Sex- and Genotype-Dependent Nicotine-Induced Behaviors in Adolescent Rats with a Human Polymorphism (rs2304297) in the 3′-UTR of the CHRNA6 Gene
Abstract
1. Introduction
2. Results
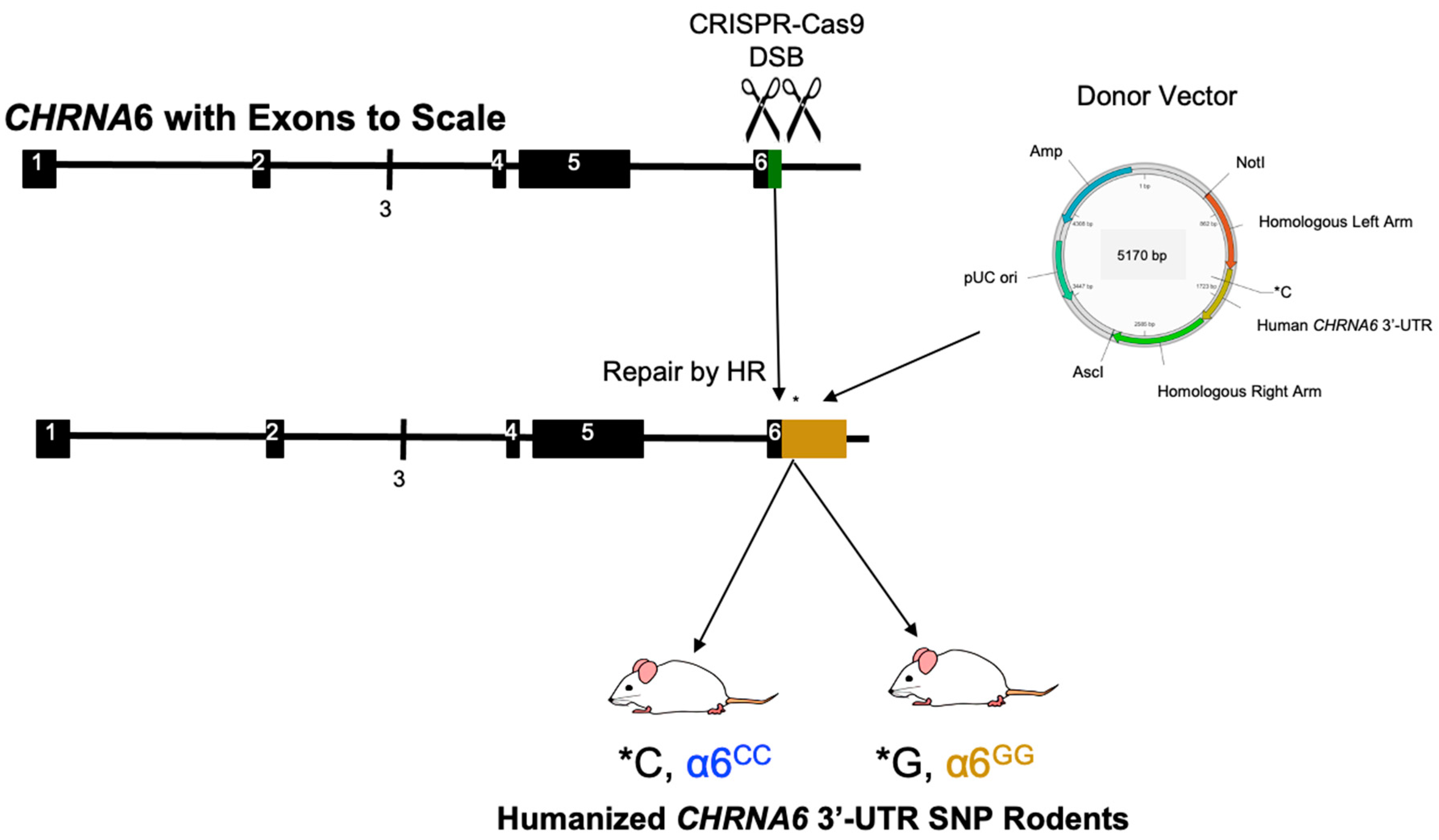
2.1. Generation and Validation of Humanized CHRNA6 3′-UTR SNP Rodents
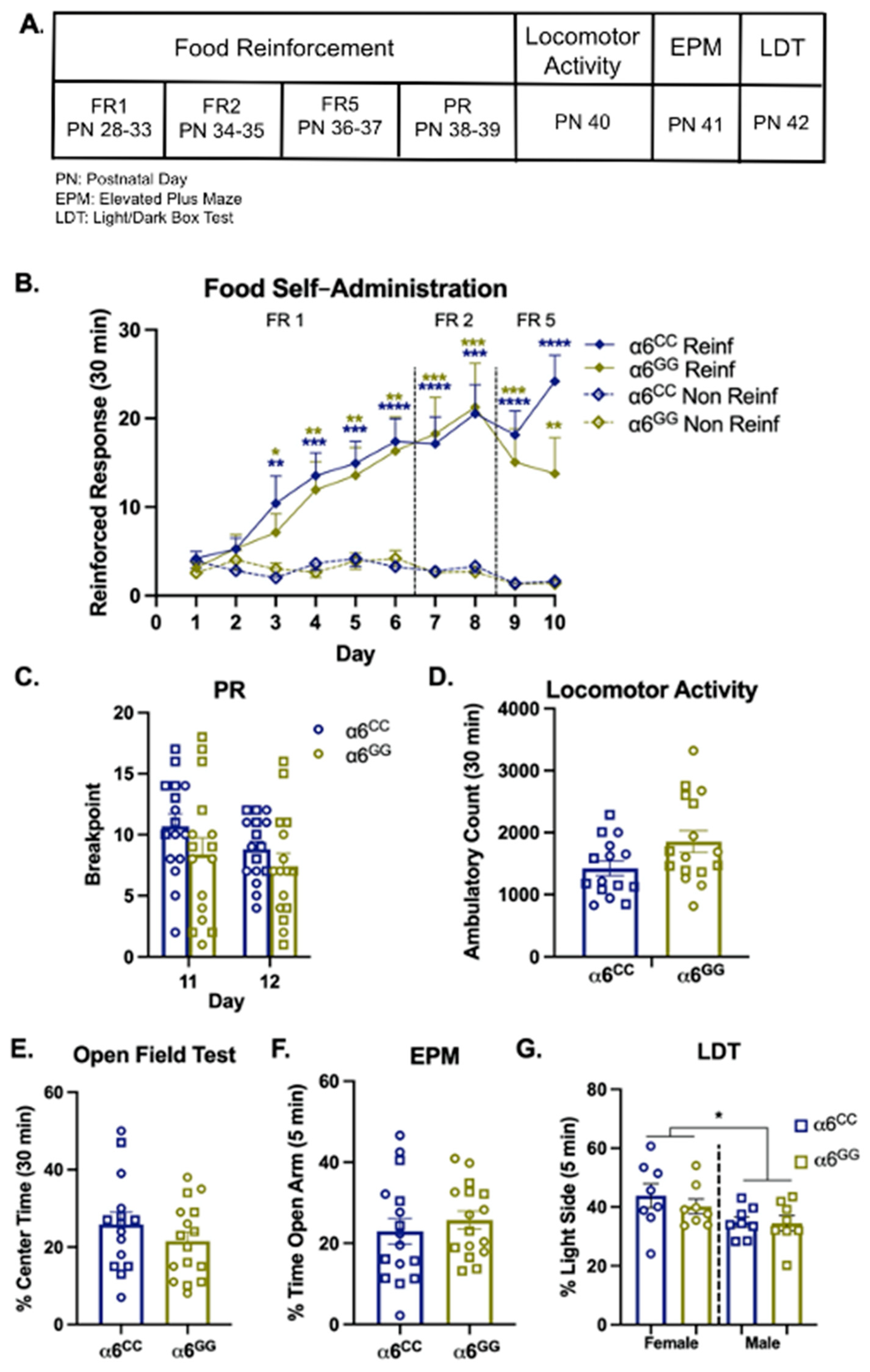
2.2. The Human CHRNA6 3′-UTR Knock-In Does Not Impact Baseline Behavior
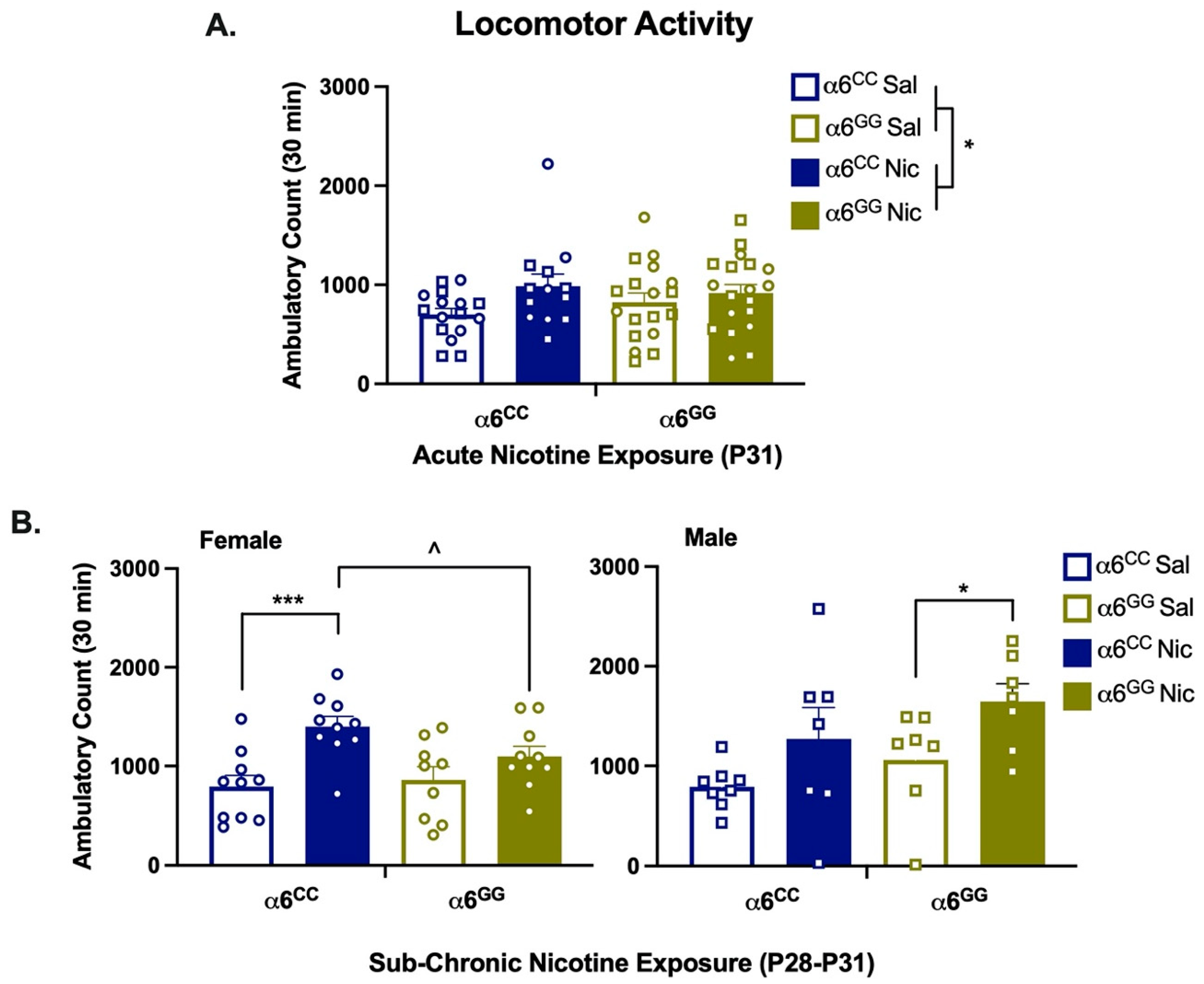
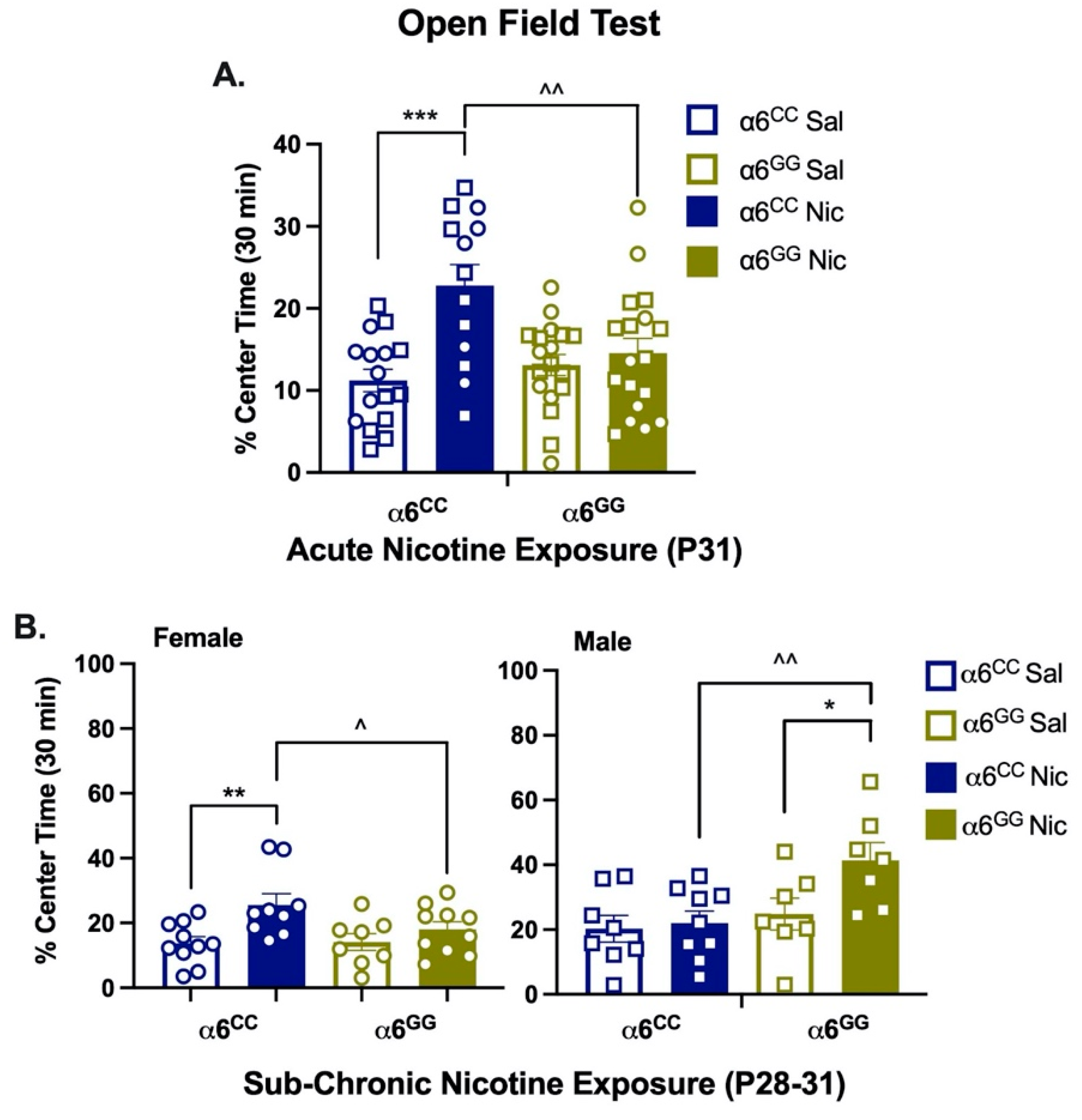
2.3. Nicotine Increases Locomotor Activity in Humanized CHRNA6 3′-UTR SNP Rats
2.4. Nicotine Increases Anxiolytic Behavior in Humanized CHRNA6 3′-UTR SNP Rats
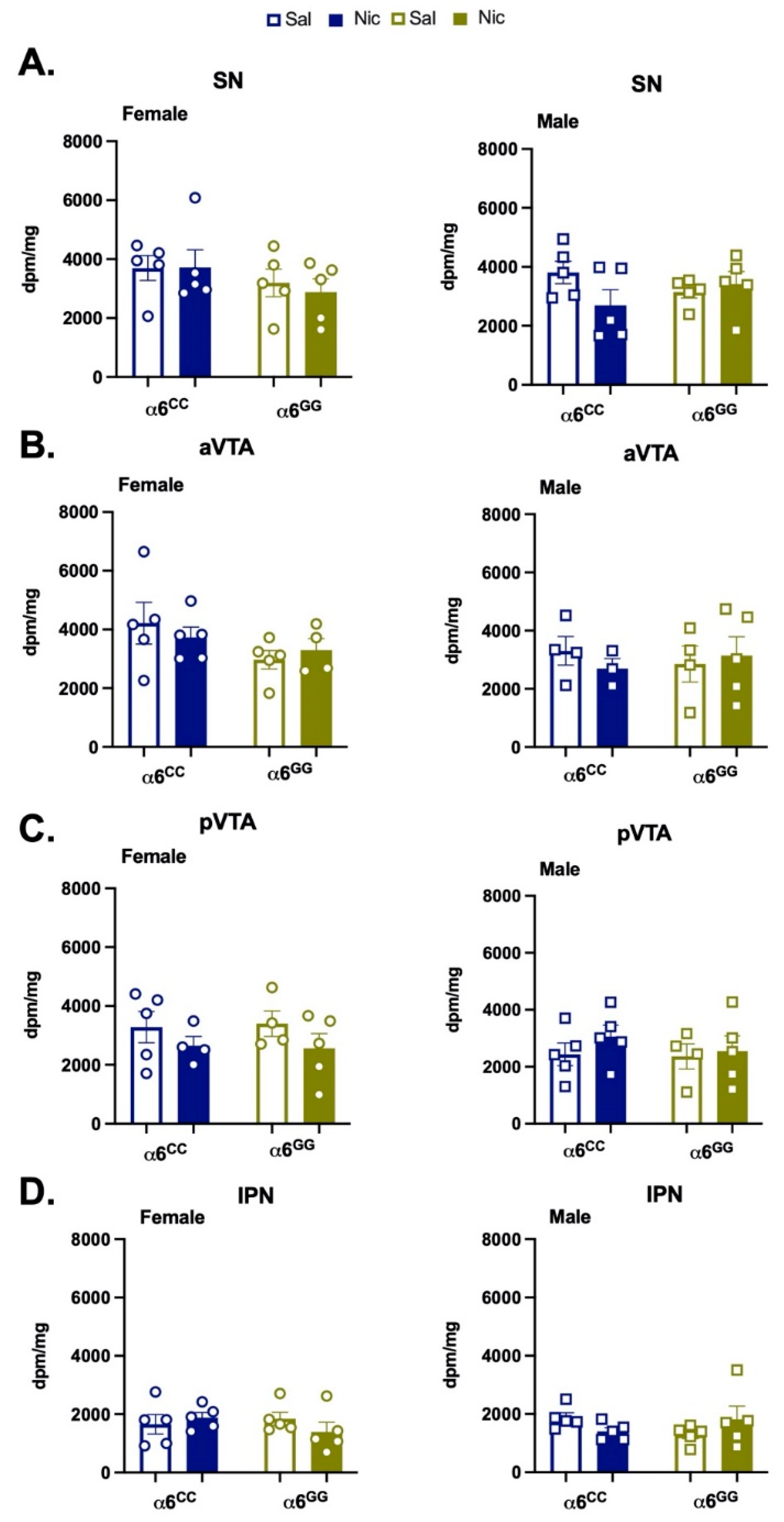
2.5. Nicotine Pretreatment Does Not Alter mRNA Expression in Humanized CHRNA6 3′-UTR SNP Rats
3. Discussion
3.1. Baseline Behaviors
3.2. Acute Nicotine Effects
3.3. Sub-Chronic Nicotine Effects
3.4. Sex Effects
3.5. Mechanisms
4. Conclusions
5. Materials and Methods
5.1. Generation of Human CHRNA6 3′-UTR SNP Rodents
5.2. Animals
5.3. Food Reinforcement
5.4. Locomotor Apparatus
5.5. Baseline Locomotor Activity & Open Field Test
5.6. Elevated Plus Maze
5.7. Light/Dark Box Test
5.8. Drugs & Reagents
5.9. Surgical Procedure
5.10. Acute Nicotine-Induced Locomotor Activity & Open Field Test
5.11. Sub-Chronic Nicotine-Induced Locomotor Activity & Open Field Test
5.12. Tissue Collection & Preparation
Tissue Preparation
5.13. In Situ Hybridization
5.14. Statistical Analysis
5.14.1. Baseline Behavior
5.14.2. Nicotine-Induced Behaviors
5.14.3. Autoradiography
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; US Department of Health and Human Services: Washington, DC, USA, 2014.
- NIDA. “What Is the Scope of Tobacco, Nicotine, and e-Cigarette Use in the United States?” National Institute on Drug Abuse. 8 February 2022. Available online: https://nida.nih.gov/publications/research-reports/tobacco-nicotine-e-cigarettes/what-scope-tobacco-use-its-cost-to-society (accessed on 13 March 2022).
- Yuan, M.; Cross, S.J.; Loughlin, S.E.; Leslie, F.M. Nicotine and the adolescent brain. J. Physiol. 2015, 593, 3397–3412. [Google Scholar] [CrossRef] [PubMed]
- Park-Lee, E.R.C.; Sawdey, M.D.; Gentzke, A.S.; Cornelius, M.; Jamal, A.; Cullen, K.A. Notes from the Field: E-Cigarette Use Among Middle and High School Students—National Youth Tobacco Survey. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Portugal, G.S.; Gould, T.J. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: Converging evidence from human and animal research. Behav. Brain Res. 2008, 193, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.B.; McQuown, S.C.; Leslie, F.M. The dynamic effects of nicotine on the developing brain. Pharmacol. Ther. 2009, 122, 125–139. [Google Scholar] [CrossRef]
- Leslie, F.M. Unique, long-term effects of nicotine on adolescent brain. Pharmacol. Biochem. Behav. 2020, 197, 173010. [Google Scholar] [CrossRef]
- Gotti, C.; Clementi, F. Neuronal nicotinic receptors: From structure to pathology. Prog. Neurobiol. 2004, 74, 363–396. [Google Scholar] [CrossRef]
- Azam, L.; Chen, Y.; Leslie, F. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience 2007, 144, 1347–1360. [Google Scholar] [CrossRef]
- Gotti, C.; Zoli, M.; Clementi, F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Lotfipour, S.; Leonard, G.; Perron, M.; Pike, B.; Richer, L.; Séguin, J.R.; Toro, R.; Veillette, S.; Pausova, Z.; Paus, T. Prenatal exposure to maternal cigarette smoking interacts with a polymorphism in the α6 nicotinic acetylcholine receptor gene to influence drug use and striatum volume in adolescence. Mol. Psychiatry 2009, 15, 6–8. [Google Scholar] [CrossRef]
- Selya, A.S.; Cannon, D.S.; Weiss, R.B.; Wakschlag, L.S.; Rose, J.S.; Dierker, L.; Hedeker, D.; Mermelstein, R.J. The role of nicotinic receptor genes (CHRN) in the pathways of prenatal tobacco exposure on smoking behavior among young adult light smokers. Addict. Behav. 2018, 84, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Pugach, O.; Cannon, D.S.; Weiss, R.B.; Hedeker, D.; Mermelstein, R.J. Classification Tree Analysis as a Method for Uncovering Relations BetweenCHRNA5A3B4andCHRNB3A6in Predicting Smoking Progression in Adolescent Smokers. Nicotine Tob. Res. 2016, 19, 410–416. [Google Scholar] [CrossRef]
- Cannon, D.S.; Mermelstein, R.J.; Hedeker, D.; Coon, H.; Cook, E.H.; McMahon, W.M.; Hamil, C.; Dunn, D.; Weiss, R.B. Effect of Neuronal Nicotinic Acetylcholine Receptor Genes (CHRN) on Longitudinal Cigarettes per Day in Adolescents and Young Adults. Nicotine Tob. Res. 2013, 16, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hoft, N.R.; Corley, R.P.; McQueen, M.B.; Schlaepfer, I.R.; Huizinga, D.; Ehringer, M.A. Genetic Association of the CHRNA6 and CHRNB3 Genes with Tobacco Dependence in a Nationally Representative Sample. Neuropsychopharmacology 2008, 34, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, J.S.; Haberstick, B.C.; Schlaepfer, I.; Collins, A.C.; Corley, R.P.; Crowley, T.J.; Hewitt, J.K.; Hopfer, C.J.; Lessem, J.; McQueen, M.B.; et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Hum. Mol. Genet. 2007, 17, 724–734. [Google Scholar] [CrossRef]
- Mayya, V.K.; Duchaine, T.F. Ciphers and Executioners: How 3′-Untranslated Regions Determine the Fate of Messenger RNAs. Front. Genet. 2019, 10, 6. [Google Scholar] [CrossRef]
- Mayr, C. What Are 3′ UTRs Doing? Cold Spring Harb. Perspect. Biol. 2019, 11, a034728. [Google Scholar] [CrossRef]
- Champtiaux, N.; Han, Z.-Y.; Bessis, A.; Rossi, F.M.; Zoli, M.; Marubio, L.; McIntosh, J.M.; Changeux, J.-P. Distribution and Pharmacology of α6-Containing Nicotinic Acetylcholine Receptors Analyzed with Mutant Mice. J. Neurosci. 2002, 22, 1208–1217. [Google Scholar] [CrossRef]
- Zoli, M.; Pistillo, F.; Gotti, C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2015, 96 Pt B, 302–311. [Google Scholar] [CrossRef]
- Adinoff, B. Neurobiologic Processes in Drug Reward and Addiction. Harv. Rev. Psychiatry 2004, 12, 305–320. [Google Scholar] [CrossRef]
- Drenan, R.M.; Grady, S.R.; Whiteaker, P.; McClure-Begley, T.; McKinney, S.; Miwa, J.M.; Bupp, S.; Heintz, N.; McIntosh, J.M.; Bencherif, M.; et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity α 6 nicotinic acetylcholine receptors. Neuron 2008, 60, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Drenan, R.M.; Grady, S.R.; Steele, A.D.; McKinney, S.; Patzlaff, N.E.; McIntosh, J.M.; Marks, M.J.; Miwa, J.M.; Lester, H.A. Cholinergic modulation of locomotion and striatal dopamine release is mediated by α6α4* nicotinic acetylcholine receptors. J. Neurosci. 2010, 30, 9877–9889. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.N.; Engle, S.E.; McIntosh, J.M.; Drenan, R.M. α6-Containing nicotinic acetylcholine receptors in midbrain dopamine neurons are poised to govern dopamine-mediated behaviors and synaptic plasticity. Neuroscience 2015, 304, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Guiducci, S.; Tedesco, V.; Corbioli, S.; Zanetti, L.; Moretti, M.; Zanardi, A.; Rimondini, R.; Mugnaini, M.; Clementi, F.; et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: Primary role of ventral tegmental area α6β2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J. Neurosci. 2010, 30, 5311–5325. [Google Scholar] [CrossRef] [PubMed]
- Le Novère, N.; Zoli, M.; Léna, C.; Ferrari, R.; Picciott, M.R.; Merlo-Pich, E.; Changeux, J.-P. Involvement of α6 nicotinic receptor subunit in nicotine-elicited locomotion, demonstrated by in vivo antisense oligonucleotide infusion. NeuroReport 1999, 10, 2497–2501. [Google Scholar] [CrossRef][Green Version]
- Quik, M.; Perez, X.A.; Grady, S.R. Role of α6 nicotinic receptors in CNS dopaminergic function: Relevance to addiction and neurological disorders. Biochem. Pharmacol. 2011, 82, 873–882. [Google Scholar] [CrossRef]
- Pons, S.; Fattore, L.; Cossu, G.; Tolu, S.; Porcu, E.; McIntosh, J.M.; Changeux, J.P.; Maskos, U.; Fratta, W. Crucial Role of 4 and 6 Nicotinic Acetylcholine Receptor Subunits from Ventral Tegmental Area in Systemic Nicotine Self-Administration. J. Neurosci. 2008, 28, 12318–12327. [Google Scholar] [CrossRef]
- Jackson, K.J.; McIntosh, J.M.; Brunzell, D.H.; Sanjakdar, S.S.; Damaj, M.I. The Role of α6-Containing Nicotinic Acetylcholine Receptors in Nicotine Reward and Withdrawal. J. Pharmacol. Exp. Ther. 2009, 331, 547–554. [Google Scholar] [CrossRef]
- Sanjakdar, S.S.; Maldoon, P.P.; Marks, M.J.; Brunzell, D.H.; Maskos, U.; McIntosh, J.M.; Bowers, M.S.; Damaj, M.I. Differential roles of α6β2* and α4β2* neuronal nicotinic receptors in nicotine- and cocaine-conditioned reward in mice. Neuropsychopharmacology 2015, 40, 350–360. [Google Scholar] [CrossRef]
- Pirmohamed, M. Pharmacogenetics: Past, present and future. Drug Discov. Today 2011, 16, 852–861. [Google Scholar] [CrossRef]
- Di Nunno, N.; Esposito, M.; Argo, A.; Salerno, M.; Sessa, F. Pharmacogenetics and Forensic Toxicology: A New Step towards a Multidisciplinary Approach. Toxics 2021, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Gieryk, A.; Ziolkowska, B.; Solecki, W.; Kubik, J.; Przewlocki, R. Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity. Psychopharmacology 2010, 208, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.G.; Izetelny, A.; Radtke, R.; Hammersley, J.; Rabinovich, N.E.; Jameson, T.R.; Huggenvik, J.I. Dopamine receptor (DRD2) genotype-dependent effects of nicotine on attention and distraction during rapid visual information processing. Nicotine Tob. Res. 2005, 7, 361–379. [Google Scholar] [CrossRef]
- McQuown, S.C.; Belluzzi, J.D.; Leslie, F.M. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol. Teratol. 2007, 29, 66–73. [Google Scholar] [CrossRef]
- McQuown, S.C.; Dao, J.M.; Belluzzi, J.D.; Leslie, F.M. Age-dependent effects of low-dose nicotine treatment on cocaine-induced behavioral plasticity in rats. Psychopharmacology 2009, 207, 143–152. [Google Scholar] [CrossRef] [PubMed]
- van Gaalen, M.M.; Steckler, T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav. Brain Res. 2000, 115, 95–106. [Google Scholar] [CrossRef]
- Steimer, T. Animal models of anxiety disorders in rats and mice: Some conceptual issues. Dialog. Clin. Neurosci. 2011, 13, 495–506. [Google Scholar]
- Jin, S.; Zhao, Y.; Jiang, Y.; Wang, Y.; Li, C.; Zhang, D.; Lian, B.; Du, Z.; Sun, H.; Sun, L. Anxiety-like behaviour assessments of adolescent rats after repeated maternal separation during early life. NeuroReport 2018, 29, 643–649. [Google Scholar] [CrossRef]
- Bishnoi, I.R.; Ossenkopp, K.; Kavaliers, M. Sex and age differences in locomotor and anxiety-like behaviors in rats: From adolescence to adulthood. Dev. Psychobiol. 2021, 63, 496–511. [Google Scholar] [CrossRef]
- Cao, J.; Belluzzi, J.D.; Loughlin, S.E.; Dao, J.M.; Chen, Y.; Leslie, F.M. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacol. Biochem. Behav. 2010, 96, 82–90. [Google Scholar] [CrossRef]
- Le Novere, N.; Zoli, M.; Changeux, J.-P. Neuronal Nicotinic Receptor a6 Subunit mRNA is Selectively Concentrated in Catecholaminergic Nuclei of the Rat Brain. Eur. J. Neurosci. 1996, 8, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Pauly, J.; Gross, S.; Deneris, E.; Hermans-Borgmeyer, I.; Heinemann, S.; Collins, A. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 1992, 12, 2765–2784. [Google Scholar] [CrossRef] [PubMed]
- Pauly, J.R.; Marks, M.J.; Robinson, S.F.; Van De Kamp, J.L.; Collins, A.C. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing α 4 or beta 2 mRNA levels. J. Pharmacol. Exp. Ther. 1996, 278, 361–369. [Google Scholar] [PubMed]
- Cardenas, A.; Elabd, M.; Lotfipour, S. Specificity of a rodent alpha(α)6 nicotinic acetylcholine receptor subunit antibody. Psychopharmacology 2020, 237, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Vailati, S.; Hanke, W.; Bejan, A.; Barabino, B.; Longhi, R.; Balestra, B.; Moretti, M.; Clementi, F.; Gotti, C. Functional α6-Containing Nicotinic Receptors Are Present in Chick Retina. Mol. Pharmacol. 1999, 56, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Moen, J.K.; DeBaker, M.C.; Myjak, J.E.; Wickman, K.; Lee, A.M. Bidirectional sex-dependent regulation of α6 and β3 nicotinic acetylcholine receptors by protein kinase Cepsilon. Addict. Biol. 2021, 26, e12954. [Google Scholar] [CrossRef]
- Moen, J.K.; Lee, A.M. Sex Differences in the Nicotinic Acetylcholine Receptor System of Rodents: Impacts on Nicotine and Alcohol Reward Behaviors. Front. Neurosci. 2021, 15, 745783. [Google Scholar] [CrossRef]
- Cross, S.J.; Linker, K.E.; Leslie, F.M. Sex-dependent effects of nicotine on the developing brain. J. Neurosci. Res. 2017, 95, 422–436. [Google Scholar] [CrossRef]
- Kanyt, L.; Stolerman, I.P.; Chandler, C.J.; Saigusa, T.; Pogun, S. Influence of Sex and Female Hormones on Nicotine-Induced Changes in Locomotor Activity in Rats. Pharmacol. Biochem. Behav. 1999, 62, 179–187. [Google Scholar] [CrossRef]
- Torres, O.V.; Natividad, L.A.; Tejeda, H.A.; Van Weelden, S.A.; O’Dell, L.E. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology 2009, 206, 303–312. [Google Scholar] [CrossRef]
- Andersen, S.L.; Thompson, A.P.; Krenzel, E.; Teicher, M.H. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology 2002, 27, 683–691. [Google Scholar] [CrossRef]
- Andersen, S.L.; Rutstein, M.; Benzo, J.M.; Hostetter, J.C.; Teicher, M.H. Sex differences in dopamine receptor overproduction and elimination. NeuroReport 1997, 8, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Egervari, G.; Jutras-Aswad, D.; Landry, J.; Miller, M.; Anderson, S.A.; Michaelides, M.; Jacobs, M.M.; Peter, C.; Yiannoulos, G.; Liu, X.; et al. A Functional 3′UTR Polymorphism (rs2235749) of Prodynorphin Alters microRNA-365 Binding in Ventral Striatonigral Neurons to Influence Novelty Seeking and Positive Reward Traits. Neuropsychopharmacology 2016, 41, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Dao, J.M.; McQuown, S.C.; Loughlin, E.S.; Belluzzi, J.D.; Leslie, F.M. Nicotine Alters Limbic Function in Adolescent Rat by a 5-HT1A Receptor Mechanism. Neuropsychopharmacology 2011, 36, 1319–1331. [Google Scholar] [CrossRef]
- Linker, K.E.; Elabd, M.G.; Tawadrous, P.; Cano, M.; Green, K.N.; Wood, M.A.; Leslie, F.M. Microglial activation increases cocaine self-administration following adolescent nicotine exposure. Nat. Commun. 2020, 11, 306. [Google Scholar] [CrossRef]
- Cardenas, A.; Martinez, M.; Mejia, A.S.; Lotfipour, S. Early adolescent subchronic low-dose nicotine exposure increases subsequent cocaine and fentanyl self-administration in Sprague–Dawley rats. Behav. Pharmacol. 2021, 32, 86–91. [Google Scholar] [CrossRef]
- Cardenas, A.; Lotfipour, S. Age- and Sex-Dependent Nicotine Pretreatment Effects on the Enhancement of Methamphetamine Self-administration in Sprague-Dawley Rats. Nicotine Tob. Res. 2021. [Google Scholar] [CrossRef]
- Ren, M.; Lotfipour, S. Nicotine Gateway Effects on Adolescent Substance Use. West. J. Emerg. Med. 2019, 20, 696–709. [Google Scholar] [CrossRef]
- Ren, M.; Lotfipour, S.; Leslie, F. Unique effects of nicotine across the lifespan. Pharmacol. Biochem. Behav. 2022, 214, 173343. [Google Scholar] [CrossRef]
- Belluzzi, J.D.; Wang, R.; Leslie, F.M. Acetaldehyde Enhances Acquisition of Nicotine Self-Administration in Adolescent Rats. Neuropsychopharmacology 2004, 30, 705–712. [Google Scholar] [CrossRef]
- Azam, L.; Winzer-Serhan, U.H.; Chen, Y.; Leslie, F.M. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J. Comp. Neurol. 2002, 444, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Winzer-Serhan, U.H.; Broide, R.S.; Chen, Y.; Leslie, F.M. Highly sensitive radioactive in situ hybridization using full length hydrolyzed riboprobes to detect α2 adrenoceptor subtype mRNAs in adult and developing rat brain. Brain Res. Protoc. 1999, 3, 229–241. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Sterotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardenas, A.; Bai, Y.; Hajy Heydary, Y.; Li, J.; Leslie, F.M.; Lotfipour, S. Sex- and Genotype-Dependent Nicotine-Induced Behaviors in Adolescent Rats with a Human Polymorphism (rs2304297) in the 3′-UTR of the CHRNA6 Gene. Int. J. Mol. Sci. 2022, 23, 3145. https://doi.org/10.3390/ijms23063145
Cardenas A, Bai Y, Hajy Heydary Y, Li J, Leslie FM, Lotfipour S. Sex- and Genotype-Dependent Nicotine-Induced Behaviors in Adolescent Rats with a Human Polymorphism (rs2304297) in the 3′-UTR of the CHRNA6 Gene. International Journal of Molecular Sciences. 2022; 23(6):3145. https://doi.org/10.3390/ijms23063145
Chicago/Turabian StyleCardenas, Anjelica, Yu Bai, Yasamin Hajy Heydary, Jiaqi Li, Frances M. Leslie, and Shahrdad Lotfipour. 2022. "Sex- and Genotype-Dependent Nicotine-Induced Behaviors in Adolescent Rats with a Human Polymorphism (rs2304297) in the 3′-UTR of the CHRNA6 Gene" International Journal of Molecular Sciences 23, no. 6: 3145. https://doi.org/10.3390/ijms23063145
APA StyleCardenas, A., Bai, Y., Hajy Heydary, Y., Li, J., Leslie, F. M., & Lotfipour, S. (2022). Sex- and Genotype-Dependent Nicotine-Induced Behaviors in Adolescent Rats with a Human Polymorphism (rs2304297) in the 3′-UTR of the CHRNA6 Gene. International Journal of Molecular Sciences, 23(6), 3145. https://doi.org/10.3390/ijms23063145




