Impact of Endocrine Disruptors upon Non-Genetic Inheritance
Abstract
:1. Introduction
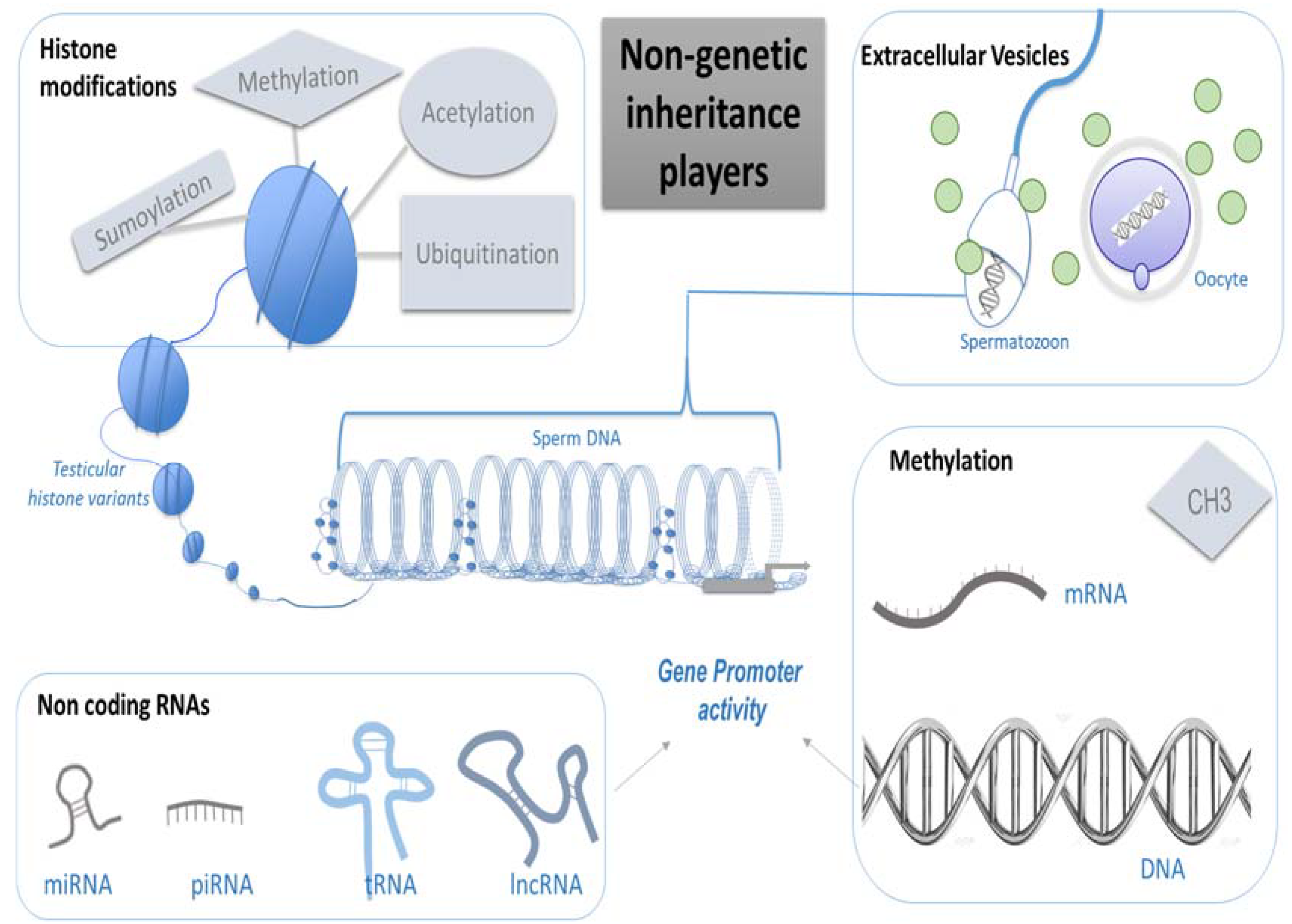
Non Genetic Inheritance Players
- (a)
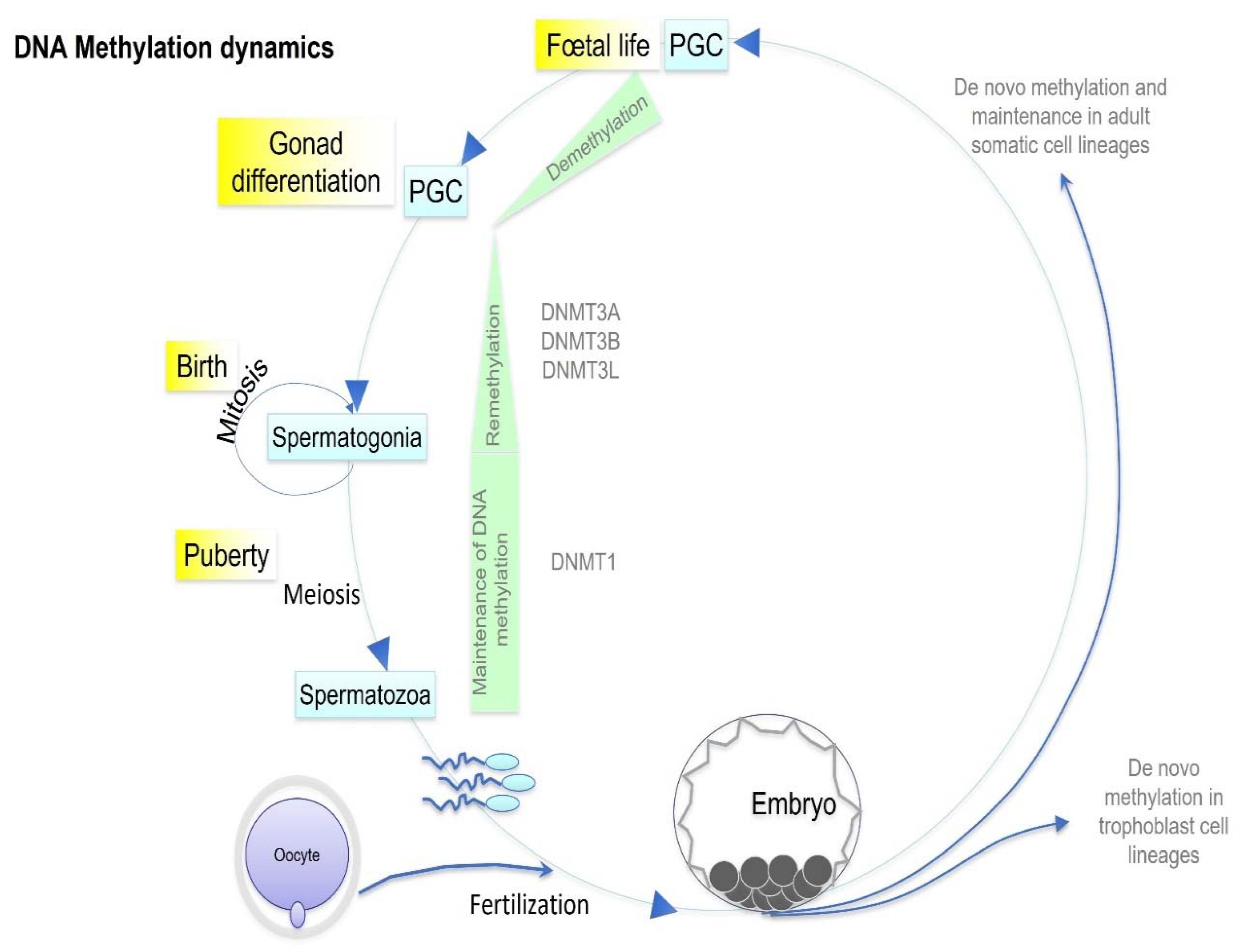
- Methylation
- (b)
- Histone Modifications
- (c)
- Non-coding RNAs
- (d)
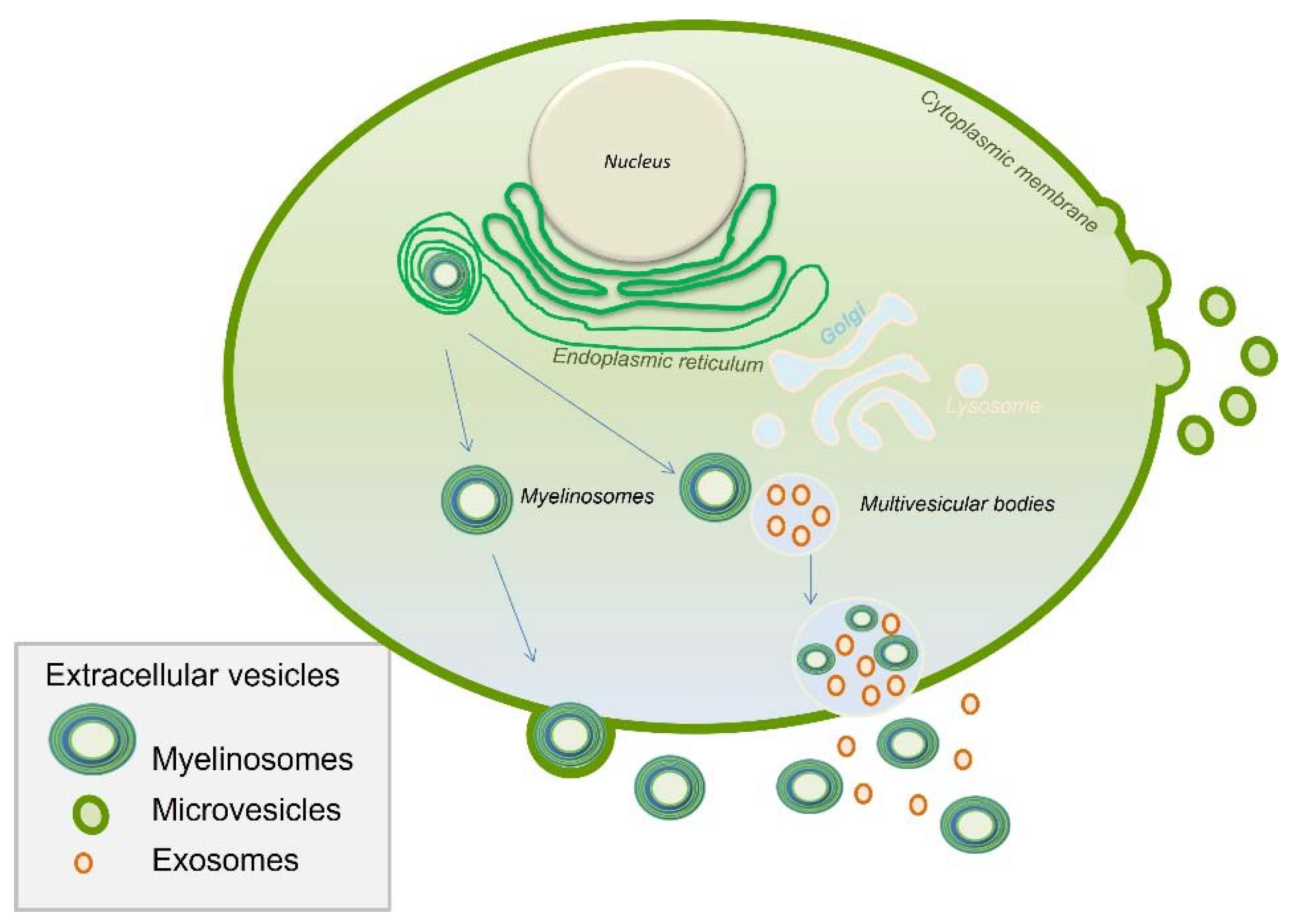
- Extracellular Vesicles
2. Endocrine-Disrupting Chemicals (EDCs) Transgenerational Impact
2.1. Dioxin
2.2. Diethylstilbestrol
2.3. Fungicides
2.4. Organochlorine Pesticides
2.5. Bisphenol A
2.6. PolyChlorinated Biphenyls
2.7. Phtalates
2.8. Perfluoroalkyl and Polyfluoroalkyl Substances
2.9. Flame Retardants
2.10. Polycyclic Aromatic Hydrocarbons
3. Epigenetic Inheritance
4. After-Effects of EDCs Exposure on Germ Cells
4.1. Epigenetic Sperm Modifications
4.2. Epigenetic Oocyte Modifications
5. Embryo Development and Critical Window
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMH | Anti-Mullerian hormone |
| ART | Assisted reproductive techniques |
| BDE-47 | 2,2′,4,4′-tetrabromodiphenyl ether |
| BPA | Bisphenol A |
| DES | Diethylstilbestrol |
| DMRs | Differentially methylated regions |
| DDT | 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane |
| DEHP | Di(2-ethylhexyl) phthalate |
| EE2 | Ethinylestradiol |
| EDCs | Endocrine-disrupting chemicals |
| Endo-siRNAs | Endogenous-small interfering RNAs |
| EVs | Extracellular vesicles |
| FDA | U.S. Food and Drug Administration |
| H3K9me3 | H3K9 trimethylation |
| HAT | Histone acetyltransferase |
| HPTE | 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane |
| Igf2 | Insulin-like growth factor 2 |
| lincRNAs | Large intergenic non-coding RNAs |
| LIF | Leukemia inhibitory factor |
| lncRNA | Long non-coding RNA |
| miRNAs | MicroRNAs |
| mRNA | Messenger RNA |
| MXC | Methoxychlor (1,1,1-trichloro-2,2-bis(p-methoxyphenyl) ethane) |
| NOAEL | No-observed-adverse-effect level |
| OP | Organophosphate |
| OPFRs | Organophosphate flame retardants, |
| piRNAs | Piwi-interacting RNAs |
| PBDEs | Polybrominated diphenyl ethers |
| PAHs | Polycyclic aromatic hydrocarbons |
| PCBs | Polychlorinated biphenyls |
| PCOS | Polycystic ovary syndrome |
| PFOS | Perfluorooctane sulfonate |
| PFOA | Perfluorooctanoate |
| PGC | Primordial germ cells |
| PPAR | Peroxisome proliferator-activated receptor |
| snoRNAs | Small nucleolar RNAs |
| sncRNA | Small non-coding RNAs |
| SUV39H2 | SUppressor of Variegation 3-9 homolog 2 Histone Lysine Methyltransferase |
| TBBPA | Tetrabromobisphenol A |
| TBT | Tributyltin |
| TCDD | 2,3,7,8-tetrachlordibenzo-p-dioxin |
| TPT | Triphenyltin |
| Xist | X-inactive specific transcript |
References
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Ravel, C.; Kah, O. Endocrine disrupters: Towards an unsatisfying regulation. Presse Med. 2018, 47 Pt 1, 943–949. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440. [Google Scholar] [CrossRef]
- MacLean, J.A.; Hayashi, K. Progesterone Actions and Resistance in Gynecological Disorders. Cells 2022, 11, 647. [Google Scholar] [CrossRef]
- Siemienowicz, K.J.; Wang, Y.; Marečková, M.; Nio-Kobayashi, J.; Fowler, P.A.; Rae, M.T.; Duncan, W.C. Early pregnancy maternal progesterone administration alters pituitary and testis function and steroid profile in male fetuses. Sci. Rep. 2020, 10, 21920. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Major, K.M.; Decourten, B.M.; Li, J.; Britton, M.; Settles, M.L.; Mehinto, A.C.; Connon, R.E.; Brander, S.M. Early Life Exposure to Environmentally Relevant Levels of Endocrine Disruptors Drive Multigenerational and Transgenerational Epigenetic Changes in a Fish Model. Front. Mar. Sci. 2020, 7, 471. [Google Scholar] [CrossRef]
- Neyroud, A.-S.; Chiechio, R.; Yefimova, M.; Faro, M.J.L.; Dejucq-Rainsford, N.; Jaillard, S.; Even-Hernandez, P.; Marchi, V.; Ravel, C. Extra-cellular vesicles of the male genital tract: New actors in male fertility? Basic Clin. Androl. 2021, 31, 25. [Google Scholar] [CrossRef]
- Brieño-Enriquez, M.A.; Lopez, J.G.; Cárdenas, D.B.; Guibert, S.; Cleroux, E.; Děd, L.; Hourcade, J.D.D.; Pěknicová, J.; Weber, M.; DEL Mazo, J. Exposure to Endocrine Disruptor Induces Transgenerational Epigenetic Deregulation of MicroRNAs in Primordial Germ Cells. PLoS ONE 2015, 10, e0124296. [Google Scholar] [CrossRef] [Green Version]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [Green Version]
- Ben Maamar, M.; Sadler-Riggleman, I.; Beck, D.; McBirney, M.; Nilsson, E.; Klukovich, R.; Xie, Y.; Tang, C.; Yan, W.; Skinner, M.K. Alterations in sperm DNA methylation, non-coding RNA expression, and histone retention mediate vinclozolin-induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2018, 4, dvy010. [Google Scholar] [CrossRef]
- Skinner, M.K. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol. Cell. Endocrinol. 2014, 398, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.L.; Grant, P.A. The Role of DNA Methylation and Histone Modifications in Transcriptional Regulation in Humans. Subcell Biochem. 2013, 61, 289–317. [Google Scholar] [CrossRef]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef]
- Guéant, J.-L.; Chéry, C.; Oussalah, A.; Nadaf, J.; Coelho, D.; Josse, T.; Flayac, J.; Robert, A.; Koscinski, I.; Gastin, I.; et al. A PRDX1 mutant allele causes a MMACHC secondary epimutation in cblC patients. Nat. Commun. 2018, 9, 67. [Google Scholar] [CrossRef]
- Skinner, M.K.; Guerrero-Bosagna, C. Role of CpG deserts in the epigenetic transgenerational inheritance of differential DNA methylation regions. BMC Genom. 2014, 15, 692. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhao, P.; Xia, Q. Epigenetic Methylations on N6-Adenine and N6-Adenosine with the same Input but Different Output. Int. J. Mol. Sci. 2019, 20, 2931. [Google Scholar] [CrossRef] [Green Version]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef]
- Bao, J.; Bedford, M.T. Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction 2016, 151, R55–R70. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, V. The Expanding Constellation of Histone Post-Translational Modifications in the Epigenetic Landscape. Genes 2021, 12, 1596. [Google Scholar] [CrossRef]
- Osada, S.; Nishikawa, J.; Nakanishi, T.; Tanaka, K.; Nishihara, T. Some organotin compounds enhance histone acetyltransferase activity. Toxicol. Lett. 2005, 155, 329–335. [Google Scholar] [CrossRef]
- Hammoud, S.S.; Nix, D.A.; Hammoud, A.O.; Gibson, M.; Cairns, B.R.; Carrell, D.T. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum. Reprod. 2011, 26, 2558–2569. [Google Scholar] [CrossRef] [Green Version]
- Legoff, L.; D’Cruz, S.C.; Lebosq, M.; Gely-Pernot, A.; Bouchekhchoukha, K.; Monfort, C.; Kernanec, P.-Y.; Tevosian, S.; Multigner, L.; Smagulova, F. Developmental exposure to chlordecone induces transgenerational effects in somatic prostate tissue which are associated with epigenetic histone trimethylation changes. Environ. Int. 2021, 152, 106472. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Ben Maamar, M.; Skinner, M.K. Role of epigenetic transgenerational inheritance in generational toxicology. Environ. Epigenet. 2022, 8, dvac001. [Google Scholar] [CrossRef]
- Yan, W. Potential roles of noncoding RNAs in environmental epigenetic transgenerational inheritance. Mol. Cell. Endocrinol. 2014, 398, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Rutenberg-Schoenberg, M.; Sexton, A.N.; Simon, M.D. The Properties of Long Noncoding RNAs That Regulate Chromatin. Annu. Rev. Genom. Hum. Genet. 2016, 17, 69–94. [Google Scholar] [CrossRef]
- Joshi, M.; Rajender, S. Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod. Biol. Endocrinol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Oberbauer, V.; Schaefer, M.R. tRNA-Derived Small RNAs: Biogenesis, Modification, Function and Potential Impact on Human Disease Development. Genes 2018, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.-H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Höög, J.L.; Lötvall, J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Yefimova, M.G.; Béré, E.; Cantereau-Becq, A.; Meunier-Balandre, A.-C.; Merceron, B.; Burel, A.; Merienne, K.; Ravel, C.; Becq, F.; Bourmeyster, N. Myelinosome Organelles in the Retina of R6/1 Huntington Disease (HD) Mice: Ubiquitous Distribution and Possible Role in Disease Spreading. Int. J. Mol. Sci. 2021, 22, 12771. [Google Scholar] [CrossRef]
- Yefimova, M.; Ravel, C.; Neyroud, A.-S.; Béré, E.; Bourmeyster, N. Myelinosomes: A new pathway of protein quality control. Med. Sci. 2020, 36, 1012–1017. [Google Scholar] [CrossRef]
- Asaadi, A.; Dolatabad, N.A.; Atashi, H.; Raes, A.; Van Damme, P.; Hoelker, M.; Hendrix, A.; Pascottini, O.B.; Van Soom, A.; Kafi, M.; et al. Extracellular Vesicles from Follicular and Ampullary Fluid Isolated by Density Gradient Ultracentrifugation Improve Bovine Embryo Development and Quality. Int. J. Mol. Sci. 2021, 22, 578. [Google Scholar] [CrossRef]
- Bridi, A.; Perecin, F.; Da Silveira, J.C. Extracellular Vesicles Mediated Early Embryo–Maternal Interactions. Int. J. Mol. Sci. 2020, 21, 1163. [Google Scholar] [CrossRef] [Green Version]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Fischer, S.; Cornils, K.; Speiseder, T.; Badbaran, A.; Reimer, R.; Indenbirken, D.; Grundhoff, A.; Brunswig-Spickenheier, B.; Alawi, M.; Lange, C. Indication of Horizontal DNA Gene Transfer by Extracellular Vesicles. PLoS ONE 2016, 11, e0163665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Goff, M.; Lagadic-Gossmann, D.; Latour, R.; Podechard, N.; Grova, N.; Gauffre, F.; Chevance, S.; Burel, A.; Appenzeller, B.M.R.; Ulmann, L.; et al. PAHs increase the production of extracellular vesicles both in vitro in endothelial cells and in vivo in urines from rats. Environ. Pollut. 2019, 255 Pt 1, 113171. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.A.; Jayasooriah, N.; Buckland, M.E.; Martin, D.I.; Cropley, J.E.; Suter, C.M. Roll over Weismann: Extracellular vesicles in the transgenerational transmission of environmental effects. Epigenomics 2015, 7, 1165–1171. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.P.; Chan, J.C.; Bale, T.L. Driving the Next Generation: Paternal Lifetime Experiences Transmitted via Extracellular Vesicles and Their Small RNA Cargo. Biol. Psychiatry 2019, 85, 164–171. [Google Scholar] [CrossRef]
- Bokobza, E.; Hinault, C.; Tiroille, V.; Clavel, S.; Bost, F.; Chevalier, N. The Adipose Tissue at the Crosstalk Between EDCs and Cancer Development. Front. Endocrinol. 2021, 12, 691658. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Ames, J.; Rauch, S.; Signorini, S.; Brambilla, P.; Mocarelli, P.; Siracusa, C.; Holland, N.; Warner, M. Dioxin exposure associated with fecundability and infertility in mothers and daughters of Seveso, Italy. Hum. Reprod. 2021, 36, 794–807. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Falandysz, J. Polybrominated dibenzo-p-dioxins and furans (PBDD/Fs): Contamination in food, humans and dietary exposure. Sci. Total Environ. 2021, 761, 143191. [Google Scholar] [CrossRef]
- Akinola, L.K.; Uzairu, A.; Shallangwa, G.A.; Abechi, S.E. Theoretical study on endocrine disrupting effects of polychlorinated dibenzo- p -dioxins using molecular docking simulation. J. Appl. Toxicol. 2021, 41, 233–246. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Dioxin (TCDD) Induces Epigenetic Transgenerational Inheritance of Adult Onset Disease and Sperm Epimutations. PLoS ONE 2012, 7, e46249. [Google Scholar] [CrossRef]
- Gaspari, L.; Paris, F.; Kalfa, N.; Soyer-Gobillard, M.-O.; Sultan, C.; Hamamah, S. Experimental Evidence of 2,3,7,8-Tetrachlordibenzo-p-Dioxin (TCDD) Transgenerational Effects on Reproductive Health. Int. J. Mol. Sci. 2021, 22, 9091. [Google Scholar] [CrossRef]
- Gaspari, L.; Soyer-Gobillard, M.-O.; Paris, F.; Kalfa, N.; Hamamah, S.; Sultan, C. Multigenerational endometriosis: Consequence of fetal exposure to diethylstilbestrol? Environ. Health 2021, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Titus, L.; Hatch, E.E.; Drake, K.M.; Parker, S.E.; Hyer, M.; Palmer, J.R.; Strohsnitter, W.C.; Adam, E.; Herbst, A.L.; Huo, D.; et al. Reproductive and hormone-related outcomes in women whose mothers were exposed in utero to diethylstilbestrol (DES): A report from the US National Cancer Institute DES Third Generation Study. Reprod. Toxicol. 2019, 84, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Paris, F.; Cassel-Knipping, N.; Villeret, J.; Verschuur, A.; Soyer-Gobillard, M.-O.; Carcopino-Tusoli, X.; Hamamah, S.; Kalfa, N.; Sultan, C. Diethylstilbestrol exposure during pregnancy with primary clear cell carcinoma of the cervix in an 8-year-old granddaughter: A multigenerational effect of endocrine disruptors? Hum. Reprod. 2020, 36, deaa267. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Paris, F.; Soyer-Gobillard, M.-O.; Hamamah, S.; Kalfa, N.; Sultan, C. “Idiopathic” partial androgen insensitivity syndrome in 11 grandsons of women treated by diethylstilbestrol during gestation: A multi-generational impact of endocrine disruptor contamination? J. Endocrinol. Investig. 2021, 44, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Soyer-Gobillard, M.-O.; Gaspari, L.; Paris, F.; Kalfa, N.; Hamamah, S.; Courtet, P.; Sultan, C. Prenatal Exposure to Diethylstilbestrol and Multigenerational Psychiatric Disorders: An Informative Family. Int. J. Environ. Res. Public Heal. 2021, 18, 9965. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; King, S.; McBirney, M.; Kubsad, D.; Pappalardo, M.; Beck, D.; Sadler-Riggleman, I.; Skinner, M.K. Vinclozolin induced epigenetic transgenerational inheritance of pathologies and sperm epimutation biomarkers for specific diseases. PLoS ONE 2018, 13, e0202662. [Google Scholar] [CrossRef]
- Nilsson, E.; Larsen, G.; Manikkam, M.; Guerrero-Bosagna, C.; Savenkova, M.I.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Ovarian Disease. PLoS ONE 2012, 7, e36129. [Google Scholar] [CrossRef] [Green Version]
- Giambò, F.; Leone, G.M.; Gattuso, G.; Rizzo, R.; Cosentino, A.; Cinà, D.; Teodoro, M.; Costa, C.; Tsatsakis, A.; Fenga, C.; et al. Genetic and Epigenetic Alterations Induced by Pesticide Exposure: Integrated Analysis of Gene Expression, microRNA Expression, and DNA Methylation Datasets. Int. J. Environ. Res. Public Health 2021, 18, 8697. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Fenton, S.E.; Aylor, D. Animal models of endocrine disruption. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 283–297. [Google Scholar] [CrossRef]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Rios-Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The impact of endocrine-disrupting chemical exposure in the mammalian hypothalamic-pituitary axis. Mol. Cell. Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Mehinto, A.C.; Denslow, N.D. Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Mol. Cell. Endocrinol. 2020, 507, 110764. [Google Scholar] [CrossRef] [PubMed]
- Torres-Flores, U.; Hernández-Hernández, A. The Interplay Between Replacement and Retention of Histones in the Sperm Genome. Front. Genet. 2020, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Ben Maamar, M.; Sadler-Riggleman, I.; Beck, D.; Nilsson, E.; McBirney, M.; Klukovich, R.; Xie, Y.; Tang, C.; Yan, W. Alterations in sperm DNA methylation, non-coding RNA and histone retention associate with DDT-induced epigenetic transgenerational inheritance of disease. Epigenet. Chromatin 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiemann, U. In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: A review. Reprod. Toxicol. 2008, 25, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.G.; Babayev, S.N.; Chen, L.X.; Carr, B.R.; Word, R.A.; Jimenez, P.T. Estrogen-Regulated miRNA-27b is altered by bisphenol a in endometrial stromal cells. Reproduction 2018, 156, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Machtinger, R.; Combelles, C.M.; Missmer, S.A.; Correia, K.F.; Williams, P.; Hauser, R.; Racowsky, C. Bisphenol-A and human oocyte maturation in vitro. Hum. Reprod. 2013, 28, 2735–2745. [Google Scholar] [CrossRef] [Green Version]
- Ziv-Gal, A.; Wang, W.; Zhou, C.; Flaws, J.A. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol. Appl. Pharmacol. 2015, 284, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2018, 4, dvy016. [Google Scholar] [CrossRef] [Green Version]
- Arambula, S.E.; Jima, D.; Patisaul, H.B. Prenatal bisphenol A (BPA) exposure alters the transcriptome of the neonate rat amygdala in a sex-specific manner: A CLARITY-BPA consortium study. NeuroToxicology 2018, 65, 207–220. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Rodosthenous, R.S.; Baccarelli, A.A.; Mansour, A.; Adir, M.; Israel, A.; Racowsky, C.; Hauser, R.; Bollati, V.; Machtinger, R. Supraphysiological Concentrations of Bisphenol A Alter the Expression of Extracellular Vesicle-Enriched miRNAs From Human Primary Granulosa Cells. Toxicol. Sci. 2019, 169, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.X.; Hornbuckle, K.C.; Thorne, P.S. Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures. Environ. Sci. Technol. 2015, 49, 8105–8112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Saktrakulkla, P.; Tuttle, K.; Marek, R.F.; Lehmler, H.-J.; Wang, K.; Hornbuckle, K.C.; Duffel, M.W. Detection and Quantification of Polychlorinated Biphenyl Sulfates in Human Serum. Environ. Sci. Technol. 2021, 55, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, M.H.; Mlynarczuk, J. The effect of polychlorinated biphenyls (PCBs) on bovine oviductal contractions and LIF synthesis during estrous cycle, in vitro studies. Res. Vet. Sci. 2020, 133, 188–193. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Bellavia, A.; Gaskins, A.J.; Chavarro, J.E.; Ford, J.B.; Souter, I.; Calafat, A.M.; Hauser, R.; Williams, P.L. Paternal mixtures of urinary concentrations of phthalate metabolites, bisphenol A and parabens in relation to pregnancy outcomes among couples attending a fertility center. Environ. Int. 2021, 146, 106171. [Google Scholar] [CrossRef]
- Tando, Y.; Hiura, H.; Takehara, A.; Ito-Matsuoka, Y.; Arima, T.; Matsui, Y. Epi-mutations for spermatogenic defects by maternal exposure to di(2-ethylhexyl) phthalate. eLife 2021, 10, e70322. [Google Scholar] [CrossRef]
- Oluwayiose, O.A.; Marcho, C.; Wu, H.; Houle, E.; Krawetz, S.A.; Suvorov, A.; Mager, J.; Pilsner, J.R. Paternal preconception phthalate exposure alters sperm methylome and embryonic programming. Environ. Int. 2021, 155, 106693. [Google Scholar] [CrossRef]
- Barakat, R.; Lin, P.-C.; Park, C.J.; Zeineldin, M.; Zhou, S.; Rattan, S.; Brehm, E.; Flaws, J.A.; Ko, C.J. Germline-dependent transmission of male reproductive traits induced by an endocrine disruptor, di-2-ethylhexyl phthalate, in future generations. Sci. Rep. 2020, 10, 5705. [Google Scholar] [CrossRef]
- Hatcher, K.M.; Willing, J.; Chiang, C.; Rattan, S.; Flaws, J.A.; Mahoney, M.M. Exposure to di-(2-ethylhexyl) phthalate transgenerationally alters anxiety-like behavior and amygdala gene expression in adult male and female mice. Physiol. Behav. 2019, 207, 7–14. [Google Scholar] [CrossRef]
- Liu, S.; Wang, K.; Svoboda, L.K.; Rygiel, C.A.; Neier, K.; Jones, T.R.; Cavalcante, R.G.; Colacino, J.A.; Dolinoy, D.C.; Sartor, M.A. Perinatal DEHP exposure induces sex- and tissue-specific DNA methylation changes in both juvenile and adult mice. Environ. Epigenet. 2021, 7, dvab004. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Wen, S.; Shen, L.; Peng, J.; Yan, C.; Cao, X.; Zhou, Y.; Long, C.; Lin, T.; et al. The Mechanism of Environmental Endocrine Disruptors (DEHP) Induces Epigenetic Transgenerational Inheritance of Cryptorchidism. PLoS ONE 2015, 10, e0126403. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Per- and Polyfluoroalkyl Substances (PFASs) in Food and Human Dietary Intake: A Review of the Recent Scientific Literature. J. Agric. Food Chem. 2017, 65, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.S.; Harlow, D.; Randolph, J.F., Jr.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef] [PubMed]
- Roepke, T.A.; Sadlier, N.C. REPRODUCTIVE TOXICOLOGY: Impact of endocrine disruptors on neurons expressing GnRH or kisspeptin and pituitary gonadotropins. Reproduction 2021, 162, F131–F145. [Google Scholar] [CrossRef]
- Kang, J.S.; Ahn, T.-G.; Park, J.-W. Perfluorooctanoic acid (PFOA) and perfluooctane sulfonate (PFOS) induce different modes of action in reproduction to Japanese medaka (Oryzias latipes). J. Hazard. Mater. 2019, 368, 97–103. [Google Scholar] [CrossRef]
- Percy, Z.; La Guardia, M.J.; Xu, Y.; Hale, R.C.; Dietrich, K.N.; Lanphear, B.P.; Yolton, K.; Vuong, A.; Cecil, K.; Braun, J.M.; et al. Concentrations and loadings of organophosphate and replacement brominated flame retardants in house dust from the home study during the PBDE phase-out. Chemosphere 2020, 239, 124701. [Google Scholar] [CrossRef]
- Blum, A.; Behl, M.; Birnbaum, L.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Butt, C.M.; Fieuws, S.; Price, T.; Murphy, S.K.; Stapleton, H.M. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ. Epigenet. 2017, 3, dvx003. [Google Scholar] [CrossRef] [Green Version]
- Giraudo, M.; Dubé, M.; Lépine, M.; Gagnon, P.; Douville, M.; Houde, M. Multigenerational effects evaluation of the flame retardant tris(2-butoxyethyl) phosphate (TBOEP) using Daphnia magna. Aquat. Toxicol. 2017, 190, 142–149. [Google Scholar] [CrossRef]
- Yu, Y.; Hou, Y.; Dang, Y.; Zhu, X.; Li, Z.; Chen, H.; Xiang, M.; Li, Z.; Hu, G. Exposure of adult zebrafish (Danio rerio) to Tetrabromobisphenol A causes neurotoxicity in larval offspring, an adverse transgenerational effect. J. Hazard. Mater. 2021, 414, 125408. [Google Scholar] [CrossRef]
- Cordier, S.; Monfort, C.; Filippini, G.; Preston-Martin, S.; Lubin, F.; Mueller, B.A.; Holly, E.A.; Peris-Bonet, R.; McCredie, M.; Choi, W.; et al. Parental Exposure to Polycyclic Aromatic Hydrocarbons and the Risk of Childhood Brain Tumors: The SEARCH International Childhood Brain Tumor Study. Am. J. Epidemiol. 2004, 159, 1109–1116. [Google Scholar] [CrossRef]
- Kubincová, P.; Sychrová, E.; Raška, J.; Basu, A.; Yawer, A.; Dydowiczová, A.; Babica, P.; Sovadinová, I. Polycyclic Aromatic Hydrocarbons and Endocrine Disruption: Role of Testicular Gap Junctional Intercellular Communication and Connexins. Toxicol. Sci. 2019, 169, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Sahay, D.; Lloyd, S.E.; Rivera, J.A.; Jezioro, J.; McDonald, J.D.; Pitiranggon, M.; Yan, B.; Szabolcs, M.; Terry, M.B.; Miller, R.L. Prenatal polycyclic aromatic hydrocarbons, altered ERα pathway-related methylation and expression, and mammary epithelial cell proliferation in offspring and grandoffspring adult mice. Environ. Res. 2021, 196, 110961. [Google Scholar] [CrossRef] [PubMed]
- Lacal, I.; Ventura, R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, M.K.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Ben Maamar, M.; McCarrey, J.R. Transgenerational sperm DNA methylation epimutation developmental origins following ancestral vinclozolin exposure. Epigenetics 2019, 14, 721–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heard, E.; Martienssen, R.A. Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Skinner, M.K.; Nilsson, E.E. Role of environmentally induced epigenetic transgenerational inheritance in evolutionary biology: Unified Evolution Theory. Environ. Epigenet. 2021, 7, dvab012. [Google Scholar] [CrossRef]
- Brehm, E.; Flaws, J.A. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef] [Green Version]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [Green Version]
- Lumey, L.H.; Ekamper, P.; Bijwaard, G.; Conti, G.; van Poppel, F. Overweight and obesity at age 19 after pre-natal famine exposure. Int. J. Obes. 2021, 45, 1668–1676. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, Q.; Liu, L.; Chen, G.; Tang, S.; He, Z.; Tan, Z. Maternal undernutrition alters the skeletal muscle development and methylation of myogenic factors in goat offspring. Anim. Biosci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lecorguillé, M.; Teo, S.; Phillips, C.M. Maternal Dietary Quality and Dietary Inflammation Associations with Offspring Growth, Placental Development, and DNA Methylation. Nutrients 2021, 13, 3130. [Google Scholar] [CrossRef]
- Sharp, G.C.; Lawlor, D.A.; Richmond, R.C.; Fraser, A.; Simpkin, A.; Suderman, M.; Shihab, H.A.; Lyttleton, O.; McArdle, W.; Ring, S.M.; et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: Findings from the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiology 2015, 44, 1288–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manikkam, M.; Haque, M.M.; Guerrero-Bosagna, C.; Nilsson, E.E.; Skinner, M.K. Pesticide Methoxychlor Promotes the Epigenetic Transgenerational Inheritance of Adult-Onset Disease through the Female Germline. PLoS ONE 2014, 9, e102091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senaldi, L.; Smith-Raska, M. Evidence for germline non-genetic inheritance of human phenotypes and diseases. Clin. Epigenet. 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Kobaly, K.; Vellanki, P.; Sisk, R.K.; Armstrong, L.; Lee, J.Y.; Lee, J.; Hayes, M.G.; Urbanek, M.; Legro, R.; Dunaif, A. Parent-of-Origin Effects on Glucose Homeostasis in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 2961–2966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinola, P.; Piltonen, T.T.; Puurunen, J.; Vanky, E.; Sundström-Poromaa, I.; Stener-Victorin, E.; Ruokonen, A.; Puukka, K.; Tapanainen, J.S.; Morin-Papunen, L. Androgen profile through life in women with polycystic ovary syndrome: A Nordic multicenter collaboration study. J. Clin. Endocrinol. Metab. 2015, 100, 3400–3407. [Google Scholar] [CrossRef] [Green Version]
- Morris, A. Transgenerational effects of polycystic ovary syndrome identified. Nat. Rev. Endocrinol. 2020, 16, 67. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Deng, Q. Epigenetic inheritance of polycystic ovary syndrome—Challenges and opportunities for treatment. Nat. Rev. Endocrinol. 2021, 17, 521–533. [Google Scholar] [CrossRef]
- Mimouni, N.E.H.; Paiva, I.; Barbotin, A.-L.; Timzoura, F.E.; Plassard, D.; Le Gras, S.; Ternier, G.; Pigny, P.; Catteau-Jonard, S.; Simon, V.; et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021, 33, 513–530. [Google Scholar] [CrossRef]
- Ben Maamar, M.; King, S.E.; Nilsson, E.; Beck, D.; Skinner, M.K. Epigenetic transgenerational inheritance of parent-of-origin allelic transmission of outcross pathology and sperm epimutations. Dev. Biol. 2020, 458, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, Z.; Wang, G.; Wang, H.; Zhou, Y.; Zhao, X.; Cheng, C.Y.; Qiao, Y.; Sun, F. Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming. Cell Discov. 2021, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Rolland, A.D.; Ravel, C. Epigenetics of Male Infertility. In Epigenetics in Human Reproduction and Development; World Scientific: Singapore, 2017; pp. 87–111. [Google Scholar] [CrossRef]
- Berthaut, I.; Montjean, D.; Dessolle, L.; Morcel, K.; Deluen, F.; Poirot, C.; Bashamboo, A.; Mcelreavey, K.; Ravel, C. Effect of temozolomide on male gametes: An epigenetic risk to the offspring? J. Assist. Reprod. Genet. 2013, 30, 827–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montjean, D.; Ravel, C.; Benkhalifa, M.; Cohen-Bacrie, P.; Berthaut, I.; Bashamboo, A.; McElreavey, K. Methylation changes in mature sperm deoxyribonucleic acid from oligozoospermic men: Assessment of genetic variants and assisted reproductive technology outcome. Fertil. Steril. 2013, 100, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Zini, A.; Ravel, C.; Belloc, S.; Dalleac, A.; Copin, H.; Boyer, P.; McElreavey, K.; Benkhalifa, M. Sperm global DNA methylation level: Association with semen parameters and genome integrity. Andrology 2015, 3, 235–240. [Google Scholar] [CrossRef]
- Santi, D.; De Vincentis, S.; Magnani, E.; Spaggiari, G. Impairment of sperm DNA methylation in male infertility: A meta-analytic study. Andrology 2017, 5, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dottermusch-Heidel, C.; Klaus, E.S.; Gonzalez, N.H.; Bhushan, S.; Meinhardt, A.; Bergmann, M.; Renkawitz-Pohl, R.; Rathke, C.; Steger, K. H3K79 methylation directly precedes the histone-to-protamine transition in mammalian spermatids and is sensitive to bacterial infections. Andrology 2014, 2, 655–665. [Google Scholar] [CrossRef]
- Denham, J.; O’Brien, B.J.; Harvey, J.T.; Charchar, F.J. Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics 2015, 7, 717–731. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; Pflueger, C.; Cairns, B.R.; Carrell, D.T. Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility. PLoS Genet. 2014, 10, e1004458. [Google Scholar] [CrossRef]
- Maurice, C.; Dalvai, M.; Lambrot, R.; Deschênes, A.; Scott-Boyer, M.-P.; McGraw, S.; Chan, D.; Côté, N.; Ziv-Gal, A.; Flaws, J.; et al. Early-Life Exposure to Environmental Contaminants Perturbs the Sperm Epigenome and Induces Negative Pregnancy Outcomes for Three Generations via the Paternal Lineage. Epigenomes 2021, 5, 10. [Google Scholar] [CrossRef]
- Okada, Y.; Yamaguchi, K. Epigenetic modifications and reprogramming in paternal pronucleus: Sperm, preimplantation embryo, and beyond. Cell Mol. Life Sci. 2017, 74, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fan, Y.; Xiao, W.; Ding, X.; Hu, W.; Xia, Y. Glufosinate-Ammonium Induced Aberrant Histone Modifications in Mouse Sperm Are Concordant with Transcriptome in Preimplantation Embryos. Front. Physiol. 2022, 12, 819856. [Google Scholar] [CrossRef] [PubMed]
- Ben Maamar, M.; Beck, D.; Nilsson, E.; McCarrey, J.R.; Skinner, M.K. Developmental origins of transgenerational sperm histone retention following ancestral exposures. Dev. Biol. 2020, 465, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, W.; Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 2016, 17, 733–743. [Google Scholar] [CrossRef]
- Chan, J.C.; Morgan, C.P.; Leu, N.A.; Shetty, A.; Cisse, Y.M.; Nugent, B.M.; Morrison, K.E.; Jašarević, E.; Huang, W.; Kanyuch, N.; et al. Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat. Commun. 2020, 11, 1499. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Alves, M.B.R.; Belleannée, C. Contribution of epididymal epithelial cell functions to sperm epigenetic changes and the health of progeny. Hum. Reprod. Update 2021, 28, 51–66. [Google Scholar] [CrossRef]
- Cafe, S.L.; Nixon, B.; Ecroyd, H.; Martin, J.H.; Skerrett-Byrne, D.A.; Bromfield, E.G. Proteostasis in the Male and Female Germline: A New Outlook on the Maintenance of Reproductive Health. Front. Cell Dev. Biol. 2021, 9, 660626. [Google Scholar] [CrossRef]
- Vabre, P.; Gatimel, N.; Moreau, J.; Gayrard, V.; Picard-Hagen, N.; Parinaud, J.; Léandri, R. Environmental pollutants, a possible etiology for premature ovarian insufficiency: A narrative review of animal and human data. Environ. Health 2017, 16, 37. [Google Scholar] [CrossRef] [Green Version]
- Manikkam, M.; Guerrero-Bosagna, C.; Tracey, R.; Haque, M.M.; Skinner, M.K. Transgenerational Actions of Environmental Compounds on Reproductive Disease and Identification of Epigenetic Biomarkers of Ancestral Exposures. PLoS ONE 2012, 7, e31901. [Google Scholar] [CrossRef] [Green Version]
- Cabry, R.; Merviel, P.; Madkour, A.; Lefranc, E.; Scheffler, F.; Desailloud, R.; Bach, V.; Benkhalifa, M. The impact of endocrine disruptor chemicals on oocyte/embryo and clinical outcomes in IVF. Endocr. Connect. 2020, 9, R134–R142. [Google Scholar] [CrossRef]
- Beck, D.; Sadler-Riggleman, I.; Skinner, M.K. Generational comparisons (F1 versus F3) of vinclozolin induced epigenetic transgenerational inheritance of sperm differential DNA methylation regions (epimutations) using MeDIP-Seq. Environ. Epigenet. 2017, 3, dvx016. [Google Scholar] [CrossRef] [PubMed]
- Bogolyubova, I.; Bogolyubov, D. Heterochromatin Morphodynamics in Late Oogenesis and Early Embryogenesis of Mammals. Cells 2020, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Raman, P.; Rominger, M.C.; Young, J.M.; Molaro, A.; Tsukiyama, T.; Malik, H.S. Novel Classes and Evolutionary Turnover of Histone H2B Variants in the Mammalian Germline. Mol. Biol. Evol. 2022, 39, msac019. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, Y.; Kuji, N.; Tanaka, Y.; Tanaka, M.; Ikeda, E.; Komatsu, S.; Kato, S.; Yoshimura, Y. Expression of human oocyte-specific linker histone protein and its incorporation into sperm chromatin during fertilization. Fertil. Steril. 2010, 93, 1134–1141. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Liu, H.; Pan, Z.; Zhang, J.; Xu, Y.; Liu, H.; Pan, Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrinol. 2019, 17, 9. [Google Scholar] [CrossRef]
- Li, D.; Xu, D.; Zou, Y.; Xu, Y.; Fu, L.; Xu, X.; Liu, Y.; Zhang, X.; Zhang, J.; Ming, H.; et al. Non-coding RNAs and ovarian diseases. Mol. Med. Rep. 2017, 15, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Javadi, M.; Soleimani Rad, J.; Pashaiasl, M.; Farashah, M.S.G.; Roshangar, L. The effects of plasma-derived extracellular vesicles on cumulus expansion and oocyte maturation in mice. Reprod. Biol. 2021, 22, 100593. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vandenberg, L.N. Developmental origins of health and disease: A paradigm for understanding disease etiology and prevention. Curr. Opin. Pediatr. 2015, 27, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Bashamboo, A.; Ravel, C.; McElreavey, K. De nouveaux modèles de cellules souches embryonnaires pour étudier le dé-veloppement des cellules germinales dans leur niche. Méd. Reprod. 2011, 13, 31–39. [Google Scholar] [CrossRef]
- Burton, A.; Brochard, V.; Galan, C.; Ruiz-Morales, E.R.; Rovira, Q.; Rodriguez-Terrones, D.; Kruse, K.; Le Gras, S.; Udayakumar, V.S.; Chin, H.G.; et al. Heterochromatin establishment during early mammalian development is regulated by pericentromeric RNA and characterized by non-repressive H3K9me3. Nat. Cell Biol. 2020, 22, 767–778. [Google Scholar] [CrossRef]
- Brieño-Enríquez, M.A.; Larriba, E.; del Mazo, J. Endocrine disrupters, microRNAs, and primordial germ cells: A dangerous cocktail. Fertil. Steril. 2016, 106, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Skinner, M.K. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 2011, 6, 838–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadafora, C. Transgenerational epigenetic reprogramming of early embryos: A mechanistic model. Environ. Epigenet. 2020, 6, dvaa009. [Google Scholar] [CrossRef] [PubMed]
- Marcho, C.; Oluwayiose, O.A.; Pilsner, J.R. The preconception environment and sperm epigenetics. Andrology 2020, 8, 924–942. [Google Scholar] [CrossRef] [PubMed]
- McCarrey, J.R. Distinctions between transgenerational and non-transgenerational epimutations. Mol. Cell. Endocrinol. 2014, 398, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Po, B.H.K.; Chiu, J.M.Y. Transgenerational impairments of reproduction and development of the marine invertebrate Crepidula onyx resulted from long-term dietary exposure of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47). Environ. Pollut. 2018, 235, 730–738. [Google Scholar] [CrossRef]
- Thayil, A.J.; Wang, X.; Bhandari, P.; Saal, F.S.V.; Tillitt, D.E.; Bhandari, R.K. Bisphenol A and 17α-ethinylestradiol-induced transgenerational gene expression differences in the brain–pituitary–testis axis of medaka, Oryzias latipes†. Biol. Reprod. 2020, 103, 1324–1335. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Xue, X.; Yuan, C.; Wang, Z. BPA’s transgenerational disturbance to transcription of ovarian steroidogenic genes in rare minnow Gobiocypris rarus via DNA and histone methylation. Sci. Total Environ. 2021, 762, 143055. [Google Scholar] [CrossRef]
- Carvan, M.J.; Kalluvila, T.A.; Klingler, R.H.; Larson, J.K.; Pickens, M.; Mora-Zamorano, F.X.; Connaughton, V.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K. Mercury-induced epigenetic transgenerational inheritance of abnormal neurobehavior is correlated with sperm epimutations in zebrafish. PLoS ONE 2017, 12, e0176155. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, R.K.; Saal, F.S.V.; Tillitt, D.E. Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes. Sci. Rep. 2015, 5, 9303. [Google Scholar] [CrossRef] [Green Version]
- Leroux, S.; Gourichon, D.; Leterrier, C.; Labrune, Y.; Coustham, V.; Rivière, S.; Zerjal, T.; Coville, J.-L.; Morisson, M.; Minvielle, F.; et al. Embryonic environment and transgenerational effects in quail. Genet. Sel. Evol. 2017, 49, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akemann, C.; Meyer, D.N.; Gurdziel, K.; Baker, T.R. TCDD-induced multi- and transgenerational changes in the methylome of male zebrafish gonads. Environ. Epigenet. 2020, 6, dvaa010. [Google Scholar] [CrossRef] [PubMed]
| Model | EDC | Transgenerational Effect | Reference |
|---|---|---|---|
| Daphnia magna microcrustacean | Flame retardants Tris(2-butoxyethyl) phosphate (TBOEP) | Levels of mRNA were found to be significantly different for genes known to be involved in endocrine-mediated mechanisms such as reproduction and growth between generations F0, F1, and F2, indicating the effects of parental exposure on offspring. | [89] |
| Crepidula onyx gastropod | 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) | Bioaccumulation and maternal transfer of BDE-47 were evident in all life stages of the F0 generation and in F1 eggs, respectively. Exposure to BDE-47 reduced fecundity, delayed sexual maturity, and impeded embryonic development in F0 to F2. | [147] |
| Zebra fish (danio rerio) | Flame retardant Tetrabromobisphenol A (TBBPA) | Neurotoxicity and decreased content of dopamine in larval offspring. | [90] |
| Medaka | BPA EE2 | BPA or EE2-induced transgenerational reproductive impairment in the F2 generation was associated with alterations in reproductive gene expression in brain and testis and global DNA methylation in testis. | [148] |
| Gobiocypris rarus | BPA | Parental BPA exposure inhibited the ovary development of the offspring. | [149] |
| Fish | Dioxin | Exposure to the environmental toxicants methylmercury or dioxin transmit to their grand-offspring behavioral changes, visual defects, increased body mass, skeletal abnormalities and/or decreased fertility, sometimes associated with changes in DNA methylation. | [150] |
| Medaka | BPA EE2 | Medaka exposed to the endocrine disruptors BPA or ethinylestradiol produce grand-offspring and great-grand-offspring with reduced fertility. | [151] |
| Bird | Genistein | In quail eggs exposed to the environmental estrogen genistein, the great-grand offspring age at which the first egg was laid was significantly greater. Embryonic environment affects the phenotype of offspring three generations later in quail. | [152] |
| Rodent | Vinclozoline | Increased obesity risk in rats is inherited transgenerationally after ancestral exposure to DDT, plastic compounds, hydrocarbons and methoxychlor. | [130] |
| Rodent | Vinclozoline | Endocrine disruptors have been shown in mouse models to induce transmissible changes over several generations, altering the quality of spermatogenesis in adulthood. | [11,130] |
| Rodent | Chlordecone | Chlordecone increases prostatic epithelial neoplasia in F1 and F3 mice. Hoxa genes are affected both in the prostate and in sperm of F1 and F3 generations. | [24] |
| Fish M. beryllina | Bifenthrin (pyrethroid insecticide) Levonorgestrel (synthetic progestin), Ethinylestradiol (synthetic estrogen), Trenbolone (synthetic androgen) | Differential methylation of EDC-responsive genes is inherited by the offspring of EDC-treated animals, sometimes in the F2 generation that was never exposed. Low environmentally relevant levels of EDCs can cause altered methylation in genes that are functionally relevant to impaired phenotypes documented in EDC-exposed animals. EDC exposure has the potential to affect epigenetic regulation in future generations of fish that have never been exposed. | [8] |
| Zebrafish | TCDD (dioxin) | Multi- and transgenerational methylomic changes in testicular tissue and decreased reproductive capacity, significantly in the indirectly exposed F1 generation. Histone modification genes were both differentially methylated and expressed in all generations, and many differentially methylated genes overlapped between multiple generations. | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montjean, D.; Neyroud, A.-S.; Yefimova, M.G.; Benkhalifa, M.; Cabry, R.; Ravel, C. Impact of Endocrine Disruptors upon Non-Genetic Inheritance. Int. J. Mol. Sci. 2022, 23, 3350. https://doi.org/10.3390/ijms23063350
Montjean D, Neyroud A-S, Yefimova MG, Benkhalifa M, Cabry R, Ravel C. Impact of Endocrine Disruptors upon Non-Genetic Inheritance. International Journal of Molecular Sciences. 2022; 23(6):3350. https://doi.org/10.3390/ijms23063350
Chicago/Turabian StyleMontjean, Debbie, Anne-Sophie Neyroud, Marina G. Yefimova, Moncef Benkhalifa, Rosalie Cabry, and Célia Ravel. 2022. "Impact of Endocrine Disruptors upon Non-Genetic Inheritance" International Journal of Molecular Sciences 23, no. 6: 3350. https://doi.org/10.3390/ijms23063350
APA StyleMontjean, D., Neyroud, A.-S., Yefimova, M. G., Benkhalifa, M., Cabry, R., & Ravel, C. (2022). Impact of Endocrine Disruptors upon Non-Genetic Inheritance. International Journal of Molecular Sciences, 23(6), 3350. https://doi.org/10.3390/ijms23063350








