The Role of Testosterone in the Elderly: What Do We Know?
Abstract
1. Introduction
2. Clinical Findings
2.1. Late-Onset Hypogonadism: The Aging Male
2.2. Testosterone and Body Composition—Fat and Lean Mass
2.3. Testosterone and Bone Density
2.4. Testosterone and Sexual Activity
2.5. Testosterone and Glycometabolic Profile—The Effects on Insulin Sensitivity
2.6. Testosterone and Cardiovascular Disease
2.7. Testosterone and Mental Activity
2.8. Testosterone and Quality of Life
2.9. Testosterone and Prostate Cancer
3. Safety of TRT
4. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| TRT | Testosterone Replacement Therapy |
| PADAM | Partial Androgen Deficiency of the Aging Male |
| LOH | Late-Onset Hypogonadism |
| LH | Luteinizing hormone |
| COX2 | cyclooxygenase-2 |
| StAR | steroidogenic acute regulatory protein |
| BMI | Body Mass Index |
| AR | androgen receptor |
| PPARγ | Peroxisome Proliferator-Activated Receptor-gamma |
| CEBPα | CAT/enhancer-binding protein alpha |
| IM | intramuscular |
| RCT | Randomized Clinical Trial |
| DHEA | Dehydroepiandrosterone |
| DHEAS | Dehydroepiandrosterone sulfate |
| BMD | bone mineral density |
| IIEF | International Index of Erectile Function |
| EFD | Erectile Function Domain |
| PDE-5i | phosphodiesterase type 5 inhibitors |
| SAID | Sexual Arousal, Interest and Drive Scale |
| HED | Hypogonadism Energy Diary |
| T2DM | Type 2 diabetes mellitus |
| HbA1c | glycated hemoglobin |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| IL-18 | interleukin 18 |
| ADT | Androgen deprivation therapy |
| CV | cardiovascular |
| FDA | Food and drug administration |
| QoL | Quality of life |
| AMS | Aging Males’ Symptom |
| ED | Erectile dysfunction |
References
- Abbasi, A.A.; Drinka, P.J.; Mattson, D.E.; Rudman, D. Low circulating levels of insulin-like growth factors and testosterone in chronically institutionalized elderly men. J. Am. Geriatr. Soc. 1993, 41, 975–982. [Google Scholar] [CrossRef]
- Baum, N.H.; Crespi, C.A. Testosterone replacement in elderly men. Geriatrics 2007, 62, 15–18. [Google Scholar] [PubMed]
- Moncada, I. Testosterone and men’s quality of life. Aging Male 2006, 9, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.M.; Vermeulen, A. The Decline of Androgen Levels in Elderly Men and Its Clinical and Therapeutic Implications. Endocr. Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef] [PubMed]
- Simanainen, U.; Brogley, M.; Gao, Y.R.; Jimenez, M.; Harwood, D.T.; Handelsman, D.J.; Robins, D.M. Length of the human androgen receptor glutamine tract determines androgen sensitivity in vivo. Mol. Cell. Endocrinol. 2011, 342, 81–86. [Google Scholar] [CrossRef]
- Matsumoto, T.; Shiina, H.; Kawano, H.; Sato, T.; Kato, S. Androgen receptor functions in male and female physiology. J. Steroid Biochem. Mol. Biol. 2008, 109, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, M.; Depenbusch, M.; Gromoll, J.; Nieschlag, E. Prostate Volume and Growth in Testosterone-Substituted Hypogonadal Men Are Dependent on the CAG Repeat Polymorphism of the Androgen Receptor Gene: A Longitudinal Pharmacogenetic Study. J. Clin. Endocrinol. Metab. 2003, 88, 2049–2054. [Google Scholar] [CrossRef][Green Version]
- Attard, C.C.; Fava, S. Benefits and risks of testosterone therapy in older men. Minerva Urol. Nefrol. 2019, 71, 217–229. [Google Scholar] [CrossRef]
- Beauchet, O. Testosterone and cognitive function: Current clinical evidence of a relationship. Eur. J. Endocrinol. 2006, 155, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.T.; Hildreth, K.L.; Pelak, V.S. Effects of Testosterone Therapy on Cognitive Function in Aging: A Systematic Review. Cogn. Behav. Neurol. 2016, 29, 122–138. [Google Scholar] [CrossRef]
- Wu, F.C.W.; Tajar, A.; Pye, S.; Silman, A.J.; Finn, J.D.; O’Neill, T.; Bartfai, G.; Casanueva, F.; Forti, G.; Giwercman, A.; et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: The european male aging study. J. Clin. Endocrinol. Metab. 2008, 93, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Perheentupa, A.; Huhtaniemi, I. Aging of the human ovary and testis. Mol. Cell. Endocrinol. 2009, 299, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Well, D.; Yang, H.; Houseni, M.; Iruvuri, S.; Alzeair, S.; Sansovini, M.; Wintering, N.; Alavi, A.; Torigian, D.A. Age-Related Structural and Metabolic Changes in the Pelvic Reproductive End Organs. Semin. Nucl. Med. 2007, 37, 173–184. [Google Scholar] [CrossRef]
- Kidd, S.A.; Eskenazi, B.; Wyrobek, A. Effects of male age on semen quality and fertility: A review of the literature. Fertil. Steril. 2001, 75, 237–248. [Google Scholar] [CrossRef]
- Di Guardo, F.; Vloeberghs, V.; Bardhi, E.; Blockeel, C.; Verheyen, G.; Tournaye, H.; Drakopoulos, P. Low Testosterone and Semen Parameters in Male Partners of Infertile Couples Undergoing IVF with a Total Sperm Count Greater than 5 Million. J. Clin. Med. 2020, 9, 3824. [Google Scholar] [CrossRef]
- Fusco, F.; Verze, P.; Capece, M.; Napolitano, L. Suppression of Spermatogenesis by Exogenous Testosterone. Curr. Pharm. Des. 2021, 27, 2750–2753. [Google Scholar] [CrossRef]
- Trussell, J.; Coward, R.; Santoro, N.; Stetter, C.; Kunselman, A.; Diamond, M.P.; Hansen, K.R.; Krawetz, S.A.; Legro, R.S.; Heisenleder, D.; et al. Association between testosterone, semen parameters, and live birth in men with unexplained infertility in an intrauterine insemination population. Fertil. Steril. 2019, 111, 1129–1134. [Google Scholar] [CrossRef]
- Huhtaniemi, I. Late-onset hypogonadism: Current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J. Androl. 2014, 16, 192–202. [Google Scholar] [CrossRef]
- Nieschlag, E.; Behre, H.; Nieschlag, S.; Van, A.H. Male reproductive health and dysfunction. Internistische. Praxis. Andrology 2011, 51, 751. [Google Scholar]
- Chen, H.; Ge, R.-S.; Zirkin, B.R. Leydig cells: From stem cells to aging. Mol. Cell. Endocrinol. 2009, 306, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Qu, Y.; Wang, L.; Geng, D.; Chen, W.; Li, L.; Tian, Y.; Chang, S.; Zhao, C.; et al. The roles of p38 MAPK → COX2 and NF-κB → COX2 signal pathways in age-related testosterone reduction. Sci. Rep. 2019, 9, 10556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Maresch, C.C.; Petry, S.F.; Paradowska-Dogan, A.; Bhushan, S.; Chang, Y.; Wrenzycki, C.; Schuppe, H.-C.; Houska, P.; Hartmann, M.F.; et al. Elevated CCL2 causes Leydig cell malfunction in metabolic syndrome. JCI Insight 2020, 5, e134882. [Google Scholar] [CrossRef] [PubMed]
- Guay, A.T. The emerging link between hypogonadism and metabolic syndrome. J. Androl. 2008, 30, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, V.A.; Castellana, M.; Lisco, G.; Triggiani, V. Critical evaluation of different available guidelines for late-onset hypogonadism. Andrology 2020, 8, 1628–1641. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European association of urology guidelines on sexual and reproductive health—2021 update: Male sexual dysfunction. Eur. Urol. 2021, 80, 333–357. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Tsujimura, A.; Miyoshi, M.; Miyoshi, Y.; Ogasa, T.; Hiramatsu, I.; Uesaka, Y.; Nozaki, T.; Shirai, M.; Kobayashi, K.; et al. Endocrinological and symptomatic characteristics of patients with late-onset hypogonadism classified by functional categories based on testosterone and luteinizing hormone levels. Int. J. Urol. 2020, 27, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Park, J.K. The optimal indication for testosterone replacement therapy in late onset hypogonadism. J. Clin. Med. 2019, 8, 209. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Vignozzi, L.; Mannucci, E.; Maggi, M. How to recognize late-onset hypogonadism in men with sexual dysfunction. Asian J. Androl. 2012, 14, 251–259. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Lee, J.Y.; O’Neill, E.A.; Meehan, A.G.; Kusek, J.W. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013, 16, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-K.; Lee, J.K.; Cho, B. The role of androgen in the adipose tissue of males. World J. Men’s Health 2013, 31, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef] [PubMed]
- Maneschi, E.; Morelli, A.; Filippi, S.; Cellai, I.; Comeglio, P.; Mazzanti, B.; Mello, T.; Calcagno, A.; Sarchielli, E.; Vignozzi, L.; et al. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J. Endocrinol. 2012, 215, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Haider, A.; Doros, G.; Traish, A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity 2013, 21, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.A.; McLachlan, R.I. Androgens and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 224–232. [Google Scholar] [CrossRef]
- Saad, F.; Aversa, A.; Isidori, A.M.; Gooren, L.J. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: A review. Curr. Diabetes Rev. 2012, 8, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Yassin, A.; Doros, G.; Saad, F. Effects of long-term testosterone therapy on patients with “diabesity”: Results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int. J. Endocrinol. 2014, 2014, 683515. [Google Scholar] [CrossRef]
- Wang, C.; Swerdloff, R.S.; Iranmanesh, A.; Dobs, A.; Snyder, P.J.; Cunningham, G.; Matsumoto, A.M.; Weber, T.N. Nancy berman the testosterone gel study group transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal Men. J. Clin. Endocrinol. Metab. 2000, 85, 2839–2853. [Google Scholar] [CrossRef]
- Aversa, A.; Bruzziches, R.; Francomano, D.; Rosano, G.; Isidori, A.M.; Lenzi, A.; Spera, G. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: Results from a 24-month, randomized, double-blind, placebo-controlled study. J. Sex. Med. 2010, 7, 3495–3503. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.; Giannetta, E.; Greco, E.; Gianfrilli, D.; Bonifacio, V.; Isidori, A.; Lenzi, A.; Fabbri, A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: A meta-analysis. Clin. Endocrinol. 2005, 63, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Giagulli, V.A.; Maseroli, E.; Vignozzi, L.; Aversa, A.; Zitzmann, M.; Saad, F.; Mannucci, E.; Maggi, M. THERAPY OF ENDOCRINE DISEASE: Testosterone supplementation and body composition: Results from a meta-analysis study. Eur. J. Endocrinol. 2016, 174, R99–R116. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, I.G.; Balagopal, P.; Nair, K.S. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men--a clinical research center study. J. Clin. Endocrinol. Metab. 1996, 81, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Groti, K.; Žuran, I.; Antonic, K.G.; Foršnarič, L.; Pfeifer, M. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male 2017, 21, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Wang, C.E.; Lapin, B.; Lakeman, J.C.; Helfand, B.T. Characteristics of Men Undergoing testosterone replacement therapy and adherence to follow-up recommendations in metropolitan multicenter health care system. Urology 2015, 85, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L.; Bhasin, S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.E.; Pell, J.M. Point: IGF is the major physiological regulator of muscle mass. J. Appl. Physiol. 2010, 108, 1820–1821. [Google Scholar] [CrossRef]
- Serra, C.; Tangherlini, F.; Rudy, S.; Lee, D.; Toraldo, G.; Sandor, N.L.; Zhang, A.; Jasuja, R.; Bhasin, S. Testosterone Improves the Regeneration of Old and Young Mouse Skeletal Muscle. J. Gerontol. Ser. A 2012, 68, 17–26. [Google Scholar] [CrossRef]
- Wagers, A.J.; Conboy, I.M. Cellular and molecular signatures of muscle regeneration: Current concepts and controversies in adult myogenesis. Cell 2005, 122, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Laurent, M.; Boonen, S.; Vanderschueren, D.; Claessens, F. Androgens and skeletal muscle: Cellular and molecular action mechanisms underlying the anabolic actions. Cell. Mol. Life Sci. 2011, 69, 1651–1667. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Tipton, K.D.; Doyle, D.; Phillips, S.M.; Cortiella, J.; Wolfe, R.R. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am. J. Physiol. Content 1998, 275, E864–E871. [Google Scholar] [CrossRef] [PubMed]
- Estrada, M.; Espinosa, A.; Müller, M.; Jaimovich, E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a g protein-coupled receptor in skeletal muscle cells. Endocrinology 2003, 144, 3586–3597. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Balsells, M.M.; Murad, M.H.; Lane, M.; Lampropulos, J.F.; Albuquerque, F.; Mullan, R.J.; Agrwal, N.; Elamin, M.B.; Gallegos-Orozco, J.F.; Wang, A.T.; et al. Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2010, 95, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Mänttäri, S.; Anttila, K.; Järvilehto, M. Testosterone stimulates myoglobin expression in different muscles of the mouse. J. Comp. Physiol. B 2008, 178, 899–907. [Google Scholar] [CrossRef]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Lauretani, F.; Ceda, G.P. Sex hormones and sarcopenia in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Grossmann, M.; Hoermann, R.; Angus, P.W.; Gow, P.J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J. Hepatol. 2016, 65, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Storer, T.W.; Woodhouse, L.; Magliano, L.; Singh, A.B.; Dzekov, C.; Dzekov, J.; Bhasin, S. Changes in Muscle Mass, Muscle Strength, and Power but Not Physical Function Are Related to Testosterone Dose in Healthy Older Men. J. Am. Geriatr. Soc. 2008, 56, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Page, S.T.; Amory, J.; Bowman, F.D.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Tenover, J.L. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J. Clin. Endocrinol. Metab. 2005, 90, 1502–1510. [Google Scholar] [CrossRef]
- Caminiti, G.; Volterrani, M.; Iellamo, F.; Marazzi, G.; Massaro, R.; Miceli, M.; Mammi, C.; Piepoli, M.; Fini, M.; Rosano, G.M. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure: A double-blind, placebo-controlled, randomized study. J. Am. Coll. Cardiol. 2009, 54, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.A.; Srinivas-Shankar, U.; Roberts, S.; Connolly, M.J.; Adams, J.E.; Oldham, J.A.; Wu, F.C.W.; Seynnes, O.; Stewart, C.; Maganaris, C.N.; et al. Effects of Testosterone on Skeletal Muscle Architecture in Intermediate-Frail and Frail Elderly Men. J. Gerontol. Ser. A 2010, 65, 1215–1219. [Google Scholar] [CrossRef]
- Sheffield-Moore, M.; Dillon, E.L.; Casperson, S.; Gilkison, C.R.; Paddon-Jones, D.; Durham, W.J.; Grady, J.J.; Urban, R.J. A Randomized Pilot Study of Monthly Cycled Testosterone Replacement or Continuous Testosterone ReplacementVersusPlacebo in Older Men. J. Clin. Endocrinol. Metab. 2011, 96, E1831–E1837. [Google Scholar] [CrossRef]
- Neto, W.K.; Gama, E.; Rocha, L.Y.; Ramos, C.C.; Taets, W.; Scapini, K.B.; Ferreira, J.B.; Rodrigues, B.; Caperuto, É. Effects of testosterone on lean mass gain in elderly men: Systematic review with meta-analysis of controlled and randomized studies. AGE 2015, 37, 5. [Google Scholar] [CrossRef]
- Magnussen, L.V.; Hvid, L.G.; Hermann, A.P.; Hougaard, D.M.; Gram, B.; Caserotti, P.; Andersen, M. Testosterone therapy preserves muscle strength and power in aging men with type 2 diabetes-a randomized controlled trial. Andrology 2017, 5, 946–953. [Google Scholar] [CrossRef]
- Skinner, J.W.; Otzel, D.M.; Bowser, A.; Nargi, D.; Agarwal, S.; Peterson, M.; Zou, B.; Borst, S.E.; Yarrow, J.F. Muscular responses to testosterone replacement vary by administration route: A systematic review and meta-analysis. J. Cachex-Sarcopenia Muscle 2018, 9, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2014, 99, 11–15. [Google Scholar] [CrossRef]
- Kang, H.-Y.; Cho, C.-L.; Huang, K.-L.; Wang, J.-C.; Hu, Y.-C.; Lin, H.-K.; Chang, C.; Huang, K.-E. Nongenomic androgen activation of phosphatidylinositol 3-Kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J. Bone Miner. Res. 2004, 19, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kaji, H.; Sugimoto, T.; Chihara, K. Testosterone inhibits osteoclast formation stimulated by parathyroid hormone through androgen receptor. FEBS Lett. 2001, 491, 91–93. [Google Scholar] [CrossRef]
- Fink, H.A.; Ewing, S.K.; Ensrud, K.; Barrett-Connor, E.; Taylor, B.; Cauley, J.A.; Orwoll, E. Association of Testosterone and Estradiol Deficiency with Osteoporosis and Rapid Bone Loss in Older Men. J. Clin. Endocrinol. Metab. 2006, 91, 3908–3915. [Google Scholar] [CrossRef]
- Rendina, D.; D’Elia, L.; De Filippo, G.; Abate, V.; Evangelista, M.; Giaquinto, A.; Barone, B.; Piccinocchi, G.; Prezioso, D.; Strazzullo, P. Metabolic syndrome is not associated to an increased risk of low bone mineral density in men at risk for osteoporosis. J. Endocrinol. Investig. 2021, 45, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Rendina, D.; D’Elia, L.; Evangelista, M.; De Filippo, G.; Giaquinto, A.; Abate, V.; Barone, B.; Piccinocchi, G.; Prezioso, D.; Strazzullo, P. Metabolic syndrome is associated to an increased risk of low bone mineral density in free-living women with suspected osteoporosis. J. Endocrinol. Investig. 2020, 44, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Prestwood, K.M.; Marcello, K.M.; Raisz, L.G. Determinants of bone density in healthy older men with low testosterone levels. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2000, 55, M492–M497. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Hannoush, P.; Berlin, J.A.; Loh, L.; Holmes, J.H.; Dlewati, A.; Staley, J.; Santanna, J.; Kapoor, S.C.; et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age1. J. Clin. Endocrinol. Metab. 1999, 84, 1966–1972. [Google Scholar] [CrossRef][Green Version]
- Basurto, L.; Zarate, A.; Gómez, R.; Vargas, C.; Saucedo, R.; Galvan, R. Effect of testosterone therapy on lumbar spine and hip mineral density in elderly men. Aging Male 2008, 11, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.; Vasilic, B.; Wehrli, F.W.; Bunker, B.; Wald, M.; Gomberg, B.; Wright, A.C.; Zemel, B.; Cucchiara, A.; Snyder, P.J. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J. Bone Miner. Res. 2005, 20, 1785–1791. [Google Scholar] [CrossRef]
- Bouloux, P.M.G.; Legros, J.-J.; Elbers, J.M.H.; Geurts, T.B.P.; Kaspers, M.J.G.H.; Meehan, A.G.; Meuleman, E.J.H.; for the Study 43203 Investigators. Effects of oral testosterone undecanoate therapy on bone mineral density and body composition in 322 aging men with symptomatic testosterone deficiency: A 1-year, randomized, placebo-controlled, dose-ranging study. Aging Male 2013, 16, 38–47. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhan, J.-K.; Huang, W.; Wang, Y.; Liu, Y.; Wang, S.; Tan, P.; Tang, Z.-Y.; Liu, Y.-S. Effects of low-dose testosterone undecanoate treatment on bone mineral density and bone turnover markers in elderly male osteoporosis with low serum testosterone. Int. J. Endocrinol. 2013, 2013, 570413. [Google Scholar] [CrossRef]
- Permpongkosol, S.; Khupulsup, K.; Leelaphiwat, S.; Pavavattananusorn, S.; Thongpradit, S.; Petchthong, T. Effects of 8-year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with late-onset hypogonadism. J. Sex. Med. 2016, 13, 1199–1211. [Google Scholar] [CrossRef]
- Snyder, P.J.; Kopperdahl, D.L.; Stephens-Shields, A.J.; Ellenberg, S.S.; Cauley, J.A.; Ensrud, K.; Lewis, C.E.; Barrett-Connor, E.; Schwartz, A.V.; Lee, D.C.; et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone. JAMA Intern. Med. 2017, 177, 471–479. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, D.; Li, H. The effects of testosterone on bone health in males with testosterone deficiency: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, G.; Guaraldi, F.; Reismann, Y.; Sforza, A.; Isidori, A.M.; Maggi, M.; Corona, G. Testosterone Replacement Therapy for Sexual Symptoms. Sex. Med. Rev. 2019, 7, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Arcaniolo, D.; Napolitano, L.; Barone, B.; La Rocca, R.; Capece, M.; Caputo, V.F.; Imbimbo, C.; De Sio, M.; Calace, F.P.; et al. Impact of Sexual Activity on the Risk of Male Genital Tumors: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 8500. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Granata, L.; Fusco, F.; Napolitano, L.; Cerbone, R.; Priadko, K.; Sciorio, C.; Mirone, V.; Romano, M. Sexual dysfunction in patients with chronic gastrointestinal and liver diseases: A neglected issue. Sex. Med. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, G.R.; Stephens-Shields, A.J.; Rosen, R.C.; Wang, C.; Ellenberg, S.S.; Matsumoto, A.M.; Bhasin, S.; Molitch, M.E.; Farrar, J.T.; Cella, D.; et al. Association of sex hormones with sexual function, vitality, and physical function of symptomatic older men with low testosterone levels at baseline in the testosterone trials. J. Clin. Endocrinol. Metab. 2015, 100, 1146–1155. [Google Scholar] [CrossRef]
- Rajfer, J. Relationship between testosterone and erectile dysfunction. Rev. Urol. 2000, 2, 122–128. [Google Scholar] [PubMed]
- Napolitano, L.; Barone, B.; Crocetto, F.; Capece, M.; La Rocca, R. The COVID-19 Pandemic: Is It A Wolf Consuming Fertility? Int. J. Fertil. Steril. 2020, 14, 159–160. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Morgentaler, A.; Sforza, A.; Mannucci, E.; Maggi, M. Meta-analysis of Results of Testosterone Therapy on Sexual Function Based on International Index of Erectile Function Scores. Eur. Urol. 2017, 72, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Caliber, M.; Doros, G.; Haider, K.S.; Haider, A. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male 2019, 23, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Efesoy, O.; Çayan, S.; Akbay, E. The Effect of Testosterone Replacement Therapy on Penile Hemodynamics in Hypogonadal Men with Erectile Dysfunction, Having Veno-Occlusive Dysfunction. Am. J. Men’s Health 2018, 12, 634–638. [Google Scholar] [CrossRef]
- Rizk, P.J.; Kohn, T.P.; Pastuszak, A.; Khera, M. Testosterone therapy improves erectile function and libido in hypogonadal men. Curr. Opin. Urol. 2017, 27, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Mirone, V.; Napolitano, L.; Bianca, R.D.D.V.; Mitidieri, E.; Sorrentino, R.; Vanelli, A.; Vanacore, D.; Turnaturi, C.; La Rocca, R.; Celentano, G.; et al. A new original nutraceutical formulation ameliorates the effect of Tadalafil on clinical score and cGMP accumulation. Arch. Ital. Urol. Androl. 2021, 93, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, W.; Ou, N.; Song, Y.; Kang, J.; Liang, Z.; Hu, R.; Yang, Y.; Liu, X. Do testosterone supplements enhance response to phosphodiesterase 5 inhibitors in men with erectile dysfunction and hypogonadism: A systematic review and meta-analysis. Transl. Androl. Urol. 2020, 9, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Vaucher, L.; Bolyakov, A.; Paduch, D.A. Evolving techniques to evaluate ejaculatory function. Curr. Opin. Urol. 2009, 16, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Paduch, D.; Polzer, P.K.; Ni, X.; Basaria, S. Testosterone replacement in androgen-deficient men with ejaculatory dysfunction: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2015, 100, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Jannini, E.A.; Mannucci, E.; Fisher, A.D.; Lotti, F.; Petrone, L.; Balercia, G.; Bandini, E.; Chiarini, V.; Forti, G.; et al. Different testosterone levels are associated with ejaculatory dysfunction. J. Sex. Med. 2008, 5, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, G.R.; Stephens-Shields, A.J.; Rosen, R.C.; Wang, C.; Bhasin, S.; Matsumoto, A.M.; Parsons, J.K.; Gill, T.; Molitch, M.E.; Farrar, J.T.; et al. Testosterone treatment and sexual function in older men with low testosterone levels. J. Clin. Endocrinol. Metab. 2016, 101, 3096–3104. [Google Scholar] [CrossRef] [PubMed]
- Brock, G.B.; Heiselman, D.; Maggi, M.; Kim, S.W.; Vallejo, J.M.R.; Behre, H.M.; Mcgettigan, J.; Dowsett, S.A.; Hayes, R.P.; Knorr, J.; et al. Effect of Testosterone Solution 2% on Testosterone Concentration, Sex Drive and Energy in Hypogonadal Men: Results of a Placebo Controlled Study. J. Urol. 2015, 195, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Brock, G.; Heiselman, D.; Knorr, J.; Ni, X.; Kinchen, K. 9-Month Efficacy and Safety Study of Testosterone Solution 2% for Sex Drive and Energy in Hypogonadal Men. J. Urol. 2016, 196, 1509–1515. [Google Scholar] [CrossRef]
- Pitteloud, N.; Mootha, V.K.; Dwyer, A.A.; Hardin, M.; Lee, H.; Eriksson, K.-F.; Tripathy, D.; Yialamas, M.; Groop, L.; Elahi, D.; et al. Relationship Between Testosterone Levels, Insulin Sensitivity, and Mitochondrial Function in Men. Diabetes Care 2005, 28, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Preziosi, P.; Barrett-Connor, E.; Roger, M.; Saint-Paul, M.; Nahoul, K.; Papoz, L. Interrelation between plasma testosterone and plasma insulin in healthy adult men: The Telecom Study. Diabetologia 1992, 35, 173–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, E.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Fahey, P.; Martin, S.; O’Loughlin, P.; Taylor, A.; Adams, R.J.; Shi, Z.; Wittert, G. Predictive value of serum testosterone for type 2 diabetes risk assessment in men. BMC Endocr. Disord. 2016, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Rubinow, K.B.; Snyder, C.N.; Amory, J.; Hoofnagle, A.N.; Page, S.T. Acute testosterone deprivation reduces insulin sensitivity in men. Clin. Endocrinol. 2011, 76, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Pencina, K.M.; Li, Z.; Basaria, S.; Bhasin, S.; Travison, T.G.; Storer, T.W.; Harman, S.M.; Tsitouras, P. Long-Term Testosterone Administration on Insulin Sensitivity in Older Men with Low or Low-Normal Testosterone Levels. J. Clin. Endocrinol. Metab. 2018, 103, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G.; Bracken, K.; Robledo, K.P.; Grossmann, M.; Yeap, B.B.; Handelsman, D.J.; Stuckey, B.; Conway, A.; Inder, W.; McLachlan, R.; et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): A randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021, 9, 32–45. [Google Scholar] [CrossRef]
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Tishova, Y.; Saad, F.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Testosterone and Metabolic Syndrome: A Meta-Analysis Study. J. Sex. Med. 2011, 8, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Zhao, Y.-L.; Yang, Y.-F.; Wang, X.; Nie, M.; Wu, X.-Y.; Mao, J.-F.; Veglio, F. Metabolic Effects of Testosterone Replacement Therapy in Patients with Type 2 Diabetes Mellitus or Metabolic Syndrome: A Meta-Analysis. Int. J. Endocrinol. 2020, 2020, 4732021. [Google Scholar] [CrossRef]
- Blaya, R.; Thomaz, L.D.G.R.; Guilhermano, F.; Paludo, A.D.O.; Rhoden, L.; Halmenschlager, G.; Rhoden, E.L. Total testosterone levels are correlated to metabolic syndrome components. Aging Male 2016, 19, 85–89. [Google Scholar] [CrossRef]
- Angelova, P.; Kamenov, Z.; Tsakova, A.; El-Darawish, Y.; Okamura, H. Interleukin-18 and testosterone levels in men with metabolic syndrome. Aging Male 2018, 21, 130–137. [Google Scholar] [CrossRef]
- Dandona, P.; Dhindsa, S.; Ghanim, H.; Saad, F. Mechanisms underlying the metabolic actions of testosterone in humans: A narrative review. Diabetes Obes. Metab. 2020, 23, 18–28. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Di Pasquale, G.; Sforza, A.; Mannucci, E.; Maggi, M. Testosterone and Cardiovascular Risk: Meta-Analysis of Interventional Studies. J. Sex. Med. 2018, 15, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.B.; Hyde, Z.; Almeida, O.P.; Norman, P.E.; Chubb, S.A.P.; Jamrozik, K.; Flicker, L.; Hankey, G.J. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J. Clin. Endocrinol. Metab. 2009, 94, 2353–2359. [Google Scholar] [CrossRef]
- Soisson, V.; Brailly-Tabard, S.; Helmer, C.; Rouaud, O.; Ancelin, M.-L.; Zerhouni, C.; Guiochon-Mantel, A.; Scarabin, P.-Y. A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: The French 3C cohort study. Maturitas 2013, 75, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Dixon, J.M.; Suarez, E.A.; Murad, M.H.; Guey, L.T.; Wittert, G.A. Endogenous testosterone and mortality in men: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; D’Amico, A.V.; Berger, P.; Clark, P.E.; Eckel, R.H.; Keating, N.L.; Milani, R.V.; Sagalowsky, A.I.; Smith, M.R.; Zakai, N. Androgen-Deprivation Therapy in Prostate Cancer and Cardiovascular Risk: A Science Advisory From the American Heart Association, American Cancer Society, and American Urological Association: Endorsed by the American Society for Radiation Oncology. CA A Cancer J. Clin. 2010, 60, 194–201. [Google Scholar] [CrossRef]
- Xu, L.; Freeman, G.; Cowling, B.J.; Schooling, C.M. Testosterone therapy and cardiovascular events among men: A systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Maseroli, E.; Rastrelli, G.; Isidori, A.; Sforza, A.; Mannucci, E.; Maggi, M. Cardiovascular risk associated with testosterone-boosting medications: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2014, 13, 1327–1351. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Carson, C.; Dobs, A.; Kopecky, S.; Mohler, E.R. Testosterone and Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 67, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Oskui, P.M.; French, W.J.; Herring, M.J.; Mayeda, G.S.; Burstein, S.; Kloner, R.A. Testosterone and the cardiovascular system: A Comprehensive review of the clinical literature. J. Am. Hear. Assoc. 2013, 2, e000272. [Google Scholar] [CrossRef]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse Events Associated with Testosterone Administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Finkle, W.D.; Greenland, S.; Ridgeway, G.K.; Adams, J.L.; Frasco, M.A.; Cook, M.B.; Fraumeni, J.F., Jr.; Hoover, R.N. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE 2014, 9, e85805. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, J.; Urban, R.J.; Kuo, Y.-F.; Ottenbacher, K.; Raji, M.A.; Du, F.; Lin, Y.-L.; Goodwin, J.S. Risk of myocardial infarction in older men receiving testosterone therapy. Ann. Pharmacother. 2014, 48, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Oni, O.A.; Gupta, K.; Chen, G.; Sharma, M.; Dawn, B.; Sharma, R.; Parashara, D.K.; Savin, V.J.; Ambrose, J.A.; et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur. Heart J. 2015, 36, 2706–2715. [Google Scholar] [CrossRef]
- Garnick, M.B. Testosterone Replacement Therapy Faces FDA Scrutiny. JAMA 2015, 313, 563–564. [Google Scholar] [CrossRef]
- Golightly, L.; Young, A. Sex hormones and mental health. Adv. Psychiatr. Treat. 1999, 5, 126–134. [Google Scholar] [CrossRef]
- Napolitano, L.; Barone, B.; Morra, S.; Celentano, G.; La Rocca, R.; Capece, M.; Morgera, V.; Turco, C.; Caputo, V.F.; Spena, G.; et al. Hypogonadism in Patients with Prader Willi Syndrome: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 1993. [Google Scholar] [CrossRef]
- Zitzmann, M. Testosterone, mood, behaviour and quality of life. Andrology 2020, 8, 1598–1605. [Google Scholar] [CrossRef]
- Schneider, G.; Nienhaus, K.; Gromoll, J.; Heuft, G.; Nieschlag, E.; Zitzmann, M. Aging males’ symptoms in relation to the genetically determined androgen receptor CAG polymorphism, sex hormone levels and sample membership. Psychoneuroendocrinology 2010, 35, 578–587. [Google Scholar] [CrossRef]
- Shores, M.M.; Moceri, V.M.; Sloan, K.L.; Matsumoto, A.M.; Kivlahan, D.R. Low testosterone levels predict incident depressive illness in older men: Effects of age and medical morbidity. J. Clin. Psychiatry 2005, 66, 7–14. [Google Scholar] [CrossRef]
- Amiaz, R.; Seidman, S.N. Testosterone and depression in men. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 278–283. [Google Scholar] [CrossRef]
- Fink, G.; Sumner, B.; Rosie, R.; Wilson, H.; McQueen, J. Androgen actions on central serotonin neurotransmission: Relevance for mood, mental state and memory. Behav. Brain Res. 1999, 105, 53–68. [Google Scholar] [CrossRef]
- van Honk, J.; Peper, J.S.; Schutter, D.J. Testosterone Reduces Unconscious Fear but Not Consciously Experienced Anxiety: Implications for the Disorders of Fear and Anxiety. Biol. Psychiatry 2005, 58, 218–225. [Google Scholar] [CrossRef]
- Rowe, R.; Maughan, B.; Worthman, C.M.; Costello, E.; Angold, A. Testosterone, antisocial behavior, and social dominance in boys: Pubertal development and biosocial interaction. Biol. Psychiatry 2004, 55, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Reimers, L.; Diekhof, E.K. Testosterone is associated with cooperation during intergroup competition by enhancing parochial altruism. Front. Neurosci. 2015, 9, 183. [Google Scholar] [CrossRef]
- Margolese, H.C. The male menopause and mood: Testosterone decline and depression in the aging male—Is there a link? J. Geriatr. Psychiatry Neurol. 2000, 13, 93–101. [Google Scholar] [CrossRef]
- Schneider, G.; Nienhaus, K.; Gromoll, J.; Heuft, G.; Nieschlag, E.; Zitzmann, M. Sex hormone levels, genetic androgen receptor polymorphism, and anxiety in ≥50-year-old males. J. Sex. Med. 2011, 8, 3452–3464. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tajar, A.; Beynon, J.M.; Pye, S.; Silman, A.J.; Finn, J.D.; O’Neill, T.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef]
- Giltay, E.J.; Tishova, Y.A.; Mskhalaya, G.J.; Gooren, L.J.; Saad, F.; Kalinchenko, S.Y. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J. Sex. Med. 2010, 7, 2572–2582. [Google Scholar] [CrossRef]
- Snyder, P.J.; Bhasin, S.; Cunningham, G.R.; Matsumoto, A.M.; Stephens-Shields, A.J.; Cauley, J.A.; Gill, T.M.; Barrett-Connor, E.; Swerdloff, R.S.; Wang, C.; et al. Effects of Testosterone Treatment in Older Men. N. Engl. J. Med. 2016, 374, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Breidenstein, J.; Miller, R. Association of testosterone treatment with alleviation of depressive symptoms in men: A systematic review and meta-analysis. JAMA Psychiatry 2019, 76, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zarrouf, F.A.; Artz, S.; Griffith, J.; Sirbu, C.; Kommor, M. Testosterone and depression: Systematic review and meta-analysis. J. Psychiatr. Pract. 2009, 15, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Seidman, S.N.; Rabkin, J.G. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J. Affect. Disord. 1998, 48, 157–161. [Google Scholar] [CrossRef]
- Vartolomei, M.D.; Kimura, S.; Vartolomei, L.; Shariat, S.F. Systematic Review of the Impact of Testosterone Replacement Therapy on Depression in Patients with Late-onset Testosterone Deficiency. Eur. Urol. Focus 2018, 6, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.J.; Putman, P.; Baas, J.; Koppeschaar, H.P.; van Honk, J. A Single Administration of Testosterone Reduces Fear-Potentiated Startle in Humans. Biol. Psychiatry 2006, 59, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.H.; Prytz, H.S.; Perski, A.; Svartberg, J. Testosterone levels and psychological health status in men from a general population: The Tromsø study. Aging Male 2011, 14, 37–41. [Google Scholar] [CrossRef]
- Marriott, R.J.; Murray, K.; Flicker, L.; Hankey, G.J.; Matsumoto, A.M.; Dwivedi, G.; Antonio, L.; Almeida, O.P.; Bhasin, S.; Dobs, A.S.; et al. Lower serum testosterone concentrations are associated with a higher incidence of dementia in men: The UK Biobank prospective cohort study. Alzheimer’s Dement. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E. Impact of Testosterone on Alzheimer’s Disease. World J. Men’s Health 2022, 40. [Google Scholar] [CrossRef] [PubMed]
- Bourque, M.; Soulet, D.; Di Paolo, T. Androgens and Parkinson’s Disease: A Review of Human Studies and Animal Models. Androg. Clin. Res. Ther. 2021, 2, 294–303. [Google Scholar] [CrossRef]
- Pope, H.G.; Kouri, E.M.; Hudson, J.I. Effects of Supraphysiologic Doses of Testosterone on Mood and Aggression in Normal Men: A Randomized Controlled Trial. Arch. Gen. Psychiatry 2000, 57, 133–140. [Google Scholar] [CrossRef]
- Chegeni, R.; Pallesen, S.; McVeigh, J.; Sagoe, D. Anabolic-androgenic steroid administration increases self-reported aggression in healthy males: A systematic review and meta-analysis of experimental studies. Psychopharmacology 2021, 238, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Hauger, L.E.; Havnes, I.A.; Jørstad, M.L.; Bjørnebekk, A. Anabolic androgenic steroids, antisocial personality traits, aggression and violence. Drug Alcohol Depend. 2021, 221, 108604. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.B.; Archer, J.; Hair, W.; Wu, F.C. Exogenous testosterone, aggression, and mood in eugonadal and hypogonadal men. Physiol. Behav. 2002, 75, 557–566. [Google Scholar] [CrossRef]
- Kenny, A.M.; Fabregas, G.; Song, C.; Biskup, B.; Bellantonio, S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J. Gerontol. Ser. A 2004, 59, M75–M78. [Google Scholar] [CrossRef] [PubMed]
- Geniole, S.; Bird, B.; McVittie, J.; Purcell, R.; Archer, J.; Carré, J. Is testosterone linked to human aggression? A meta-analytic examination of the relationship between baseline, dynamic, and manipulated testosterone on human aggression. Horm. Behav. 2020, 123, 104644. [Google Scholar] [CrossRef]
- Testa, M.A.; Simonson, D.C. Assessment of Quality-of-Life Outcomes. N. Engl. J. Med. 1996, 334, 835–840. [Google Scholar] [CrossRef]
- Morssinkhof, M.; van Wylick, D.; Priester-Vink, S.; van der Werf, Y.; Heijer, M.D.; Heuvel, O.V.D.; Broekman, B. Associations between sex hormones, sleep problems and depression: A systematic review. Neurosci. Biobehav. Rev. 2020, 118, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Kelly, S.; Millar, A.C.; Peterson, J.; Chen, L.; Johnston, A.; Kotb, A.; Skidmore, B.; Bai, Z.; Mamdani, M.; et al. Testosterone therapy in hypogonadal men: A systematic review and network meta-analysis. BMJ Open 2017, 7. [Google Scholar] [CrossRef]
- Amanatkar, H.R.; Chibnall, J.T.; Seo, B.-W.; Manepalli, J.N.; Grossberg, G.T. Impact of exogenous testosterone on mood: A systematic review and meta-analysis of randomized placebo-controlled trials. Ann. Clin. Psychiatry 2014, 26, 19–32. [Google Scholar] [PubMed]
- Behre, H.M.; Tammela, T.L.J.; Arver, S.; Tolrá, J.R.; Bonifacio, V.; Lamche, M.; Kelly, J.; Hiemeyer, F.; Giltay, E.J.; Gooren, L.J.; et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male 2012, 15, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.-F.; Ng, C.J.; Lee, B.-C.; Lee, V.-K.M.; Khoo, E.-M.; Lee, E.-G.; Tan, H.-M. Effect of long-acting testosterone undecanoate treatment on quality of life in men with testosterone deficiency syndrome: A double blind randomized controlled trial. Asian J. Androl. 2012, 14, 604–611. [Google Scholar] [CrossRef]
- Rosen, R.C.; Wu, F.; Behre, H.M.; Porst, H.; Meuleman, E.J.; Maggi, M.; Romero-Otero, J.; Martinez-Salamanca, J.I.; Jones, T.H.; Debruyne, F.M.; et al. Quality of Life and Sexual Function Benefits of Long-Term Testosterone Treatment: Longitudinal Results From the Registry of Hypogonadism in Men (RHYME). J. Sex. Med. 2017, 14, 1104–1115. [Google Scholar] [CrossRef]
- Yassin, A.; AlRumaihi, K.; Alzubaidi, R.; Alkadhi, S.; Al Ansari, A. Testosterone, testosterone therapy and prostate cancer. Aging Male 2019, 22, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Morgentaler, A.; Bruning, C.O.; DeWolf, W.C. Occult prostate cancer in men with low serum testosterone levels. JAMA: J. Am. Med Assoc. 1996, 276, 1904–1906. [Google Scholar] [CrossRef]
- Morgentaler, A.; Traish, A.M. Shifting the Paradigm of Testosterone and Prostate Cancer: The Saturation Model and the Limits of Androgen-Dependent Growth. Eur. Urol. 2009, 55, 310–321. [Google Scholar] [CrossRef]
- di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Zhu, Y.; Luo, J. Regulation of androgen receptor variants in prostate cancer. Asian J. Urol. 2020, 7, 251–257. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, D.; Ma, X.; Xu, K.; Ding, B.; Li, H.; Wang, Z.; Ouyang, W.; Long, G.; et al. Androgen Receptor Splice Variant 7 Predicts Shorter Response in Patients with Metastatic Hormone-sensitive Prostate Cancer Receiving Androgen Deprivation Therapy. Eur. Urol. 2021, 79, 879–886. [Google Scholar] [CrossRef]
- Cui, Y.; Zong, H.; Yan, H.; Zhang, Y. The effect of testosterone replacement therapy on prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014, 17, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.L.; Trinh, Q.; Sun, M.; Carter, S.C.; Nguyen, P.L.; Shih, Y.T.; Marks, L.S.; Hu, J.C. Testosterone replacement therapy following the diagnosis of prostate cancer: Outcomes and utilization trends. J. Sex. Med. 2014, 11, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Oefelein, M.G. Testosterone replacement therapy after primary treatment for prostate cancer. J. Urol. 2005, 173, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Lenfant, L.; Leon, P.; Cancel-Tassin, G.; Audouin, M.; Staerman, F.; Rouprêt, M.; Cussenot, O. Testosterone replacement therapy (TRT) and prostate cancer: An updated systematic review with a focus on previous or active localized prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.L.; Lenis, A.T.; Shah, A.; Rajfer, J.; Hu, J.C. Testosterone replacement therapy in men with prostate cancer: A time-varying analysis. J. Sex. Med. 2015, 12, 374–380. [Google Scholar] [CrossRef]
- Jones, S.D.; Dukovac, T.; Sangkum, P.; Yafi, F.A.; Hellstrom, W.J. Erythrocytosis and Polycythemia Secondary to Testosterone Replacement Therapy in the Aging Male. Sex. Med. Rev. 2015, 3, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kohn, T.P.; Mata, D.A.; Ramasamy, R.; Lipshultz, L.I. Effects of Testosterone Replacement Therapy on Lower Urinary Tract Symptoms: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 69, 1083–1090. [Google Scholar] [CrossRef]
- Baas, W.; Köhler, T.S. Testosterone Replacement Therapy and BPH/LUTS. What is the Evidence? Curr. Urol. Rep. 2016, 17, 46. [Google Scholar] [CrossRef]
- Accurso, V.; Santoro, M.; Mancuso, S.; Contrino, A.D.; Casimiro, P.; Sardo, M.; Raso, S.; Di Piazza, F.; Perez, A.; Bono, M.; et al. Cardiovascular risk in essential thrombocythemia and polycythemia vera: Thrombotic risk and survival. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020008. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Calogero, A.E.; Cannarella, R.; Condorelli, R.A.; Magagnini, C.; Aversa, A. Obstructive Sleep Apnea and Testosterone Replacement Therapy. Androg. Clin. Res. Ther. 2020, 1, 10–14. [Google Scholar] [CrossRef]
- Grech, A.; Breck, J.; Heidelbaugh, J. Adverse effects of testosterone replacement therapy: An update on the evidence and controversy. Ther. Adv. Drug Saf. 2014, 5, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; D’Andrea, S.; Francavilla, S. Testosterone replacement therapy. Andrology 2020, 8, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, J.J.; Wilson, M.K.; Spinner, M.L. Pharmacology of testosterone replacement therapy preparations. Transl. Androl. Urol. 2016, 5, 834–843. [Google Scholar] [CrossRef] [PubMed]

| Author | Study Design | Sample Size | Aim of the Study | T Formulation | Outcome |
|---|---|---|---|---|---|
| Saad, 2013 [34] | P | 255 | T effect on anthropometric parameters in hypogonadal men | T undecanoate | Improved BW, WC, and BMI |
| Haider, 2014 [37] | P | 156 | T effect in obese men and type 2 diabetes mellitus with T deficiency | T undecanoate | Improved HbA1c, WC, BMI |
| Snyder, 1999 [74] | RCT | 108 | T effect on bone density | T gel | Improved Lumbar BMD |
| Basurto, 2008 [75] | RCT | 48 | T effect on bone density healthy elderly men with low levels of total testosterone | T enanthate | Improved Lumbar BMD |
| Saad, 2019 [89] | P | 420 | T effect on ED in hypogonadal men | T undecanoate | Improved EF |
| Paduch, 2015 [95] | RCT | 76 | T effect on ED in hypogonadal men | T gel | Improved EF |
| Wittert, 2021 [106] | RCT | 504 | T effect on early type 2 diabetes | T undecanoate | Improved Type 2 diabetes |
| Sharma, 2015 [124] | R | 83 010 | T effect on cardiovascular system | T undecanoato/gel | Decreased All-cause mortality, MI, and stroke. |
| Giltay, 2010 [139] | RCT | 184 | T effect on depressive symptoms | T undecanoate | Decreased Depression symptoms |
| Behre, 2015 [160] | RCT | 362 | T effect on Quality of life in hypogonadal men | T gel | Improved Quality of life |
| Kaplan, 2014 [171] | O | 1181 | T effect in men with prostate cancer. | T undecanoate | No improve Cancer-specific mortality |
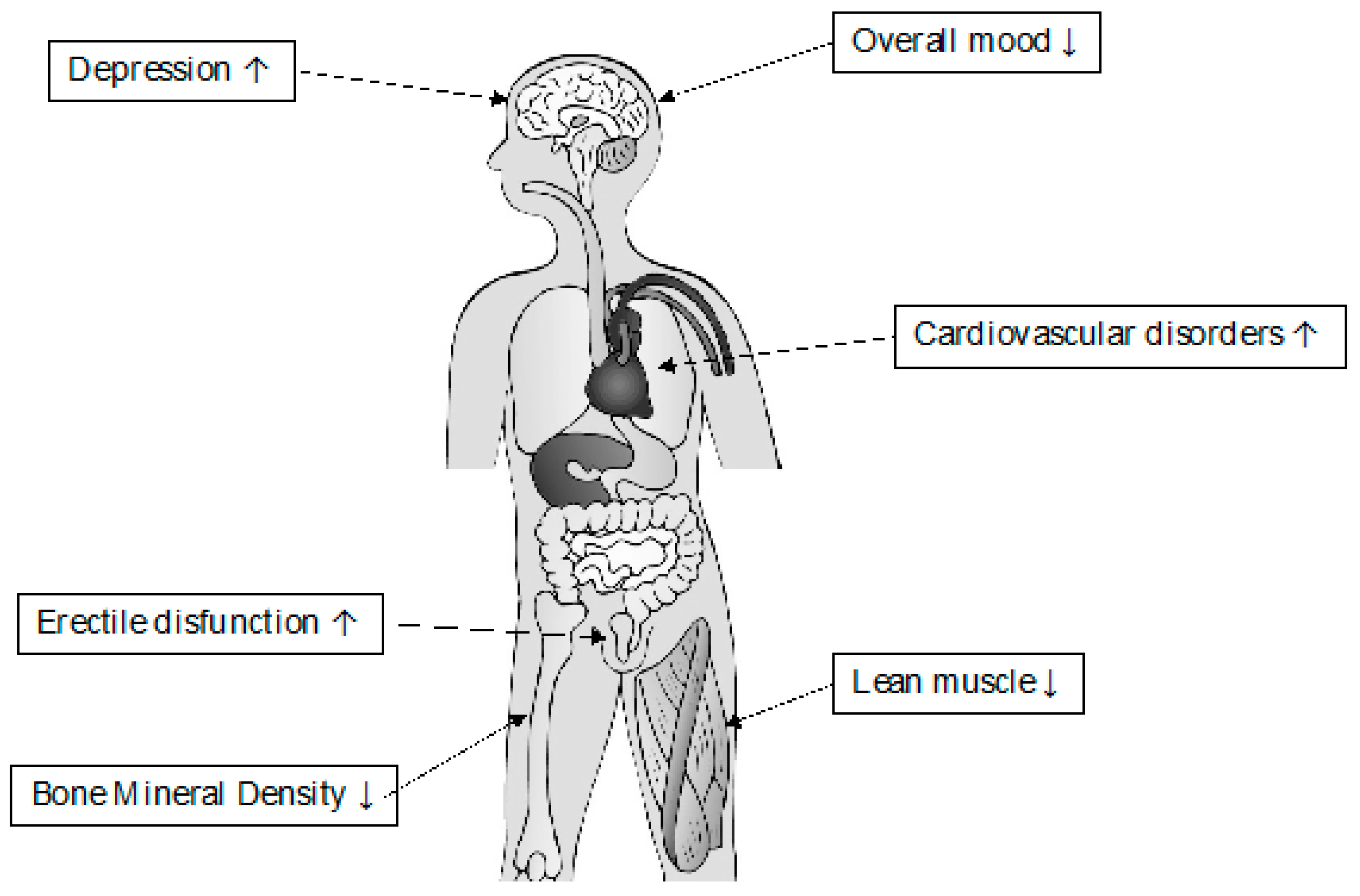
| Signs and Symptoms Related to Low Testosterone Levels | |
|---|---|
| Bone Density | Osteoporosis Decreased resistance to fractures |
| Mental Activity | Depression Poor concentration Fear Anxiety |
| Body Composition | Low muscle mass Decreased strength Increased muscular fatigue |
| Glycometabolic profile | Obesity Insulin resistance Metabolic Syndrome |
| Cardiovascular disease | Hot flushes Increased risk of atheroma development Endothelial dysfunction |
| Sexual Activity | Erectile dysfunction Low desire Ejaculation disorders |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, B.; Napolitano, L.; Abate, M.; Cirillo, L.; Reccia, P.; Passaro, F.; Turco, C.; Morra, S.; Mastrangelo, F.; Scarpato, A.; et al. The Role of Testosterone in the Elderly: What Do We Know? Int. J. Mol. Sci. 2022, 23, 3535. https://doi.org/10.3390/ijms23073535
Barone B, Napolitano L, Abate M, Cirillo L, Reccia P, Passaro F, Turco C, Morra S, Mastrangelo F, Scarpato A, et al. The Role of Testosterone in the Elderly: What Do We Know? International Journal of Molecular Sciences. 2022; 23(7):3535. https://doi.org/10.3390/ijms23073535
Chicago/Turabian StyleBarone, Biagio, Luigi Napolitano, Marco Abate, Luigi Cirillo, Pasquale Reccia, Francesco Passaro, Carmine Turco, Simone Morra, Francesco Mastrangelo, Antonio Scarpato, and et al. 2022. "The Role of Testosterone in the Elderly: What Do We Know?" International Journal of Molecular Sciences 23, no. 7: 3535. https://doi.org/10.3390/ijms23073535
APA StyleBarone, B., Napolitano, L., Abate, M., Cirillo, L., Reccia, P., Passaro, F., Turco, C., Morra, S., Mastrangelo, F., Scarpato, A., Amicuzi, U., Morgera, V., Romano, L., Calace, F. P., Pandolfo, S. D., De Luca, L., Aveta, A., Sicignano, E., Trivellato, M., ... Crocetto, F. (2022). The Role of Testosterone in the Elderly: What Do We Know? International Journal of Molecular Sciences, 23(7), 3535. https://doi.org/10.3390/ijms23073535










