Hedgehog Signalling Modulates Immune Response and Protects against Experimental Autoimmune Encephalomyelitis
Abstract
:1. Introduction
2. Results
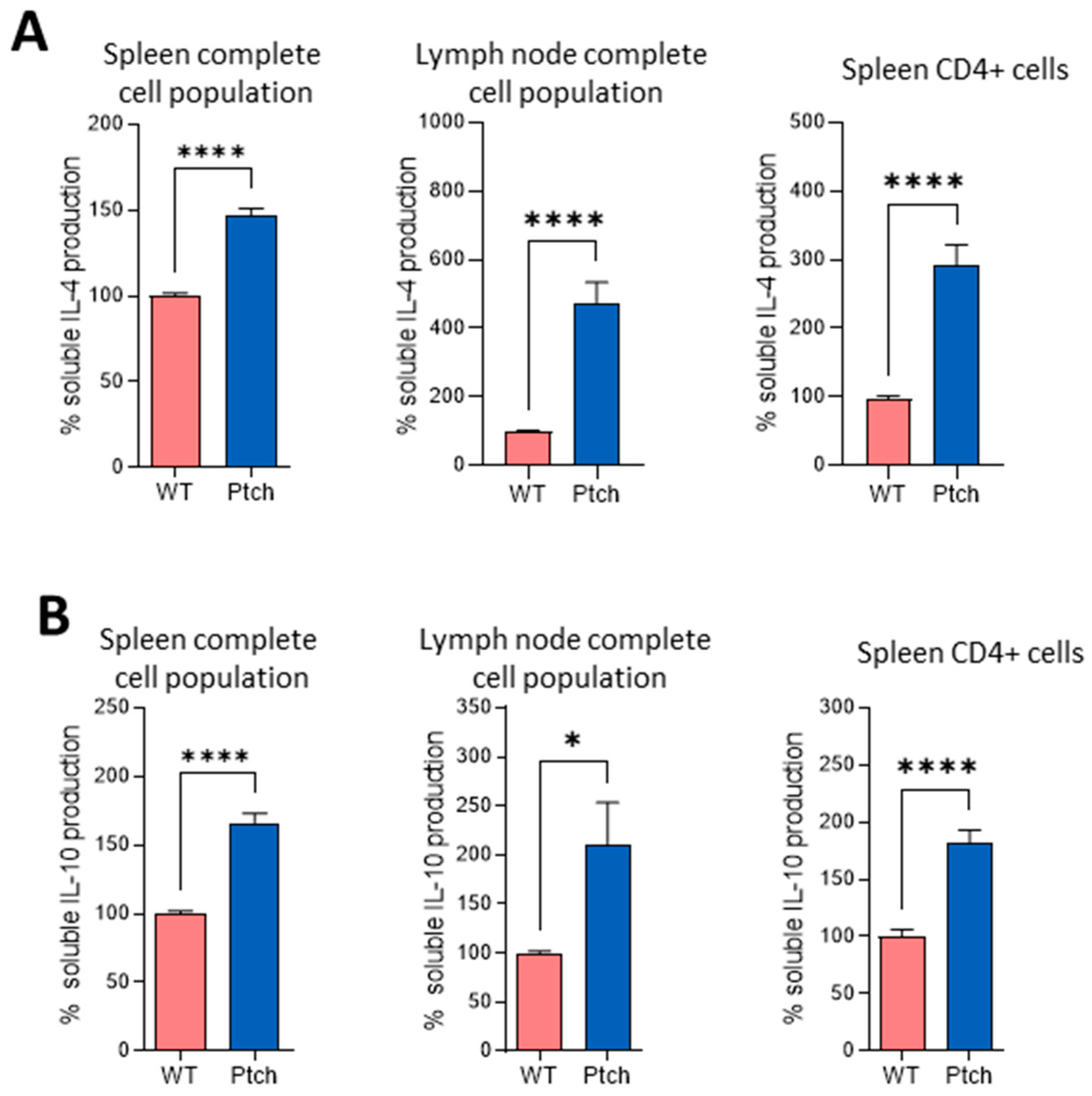
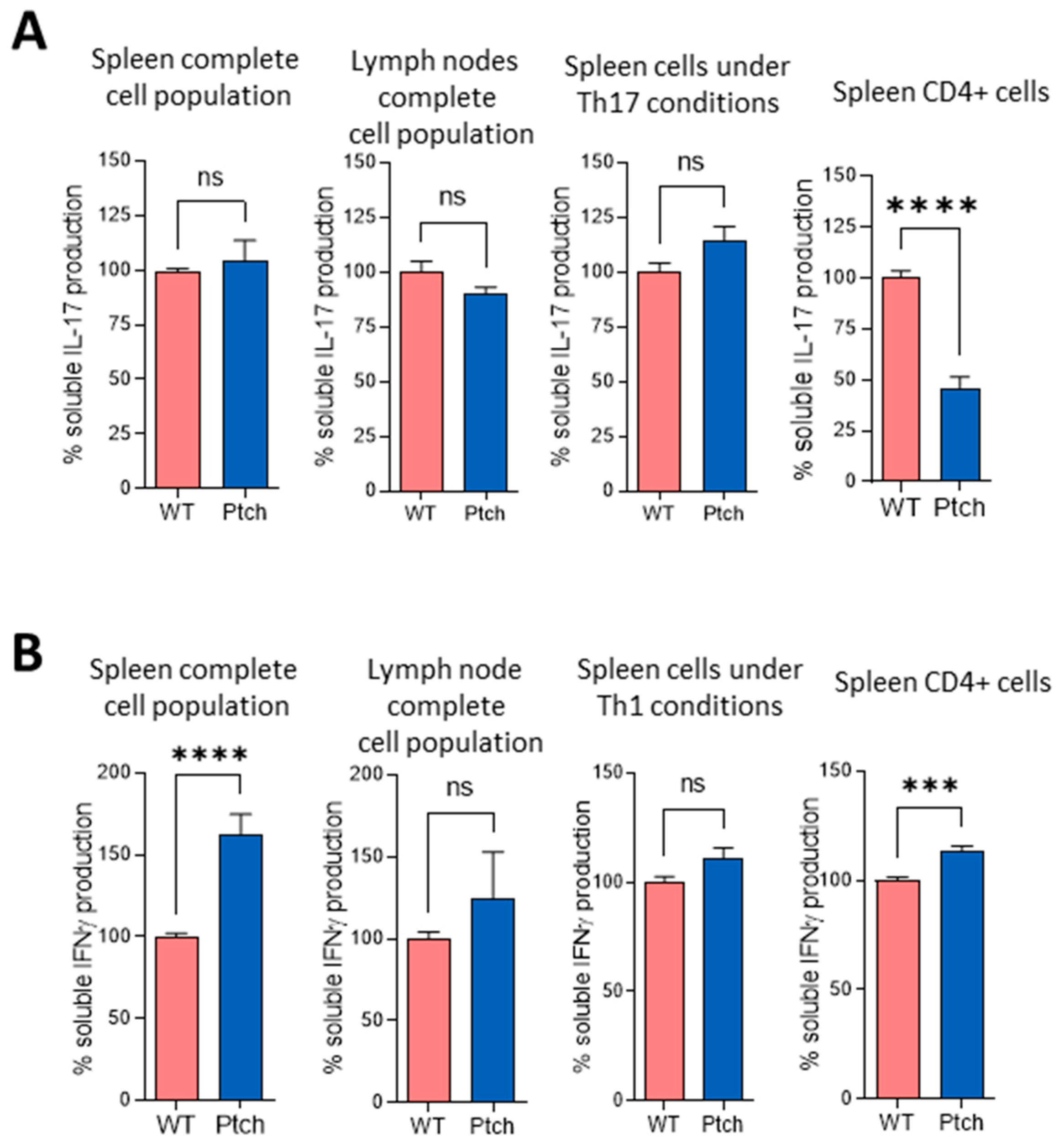
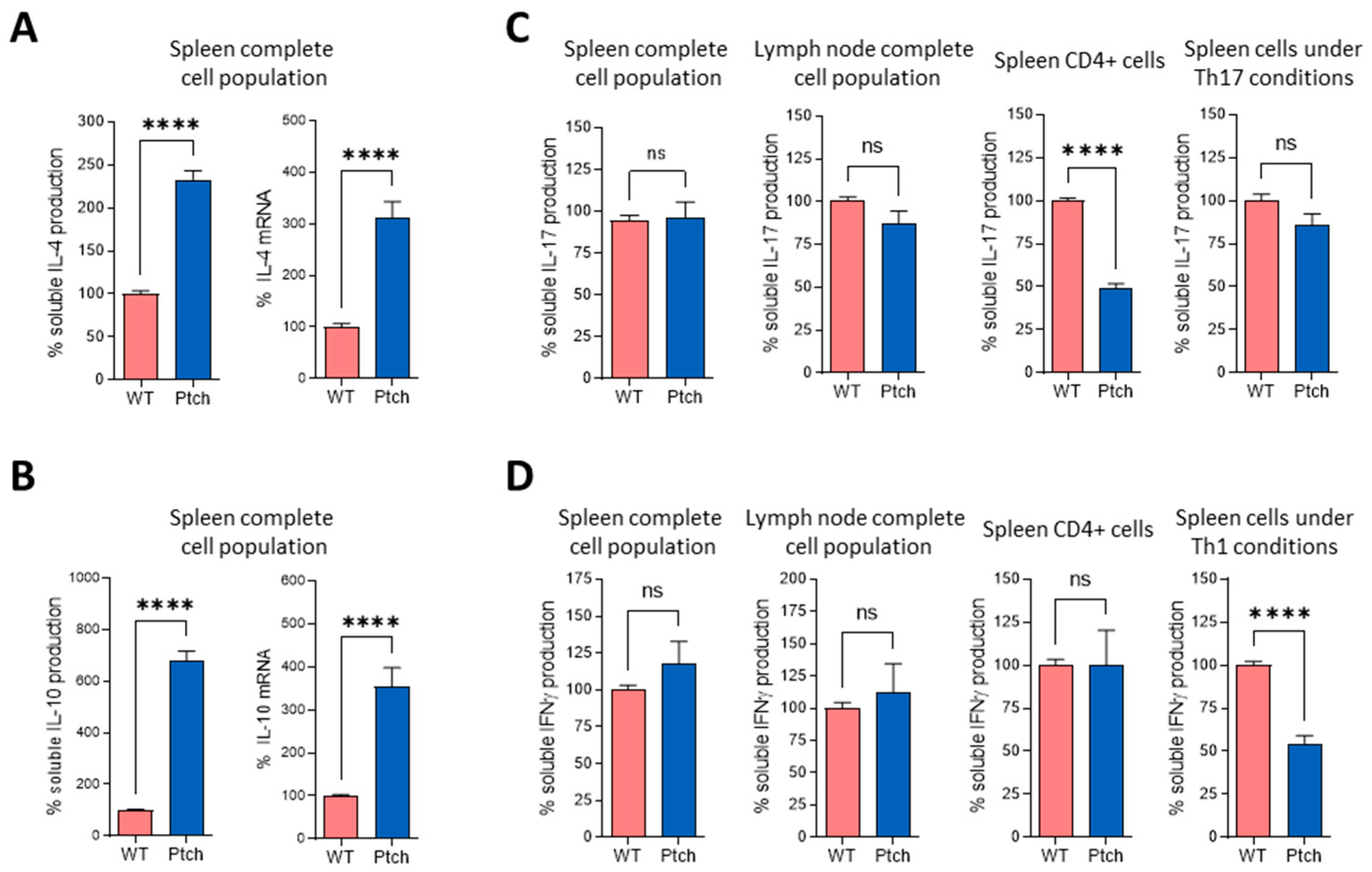
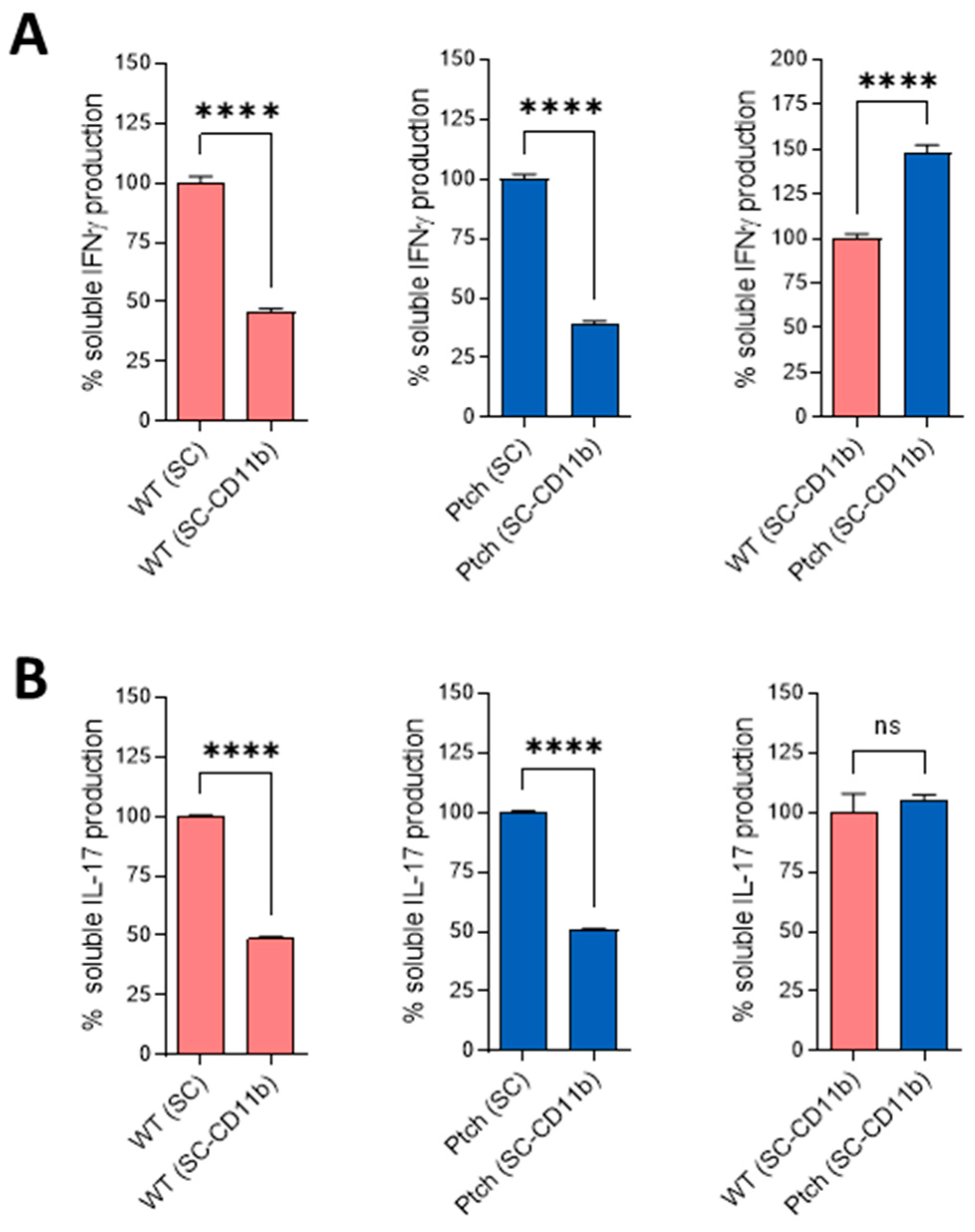
2.1. The Th2 Profile Is Favoured in T lymphocytes from ptch+/− Mice
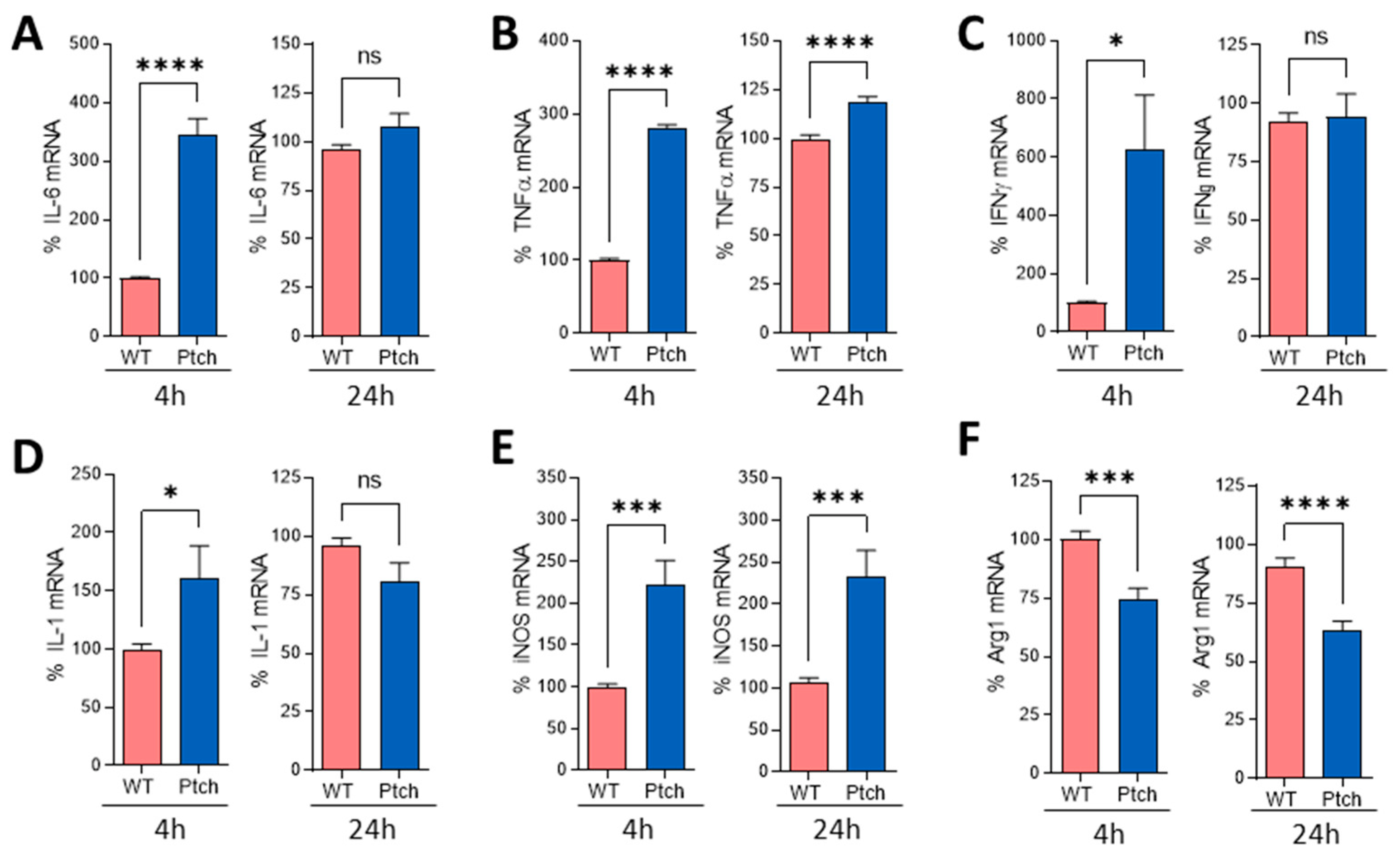
2.2. Peritoneal Macrophages Are Prone to M1 Phenotype
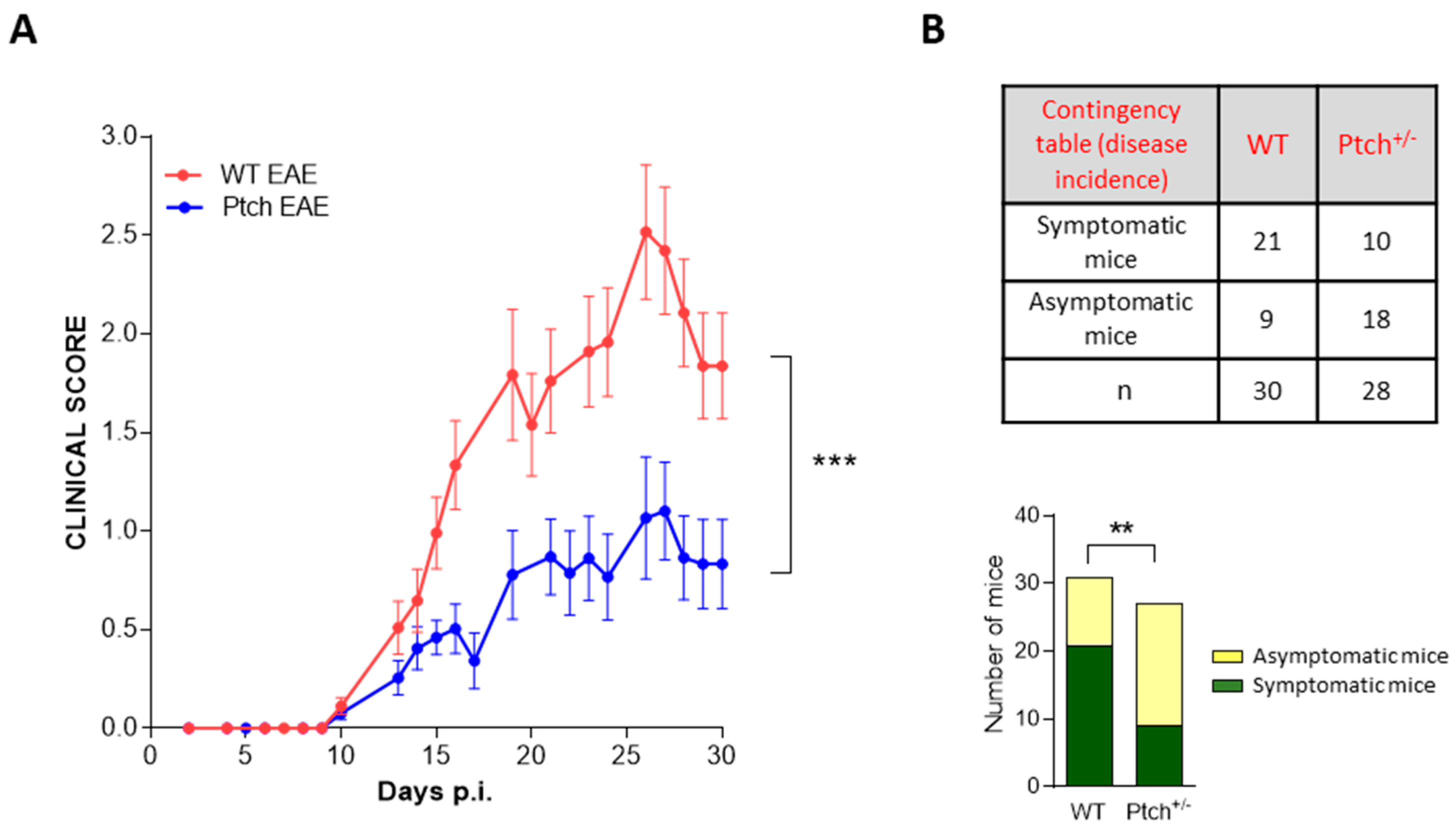
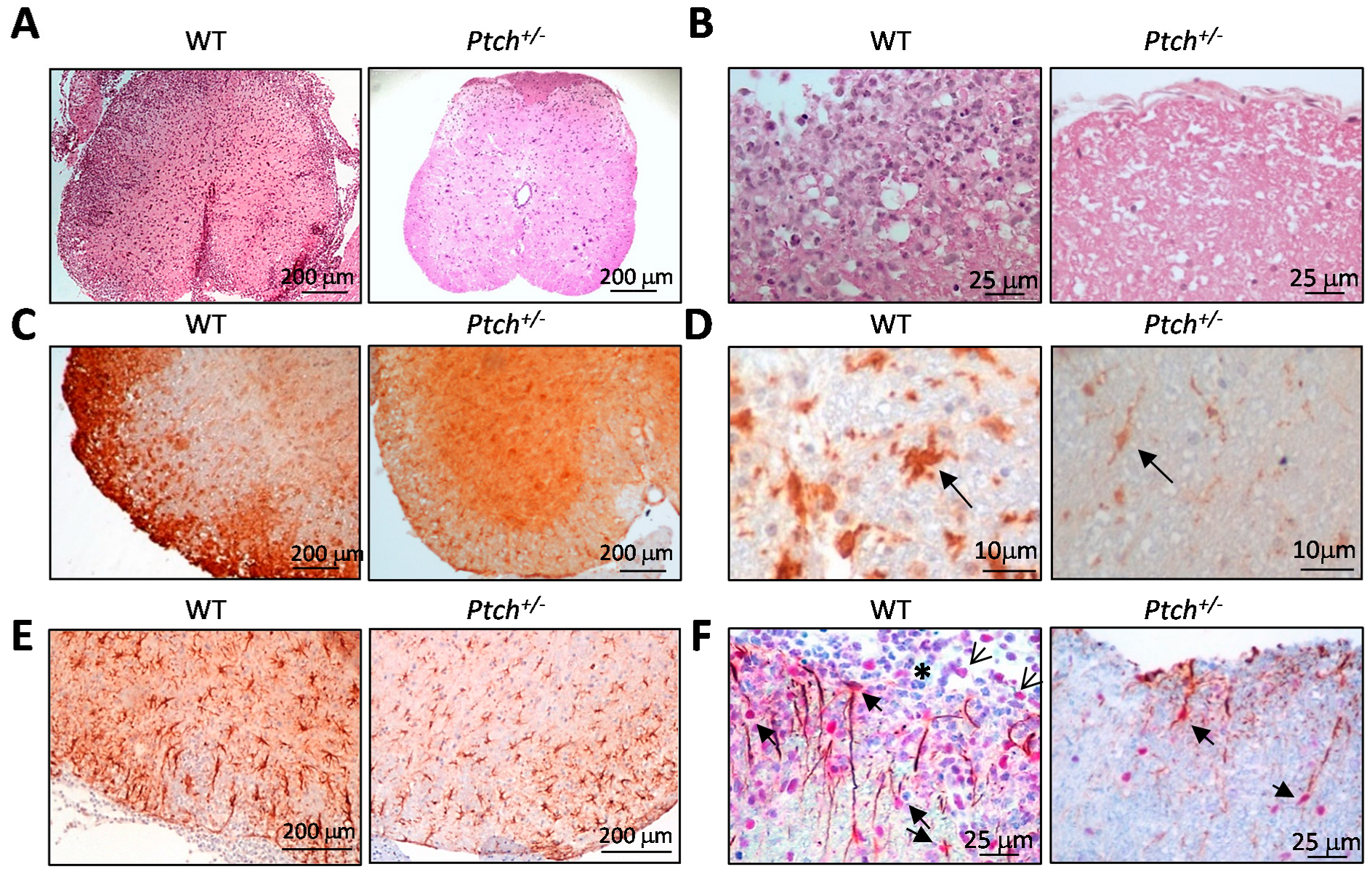
2.3. EAE Development Is Attenuated in Ptch+/− Mice
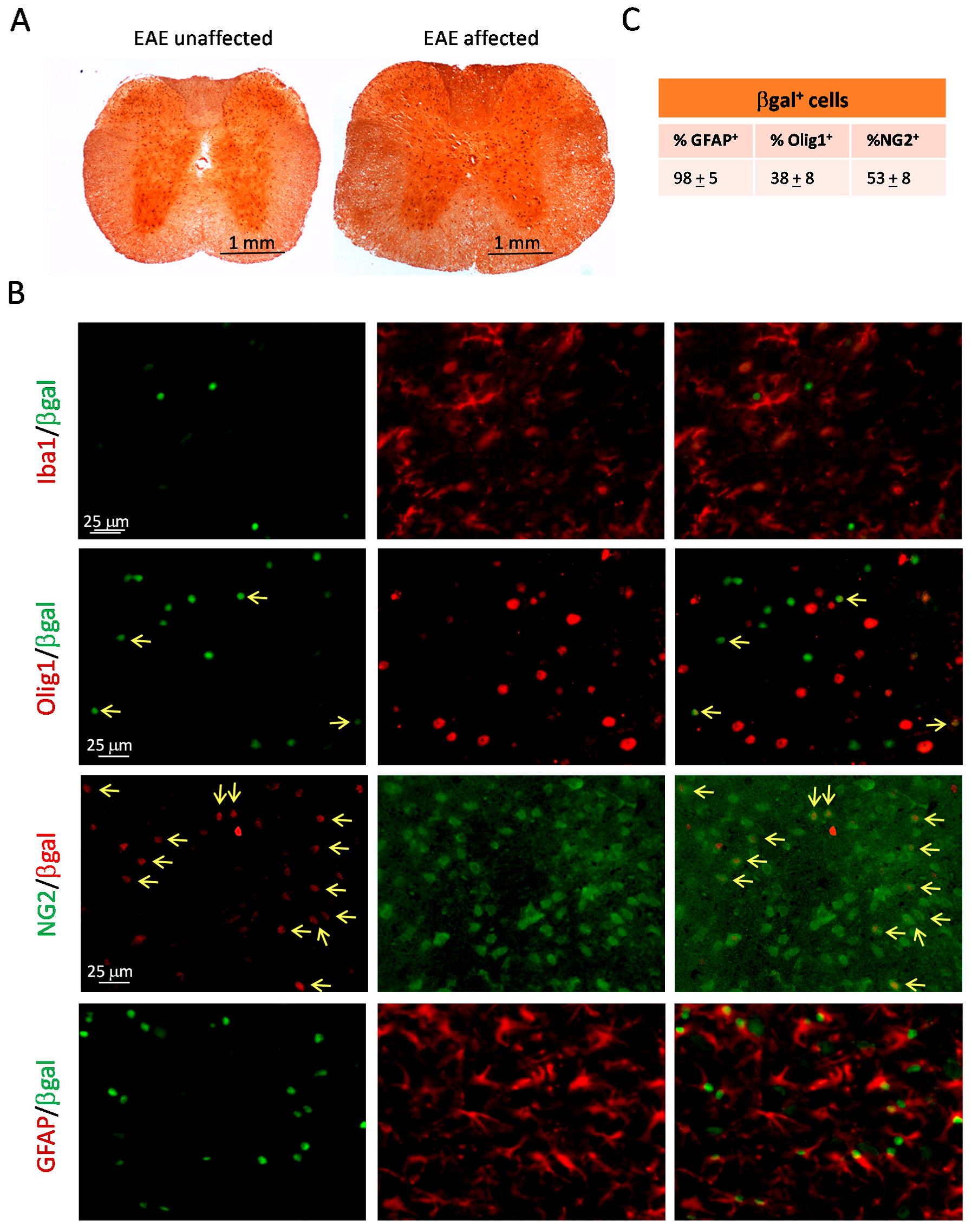
2.4. The Hh Pathway Is Activated in Glial Cells of ptch+/− Mice Inoculated with MOG
3. Discussion
4. Materials and Methods
4.1. Cell Isolations, Cultures and Stimulations
4.2. ELISA Determinations
4.3. Quantitative Real-Time RT-PCR (RT-qPCR)
4.4. Animals, EAE Inductions, Clinical Evaluations and Treatments
4.5. Histopathology
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briscoe, J.; Therond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Ingham, P.W.; McMahon, A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [Green Version]
- Crompton, T.; Outram, S.V.; Hager-Theodorides, A.L. Sonic hedgehog signalling in T-cell development and activation. Nat. Rev. Immunol. 2007, 7, 726–735. [Google Scholar] [CrossRef]
- Rowbotham, N.J.; Hager-Theodorides, A.L.; Furmanski, A.L.; Crompton, T. A novel role for Hedgehog in T-cell receptor signaling: Implications for development and immunity. Cell Cycle 2007, 6, 2138–2142. [Google Scholar] [CrossRef] [PubMed]
- Doheny, D.; Manore, S.G.; Wong, G.L.; Lo, H.W. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells 2020, 9, 2114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Struhl, G. Dual roles for patched in sequestering and transducing Hedgehog. Cell 1996, 87, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D. Hedgehog signalling: Emerging evidence for non-canonical pathways. Cell Signal. 2009, 21, 1023–1034. [Google Scholar] [CrossRef]
- Caimano, M.; Severini, L.L.; Loricchio, E.; Infante, P.; Di Marcotullio, L. Drug Delivery Systems for Hedgehog Inhibitors in the Treatment of SHH-Medulloblastoma. Front. Chem. 2021, 9, 688108. [Google Scholar] [CrossRef]
- Gonnissen, A.; Isebaert, S.; Haustermans, K. Targeting the Hedgehog signaling pathway in cancer: Beyond Smoothened. Oncotarget 2015, 6, 13899–13913. [Google Scholar] [CrossRef] [Green Version]
- Villani, A.; Cinelli, E.; Fabbrocini, G.; Lallas, A.; Scalvenzi, M. Hedgehog inhibitors in the treatment of advanced basal cell carcinoma: Risks and benefits. Expert Opin. Drug Saf. 2020, 19, 1585–1594. [Google Scholar] [CrossRef]
- Katoh, M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin. Sci. 2019, 133, 953–970. [Google Scholar] [CrossRef]
- Petty, A.J.; Li, A.; Wang, X.; Dai, R.; Heyman, B.; Hsu, D.; Huang, X.; Yang, Y. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J. Clin. Investig. 2019, 129, 5151–5162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fan, J.; Zeng, X.; Nie, M.; Luan, J.; Wang, Y.; Ju, D.; Yin, K. Hedgehog signaling in gastrointestinal carcinogenesis and the gastrointestinal tumor microenvironment. Acta Pharm. Sin. B 2021, 11, 609–620. [Google Scholar] [CrossRef]
- Benson, R.A.; Lowrey, J.A.; Lamb, J.R.; Howie, S.E. The Notch and Sonic hedgehog signalling pathways in immunity. Mol. Immunol. 2004, 41, 715–725. [Google Scholar] [CrossRef]
- Outram, S.V.; Varas, A.; Pepicelli, C.V.; Crompton, T. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity 2000, 13, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Furmanski, A.L.; Saldana, J.I.; Ono, M.; Sahni, H.; Paschalidis, N.; D’Acquisto, F.; Crompton, T. Tissue-derived hedgehog proteins modulate Th differentiation and disease. J. Immunol. 2013, 190, 2641–2649. [Google Scholar] [CrossRef] [Green Version]
- Hanna, A.; Metge, B.J.; Bailey, S.K.; Chen, D.; Chandrashekar, D.S.; Varambally, S.; Samant, R.S.; Shevde, L.A. Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. Oncoimmunology 2019, 8, 1548241. [Google Scholar] [CrossRef]
- Papaioannou, E.; Yanez, D.C.; Ross, S.; Lau, C.I.; Solanki, A.; Chawda, M.M.; Virasami, A.; Ranz, I.; Ono, M.; O’Shaughnessy, R.F.L.; et al. Sonic Hedgehog signaling limits atopic dermatitis via Gli2-driven immune regulation. J. Clin. Investig. 2019, 129, 3153–3170. [Google Scholar] [CrossRef]
- Yanez, D.C.; Lau, C.I.; Chawda, M.M.; Ross, S.; Furmanski, A.L.; Crompton, T. Hedgehog signaling promotes TH2 differentiation in naive human CD4 T cells. J. Allergy Clin. Immunol. 2019, 144, 1419–1423.e1. [Google Scholar] [CrossRef] [Green Version]
- Benallegue, N.; Kebir, H.; Kapoor, R.; Crockett, A.; Li, C.; Cheslow, L.; Abdel-Hakeem, M.S.; Gesualdi, J.; Miller, M.C.; Wherry, E.J.; et al. The hedgehog pathway suppresses neuropathogenesis in CD4 T cell-driven inflammation. Brain 2021, 144, 1670–2156. [Google Scholar] [CrossRef]
- Lee, J.J.; Rothenberg, M.E.; Seeley, E.S.; Zimdahl, B.; Kawano, S.; Lu, W.J.; Shin, K.; Sakata-Kato, T.; Chen, J.K.; Diehn, M.; et al. Control of inflammation by stromal Hedgehog pathway activation restrains colitis. Proc. Natl. Acad. Sci. USA 2016, 113, E7545–E7553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, C.W.; Zacharias, W.J.; Tremelling, M.; Noble, C.L.; Nimmo, E.R.; Tenesa, A.; Cornelius, J.; Torkvist, L.; Kao, J.; Farrington, S.; et al. Analysis of germline GLI1 variation implicates hedgehog signalling in the regulation of intestinal inflammatory pathways. PLoS Med. 2008, 5, e239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Cai, L.; Ding, J.; Hu, C.M.; Wu, T.N.; Hu, X.Y. Inhibition of hedgehog signal pathway by cyclopamine attenuates inflammation and articular cartilage damage in rats with adjuvant-induced arthritis. J. Pharm. Pharmacol. 2015, 67, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lai, K.; Xie, Y.; He, X.; Zhou, X. Gli2 Mediated Activation of Hedgehog Signaling Attenuates Acute Pancreatitis via Balancing Inflammatory Cytokines in Mice. Cell. Physiol. Biochem. 2018, 48, 120–130. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Li, Y.; Cui, X.; Zheng, X.; Roberts, C.; Lu, M.; Elias, S.B.; Chopp, M. Niaspan treatment improves neurological functional recovery in experimental autoimmune encephalomyelitis mice. Neurobiol. Dis. 2008, 32, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Goodrich, L.V.; Milenkovic, L.; Higgins, K.M.; Scott, M.P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 1997, 277, 1109–1113. [Google Scholar] [CrossRef]
- Merk, D.J.; Segal, R.A. Sonic Hedgehog Signaling is Blue: Insights from the Patched Mutant Mice. Trends Neurosci. 2018, 41, 870–872. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, J.X.; Zhang, G.X. Macrophages: A double-edged sword in experimental autoimmune encephalomyelitis. Immunol. Lett. 2014, 160, 17–22. [Google Scholar] [CrossRef]
- Herrmann, J.E.; Imura, T.; Song, B.; Qi, J.; Ao, Y.; Nguyen, T.K.; Korsak, R.A.; Takeda, K.; Akira, S.; Sofroniew, M.V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008, 28, 7231–7243. [Google Scholar] [CrossRef]
- Standing, A.S.I.; Yanez, D.C.; Ross, R.; Crompton, T.; Furmanski, A.L. Frontline Science: Shh production and Gli signaling is activated in vivo in lung, enhancing the Th2 response during a murine model of allergic asthma. J. Leukoc. Biol. 2017, 102, 965–976. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Han, L.; Chen, F.F.; Wang, D.; Wang, F.; Zhang, L.; Wang, Z.H.; Zhong, M.; Tang, M.X.; Zhang, W. Adipocyte-Derived Exosomes Carrying Sonic Hedgehog Mediate M1 Macrophage Polarization-Induced Insulin Resistance via Ptch and PI3K Pathways. Cell. Physiol. Biochem. 2018, 48, 1416–1432. [Google Scholar] [CrossRef]
- Kangwan, N.; Kim, Y.J.; Han, Y.M.; Jeong, M.; Park, J.M.; Hahm, K.B. Concerted actions of ameliorated colitis, aberrant crypt foci inhibition and 15-hydroxyprostaglandin dehydrogenase induction by sonic hedgehog inhibitor led to prevention of colitis-associated cancer. Int. J. Cancer 2016, 138, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- Heller, E.; Hurchla, M.A.; Xiang, J.; Su, X.; Chen, S.; Schneider, J.; Joeng, K.S.; Vidal, M.; Goldberg, L.; Deng, H.; et al. Hedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironment. Cancer Res. 2012, 72, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Peng, Y.; Li, H. The Injury-Related Activation of Hedgehog Signaling Pathway Modulates the Repair-Associated Inflammation in Liver Fibrosis. Front. Immunol. 2017, 8, 1450. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb. Perspect. Biol. 2018, 10, a030338. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.C.; Kim, S.; Steelman, A.J.; Tracy, K.; Zhou, B.; Michaud, D.; Hillhouse, A.E.; Konganti, K.; Li, J. STAT3 signaling in myeloid cells promotes pathogenic myelin-specific T cell differentiation and autoimmune demyelination. Proc. Natl. Acad. Sci. USA 2020, 117, 5430–5441. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, Z.; Xia, Z.; Rallo, M.; Duffy, A.; Matise, M.P. Inactivation of Hedgehog signal transduction in adult astrocytes results in region-specific blood-brain barrier defects. Proc. Natl. Acad. Sci. USA 2021, 118, e2017779118. [Google Scholar] [CrossRef]
- Ligon, K.L.; Kesari, S.; Kitada, M.; Sun, T.; Arnett, H.A.; Alberta, J.A.; Anderson, D.J.; Stiles, C.D.; Rowitch, D.H. Development of NG2 neural progenitor cells requires Olig gene function. Proc. Natl. Acad. Sci. USA 2006, 103, 7853–7858. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Bergles, D.E.; Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2008, 135, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Honsa, P.; Valny, M.; Kriska, J.; Matuskova, H.; Harantova, L.; Kirdajova, D.; Valihrach, L.; Androvic, P.; Kubista, M.; Anderova, M. Generation of reactive astrocytes from NG2 cells is regulated by sonic hedgehog. Glia 2016, 64, 1518–1531. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonniere, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef] [Green Version]
- Seifert, T.; Bauer, J.; Weissert, R.; Fazekas, F.; Storch, M.K. Differential expression of sonic hedgehog immunoreactivity during lesion evolution in autoimmune encephalomyelitis. J. Neuropathol. Exp. Neurol. 2005, 64, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Z.G.; Li, Y.; Ding, X.; Shang, X.; Lu, M.; Elias, S.B.; Chopp, M. Fingolimod treatment promotes proliferation and differentiation of oligodendrocyte progenitor cells in mice with experimental autoimmune encephalomyelitis. Neurobiol. Dis. 2015, 76, 57–66. [Google Scholar] [CrossRef]
- Bravo, B.; Gallego, M.I.; Flores, A.I.; Bornstein, R.; Puente-Bedia, A.; Hernandez, J.; de la Torre, P.; Garcia-Zaragoza, E.; Perez-Tavarez, R.; Grande, J.; et al. Restrained Th17 response and myeloid cell infiltration into the central nervous system by human decidua-derived mesenchymal stem cells during experimental autoimmune encephalomyelitis. Stem Cell Res. Ther. 2016, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Click, R.E.; Benck, L.; Alter, B.J. Enhancement of antibody synthesis in vitro by mercaptoethanol. Cell. Immunol. 1972, 3, 156–160. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, C.; Bravo, B.; Ballester, A.; Gomez-Perez, R.; Eguiluz, C.; Redondo, M.; Martinez, A.; Gil, C.; Ballester, S. Comparative assessment of PDE 4 and 7 inhibitors as therapeutic agents in experimental autoimmune encephalomyelitis. Br. J. Pharmacol. 2013, 170, 602–613. [Google Scholar] [CrossRef] [Green Version]
- Martin-Saavedra, F.M.; Flores, N.; Dorado, B.; Eguiluz, C.; Bravo, B.; Garcia-Merino, A.; Ballester, S. Beta-interferon unbalances the peripheral T cell proinflammatory response in experimental autoimmune encephalomyelitis. Mol. Immunol. 2007, 44, 3597–3607. [Google Scholar] [CrossRef]
- Martin-Saavedra, F.M.; Gonzalez-Garcia, C.; Bravo, B.; Ballester, S. Beta interferon restricts the inflammatory potential of CD4+ cells through the boost of the Th2 phenotype, the inhibition of Th17 response and the prevalence of naturally occurring T regulatory cells. Mol. Immunol. 2008, 45, 4008–4019. [Google Scholar] [CrossRef]
| Forward (5’-3’) | Reverse (5’-3’) | |
|---|---|---|
| IL-4 | ATCCTGCTCTTCTTTCTCG | GATGCTCTTTAGGCTTTCC |
| IL-10 | GCCTTATCGGAAATGATCCA | GCTCCACTGCCTTGCTCTTA |
| IFNγ | TGCATCTTGGCTTTGCAGCTCTTCCTCATGGC | TGGACCTGTGGGTTGTTGACCTCAAACTTGGC |
| IL-17 | CGCAAAAGTGAGCTCCAGAA | CCTCTTCAGGACCAGGATCT |
| IL-6 | GCCTTCTTGGGACTGATGCT | GACAGGTCTGTTGGGAGTGG |
| IL-1β | AGAGCCCATCCTCTGTGACT | GGAGCCTGTAGTGCAGTTGT |
| TNFα | CGTCAGCCGATTTGCTATCT | CGGACTCCGCAAAGTCTAAG |
| iNOS | CTCACTGGGACAGCACAGAA | GCTTGTCTCTGGGTCCTCTG |
| Arg1 | CATGGAAGTGAACCCAACTCTTGG | TCAGTCCCTGGCTTATGGTTACCC |
| β-actin | TGTTACCAACTGGGACGACA | GGGGTGTTGAAGGTCTCAAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballester, A.; Guijarro, A.; Bravo, B.; Hernández, J.; Murillas, R.; Gallego, M.I.; Ballester, S. Hedgehog Signalling Modulates Immune Response and Protects against Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2022, 23, 3171. https://doi.org/10.3390/ijms23063171
Ballester A, Guijarro A, Bravo B, Hernández J, Murillas R, Gallego MI, Ballester S. Hedgehog Signalling Modulates Immune Response and Protects against Experimental Autoimmune Encephalomyelitis. International Journal of Molecular Sciences. 2022; 23(6):3171. https://doi.org/10.3390/ijms23063171
Chicago/Turabian StyleBallester, Alicia, Adriana Guijarro, Beatriz Bravo, Javier Hernández, Rodolfo Murillas, Marta I. Gallego, and Sara Ballester. 2022. "Hedgehog Signalling Modulates Immune Response and Protects against Experimental Autoimmune Encephalomyelitis" International Journal of Molecular Sciences 23, no. 6: 3171. https://doi.org/10.3390/ijms23063171
APA StyleBallester, A., Guijarro, A., Bravo, B., Hernández, J., Murillas, R., Gallego, M. I., & Ballester, S. (2022). Hedgehog Signalling Modulates Immune Response and Protects against Experimental Autoimmune Encephalomyelitis. International Journal of Molecular Sciences, 23(6), 3171. https://doi.org/10.3390/ijms23063171






