The Inflammatory Conspiracy in Multiple Sclerosis: A Crossroads of Clues and Insights through Mast Cells, Platelets, Inflammation, Gut Microbiota, Mood Disorders and Stem Cells
Abstract
1. Introduction
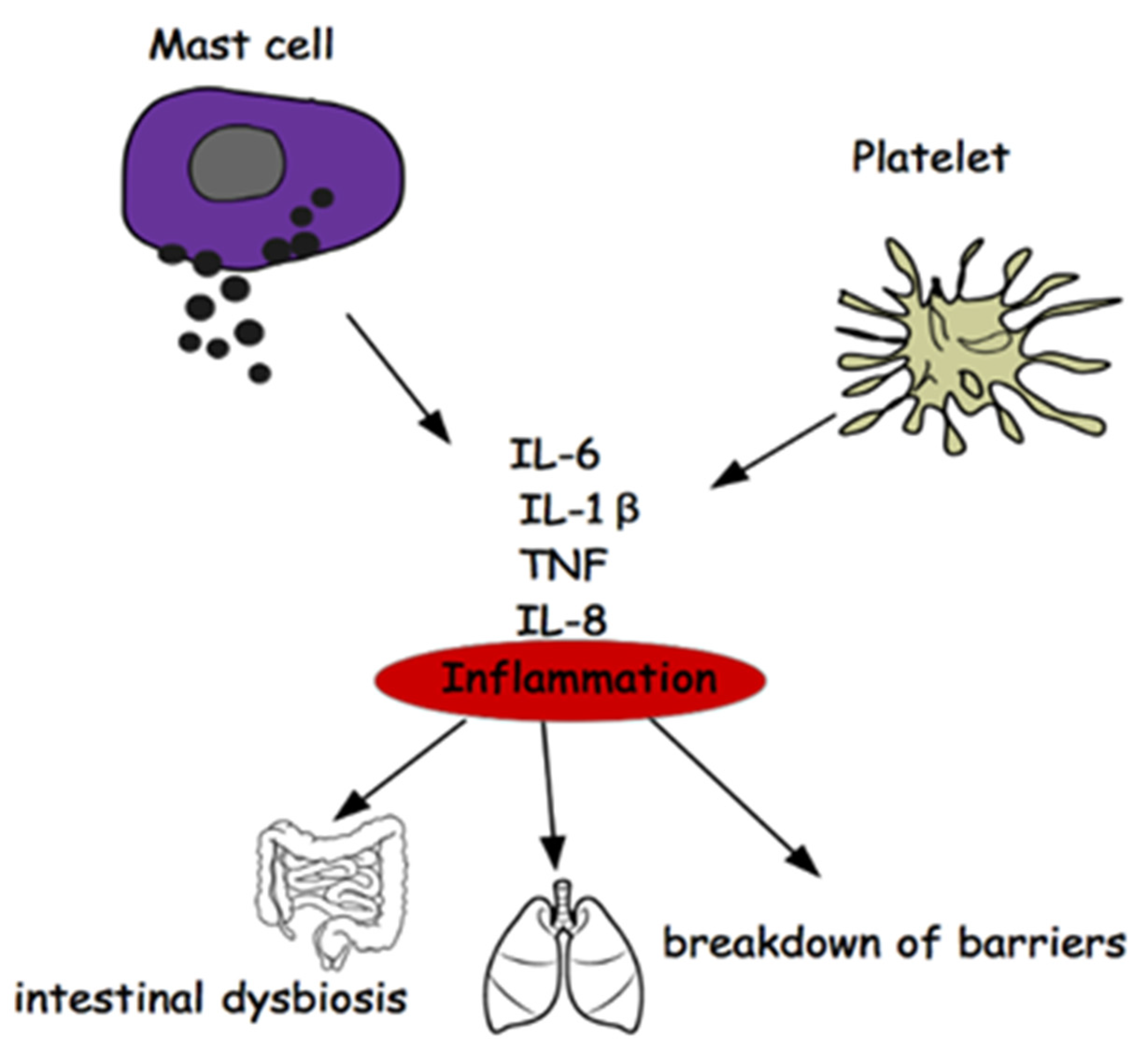
2. Mast Cells and Multiple Sclerosis
2.1. Mast Cells: From Blood to Brain
2.2. MCs, Brain Inflammation, Psychiatric Disorders
3. Platelets
4. Multiple Sclerosis and Intestinal Microbiota
5. Ariadne’s Thread of Multiple Sclerosis
“… Immunological abnormalities in cerebrospinal fluid are remarkably similar in scleroderma involving the central nervous system and in multiple sclerosis. In both conditions, a higher concentration of IgG in the cerebrospinal fluid and of the immune complex in the gamma region was observed on agarose gel electrophoresis. However, immunoglobulin deposition in brain tissue was not consistently found [111]…”
“… Careful evaluation of neurological signs in patients with systemic sclerosis may reveal a more frequent co-occurrence of these two diseases, although the association of these two diseases has rarely been reported in the literature …”
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef]
- Solaro, C.; Gamberini, G.; Masuccio, F.G. Depression in multiple sclerosis: Epide-miology, aetiology, diagnosis and treatment. CNS Drugs. 2018, 32, 117–133. [Google Scholar] [CrossRef]
- Feinstein, A.; Magalhaes, S.; Richard, J.F.; Aude, B.; Moore, C. The link between multiple sclerosis and depression. Nat. Rev. Neurol. 2014, 10, 507–517. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Michael, P.S.; Segal, E.; Eran, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021, 19, 13. [Google Scholar] [CrossRef]
- Jones, M.K.; Nair, A.; Gupta, M. Mast Cells in Neurodegenerative Disease. Front. Cell. Neurosci. 2019, 13, 171. [Google Scholar] [CrossRef]
- De Zuani, M.; Dal Secco, C.; Frossi, B. Mast cells at the crossroads of microbiota and IBD. Eur. J. Immunol. 2018, 48, 1929–1937. [Google Scholar] [CrossRef]
- Traina, G. The role of mast cells in the gut and brain. J. Integr. Neurosci. 2021, 20, 185. [Google Scholar] [CrossRef]
- Kapsenberg, M.L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003, 3, 984–993. [Google Scholar] [CrossRef]
- Traina, G. Mast cells in the brain—Old cells, new target. J. Integr. Neurosci. 2017, 16, S69–S83. [Google Scholar] [CrossRef]
- Traina, G. Mast cells in gut and brain and their potential role as an emerging therapeutic target for neural diseases. Front. Cell. Neurosci. 2019, 13, 345. [Google Scholar] [CrossRef]
- Oksaharju, A.; Kankainen, M.; Kekkonen, R.A.; Lindstedt, K.A.; Kovanen, P.T.; Korpela, R.; Miettinen, M. Probiotic Lactobacillus rhamnosus downregulates FCER1 and HRH4 expression in human mast cells. World J. Gastroenterol. 2011, 17, 750–759. [Google Scholar] [CrossRef]
- Dropp, J.J. Mast cells in the central nervous system of several rodents. Anat. Rec. 1972, 174, 227–237. [Google Scholar] [CrossRef]
- Dropp, J.J. Mast cells in mammalian brain. I. Distribution. Acta Anat. 1976, 94, 1–21. [Google Scholar] [CrossRef]
- Theoharides, T.C. Mast cells: The immune gate to the brain. Life Sci. 1990, 46, 607–617. [Google Scholar] [CrossRef]
- Neumann, J. Ueber das Vorkommen der sogenannten “Mastzellen” bei pathologischen Veränderungen des Gehirns. Arch. für Pathol. Anat. und Physiol. und für Klin. Med. 1890, 122, 378–380. [Google Scholar] [CrossRef]
- Seeldrayers, P.A.; Yasui, D.; Weiner, H.L.; Johnson, D. Treatment of experimental allergic neuritis with nedocromil sodium. J. Neuroimmunol. 1989, 25, 221–226. [Google Scholar] [CrossRef]
- Powell, H.C.; Braheny, S.L.; Myers, R.R.; Rodriguez, M.; Lampert, P.W. Early changes in experimental allergic neuritis. Lab. Investig. 1983, 48, 332–338. [Google Scholar]
- Brosnan, C.F.; Claudio, L.; Tansey, F.A.; Martiney, J. Mechanisms of autoimmune neuropathies. Ann. Neurol. 1990, 27, S75–S79. [Google Scholar] [CrossRef]
- Conti, P.; Kempuraj, D. Important role of mast cells in multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 5, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.; Manolides, L.; Balogiannis, S. Activated rat peritoneal mast cells can cause syngeneic brain demyelination in vitro. Intern. J. Immunopathol. Pharmacol. 1991, 4, 137–144. [Google Scholar]
- Johnson, D.; Seeldrayers, P.A.; Weiner, H.L. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988, 444, 195–198. [Google Scholar] [CrossRef]
- Pinke, K.; Zorzella-Pezavento, S.G.; Lara, V.; Sartori, A. Should mast cells be considered therapeutic targets in multiple sclerosis? Neural Regen Res. 2020, 15, 1995. [Google Scholar] [CrossRef] [PubMed]
- Yuzurihara, M.; Ikarashi, Y.; Ishige, A.; Sasaki, H.; Kuribara, H.; Maruyama, Y. Effects of drugs acting as histamine releasers or histamine receptor blockers on an experimental anxiety model in mice. Pharmacol. Biochem. Behav. 2000, 67, 145–150. [Google Scholar] [CrossRef]
- Goldschmidt, R.C.; Hough, L.B.; Glick, S.D. Rat Brain Mast Cells: Contribution to Brain Histamine Levels. J. Neurochem. 1985, 44, 1943–1947. [Google Scholar] [CrossRef]
- Dimitriadou, V.; Lambracht-Hall, M.; Reichler, J.; Theoharides, T.C. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48 80 and carbachol. Neuroscience 1990, 39, 209–224. [Google Scholar] [CrossRef]
- Manning, K.A.; Pienkowski, T.P.; Uhlrich, D.J. Histaminergic and non-histamine-immunoreactive mast cells within the cat lateral geniculate complex examined with light and electron microscopy. Neuroscience 1994, 63, 191–206. [Google Scholar] [CrossRef]
- Traina, G.; Cocchi, M. Mast Cells, Astrocytes, Arachidonic Acid: Do They Play a Role in Depression? Appl. Sci. 2020, 10, 3455. [Google Scholar] [CrossRef]
- Silverman, A.J.; Sutherland, A.K.; Wilhelm, M.; Silver, R. Mast cells migrate from blood to brain. J. Neurosci. 2000, 20, 401–408. [Google Scholar] [CrossRef]
- Brown, M.A.; Hatfield, J.K. Mast cells are important modifiers of autoimmune disease: With so much evidence, why is there still controversy? Front. Immunol. 2012, 3, 147. [Google Scholar] [CrossRef] [PubMed]
- Lindsberg, P.J.; Strbian, D.; Karjalainen-Lindsberg, M.L. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J. Cereb. Blood Flow Metabolism. SAGE Publ. 2010, 30, 689–702. [Google Scholar] [CrossRef]
- Nelissen, S.; Lemmens, E.; Geurts, N.; Kramer, P.; Maurer, M.; Hendriks, J.; Hendrix, S. The role of mast cells in neuroinflammation. Acta Neuropathologica. Acta Neuropathol. 2013, 125, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, H.; He, S. TNF increases expression of IL-4 and PARs in mast cells. Cell Physiol. Biochem. 2010, 26, 327–336. [Google Scholar] [CrossRef]
- Kim, D.Y.; Jeoung, D.; Ro, J.Y. Signaling Pathways in the Activation of Mast Cells Cocultured with Astrocytes and Colocalization of Both Cells in Experimental Allergic Encephalomyelitis. J. Immunol. 2010, 185, 273–283. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hong, G.U.; Ro, J.Y. Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J. Neuroinflamm. 2011, 8, 1–16. [Google Scholar] [CrossRef]
- Hösli, L.; Hösli, E.; Schneider, U.; Wiget, W. Evidence for the existence of histamine H1- and H2-receptors on astrocytes of cultured rat central nervous system. Neurosci. Lett. 1984, 48, 287–291. [Google Scholar] [CrossRef]
- Rozniecki, J.J.; Hauser, S.L.; Strein, M.; Lincoln, R.; Theoharides, T.C. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 1995, 37, 63–66. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L. Bio Molecular Considerations in Major Depression and Ischemic Cardiovascular Disease. Cent. Nerv. Syst. Agents Med. Chem. 2012, 10, 97–107. [Google Scholar] [CrossRef]
- Benedetti, S.; Bucciarelli, S.; Canestrari, F.; Catalani, S.; Mandolini, S.; Marconi, V.; Mastrogiacomo, A.M.; Silvestri, R.; Tagliamento, M.C.; Raimondo, V.; et al. Platelet’s Fatty Acids and Differential Diagnosis of Major Depression and Bipolar Disorder through the Use of an Unsupervised Competitive-Learning Network Algorithm (SOM). Open J. Depress. 2014, 3, 52–73. [Google Scholar] [CrossRef][Green Version]
- Cocchi, M.; Tonello, L. Biological, biochemical and mathematical considerations about the use of an Artificial Neural Network (ANN) for the study of the connection between platelet fatty acids and major depression. Technical report. J. Biol. Res. 2006, 81, 82–87. [Google Scholar] [CrossRef]
- Marshall, J.S.; Waserman, S. Mast cells and the nerves—Potential interactions in the context of chronic disese. Clinical and Experimental Allergy. Clin. Exp. Allergy 1995, 25, 102–110. [Google Scholar] [CrossRef]
- Bienenstock, J.; Blennerhassett, M.; Goetzl, E. Nerve–Mast Cell Interactions—Partnership in Health and Disease. In Autonomic Neuroimmunology; CRC Press: Boca Raton, FL, USA, 2003; pp. 157–188. [Google Scholar] [CrossRef]
- Purcell, W.M.; Atterwill, C.K. Mast cells in neuroimmune function: Neurotoxicological and neuropharmacological perspectives. Neurochem. Res. 1995, 20, 521–532. [Google Scholar] [CrossRef]
- Pang, X.; Boucher, W.; Triadafilopoulos, G.; Sant, G.R.; Theoharides, T.C. Mast cell and substance P-positive nerve involvement in a patient with both irritable bowel syndrome and interstitial cystitis. Urology 1996, 47, 436–438. [Google Scholar] [CrossRef]
- Arizono, N.; Matsuda, S.; Hattori, T.; Kojima, Y.; Maeda, T.; Galli, S. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab. Investig. A J. Tech. Methods Pathol. 1990, 62, 626–634. [Google Scholar]
- Bauer, O.; Razin, E. Mast Cell-Nerve Interactions. Physiology 2000, 15, 213–218. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kops, S.K.; Bondy, P.K.; Askenase, P.W. Differential release of serotonin without comparable histamine under diverse conditions in the rat mast cell. Biochem. Pharmacol. 1985, 34, 1389–1398. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Klutke, C.G.; Hofmeister, M.; He, F.; Russell, J.H.; Becich, M.J. Role of the Immune Response in Interstitial Cystitis. Clin. Immunol. Immunopathol. 1995, 74, 209–216. [Google Scholar] [CrossRef]
- Kandere-Grzybowska, K.; Letourneau, R.; Kempuraj, D.; Donelan, J.; Poplawski, S.; Boucher, W.; Athanassiou, A.; Theoharis, C.T. IL-1 Induces Vesicular Secretion of IL-6 without Degranulation from Human Mast Cells. J. Immunol. 2003, 171, 4830–4836. [Google Scholar] [CrossRef]
- Kops, S.K.; Theoharides, T.C.; Cronin, C.T.; Kashgarian, M.G.; Askenase, P.W. Ultrastructural characteristics of rat peritoneal mast cells undergoing differential release of serotonin without histamine and without degranulation. Cell Tissue Res. 1990, 262, 415–424. [Google Scholar] [CrossRef]
- Lafaille, J.J.; Van de Keere, F.; Hsu, A.L.; Baron, J.L.; Haas, W.; Raine, C.S.; Tonegawa, S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 1997, 186, 307–312. [Google Scholar] [CrossRef]
- Brenner, T.; Soffer, D.; Shalit, M.; Levi-Schaffer, F. Mast cells in experimental allergic encephalomyelitis: Characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J. Neurol. Sci. 1994, 122, 210–213. [Google Scholar] [CrossRef]
- Secor, V.H.; Secor, E.W.; Gutekunst, C.A.; Brown, M.A. Mast Cells Are Essential for Early Onset and Severe Disease in a Murine Model of Multiple Sclerosis. J. Exp. Med. 2000, 191, 813–821. [Google Scholar] [CrossRef]
- Silver, R.; Curley, J.P. Mast cells on the mind: New insights and opportunities. Trends in Neurosciences. Trends Neurosci. 2013, 36, 513–521. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Donelan, J.M.; Papadopoulou, N.; Cao, J.; Kempuraj, D.; Conti, P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol. Sci. 2004, 25, 563–568. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Amodeo, G.; Allegra, T.M.; Fagiolini, A. Depression and Inflammation: Disentangling a Clear Yet Complex and Multifaceted Link. Neuropsychiatry 2018, 7, 448–457. [Google Scholar] [CrossRef]
- Stertz, L.; Magalhães, P.V.S.; Kapczinski, F. Is bipolar disorder an inflammatory condition? the relevance of microglial activation. Curr. Opin Psychiatry 2013, 26, 19–26. [Google Scholar] [CrossRef]
- Kempuraj, D.; Mentor, S.; Thangavel, R.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Dubova, I.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer’s Disease. Front. Cell. Neurosci. 2019, 13, 54. [Google Scholar] [CrossRef]
- Verri, M.; Pastoris, O.; Dossena, M.; Aquilani, R.; Guerriero, F.; Cuzzoni, G.; Venturini, L.; Ricevuti, G.; Bongiorno, A.I. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int. J. Immunopathol. Pharmacol. 2012, 25, 345–353. [Google Scholar] [CrossRef]
- Kritas, S.K.; Saggini, A.; Cerulli, G.; Caraffa, A.; Antinolfi, P.; Pantalone, A.; Rosati, M.; Tei, M.; Speziali, A.; Saggini, R.; et al. Impact of Mast Cells on Multiple Sclerosis: Inhibitory Effect of Natalizumab. Int. J. Immunopathol. Pharmacol. 2014, 27, 331–335. [Google Scholar] [CrossRef]
- Costanza, M.; Colombo, M.P.; Pedotti, R. Mast Cells in the Pathogenesis of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2012, 13, 15107–15125. [Google Scholar] [CrossRef]
- Nautiyal, K.M.; Ribeiro, A.C.; Pfaff, D.W.; Silver, R. Brain mast cells link the immune system to anxiety-like behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 18053–18057. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Isenberg, S.; Hochron, S.M. Asthma and emotion: A review. J. Asthma 1993, 30, 5–21. [Google Scholar] [CrossRef]
- Costa-Pinto, F.A.; Basso, A.S.; Russo, M. Role of mast cell degranulation in the neural correlates of the immediate allergic reaction in a murine model of asthma. Brain Behav. Immun. 2007, 21, 783–790. [Google Scholar] [CrossRef]
- Costa-Pinto, F.A.; Basso, A.S.; De Sá-Rocha, L.C.; Britto, L.R.G.; Russo, M.; Palermo-Neto, J. Neural correlates of IgE-mediated allergy. In Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2006; pp. 116–131. [Google Scholar] [CrossRef]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef]
- Donoso, A.O.; Broitman, S.T. Effects of a histamine synthesis inhibitor and antihistamines on the sexual behavior of female rats. Psychopharmacology 1979, 66, 251–255. [Google Scholar] [CrossRef]
- Ikarashi, Y.; Yuzurihara, M. Experimental anxiety induced by histaminergics in mast cell-deficient and congenitally normal mice. Pharmacol. Biochem. Behav. 2002, 72, 437–441. [Google Scholar] [CrossRef]
- Dere, E.; De Souza-Silva, M.A.; Spieler, R.E.; Lin, J.S.; Ohtsu, H.; Haas, H.L.; Huston, J.P. Changes in motoric, exploratory and emotional behaviours and neuronal acetylcholine content and 5-HT turnover in histidine decarboxylase-KO mice. Eur. J. Neurosci. 2004, 20, 1051–1058. [Google Scholar] [CrossRef]
- Lucki, I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 1998, 44, 151–162. [Google Scholar] [CrossRef]
- Gaspar, P.; Cases, O.; Maroteaux, L. The developmental role of serotonin: News from mouse molecular genetics. Nat. Rev. Neurosci. 2003, 4, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J. Cytokine activation of the HPA axis. Ann. N. Y. Acad. Sci. 2000, 917, 608–817. [Google Scholar] [CrossRef]
- Conti, B.; Tabarean, I.; Andrei, C.; Bartfai, T. Cytokines and fever. Front. Biosci. 2004, 9, 1433–1449. [Google Scholar] [CrossRef]
- Krueger, J.M.; Obál, F.; Fang, J.; Kubota, T.; Taishi, P. The role of cytokines in physiological sleep regulation. N. Y. Acad. Sci. 2001, 993, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ueno, R.; Honda, K.; Inoue, S.; Hayaishi, O. Prostaglandin D2, a cerebral sleep-inducing substance in rats. Proc. Natl. Acad. Sci. USA 1983, 80, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Devidze, N.; Fujimori, K.; Urade, Y.; Pfaff, D.W.; Mong, J.A. Estradiol regulation of lipocalin-type prostaglandin D synthase promoter activity: Evidence for direct and indirect mechanisms. Neurosci. Lett. 2010, 474, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhao, W.; Beers, D.R.; Henkel, J.S.; Appel, S.H. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol. 2012, 237, 147–152. [Google Scholar] [CrossRef]
- Corcia, P.; Tauber, C.; Vercoullie, J.; Arlicot, N.; Prunier, C.; Praline, J.; Nicolas, G.; Venel, Y.; Hommet, C.; Baulieu, J.L.; et al. Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e52941. [Google Scholar] [CrossRef]
- Ehrhart, J.; Smith, A.J.; Kuzmin-Nichols, N.; Zesiewicz, T.A.; Jahan, I.; Shytle, R.D.; Kim, S.H.; Sanberg, C.D.; Vu, T.H.; Gooch, C.L.; et al. Humoral factors in ALS patients during disease progression. J. Neuroinflammation 2015, 12, 127. [Google Scholar] [CrossRef]
- Leiter, O.; Walker, T.L. Platelets in Neurodegenerative Conditions—Friend or Foe? Front. Immunol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Ponomarev, E.D. Fresh evidence for platelets as neuronal and innate immune cells: Their role in the activation, differentiation, and deactivation of Th1, Th17, and tregs during tissue inflammation. Front. Immunol. 2018, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Orian, J.M.; D’Souza, C.S.; Kocovski, P.; Krippner, G.; Hale, M.W.; Wang, X.; Peter, K. Platelets in Multiple Sclerosis: Early and Central Mediators of Inflammation and Neurodegeneration and Attractive Targets for Molecular Imaging and Site-Directed Therapy. Front. Immunol. 2021, 12, 620963. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Q.; Xu, J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020, 99, 1205–1208. [Google Scholar] [CrossRef]
- Sheremata, W.A.; Jy, W.; Horstman, L.L.; Ahn, Y.S.; Alexander, J.S.; Minagar, A. Evidence of platelet activation in multiple sclerosis. J. Neuroinflamm. 2008, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Karhausen, J.; Choi, H.W.; Maddipati, K.R.; Mathew, J.P.; Ma, Q.; Boulaftali, Y.; Lee, R.B.; Bergmeir, W.; Abraham, S.N. Platelets trigger perivascular mast cell degranulation to cause inflammatory responses and tissue injury. Sci. Adv. 2020, 6, eaay6314. [Google Scholar] [CrossRef] [PubMed]
- Horstman, L.L.; Jy, W.; Ahn, Y.S.; Zivadinov, R.; Maghzi, A.H.; Etemadifar, M.; Alexander, J.A.; Minagar, A. Role of platelets in neuroinflammation: A wide-angle perspective. J. Neuroinflamm. 2010, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Choi, E.Y.; Zhou, H.; Schleicher, R.; Chung, K.J.; Tang, Z. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ. Res. 2012, 110, 1202–1210. [Google Scholar] [CrossRef]
- Vogel, D.Y.S.; Vereyken, E.J.F.; Glim, J.E.; Heijnen, P.D.A.M.; Moeton, M.; van der Valk, P.; Amor, S.; Teunissen, E.C.; van Horssen, J.; Dijkstra, C.D. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 2013, 10, 1–12. [Google Scholar] [CrossRef]
- Ehrlich, D. Platelets in psychiatric disorders. World J. Psychiatry 2012, 2, 91. [Google Scholar] [CrossRef]
- Williams, M.S. Platelets and depression in cardiovascular disease: A brief review of the current literature. World J. Psychiatry 2012, 2, 114. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L.; Tsaluchidu, S.; Puri, B.K. The use of artificial neural networks to study fatty acids in neuropsychiatric disorders. BMC Psychiatry 2008, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Izzi, B.; Tirozzi, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Hoylaerts, M.F.; Icaoviello, L.; Gialluisi, A. Beyond haemostasis and thrombosis: Platelets in depression and its co-morbidities. Int. J. Mol. Sci. 2020, 21, 8817. [Google Scholar] [CrossRef] [PubMed]
- Heron, D.S.; Shinitzky, M.; Hershkowitz, M.; Samuel, D. Lipid fluidity markedly modulates the binding of serotonin to mouse brain membranes. Proc. Natl. Acad. Sci. USA 1980, 77, 7463–7467. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Traina, G. Tryptophan and membrane mobility as conditioners and brokers of gut-brain axis in depression. Appl. Sci. 2020, 10, 4933. [Google Scholar] [CrossRef]
- Hanage, W.P. Microbiology: Microbiome science needs a healthy dose of scepticism. Nature 2014, 512, 247–248. [Google Scholar] [CrossRef]
- Briones, M.R.S.; Snyder, A.M.; Ferreira, R.C.; Neely, E.B.; Connor, J.R.; Broach, J.R. A Possible Role for Platelet-Activating Factor Receptor in Amyotrophic Lateral Sclerosis Treatment. Front. Neurol. 2018, 9, 39. [Google Scholar] [CrossRef]
- Branton, W.G.; Lu, J.Q.; Surette, M.G.; Holt, R.A.; Lind, J.; Laman, J.D.; Power, C. Brain microbiota disruption within inflammatory demyelinating lesions in multiple sclerosis. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Schrijver, I.A.; Van Meurs, M.; Melief, M.J.; Ang, C.W.; Buljevac, D.; Ravid, R.; Hazenberg, M.P.; Laman, J.D. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain 2001, 124, 1544–1554. [Google Scholar] [CrossRef]
- Visser, L.; Melief, M.J.; Van Riel, D.; Van Meurs, M.; Sick, E.A.; Inamura, S.; Bajramovic, J.J.; Amor, S.; Hintzen, R.Q.; Boven, L.A.; et al. Phagocytes containing a disease-promoting toll-like receptor/nod ligand are present in the brain during demyelinating disease in primates. Am. J. Pathol. 2006, 169, 1671–1685. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Pellegrini, C.; Colucci, R.; Antonioli, L.; Barocelli, E.; Ballabeni, V.; Bernardini, N.; Blandizzi, C.; de Jonge, W.J.; Fornai, M. Intestinal dysfunction in Parkinson’s disease: Lessons learned from translational studies and experimental models. Neurogastroenterol. Motil. 2016, 28, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Aletta, D.; KraneveldRietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.A.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Walsh, J.G.; Sinclair, D.B.; Johnson, E.; Tang-Wai, R.; Wheatley, B.M.; Branton, W.; Maingat, F.; Snyder, T.; Gross, D.W.; et al. Inflammasome induction in Rasmussen’s encephalitis: Cortical and associated white matter pathogenesis. J. Neuroinflamm. 2013, 10, 918. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 27, 28484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Trostle, D.C.; Helfrich, D.; Medsger, T.A. Systemic sclerosis (scleroderma) and multiple sclerosis. Arthritis Rheum. 1986, 29, 124–127. [Google Scholar] [CrossRef]
- Bernheimer, H.; Budka, H.; Müller, P. Brain tissue immunoglobulins in adrenoleukodystrophy: A comparison with multiple sclerosis and systemic lupus erythematosus. Acta Neuropathol. 1983, 59, 95–102. [Google Scholar] [CrossRef]
- McFarlin, D.E.; McFarland, H.F. Multiple sclerosis (first of two parts). N. Engl. J. Med. 1982, 307, 1183–1188. [Google Scholar] [CrossRef]
- Pelidou, S.H.; Tsifetaki, N.; Giannopoulos, S.; Deretzi, G.; Voulgari, P.; Kyritsis, A. Multiple sclerosis associated with systemic sclerosis. Rheumatol. Int. 2007, 27, 771–773. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L.; Gabrielli, F. Hypothesis of stem cells involvement in depressive disorders: A possible link to human evolution and to philosophical reflection? Hum. Evol. 2011, 26, 1–12. Available online: https://www.researchgate.net/publication/286720769_Hypothesis_of_stem_cells_involvement_in_depressive_disorders_A_possible_link_to_human_evolution_and_to_philosophical_reflection (accessed on 28 January 2022).
- Kohonen Self-Organizing Maps. A special type of Artificial Neural Network. Available online: https://towardsdatascience.com/kohonen-self-organizing-maps-a29040d688da (accessed on 25 April 2021).
- Jangi, S.; Gandhi, R.; Cox, L.; Li, N.; Von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [PubMed]
- Sigeeson, B.J.; Socicty, S. Lectures on the Diseases of the Nervous System.1-_This volume will be highly prized by the members of the Sydenham 1 Lectures on the Diseases of the Nervous System, delivered at La Salpetrie>’e? The British and Foreign Medico-Chirurgical Review. Biomed. J. Digit. Proj. 1877, 60, 271. [Google Scholar]
- Butler, M.A.; Bennett, T.L. In Search of a Conceptualization of Multiple Sclerosis: A Historical Perspective. Neuropsychol. Rev. 2003, 13, 93–112. [Google Scholar] [CrossRef]
- Ebers, G.C. Neurobehavioral Aspects of Multiple Sclerosis. Arch. Neur. 1991, 48, 897. [Google Scholar] [CrossRef]
- Patten, S.B.; Beck, C.A.; Williams, J.V.A.; Barbui, C.; Metz, L.M. Major depression in multiple sclerosis: A population-based perspective. Neurology 2003, 62, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Tauil, C.B.; Grippe, T.C.; Dias, R.M.; Dias-Carneir, R.P.C.; Carneiro, N.M.; Aguilar, A.C.R.; da Silvia, F.M.; Bezerra, F.; de Almeida, L.K.; Massarente, V.L.; et al. Suicidal ideation, anxiety, and depression in patients with multiple sclerosis. Arq. Neuropsiquiatr. 2018, 76, 296–301. [Google Scholar] [CrossRef]
- Mikula, P.; Timkova, V.; Linkova, M.; Vitkova, M.; Szilasiova, J.; Nagyova, I. Fatigue and Suicidal Ideation in People With Multiple Sclerosis: The Role of Social Support. Front. Psychol. 2020, 11, 504. [Google Scholar] [CrossRef]
- Le-Niculescu, H.; Roseberry, K.; Gill, S.S.; Levey, D.F.; Phalen, P.L.; Mullen, J.; Bhairo, S.; Voegtline, T.; Davis, H.; Shekhar, A.; et al. Precision medicine for mood disorders: Objective assessment, risk prediction, pharmacogenomics, and repurposed drugs. Mol. Psychiatry 2021, 26, 2776–2804. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchi, M.; Mondo, E.; Romeo, M.; Traina, G. The Inflammatory Conspiracy in Multiple Sclerosis: A Crossroads of Clues and Insights through Mast Cells, Platelets, Inflammation, Gut Microbiota, Mood Disorders and Stem Cells. Int. J. Mol. Sci. 2022, 23, 3253. https://doi.org/10.3390/ijms23063253
Cocchi M, Mondo E, Romeo M, Traina G. The Inflammatory Conspiracy in Multiple Sclerosis: A Crossroads of Clues and Insights through Mast Cells, Platelets, Inflammation, Gut Microbiota, Mood Disorders and Stem Cells. International Journal of Molecular Sciences. 2022; 23(6):3253. https://doi.org/10.3390/ijms23063253
Chicago/Turabian StyleCocchi, Massimo, Elisabetta Mondo, Marcello Romeo, and Giovanna Traina. 2022. "The Inflammatory Conspiracy in Multiple Sclerosis: A Crossroads of Clues and Insights through Mast Cells, Platelets, Inflammation, Gut Microbiota, Mood Disorders and Stem Cells" International Journal of Molecular Sciences 23, no. 6: 3253. https://doi.org/10.3390/ijms23063253
APA StyleCocchi, M., Mondo, E., Romeo, M., & Traina, G. (2022). The Inflammatory Conspiracy in Multiple Sclerosis: A Crossroads of Clues and Insights through Mast Cells, Platelets, Inflammation, Gut Microbiota, Mood Disorders and Stem Cells. International Journal of Molecular Sciences, 23(6), 3253. https://doi.org/10.3390/ijms23063253







