Autosomal Dominant Polycystic Kidney Disease: From Pathophysiology of Cystogenesis to Advances in the Treatment
Abstract
1. Introduction
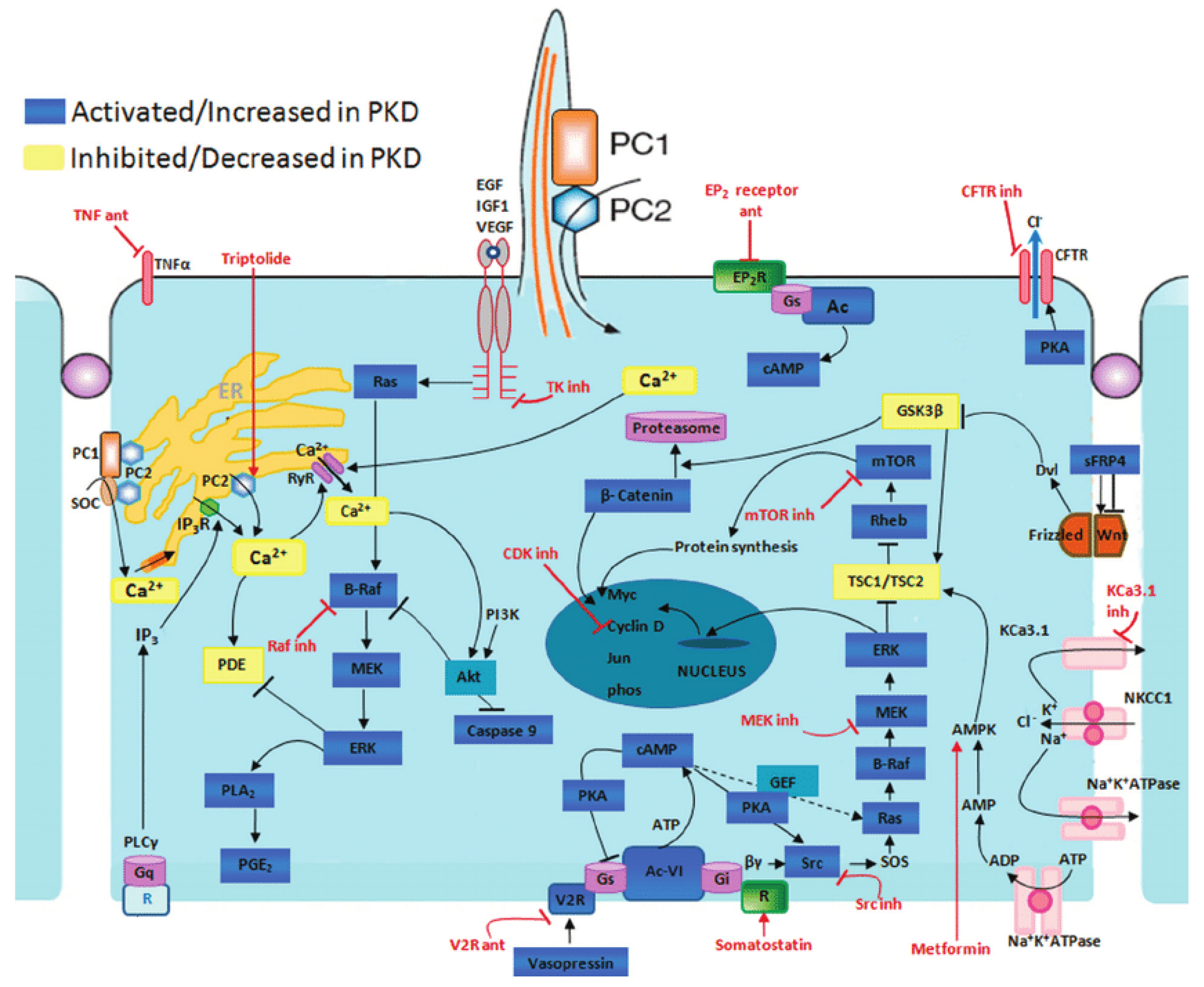
2. Pathogenesis of ADPKD
2.1. Polycystins and Signaling Pathways
2.2. Cyclic AMP Pathway
2.3. The TSC-mTOR Pathway
2.4. PI3K/Akt Pathway
2.5. The JAK-STAT Pathway
2.6. The Id Pathway
2.7. G-Protein Signaling Pathway
2.8. Wnt Signaling Pathway
3. Treatments
3.1. Current Therapeutical Options
3.2. Tolvaptan
3.3. Somatostatin Analogues
4. Future Options
4.1. Targeting the cAMP Pathway
4.1.1. Lixivaptan
4.1.2. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and Potassium Channel Inhibitors
4.1.3. TMEM16A Inhibitors
4.2. Targeting the EGF Receptor Pathway
4.2.1. Bosutinib
4.2.2. Tesevatinib
4.2.3. Thiazolidinediones
4.3. Targeting AMP-Activator Protein Kinase
4.3.1. Metformin
4.3.2. Statins
4.4. Targeting MAPK Pathway-Raf Kinase Inhibitors
4.5. Dietary Interventions in ADPKD
4.5.1. Caloric Restriction
4.5.2. 2-Deoxyglucose (2DG)
4.5.3. mTOR Inhibitors
4.6. Targeting the KEAP1-Nrf2 Pathway
4.7. Targeting Intracellular Calcium Regulation and Cell Cycle Regulation
4.7.1. Calcimimetics
4.7.2. Triptolide
4.7.3. Cyclin Dependent Kinases (CDK) Inhibitors
4.7.4. Histon Deacetylase 6 Inhibitors (HDACi)
4.8. Targeting Interstitial Changes
4.8.1. Venglustat
4.8.2. Inflammation Inhibitors
4.9. MicroRNAs Blockers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Willey, C.J.; Blais, J.D.; Hall, A.K.; Krasa, H.B.; Makin, A.J.; Czerwiec, F.S. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol. Dial. Transplant. 2017, 32, 1356–1363. [Google Scholar] [CrossRef]
- Lanktree, M.B.; Haghighi, A.; Guiard, E.; Iliuta, I.A.; Song, X.; Harris, P.C.; Paterson, A.D.; Pei, Y. Prevalence Estimates of Polycystic Kidney and Liver Disease by Population Sequencing. J. Am. Soc. Nephrol. 2018, 29, 2593–2600. [Google Scholar] [CrossRef]
- Neumann, H.P.; Jilg, C.; Bacher, J.; Nabulsi, Z.; Malinoc, A.; Hummel, B.; Hoffmann, M.M.; Ortiz-Bruechle, N.; Glasker, S.; Pisarski, P. Epidemiology of autosomal-dominant polycystic kidney disease: An in-depth clinical study for south-western Germany. Nephrol. Dial. Transplant. 2013, 28, 1472–1487. [Google Scholar] [CrossRef]
- Spithoven, E.M.; Kramer, A.; Meijer, E.; Orskov, B.; Wanner, C.; Abad, J.M.; Aresté, N.; De La Torre, R.A.; Caskey, F.; Couchoud, C.; et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: Prevalence and survival—An analysis of data from the ERA-EDTA Registry. Nephrol. Dial. Transplant. 2014, 29, iv15–iv25. [Google Scholar] [CrossRef]
- Chapman, A.B.; Guay-Woodford, L.M.; Grantham, J.J.; Torres, V.E.; Bae, K.T.; Baumgarten, D.A.; Kenney, P.J.; King, B.F.; Glockner, J.F.; Wetzel, L.H.; et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort1. Kidney Int. 2003, 64, 1035–1045. [Google Scholar] [CrossRef]
- Su, Q.; Hu, F.; Ge, X.; Lei, J.; Yu, S.; Wang, T.; Zhou, Q.; Mei, C.; Shi, Y. Structure of the human PKD1-PKD2 complex. Science 2018, 80, eaat9819. [Google Scholar] [CrossRef]
- Terryn, S.; Ho, A.; Beauwens, R.; Devuyst, O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1314–1321. [Google Scholar] [CrossRef]
- Clark, W.F.; Devuyst, O.; Roussel, R. The vasopressin system: New insights for patients with kidney diseases: Epidemiological evidence and therapeutic perspectives. J. Intern. Med. 2017, 282, 310–321. [Google Scholar] [CrossRef]
- Bastos, A.P.A.; Onuchic, L. Molecular and cellular pathogenesis of autosomal dominant polycystic kidney disease. Braz. J. Med. Biol. Res. 2011, 44, 606–617. [Google Scholar] [CrossRef]
- Hanaoka, K.; Guggino, W.B. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J. Am. Soc. Nephrol. 2000, 11, 1179–1187. [Google Scholar] [CrossRef]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef]
- Boletta, A. Emerging evidence of a link between the polycystins and the mTOR pathways. Pathogenetics 2009, 2, 6. [Google Scholar] [CrossRef]
- Dere, R.; Wilson, P.D.; Sandford, R.N.; Walker, C.L. Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR. PLoS ONE 2010, 5, e9239. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Li, Y.; Santoso, N.G.; Yu, S.; Woodward, O.M.; Qian, F.; Guggino, W.B. Polycystin-1 interacts with inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling with implications for polycystic kidney disease. J. Biol. Chem. 2009, 284, 36431–36441. [Google Scholar] [CrossRef]
- Santoso, N.G.; Cebotaru, L.; Guggino, W.B. Polycystin-1,2, and STIM1 interact with IP 3 R to Modulate ER Ca2+ release through the PI3K/Akt pathway. Cell. Physiol. Biochem. 2011, 27, 715–726. [Google Scholar] [CrossRef]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect Biol. 2012, 4, a011189. [Google Scholar] [CrossRef]
- Boca, M.; Distefano, G.; Qian, F.; Bhunia, A.K.; Germino, G.G.; Boletta, A. Polycystin-1 Induces Resistance to Apoptosis through the Phosphatidylinositol 3-Kinase/Akt Signaling Pathway. J. Am. Soc. Nephrol. 2006, 17, 637–647. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Piontek, K.; Boletta, A.; Liu, L.; Qian, F.; Xu, P.-N.; Germino, F.; Germino, G. PKD1 Induces p21waf1 and Regulation of the Cell Cycle via Direct Activation of the JAK-STAT Signaling Pathway in a Process Requiring PKD2. Cell 2002, 109, 157–168. [Google Scholar] [CrossRef]
- Low, S.H.; Vasanth, S.; Larson, C.H.; Mukherjee, S.; Sharma, N.; Kinter, M.T.; Kane, M.E.; Obara, T.; Weimbs, T. Polycystin-1, STAT6, and P100 Function in a Pathway that Transduces Ciliary Mechanosensation and is Activated in Polycystic Kidney Disease. Dev. Cell 2006, 10, 57–69. [Google Scholar] [CrossRef]
- Weimbs, T.; Olsan, E.E.; Talbot, J.J. Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. JAK STAT 2013, 2, e23650. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; Starremans, P.G.; McNamara, C.A.; Pei, Y.; Zhou, J. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat. Cell Biol. 2005, 7, 1202–1212. [Google Scholar] [CrossRef]
- Arnould, T.; Kim, E.; Tsiokas, L.; Jochimsen, F.; Grüning, W.; Chang, J.D.; Walz, G. The Polycystic Kidney Disease 1 Gene Product Mediates Protein Kinase C α-dependent and c-Jun N-terminal Kinase-dependent Activation of the Transcription Factor AP-1. J. Biol. Chem. 1998, 273, 6013–6018. [Google Scholar] [CrossRef]
- Arnould, T.; Sellin, L.; Benzing, T.; Tsiokas, L.; Cohen, H.T.; Kim, E.; Walz, G. Cellular Activation Triggered by the Autosomal Dominant Polycystic Kidney Disease Gene Product PKD2. Mol. Cell. Biol. 1999, 19, 3423–3434. [Google Scholar] [CrossRef][Green Version]
- Horsley, V.; Pavlath, G.K. NFAT: Ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 2002, 156, 771–774. [Google Scholar] [CrossRef]
- Puri, S.; Magenheimer, B.S.; Maser, R.L.; Ryan, E.M.; Zien, C.A.; Walker, D.D.; Wallace, D.P.; Hempson, S.J.; Calvet, J.P. Polycystin-1 Activates the Calcineurin/NFAT (Nuclear Factor of Activated T-cells) Signaling Pathway. J. Biol. Chem. 2004, 279, 55455–55464. [Google Scholar] [CrossRef]
- Macián, F.; López-Rodríguez, C.; Rao, A. Partners in transcription: NFAT and AP-1. Oncogene 2001, 20, 2476–2489. [Google Scholar] [CrossRef]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Sugimura, R.; Li, L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res. Part C Embryo Today Rev. 2010, 90, 243–256. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Gleeson, J.G. Cystic kidney disease: The role of Wnt signaling. Trends Mol. Med. 2010, 16, 349–360. [Google Scholar] [CrossRef]
- Schrier, R.W.; Abebe, K.; Perrone, R.D.; Torres, V.E.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Blood Pressure in Early Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2014, 371, 2255–2266. [Google Scholar] [CrossRef]
- Torres, V.E.; Abebe, K.; Chapman, A.B.; Schrier, R.W.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Angiotensin Blockade in Late Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2014, 371, 2267–2276. [Google Scholar] [CrossRef]
- Torres, V.E.; Wang, X.; Qian, Q.; Somlo, S.; Harris, P.C.; Gattone, V.H., 2nd. Effective treatment of an orthologuous model of autosomal dominant polycystic kidney disease. Nat. Med. 2004, 10, 363–364. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Ward, C.J.; Harris, P.C.; Torres, V.E. Vasopressin dierectly regulates cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 102–108. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Dandurand, A.; Ouyang, J.; Czerwiec, F.S.; Blais, J.D.; for the TEMPO 4:4 Trial Investigators. Multicentric, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 trial. Nephrol. Dial. Transplant. 2017, 33, 477–489. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef]
- Torres, V.E.; Gansevoort, R.T.; Perrone, R.D.; Chapman, A.B.; Ouyang, J.; Lee, J.; Japes, H.; Nourbakhsh, A.; Wang, T. Tolvaptan in ADPKD Patients with Very Low Kidney Function. Kidney Int. Rep. 2021, 6, 2171–2178. [Google Scholar] [CrossRef]
- Chapman, A.B.; Bost, J.E.; Torres, V.E.; Guay-Woodford, L.; Bae, K.T.; Landsittel, D.; Li, J.; King, B.F.; Martin, D.; Wetzel, L.H.; et al. Kidney Volume and Functional Outcomes in Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 479–486. [Google Scholar] [CrossRef]
- Cornec-Le Gall, E.; Audrezet, M.-P.; Rousseau, A.; Hourmant, M.; Renaudineau, E.; Charasse, C.; Morin, M.-P.; Moal, M.-C.; Dantal, J.; Wehbe, B. The PROPKD score: A new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2016, 6, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Blais, J.D.; Perrone, R.D.; Gansevoort, R.T.; Chapman, A.B.; Devuyst, O.; Higashihara, E.; Harris, P.C.; Zhou, W.; Ouyang, J.; et al. Prognostic Enrichment Design in Clinical Trials for Autosomal Dominant Polycystic Kidney Disease: The TEMPO 3:4 Clinical Trial. Kidney Int. Rep. 2016, 1, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.C.; Masyuk, T.V.; Page, L.J.; Kubly, V.J.; Bergstralh, E.J.; Li, X.; Kim, B.; King, B.F.; Glockner, J.; Holmes, D.R.; et al. Randomized Clinical Trial of Long-Acting Somatostatin for Autosomal Dominant Polycystic Kidney and Liver Disease. J. Am. Soc. Nephrol. 2010, 21, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Remuzzi, A.; Ondei, P.; Fasolini, G.; Antiga, L.; Ene-Iordache, B.; Remuzzi, G.; Epstein, F.H. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Perico, N.; Perna, A.; Antiga, L.; Brambilla, P.; Pisani, A.; Visciano, B.; Imbriaco, M.; Messa, P.; Cerutti, R.; et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): A randomized placebo-controlled, multicentre trial. Lancet 2013, 382, 1485–1495. [Google Scholar] [CrossRef]
- Meijer, E.; Drenth, J.P.; d’Agnolo, H.; Casteleijn, N.F.; de Fijter, J.W.; Gevers, T.J.; Kappert, P.; Peters, D.J.; Salih, M.; Soonawala, D.; et al. Rationale and design of the DIPAK 1 study: A randomized controlled clinical trial assessing the efficacy of lanreotide to Halt disease progression in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2014, 63, 446–455. [Google Scholar] [CrossRef] [PubMed]
- van Keimpema, L.; Nevens, F.; Vanslembrouck, R.; van Oijen, M.G.; Hoffmann, A.L.; Dekker, H.M.; de Man, R.A.; Drenth, J.P. Lanreotide reduces volume of polycystic liver: A randomized, double-blind, placebo-controlled trial. Gastroenterology 2009, 137, 1661–1668. [Google Scholar] [CrossRef]
- Chrispijn, M.; Nevens, F.; Gevers, T.J.G.; Vanslembrouck, R.; van Oijen, M.G.H.; Coudyzer, W.; Hoffmann, A.L.; Dekker, H.M.; de Man, R.A.; van Keimpema, L.; et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment. Pharmacol. Ther. 2012, 35, 266–274. [Google Scholar] [CrossRef]
- Wang, X.; Constans, M.M.; Chebib, F.T.; Torres, V.E.; Pellegrini, L. Effect of a vasopressin V2 receptor antagonist on polycystic kidney disease development in a rat model. Am. J. Nephrol. 2019, 49, 487–493. [Google Scholar] [CrossRef]
- Woodhead, J.L.; Pellegrini, L.; Shoda, L.K.M.; Howell, B.A. Comparison of the Hepatotoxic Potential of Two Treatments for Autosomal-Dominant Polycystic Kidney Disease Using Quantitative Systems Toxicology Modeling. Pharm. Res. 2020, 37, 24. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Glockner, J.F.; Rossetti, S.; Babovich-Vuksanovic, D.; Harris, P.C.; Torres, V.E. Autosomal dominant polycystic kidney disease coexisting with cystic fibrosis. J. Nephrol. 2006, 19, 529–534. [Google Scholar] [PubMed]
- Yanda, M.K.; Liu, Q.; Cebotaru, L. A potential strategy for reducing cysts in autosomal dominant polycystic kidney disease with a CFTR corrector. J. Biol. Chem. 2018, 293, 11513–11526. [Google Scholar] [CrossRef] [PubMed]
- Yuajit, C.; Homvisasevongsa, S.; Chatsudthipong, L.; Soodvilai, S.; Muanprasat, C.; Chatsudthipong, V. Steviol Reduces MDCK Cyst Formation and Growth by Inhibiting CFTR Channel Activity and Promoting Proteasome-Mediated CFTR Degradation. PLoS ONE 2013, 8, e58871. [Google Scholar] [CrossRef] [PubMed]
- Nantavishit, J.; Chatsudthipong, V.; Soodvilai, S. Lansoprazole reduces renal cyst in polycystic kidney disease via inhibition of cell proliferation and fluid secretion. Biochem. Pharmacol. 2018, 154, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Albaqumi, M.; Srivastava, S.; Li, Z.; Zhdnova, O.; Wulff, H.; Itani, O.; Wallace, D.P.; Skolnik, E.Y. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal dominant polycystic kidney disease. Kidney Int. 2008, 74, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, I.; Kraus, A.; Scholz, J.K.; Skoczynski, K.; Schreiber, R.; Kunzelmann, K.; Buchholz, B. Cyst growth in ADPKD is prevented by pharmacological and genetic inhibition of TMEM16A in vivo. Nat. Commun. 2020, 11, 4320. [Google Scholar] [CrossRef] [PubMed]
- Miner, K.; Labitzke, K.; Liu, B.; Wang, P.; Henckels, K.; Gaida, K.; Elliott, R.; Chen, J.J.; Liu, L.; Leith, A.; et al. Drug repurposing: The anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways. Front. Pharmacol. 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, J.; Chang, J.; Kim, S.S.; Namkung, W.; Kim, I. Synthesis and biological evaluation of novel Ani9 derivatives as potent and selective ANO1 inhibitors. Eur. J. Med. Chem. 2018, 160, 245–255. [Google Scholar] [CrossRef]
- Elliot, J.; Zheleznova, N.N.; Wilson, P.D. c-Src inactivation reduces renal peithelial cell-matrix adhesion, proliferation, and cyst formation. Am. J. Physiol. Cell Physiol. 2011, 302, C522–C529. [Google Scholar] [CrossRef]
- Tesar, V.; Ciechanowski, K.; Pei, Y.; Barash, I.; Shannon, M.; Li, R.; Williams, J.H.; Levisetti, M.; Arkin, S.; Serra, A. Bosutinib versus Placebo for Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2017, 28, 3404–3413. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, W.E.; Frost, P.; Avner, E.D. Tesevatinib ameliorates progression of polycystic kidney disease in rodent models of autosomal recessive polycystic kidney disease. World J. Nephrol. 2017, 6, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Masaki, T.; Doi, S.; Arakawa, T.; Yokoyama, Y.; Doi, T.; Kohno, N.; Yorioka, N. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab. Investig. 2009, 89, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Nofziger, C.; Brown, K.K.; Smith, C.D.; Harrington, W.; Murray, D.; Bisi, J.; Ashton, T.T.; Maurio, F.P.; Kalsi, K.; West, T.A.; et al. PPARgamma agonists inhibit vasopressin-mediated anion transport in the MDCK-C7 cell line. Am. J. Physiol. Renal. Physiol. 2009, 297, F55–F62. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, A.A.; Bange, H.; Verburg, L.; Dijkstra, K.L.; Price, L.S.; Peters, D.J.M.; Leonhard, W.N. Renal cyst growth is attenuated by a combination of tolvaptan and pioglitazon, while pioglitazone treatment alone is not effective. Sci. Rep. 2020, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Takiar, V.; Nishio, S.; Seo-Mayer, P.; King, J.D., Jr.; Li, H.; Zhang, L.; Karihaloo, A.; Hallows, K.R.; Somlo, S.; Caplan, M.J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.F.; Lin, C.-C.; Zhou, F.; Germino, G.G. Fatty Acid Oxidation is Impaired in An Orthologous Mouse Model of Autosomal Dominant Polycystic Kidney Disease. eBioMedicine 2016, 5, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Ma, T.-L.; Hung, C.-C.; Tian, Y.-C.; Chen, Y.-C.; Ming-Yang, C.; Cheng, Y.-C. Metformin Inhibits Cyst Formation in a Zebrafish Model of Polycystin-2 Deficiency. Sci. Rep. 2017, 7, 7161. [Google Scholar] [CrossRef] [PubMed]
- Perrone, R.D.; Abebe, K.Z.; Watnick, T.J.; Althouse, A.D.; Hallows, K.R.; Lalama, C.M.; Miskulin, D.C.; Seliger, S.L.; Tao, C.; Harris, P.C.; et al. Primary results of the randomized trial of metformin administration in polycystic kideny disease (TAME PKD). Kidney Int. 2021, 100, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S.; Packham, D.; Fairley, K.F.; Kincaid-Smith, P. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease. Scand. J. Urol. Nephrol. 2009, 44, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Cadnapaphornchai, M.A.; George, D.M.; McFann, K.; Wang, W.; Gitomer, B.; Strain, J.D.; Schrier, R.W. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2014, 9, 889–896. [Google Scholar] [CrossRef]
- Brosnahan, G.M.; Abebe, K.Z.; Rahbari-Oskoui, F.F.; Patterson, C.G.; Bae, K.T.; Schrier, R.W.; Braun, W.E.; Chapman, A.B.; Flessner, M.F.; Harris, P.C.; et al. Effect of statin therapy on the progression of autosomal dominant polycystic kidney disease, a secondary analysis of the HALT PKD Trials. Curr. Hypertens. Rev. 2017, 13, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Pelling, J.C.; Ramaswamy, N.T.; Eppler, J.W.; Wallace, D.P.; Nagao, S.; Rome, L.A.; Sullivan, L.P.; Grantham, J.J. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracelllular signal-regulated kinase pathway. Kidney Int. 2000, 57, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.; Klanke, B.; Schley, G.; Bollag, G.; Tsai, J.; Kroening, S.; Yoshihara, D.; Wallace, D.P.; Kraenzlin, B.; Gretz, N.; et al. The Raf kinase inhibitor PLX5568 slows cyst proliferation in rat polycystic kidney disease but promotes renal and hepatic fibrosis. Nephrol. Dial. Transplant. 2011, 26, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Podrini, C.; Cassina, L.; Boletta, A. Metabolic reprogramming and the role of mitochondria in polycystic kidney disease. Cell. Signal. 2019, 67, 109495. [Google Scholar] [CrossRef] [PubMed]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Kipp, K.R.; Rezaei, M.; Lin, L.; Dewey, E.C.; Weimbs, T. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2016, 310, F726–F731. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.; Hein, K.Z.; Nin, V.; Edwards, M.; Chini, C.C.; Hopp, K.; Harris, P.C.; Torres, V.E.; Chini, E.N. Food Restriction Ameliorates the Development of Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2015, 27, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; You, Z.; Gitomer, B.; Brosnahan, G.; Torres, V.E.; Chapman, A.B.; Perrone, R.D.; Steinman, T.I.; Abebe, K.Z.; Rahbari-Oskoui, F.F.; et al. Overweight and obesity are predictors of progression in early autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2018, 29, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Abebe, K.Z.; Schrier, R.W.; Perrone, R.D.; Chapman, A.B.; Yu, A.S.; Braun, W.E.; Steinman, T.I.; Brosnahan, G.; Hogan, M.C.; et al. Dietary salt restriction is beneficial to the management of autosomal dominant polycystic kidney disease. Kidney Int. 2017, 91, 493–500. [Google Scholar] [CrossRef]
- Riwanto, M.; Kapoor, S.; Rodriguez, D.; Edenhofer, I.; Segerer, S.; Wüthrich, R.P. Inhibition of Aerobic Glycolysis Attenuates Disease Progression in Polycystic Kidney Disease. PLoS ONE 2016, 11, e0146654. [Google Scholar] [CrossRef] [PubMed]
- Walz, G.; Budde, K.; Mannaa, M.; Nürnberger, J.; Wanner, C.; Sommerer, C.; Kunzendorf, U.; Banas, B.; Hörl, W.H.; Obermüller, N.; et al. Everolimus in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2010, 363, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.L.; Poster, D.; Kistler, A.D.; Krauer, F.; Raina, S.; Young, J.; Rentsch, K.M.; Spanaus, K.S.; Senn, O.; Kristanto, P.; et al. Sirolimus and Kidney Growth in Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2010, 363, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone Methyl and Kidney Function in CKD with Type 2 Diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013, 329, 2492–2503. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Y.; Liu, Z.; Lu, Y.; Zhu, X.; Lan, B.; Mi, Z.; Dang, L.; Li, N.; Zhan, W.; et al. Activation of NRF2 ameliorates oxidative stress and cystogenesis in autosomal dominant polycystic kidney disease. Sci. Transl. Med. 2020, 12, eaba3613. [Google Scholar] [CrossRef] [PubMed]
- Gattone, V.H.; Chen, N.X.; Sinders, R.M.; Seifert, M.F.; Duan, D.; Martin, D.; Henley, C.; Moe, S.M. Calcimimetic Inhibits Late-Stage Cyst Growth in ADPKD. J. Am. Soc. Nephrol. 2009, 20, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Di Mise, A.; Wang, X.; Ye, H.; Pellegrini, L.; Torres, V.E.; Valenti, G. Pre-clinical evaluation of dual targeting of the GPCRs CaSR and V2R as therapeutic strategy for autosomal dominant polycystic kidney disease. FASEB J. 2021, 35, e21874. [Google Scholar] [CrossRef]
- He, S.; Chen, M.; Lin, X.; Lv, Z.; Liang, R.; Huang, L. Triptolide inhibits PDGF-induced proliferation of ASMCs therough G0/G1 cell cycle arrest and suppression of the AKT/NK-κB/cyclinD1 signaling pathway. Eur. J. Pharmacol. 2020, 867, e172811. [Google Scholar] [CrossRef]
- Chen, D.; Ma, Y.; Wang, X.; Yu, S.; Li, L.; Dai, B.; Mao, Z.; Liu, H.; Liu, S.; Mei, C. Triptolide-Containing Formulation in Patients With Autosomal Dominant Polycystic Kidney Disease and Proteinuria: An Uncontrolled Trial. Am. J. Kidney Dis. 2014, 63, 1070–1072. [Google Scholar] [CrossRef]
- Bukanov, N.O.; Moreno, S.E.; Natoli, T.A.; Rogers, K.A.; Smith, L.A.; Ledbetter, S.R.; Oumata, N.; Galons, H.; Meijer, L.; Ibraghimov-Beskrovnaya, O. CDK inhibitors R-roscovitine and S-CR8 effectively block renal and hepatic cystogenesis in an orthologous model of ADPKD. Cell Cycle 2012, 11, 4040–4046. [Google Scholar] [CrossRef]
- Cebotaru, L.; Liu, Q.; Yanda, M.K.; Boinot, C.; Outeda, P.; Huso, D.L.; Watnick, T.; Guggino, W.B.; Cebotaru, V. Inhibition of histone deacetylase 6 activity reduces cyst growth in polycystic kidney disease. Kidney Int. 2016, 90, 90–99. [Google Scholar] [CrossRef]
- Cao, Y.; Semanchik, N.; Lee, S.H.; Somlo, S.; Barbano, P.E.; Coifman, R.; Sun, Z. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc. Natl. Acad. Sci. USA 2009, 106, 21819–21824. [Google Scholar] [CrossRef] [PubMed]
- Natoli, T.A.; Modur, V.; Ibraghimov-Beskrovnaya, O. Glycosphingolipid metabolism and polycystic kidney disease. Cell. Signal. 2020, 69, 109526. [Google Scholar] [CrossRef] [PubMed]
- Natoli, T.A.; Smith, L.A.; Rogers, K.A.; Wang, B.; Komarnitsky, S.; Budman, Y.; Belenky, A.; Bukanov, N.O.; Dackowski, W.R.; Husson, H.; et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat. Med. 2010, 16, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Corna, D.; Locatelli, M.; Rottoli, D.; Pezzotta, A.; Morigi, M.; Zanchi, C.; Buelli, S.; Guglielmotti, A.; Perico, N.; et al. Effects of MCP-1 Inhibition by Bindarit Therapy in a Rat Model of Polycystic Kidney Disease. Nephron 2014, 129, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Magenheimer, B.S.; Xia, S.; Johnson, T.; Wallace, D.P.; Calvet, J.P.; Li, R. A tumor necrosis factor-α–mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 2008, 14, 863–868. [Google Scholar] [CrossRef]
- Cordido, A.; Nuñez-Gonzalez, L.; Martinez-Moreno, J.M.; Lamas-Gonzalez, O.; Rodriguez-Osorio, L.; Perez-Gomez, M.V.; Martin-Sanchez, D.; Outeda, P.; Chiaravalli, M.; Watnick, T.; et al. TWEAK signaling pathway blockade slows cyst growth and disease progression in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2021, 32, 1913–1932. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Williams, D.; Hajarnis, S.; Hunter, R.; Pontoglio, M.; Somlo, S.; Igarashi, P. miR-17 92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2013, 110, 10765–10770. [Google Scholar] [CrossRef]
- Lee, E.C.; Valencia, T.; Allerson, C.; Schairer, A.; Flaten, A.; Yheskel, M.; Kersjes, K.; Li, J.; Gatto, S.; Takhar, M.; et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 fot the treatment of polycystic kidney disease. Nat. Commun. 2019, 10, 4148. [Google Scholar] [CrossRef]
- Kim, D.Y.; Woo, Y.M.; Lee, S.; Oh, S.; Shin, Y.; Shin, J.-O.; Park, E.Y.; Ko, J.Y.; Lee, E.J.; Bok, J.; et al. Impact of miR-192 and miR-194 on cyst enlargement through EMT in autosomal dominant polycystic kidney disease. FASEB J. 2018, 33, 2870–2884. [Google Scholar] [CrossRef]
| Study Title | Intervention | ADPKD Patients | Duration |
|---|---|---|---|
| Statin therapy in patients with early stage ADPKD (NCT03273413) | pravastatin 40 mg | 200 | 2 years |
| A trial of bardoxolone methyl in patients with ADPKD (FALCON) (NCT03918447) | bardoxolone mehyl placebo | 550 | 52 weeks |
| An extended access program for bardoxolone methyl in patients with CKD (EAGLE) (NCT03749447) | bardoxolone methyl (open label) | 480 | 5 years |
| To evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of oral ALO1211 in healhy volunteers and in ADPKD subjects (NCT04908462) | AL01211 placebo | 98 | 6 months |
| Efficacy and safety of Lixivaptan in the treatment of ADPKD (NCT04064346) | Lixivaptan placebo | 1200 | 2 years |
| Safety of lixivaptan in subjects previously treated with tolvaptan fo ADPKD (ALERT) (NCT04152837) | lixivaptan (open label) | 50 | 52 weeks |
| Study | Title ADPKD Patients | Duration |
|---|---|---|
| Ketogenic dietary interventions in ADPKD (Keto-ADPKD) (NCT04680780) | 63 | 3 months |
| Daily caloric restriction in ADPKD (NCT04907799) | 126 | 2 years |
| Dietary intervention in ADPKD on tolvaptan (NCT03858439) | all on tolvaptan form Hamilton Nephrology clinic (Canada) | 3 months |
| Time restricted feeding in ADPKD (NCT04534985) | 30 | 2 years |
| Study Title | Interventions | ADPKD Patients | Duration |
|---|---|---|---|
| Metformin vs. Tolvaptan for treatment of ADPKD (NCT03764605) | drug: metformin drug: tolvaptan | 150 non-diabetic with PKD1 truncating mutation | 25 months |
| Implementation of Metformin herapy to ease decline of kidney function in polycystic kidney disease (IMPEDE-PKD) (NCT04939935) | drug:metformin drug:placebo | 1164 | 104 weeks |
| PB to treat hereditary nephrogenic diabetes insipidus, ADPKD treated with tolvaptan, and severely polyuric patients with previous lithium administration (SerependityPB1) (NCT05190744) | drug: PB | 20 on tolvaptan | 1 year |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiterová, J.; Tesař, V. Autosomal Dominant Polycystic Kidney Disease: From Pathophysiology of Cystogenesis to Advances in the Treatment. Int. J. Mol. Sci. 2022, 23, 3317. https://doi.org/10.3390/ijms23063317
Reiterová J, Tesař V. Autosomal Dominant Polycystic Kidney Disease: From Pathophysiology of Cystogenesis to Advances in the Treatment. International Journal of Molecular Sciences. 2022; 23(6):3317. https://doi.org/10.3390/ijms23063317
Chicago/Turabian StyleReiterová, Jana, and Vladimír Tesař. 2022. "Autosomal Dominant Polycystic Kidney Disease: From Pathophysiology of Cystogenesis to Advances in the Treatment" International Journal of Molecular Sciences 23, no. 6: 3317. https://doi.org/10.3390/ijms23063317
APA StyleReiterová, J., & Tesař, V. (2022). Autosomal Dominant Polycystic Kidney Disease: From Pathophysiology of Cystogenesis to Advances in the Treatment. International Journal of Molecular Sciences, 23(6), 3317. https://doi.org/10.3390/ijms23063317







