https://journals.biologists.com/jcs/article/122/20/3589/30940/mTOR-signaling-at-a-glance (accessed on 18 March 2022). [CrossRef] [PubMed] [Green Version]
- Correia, B.; Sousa, M.I.; Ramalho-Santos, J. The MTOR Pathway in Reproduction: From Gonadal Function to Developmental Coordination. Reproduction 2020, 159, R173–R188. [Google Scholar] [CrossRef]
- Coller, H.A. The Paradox of Metabolism in Quiescent Stem Cells. FEBS Lett. 2019, 593, 2817–2839. [Google Scholar] [CrossRef]
- Sousa, M.I.; Correia, B.; Rodrigues, A.S.; Ramalho-Santos, J. Metabolic Characterization of a Paused-like Pluripotent State. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2020, 1864, 129612. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Shaw, G.; Duffy, N.; Islam, M.N.; O’Shea, P.M.; O’Toole, D.; Howard, L.; Murphy, J.M. Survival/Adaptation of Bone Marrow-Derived Mesenchymal Stem Cells After Long-Term Starvation Through Selective Processes. Stem Cells 2019, 37, 813–827. [Google Scholar] [CrossRef]
- Bhandi, S.; Al Kahtani, A.; Mashyakhy, M.; Alsofi, L.; Maganur, P.C.; Vishwanathaiah, S.; Testarelli, L.; Del Giudice, A.; Mehta, D.; Vyas, N.; et al. Modulation of the Dental Pulp Stem Cell Secretory Profile by Hypoxia Induction Using Cobalt Chloride. J. Pers. Med. 2021, 11, 247. [Google Scholar] [CrossRef]
- Valcourt, J.; Lemons, J.; Haley, E.; Kojima, M.; Demuren, O.; Coller, H.A. Staying Alive: Metabolic adaptations to quiescence. Cell Cycle 2012, 11, 1680–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the MTOR Complex 1 Pathway by Nutrients, Growth Factors, and Stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, W.-K.; Cheung, T.H. Molecular Regulation of Cellular Quiescence: A Perspective from Adult Stem Cells and Its Niches. In Methods in Molecular Biologyl; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1686, pp. 1–25. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The Use of Cobalt Chloride as a Chemical Hypoxia Model. J. Appl. Toxicol. 2019, 39, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-L.; Zhong, Q.; Qin, Y.-L.; Bu, Q.-Q.; Han, X.-A.; Jia, H.-T.; Liu, H.-W. Hypoxia-Mimetic Agents Inhibit Proliferation and Alter the Morphology of Human Umbilical Cord-Derived Mesenchymal Stem Cells. BMC Cell Biol. 2011, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Cao, Y.; Zhao, Q.; Li, J.; Zhou, C.; Liao, L.; Jia, M.; Zhao, Q.; Cai, H.; Han, Z.C.; et al. Proliferation and Differentiation of Bone Marrow Stromal Cells under Hypoxic Conditions. Biochem. Biophys. Res. Commun. 2006, 347, 12–21. [Google Scholar] [CrossRef]
- Pacary, E.; Legros, H.; Valable, S.; Duchatelle, P.; Lecocq, M.; Petit, E.; Nicole, O.; Bernaudin, M. Synergistic Effects of CoCl2 and ROCK Inhibition on Mesenchymal Stem Cell Differentiation into Neuron-like Cells. J. Cell Sci. 2006, 119, 2667–2678. [Google Scholar] [CrossRef] [Green Version]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 Mediates Adaptation to Hypoxia by Actively Downregulating Mitochondrial Oxygen Consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Dynamic Regulation of Stem Cell Specification and Maintenance by Hypoxia-Inducible Factors. Mol. Asp. Med. 2016, 47–48, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.S.; Correia, M.; Gomes, A.; Pereira, S.L.; Perestrelo, T.; Sousa, M.I.; Ramalho-Santos, J. Dichloroacetate, the Pyruvate Dehydrogenase Complex and the Modulation of MESC Pluripotency. PLoS ONE 2015, 10, e0131663. [Google Scholar] [CrossRef]
- Kim, J.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Sart, S.; Song, L.; Li, Y. Controlling Redox Status for Stem Cell Survival, Expansion, and Differentiation. Oxid. Med. Cell. Longev. 2015, 2015, 105135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scognamiglio, R.; Cabezas-Wallscheid, N.; Thier, M.C.; Altamura, S.; Reyes, A.; Prendergast, Á.M.; Baumgärtner, D.; Carnevalli, L.S.; Atzberger, A.; Haas, S.; et al. Myc Depletion Induces a Pluripotent Dormant State Mimicking Diapause. Cell 2016, 164, 668–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, K.; Jolly, M.K. Acute vs. Chronic vs. Cyclic Hypoxia: Their Differential Dynamics, Molecular Mechanisms, and Effects on Tumor Progression. Biomolecules 2019, 9, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulut-Karslioglu, A.; Biechele, S.; Jin, H.; Macrae, T.A.; Hejna, M.; Gertsenstein, M.; Song, J.S.; Ramalho-Santos, M. Inhibition of mTOR induces a paused pluripotent state. Nature 2016, 540, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, J.H.; Allgeier, D.L. Safe Self-Contained Carbon Dioxide-Hydrogen Anaerobic System. Appl. Microbiol. 1966, 14, 985–988. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Paradyse, A.; Ferrick, D.A.; Murphy, A.N.; Jastroch, M. Chapter Sixteen—Analysis and Interpretation of Microplate-Based Oxygen Consumption and PH Data. In Methods in Enzymology; Murphy, A.N., Chan, D.C., Eds.; Mitochondrial Function; Academic Press: New York, NY, USA, 2014; Volume 547, pp. 309–354. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moniz, I.; Ramalho-Santos, J.; Branco, A.F. Differential Oxygen Exposure Modulates Mesenchymal Stem Cell Metabolism and Proliferation through mTOR Signaling. Int. J. Mol. Sci. 2022, 23, 3749. https://doi.org/10.3390/ijms23073749
Moniz I, Ramalho-Santos J, Branco AF. Differential Oxygen Exposure Modulates Mesenchymal Stem Cell Metabolism and Proliferation through mTOR Signaling. International Journal of Molecular Sciences. 2022; 23(7):3749. https://doi.org/10.3390/ijms23073749
Chicago/Turabian StyleMoniz, Inês, João Ramalho-Santos, and Ana F. Branco. 2022. "Differential Oxygen Exposure Modulates Mesenchymal Stem Cell Metabolism and Proliferation through mTOR Signaling" International Journal of Molecular Sciences 23, no. 7: 3749. https://doi.org/10.3390/ijms23073749
APA StyleMoniz, I., Ramalho-Santos, J., & Branco, A. F. (2022). Differential Oxygen Exposure Modulates Mesenchymal Stem Cell Metabolism and Proliferation through mTOR Signaling. International Journal of Molecular Sciences, 23(7), 3749. https://doi.org/10.3390/ijms23073749






