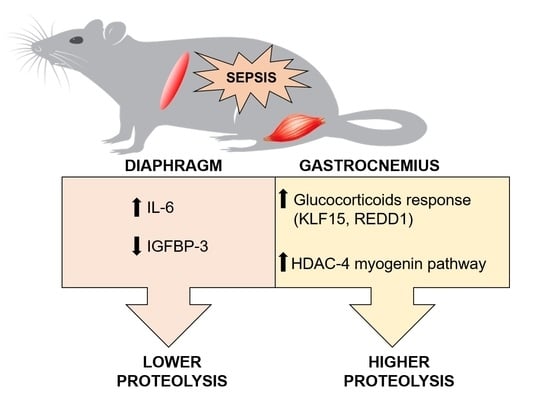
Role of Glucocorticoid Signaling and HDAC4 Activation in Diaphragm and Gastrocnemius Proteolytic Activity in Septic Rats
Abstract
1. Introduction
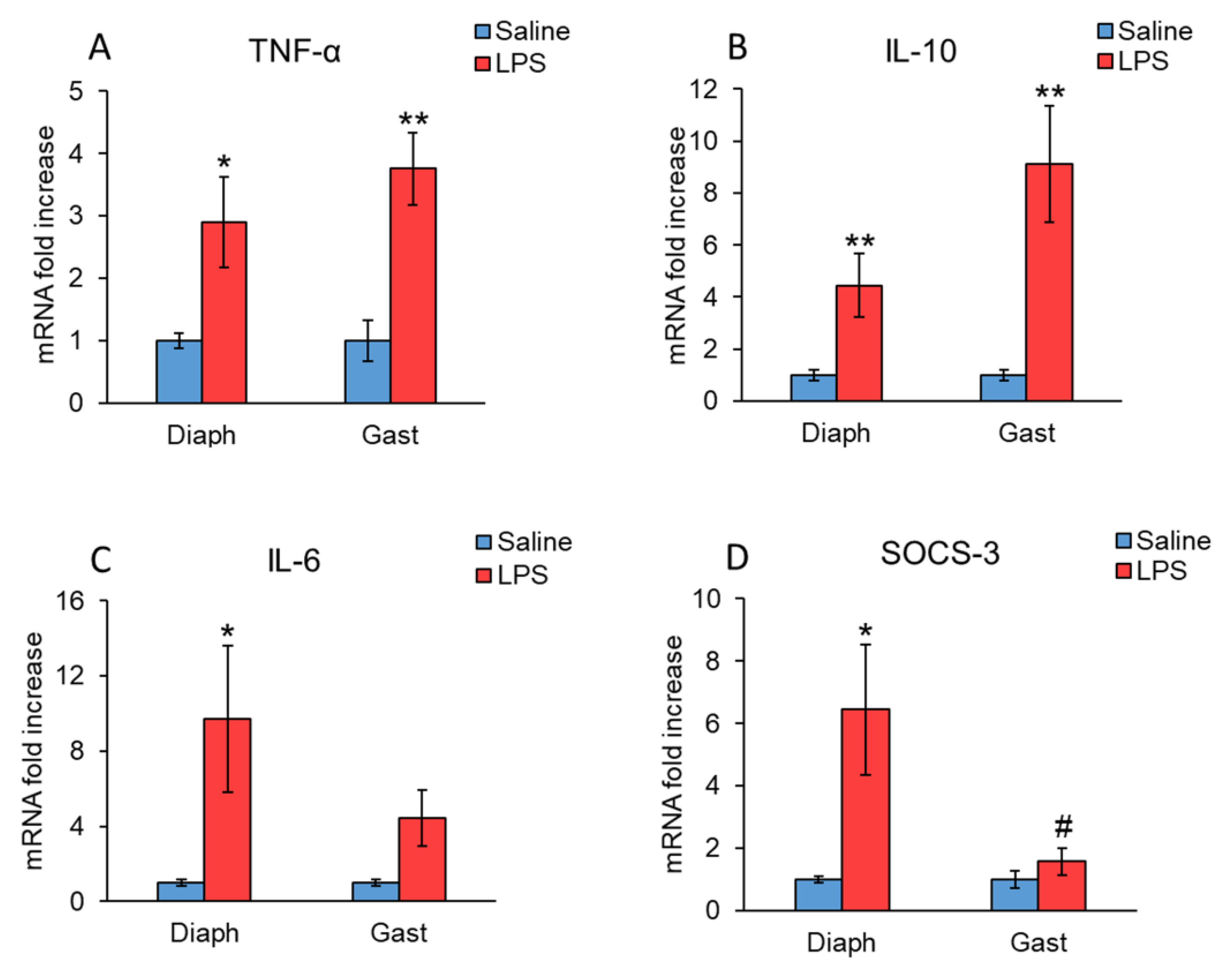
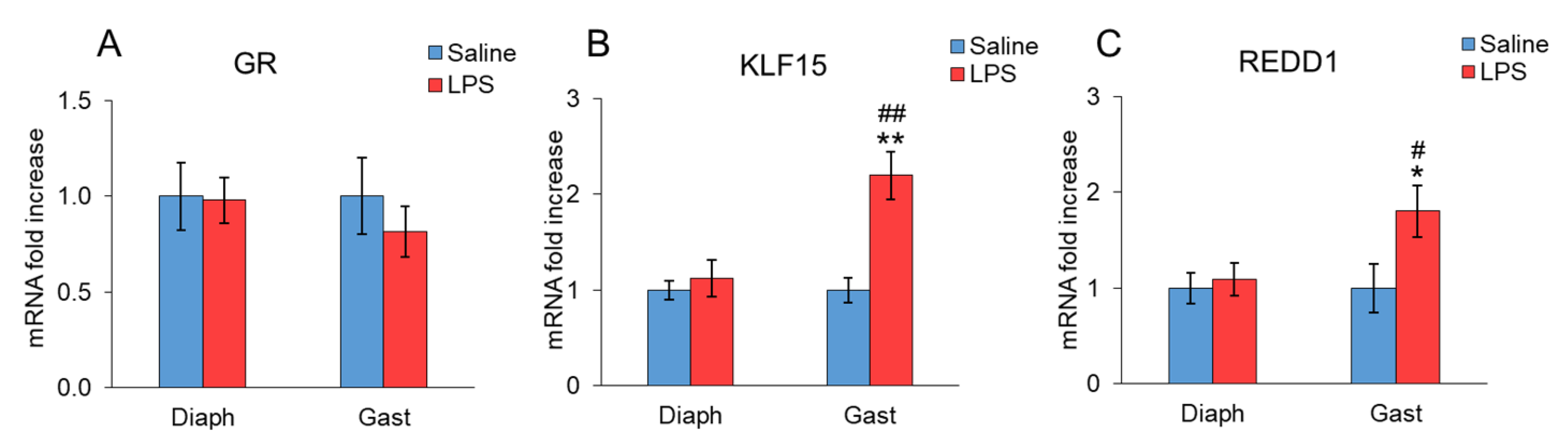
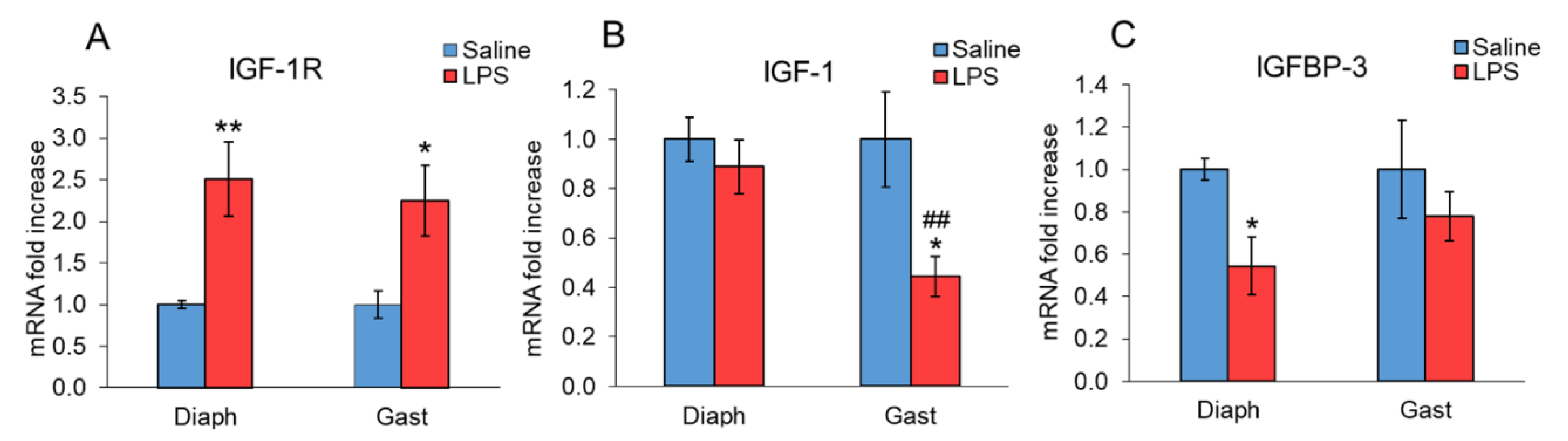
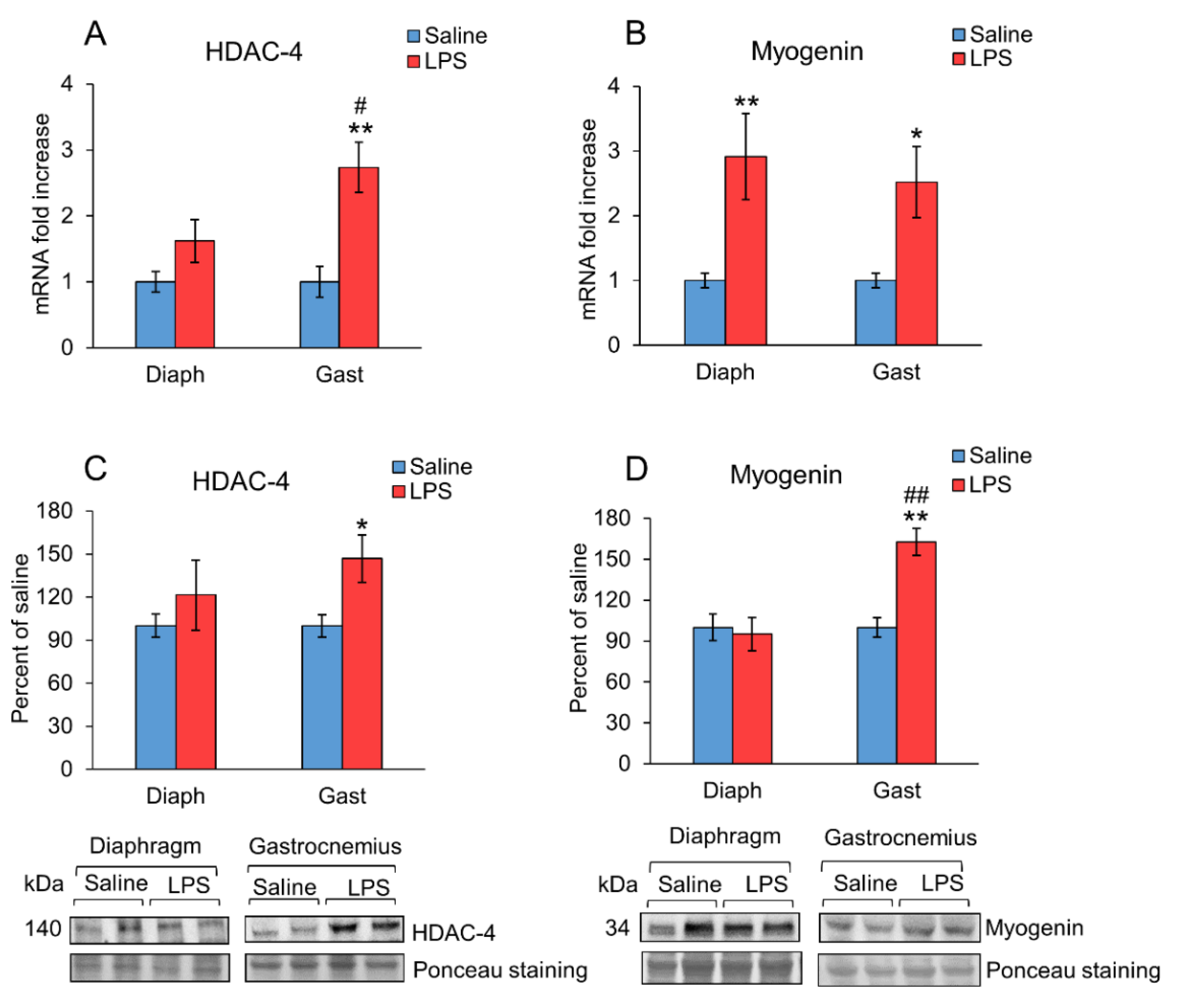
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.2. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.3. Protein Analysis by Western Blot
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakshmikanth, C.L.; Jacob, S.P.; Chaithra, V.H.; de Castro-Faria-Neto, H.C.; Marathe, G.K. Sepsis: In search of cure. Inflamm. Res. 2016, 65, 587–602. [Google Scholar] [CrossRef]
- Dumitru, A.; Radu, B.M.; Radu, M.; Cretoiu, S.M. Muscle Changes During Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Klaude, M.; Mori, M.; Tjader, I.; Gustafsson, T.; Wernerman, J.; Rooyackers, O. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin. Sci. 2012, 122, 133–142. [Google Scholar] [CrossRef]
- Morel, J.; Palao, J.C.; Castells, J.; Desgeorges, M.; Busso, T.; Molliex, S.; Jahnke, V.; Del Carmine, P.; Gondin, J.; Arnould, D.; et al. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in locomotor and respiratory muscles during experimental sepsis in mice. Sci. Rep. 2017, 7, 10866. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, M.; Muthny, T.; Sispera, L.; Holecek, M. Effects of beta-hydroxy-beta-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J. Physiol. Biochem. 2010, 66, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Stana, F.; Vujovic, M.; Mayaki, D.; Leduc-Gaudet, J.P.; Leblanc, P.; Huck, L.; Hussain, S.N.A. Differential Regulation of the Autophagy and Proteasome Pathways in Skeletal Muscles in Sepsis. Crit. Care Med. 2017, 45, e971–e979. [Google Scholar] [CrossRef] [PubMed]
- Moarbes, V.; Mayaki, D.; Huck, L.; Leblanc, P.; Vassilakopoulos, T.; Petrof, B.J.; Hussain, S.N.A. Differential regulation of myofibrillar proteins in skeletal muscles of septic mice. Physiol. Rep. 2019, 7, e14248. [Google Scholar] [CrossRef]
- Owen, A.M.; Patel, S.P.; Smith, J.D.; Balasuriya, B.K.; Mori, S.F.; Hawk, G.S.; Stromberg, A.J.; Kuriyama, N.; Kaneki, M.; Rabchevsky, A.G.; et al. Chronic muscle weakness and mitochondrial dysfunction in the absence of sustained atrophy in a preclinical sepsis model. Elife 2019, 8, e49920. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.; Austin, S.; Rhodes, A.; Finfer, S.; Opal, S.; Thompson, T.; Bozza, F.A.; LaRosa, S.P.; Ranieri, V.M.; Angus, D.C. Long-Term Quality of Life Among Survivors of Severe Sepsis: Analyses of Two International Trials. Crit. Care Med. 2016, 44, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.E.; Bersten, A.D. Alterations in Respiratory and Limb Muscle Strength and Size in Patients With Sepsis Who Are Mechanically Ventilated. Phys. Ther. 2014, 94, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Paraskevas, T.; Velissaris, D. Sepsis and the muscle tissue. A narrative review. Rom. J. Intern. Med. 2021, 59, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Pai, M.H.; Wu, J.M.; Yang, P.J.; Lee, P.C.; Chen, K.Y.; Yeh, S.L.; Lin, M.T. Protective Effects of Glutamine and Leucine Supplementation on Sepsis-Induced Skeletal Muscle Injuries. Int. J. Mol. Sci. 2021, 22, 13003. [Google Scholar] [CrossRef] [PubMed]
- Jude, B.; Tissier, F.; Dubourg, A.; Droguet, M.; Castel, T.; Leon, K.; Giroux-Metges, M.A.; Pennec, J.P. TGF-beta Pathway Inhibition Protects the Diaphragm From Sepsis-Induced Wasting and Weakness in Rat. Shock 2020, 53, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef]
- Martin, A.I.; Priego, T.; Lopez-Calderon, A. Hormones and Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 207–233. [Google Scholar] [CrossRef]
- Soto, L.; Martin, A.I.; Millan, S.; Vara, E.; Lopez-Calderon, A. Effects of endotoxin lipopolysaccharide administration on the somatotropic axis. J. Endocrinol. 1998, 159, 239–246. [Google Scholar] [CrossRef][Green Version]
- Martin, A.I.; Gomez-SanMiguel, A.B.; Priego, T.; Lopez-Calderon, A. Formoterol treatment prevents the effects of endotoxin on muscle TNF/NF-kB, Akt/mTOR, and proteolytic pathways in a rat model. Role of IGF-I and miRNA 29b. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E705–E714. [Google Scholar] [CrossRef]
- Martin, A.I.; Priego, T.; Moreno-Ruperez, A.; Gonzalez-Hedstrom, D.; Granado, M.; Lopez-Calderon, A. IGF-1 and IGFBP-3 in Inflammatory Cachexia. Int. J. Mol. Sci. 2021, 22, 9469. [Google Scholar] [CrossRef]
- Gomez-SanMiguel, A.B.; Villanua, M.A.; Martin, A.I.; Lopez-Calderon, A. D-TRP(8)-gammaMSH Prevents the Effects of Endotoxin in Rat Skeletal Muscle Cells through TNFalpha/NF-KB Signalling Pathway. PLoS ONE 2016, 11, e0155645. [Google Scholar] [CrossRef][Green Version]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbe, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Minetto, M.A.; Lanfranco, F.; Motta, G.; Allasia, S.; Arvat, E.; D’Antona, G. Steroid myopathy: Some unresolved issues. J. Endocrinol. Investig. 2011, 34, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Van Remmen, H. Emerging roles for histone deacetylases in age-related muscle atrophy. Nutr. Healthy Aging 2016, 4, 17–30. [Google Scholar] [CrossRef]
- Luo, L.; Martin, S.C.; Parkington, J.; Cadena, S.M.; Zhu, J.; Ibebunjo, C.; Summermatter, S.; Londraville, N.; Patora-Komisarska, K.; Widler, L.; et al. HDAC4 Controls Muscle Homeostasis through Deacetylation of Myosin Heavy Chain, PGC-1alpha, and Hsc70. Cell Rep. 2019, 29, 749–763.e12. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hedstrom, D.; Priego, T.; Lopez-Calderon, A.; Amor, S.; de la Fuente-Fernandez, M.; Inarejos-Garcia, A.M.; Garcia-Villalon, A.L.; Martin, A.I.; Granado, M. Beneficial Effects of a Mixture of Algae and Extra Virgin Olive Oils on the Age-Induced Alterations of Rodent Skeletal Muscle: Role of HDAC-4. Nutrients 2020, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, M.; Zielonka, D.; Carnemolla, A.; Marcinkowski, J.T.; Guidez, F. HDAC4 as a potential therapeutic target in neurodegenerative diseases: A summary of recent achievements. Front. Cell. Neurosci. 2015, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Bricceno, K.V.; Sampognaro, P.J.; Van Meerbeke, J.P.; Sumner, C.J.; Fischbeck, K.H.; Burnett, B.G. Histone deacetylase inhibition suppresses myogenin-dependent atrogene activation in spinal muscular atrophy mice. Hum. Mol. Genet 2012, 21, 4448–4459. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Karl, I.E. Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. JAMA 1992, 267, 1503–1510. [Google Scholar] [CrossRef]
- Levy, B.; Gibot, S.; Franck, P.; Cravoisy, A.; Bollaert, P.E. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: A prospective study. Lancet 2005, 365, 871–875. [Google Scholar] [CrossRef]
- Garcia-Alvarez, M.; Marik, P.; Bellomo, R. Sepsis-associated hyperlactatemia. Crit. Care 2014, 18, 503. [Google Scholar] [CrossRef]
- de Morais, H.; de Fatima Silva, F.; da Silva, F.G.; Silva, M.O.; Graciano, M.F.R.; Martins, M.I.L.; Carpinelli, Â.R.; Mazucco, T.L.; Bazotte, R.B.; de Souza, H.M. Insulin, not glutamine dipeptide, reduces lipases expression and prevents fat wasting and weight loss in Walker 256 tumor-bearing rats. Eur. J. Pharmacol. 2017, 806, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mofarrahi, M.; Sigala, I.; Guo, Y.; Godin, R.; Davis, E.C.; Petrof, B.; Sandri, M.; Burelle, Y.; Hussain, S.N. Autophagy and skeletal muscles in sepsis. PLoS ONE 2012, 7, e47265. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.N. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc. Soc. Exp. Biol. Med. 1994, 205, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, J.; Li, M.; Qian, L.; Zhang, L.; Huang, Z.; Shen, Y.; Law, B.Y.; Liu, L.; Gu, X. Transcriptome Analysis of Immune Receptor Activation and Energy Metabolism Reduction as the Underlying Mechanisms in Interleukin-6-Induced Skeletal Muscle Atrophy. Front. Immunol. 2021, 12, 730070. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Bruells, C.S.; Duschner, P.; Marx, G.; Gayan-Ramirez, G.; Frank, N.; Breuer, T.; Krenkel, O.; Tacke, F.; Mossanen, J.C. Acute liver injury following acetaminophen administration does not activate atrophic pathways in the mouse diaphragm. Sci. Rep. 2021, 11, 6302. [Google Scholar] [CrossRef]
- Garcia-Martinez, C.; Lopez-Soriano, F.J.; Argiles, J.M. Interleukin-6 does not activate protein breakdown in rat skeletal muscle. Cancer Lett. 1994, 76, 1–4. [Google Scholar] [CrossRef]
- Gao, S.; Durstine, J.L.; Koh, H.J.; Carver, W.E.; Frizzell, N.; Carson, J.A. Acute myotube protein synthesis regulation by IL-6-related cytokines. Am. J. Physiol. Cell Physiol. 2017, 313, C487–C500. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, Y.; Bao, W.; Fu, Q.; Hao, H.; Han, L.; Zhang, X.; Tian, X.; Zhang, M. STAT3 and IL-6 Contribute to Corticosteroid Resistance in an OVA and Ozone-induced Asthma Model with Neutrophil Infiltration. Front. Mol. Biosci. 2021, 8, 717962. [Google Scholar] [CrossRef]
- Baetz, A.; Frey, M.; Heeg, K.; Dalpke, A.H. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J. Biol. Chem. 2004, 279, 54708–54715. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, W.; Dai, Y. SOCS3 and its role in associated diseases. Hum. Immunol. 2015, 76, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Grossberg, A.J.; Krasnow, S.M.; Levasseur, P.R.; Szumowski, M.; Zhu, X.X.; Maxson, J.E.; Knoll, J.G.; Barnes, A.P.; Marks, D.L. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J. 2013, 27, 3572–3582. [Google Scholar] [CrossRef] [PubMed]
- Surmachevska, N.; Tiwari, V. Corticosteroid Induced Myopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shimizu, N.; Yoshikawa, N.; Ito, N.; Maruyama, T.; Suzuki, Y.; Takeda, S.; Nakae, J.; Tagata, Y.; Nishitani, S.; Takehana, K.; et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011, 13, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Wang, B.; Orihuela, Y.; Hong, E.G.; Fisch, S.; Haldar, S.; Cline, G.W.; Kim, J.K.; Peroni, O.D.; Kahn, B.B.; et al. Regulation of gluconeogenesis by Kruppel-like factor 15. Cell Metab. 2007, 5, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Britto, F.A.; Dumas, K.; Giorgetti-Peraldi, S.; Ollendorff, V.; Favier, F.B. Is REDD1 a metabolic double agent? Lessons from physiology and pathology. Am. J. Physiol. Cell Physiol. 2020, 319, C807–C824. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Britto, F.A.; Begue, G.; Rossano, B.; Docquier, A.; Vernus, B.; Sar, C.; Ferry, A.; Bonnieu, A.; Ollendorff, V.; Favier, F.B. REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E983–E993. [Google Scholar] [CrossRef]
- Schulze, P.C.; Gielen, S.; Adams, V.; Linke, A.; Mobius-Winkler, S.; Erbs, S.; Kratzsch, J.; Hambrecht, R.; Schuler, G. Muscular levels of proinflammatory cytokines correlate with a reduced expression of insulinlike growth factor-I in chronic heart failure. Basic Res. Cardiol. 2003, 98, 267–274. [Google Scholar] [CrossRef]
- Costelli, P.; Muscaritoli, M.; Bossola, M.; Penna, F.; Reffo, P.; Bonetto, A.; Busquets, S.; Bonelli, G.; Lopez-Soriano, F.J.; Doglietto, G.B.; et al. IGF-1 is downregulated in experimental cancer cachexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R674–R683. [Google Scholar] [CrossRef]
- Castillero, E.; Martin, A.I.; Lopez-Menduina, M.; Granado, M.; Villanua, M.A.; Lopez-Calderon, A. IGF-I system, atrogenes and myogenic regulatory factors in arthritis induced muscle wasting. Mol. Cell. Endocrinol. 2009, 309, 8–16. [Google Scholar] [CrossRef]
- Nystrom, G.; Pruznak, A.; Huber, D.; Frost, R.A.; Lang, C.H. Local insulin-like growth factor I prevents sepsis-induced muscle atrophy. Metabolism 2009, 58, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Celemin, L.; Pasko, N.; Blomart, V.; Thissen, J.P. Inhibition of muscle insulin-like growth factor I expression by tumor necrosis factor-alpha. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1279–E1290. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Huang, Z.L.; Yang, J.H.; Xu, Y.H.; Sun, J.S.; Zheng, Q.; Wei, C.Y.; Song, W.; Yuan, Z. Pancreatic cancer cell-derived IGFBP-3 contributes to muscle wasting. J. Exp. Clin. Cancer Res. 2016, 35, 46. [Google Scholar] [CrossRef] [PubMed]
- Yakovenko, A.; Cameron, M.; Trevino, J.G. Molecular therapeutic strategies targeting pancreatic cancer induced cachexia. World J. Gastrointest. Surg. 2018, 10, 95–106. [Google Scholar] [CrossRef]
- Lopez-Menduina, M.; Martin, A.I.; Castillero, E.; Villanua, M.A.; Lopez-Calderon, A. Systemic IGF-I administration attenuates the inhibitory effect of chronic arthritis on gastrocnemius mass and decreases atrogin-1 and IGFBP-3. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R541–R551. [Google Scholar] [CrossRef][Green Version]
- Cole, C.L.; Bachman, J.F.; Ye, J.; Murphy, J.; Gerber, S.A.; Beck, C.A.; Boyce, B.F.; Muthukrishnan, G.; Chakkalakal, J.V.; Schwarz, E.M.; et al. Increased myocellular lipid and IGFBP-3 expression in a pre-clinical model of pancreatic cancer-related skeletal muscle wasting. J. Cachexia Sarcopenia Muscle 2021, 12, 731–745. [Google Scholar] [CrossRef]
- Puig-Vilanova, E.; Aguilo, R.; Rodriguez-Fuster, A.; Martinez-Llorens, J.; Gea, J.; Barreiro, E. Epigenetic mechanisms in respiratory muscle dysfunction of patients with chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e111514. [Google Scholar] [CrossRef]
- Ha, Z.L.; Yu, Z.Y. Downregulation of miR-29b-3p aggravates podocyte injury by targeting HDAC4 in LPS-induced acute kidney injury. Kaohsiung J. Med. Sci. 2021, 37, 1069–1076. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, Y.J.; Bayliss, G.; Zhuang, S.G. Class IIa HDAC inhibitor TMP195 alleviates lipopolysaccharide-induced acute kidney injury. Am. J. Physiol.-Ren. Physiol. 2020, 319, F1015–F1026. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Qi, J. Activation of HDAC4 and GR signaling contributes to stress-induced hyperalgesia in the medial prefrontal cortex of rats. Brain Res. 2020, 1747, 147051. [Google Scholar] [CrossRef]
- Ko, J.Y.; Chuang, P.C.; Chen, M.W.; Ke, H.C.; Wu, S.L.; Chang, Y.H.; Chen, Y.S.; Wang, F.S. MicroRNA-29a ameliorates glucocorticoid-induced suppression of osteoblast differentiation by regulating beta-catenin acetylation. Bone 2013, 57, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Gao, Y.; Hu, N.; Wu, L.; Chen, Q. miR-365 Ameliorates Dexamethasone-Induced Suppression of Osteogenesis in MC3T3-E1 Cells by Targeting HDAC4. Int. J. Mol. Sci. 2017, 18, 977. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, M.; Toczek, M.; Smeets, C.J.; Franklin, S.A.; Bondulich, M.K.; Jolinon, N.; Muller, T.; Ahmed, M.; Dick, J.R.; Piotrowska, I.; et al. HDAC4-myogenin axis as an important marker of HD-related skeletal muscle atrophy. PLoS Genet 2015, 11, e1005021. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Muscle wasting in animal models of severe illness. Int. J. Exp. Pathol. 2012, 93, 157–171. [Google Scholar] [CrossRef] [PubMed]

| Control, n = 9 | LPS, n = 8 | |
|---|---|---|
| Body weight gain (g/24 h.) | −2.0 ± 0.9 | −21.9 ± 3.6 ** |
| Diaphragm (mg/100 g b.w.) | 206 ± 15 | 232 ± 17 |
| Gastrocnemius (mg/100 g b.w.) | 520 ± 20 | 517 ± 10 |
| Urea (mg/dL) | 22.1 ± 1.7 | 49.8 ± 5.9 ** |
| Creatinine (mg/dL) | 0.208 ± 0.008 | 0.331 ± 0.03 ** |
| Glucose (mg/dL) | 234 ± 8 | 180 ± 16 ** |
| Lactate (mmol/L) | 1.6 ± 0.2 | 5.6 ± 0.8 ** |
| HCO3- (mmol/L) | 26.0 ± 0.6 | 18.0 ± 2.2 ** |
| PaCO2 (mmHg) | 42.7 ± 3.9 | 37.8 ± 5.6 |
| PaO2 (mmHg) | 86.0 ± 6.1 | 88.0 ± 6.6 |
| SO2% (mmol/L) | 95.0 ± 1.3 | 95 ± 0.9 |
| pH | 7.39 ± 0.03 | 7.30 ± 0.04 |
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| 18 S | GGTGCATGGCCGTTCTTA | TCGTTCGTTATCGGAATTAACC |
| IL10 | AGTGGAGCAGGTGAAGAATGA | TCATGGCCTTGTAGACACCTT |
| IL6 | GGAAGTTGGGGTAGGAAGGA | CCTGGAGTTTGTGAAGAACAACT |
| TNFα | TGAACTTCGGGGTGATCG | GGGCTTGTCACTCGAGTTTT |
| SOCS3 | CCTCCAGCATCTTTGTCGGAAGAC | CATTCGGGAGTTCCTGGACCAGTA |
| MuRF1 | TGTCTGGAGGTCGTTTCCG | AAGTGATCATGGACCGGCAT |
| Atrogin-1 | GAACAGCAAAACCAAAACTCAGTA | GCTCCTTAGTACTCCCTTTGTGAA |
| IGF-1 Ea | GCTATGGCTCCAGCATTCG | GGATGAGTGTTGCTTCCGGA |
| IGFBP3 | GGAAAGACGACGTGCATTG | GCGTATTTGAGCTCCACGTT |
| IGF1R | GCCTCCAACTTTGTCTTTGC | TCACTGGGCCAGGAATGT |
| HDAC4 | CACACCTCTTGGAGGGTACAA | AGCCCATCAGCTGTTTTGTC |
| Myogenin | CCTTGCTCAGCTCCCTCA | TGGGAGTTGCATTCACTGG |
| LC3b | CAGGTTGCCTAGCAGAGGTC | TGTCCTATACACCTGACCTGTTTC |
| GR | AAGAGCAGTGGAAGGACAGC | GCTGGGCAGTTTTTCCTTCG |
| KLF15 | TTGTGGGCCAGAAGTTCC | TGCATTTGTGCATTTTGAGAA |
| REDD1 | CCAGAGAAGAGGGCCTTGA | CCATCCAGGTATGAGGAGTCTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Rupérez, Á.; Priego, T.; González-Nicolás, M.Á.; López-Calderón, A.; Lázaro, A.; Martín, A.I. Role of Glucocorticoid Signaling and HDAC4 Activation in Diaphragm and Gastrocnemius Proteolytic Activity in Septic Rats. Int. J. Mol. Sci. 2022, 23, 3641. https://doi.org/10.3390/ijms23073641
Moreno-Rupérez Á, Priego T, González-Nicolás MÁ, López-Calderón A, Lázaro A, Martín AI. Role of Glucocorticoid Signaling and HDAC4 Activation in Diaphragm and Gastrocnemius Proteolytic Activity in Septic Rats. International Journal of Molecular Sciences. 2022; 23(7):3641. https://doi.org/10.3390/ijms23073641
Chicago/Turabian StyleMoreno-Rupérez, Álvaro, Teresa Priego, María Ángeles González-Nicolás, Asunción López-Calderón, Alberto Lázaro, and Ana Isabel Martín. 2022. "Role of Glucocorticoid Signaling and HDAC4 Activation in Diaphragm and Gastrocnemius Proteolytic Activity in Septic Rats" International Journal of Molecular Sciences 23, no. 7: 3641. https://doi.org/10.3390/ijms23073641
APA StyleMoreno-Rupérez, Á., Priego, T., González-Nicolás, M. Á., López-Calderón, A., Lázaro, A., & Martín, A. I. (2022). Role of Glucocorticoid Signaling and HDAC4 Activation in Diaphragm and Gastrocnemius Proteolytic Activity in Septic Rats. International Journal of Molecular Sciences, 23(7), 3641. https://doi.org/10.3390/ijms23073641