Atorvastatin Decreases Renal Calcium Oxalate Stone Deposits by Enhancing Renal Osteopontin Expression in Hyperoxaluric Stone-Forming Rats Fed a High-Fat Diet
Abstract
:1. Introduction
2. Results
2.1. Serum and Urinary Biochemistry Change after Statins Use
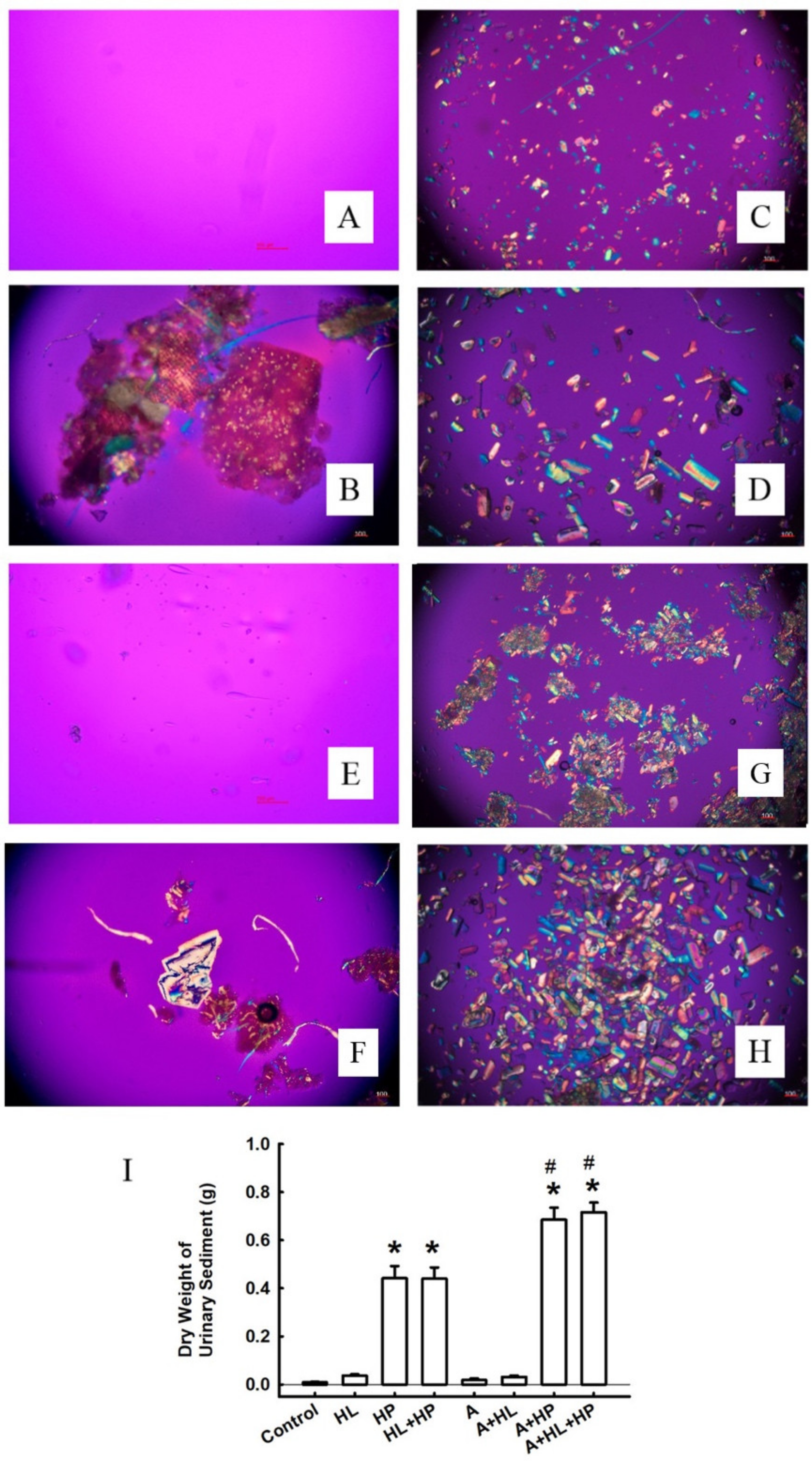
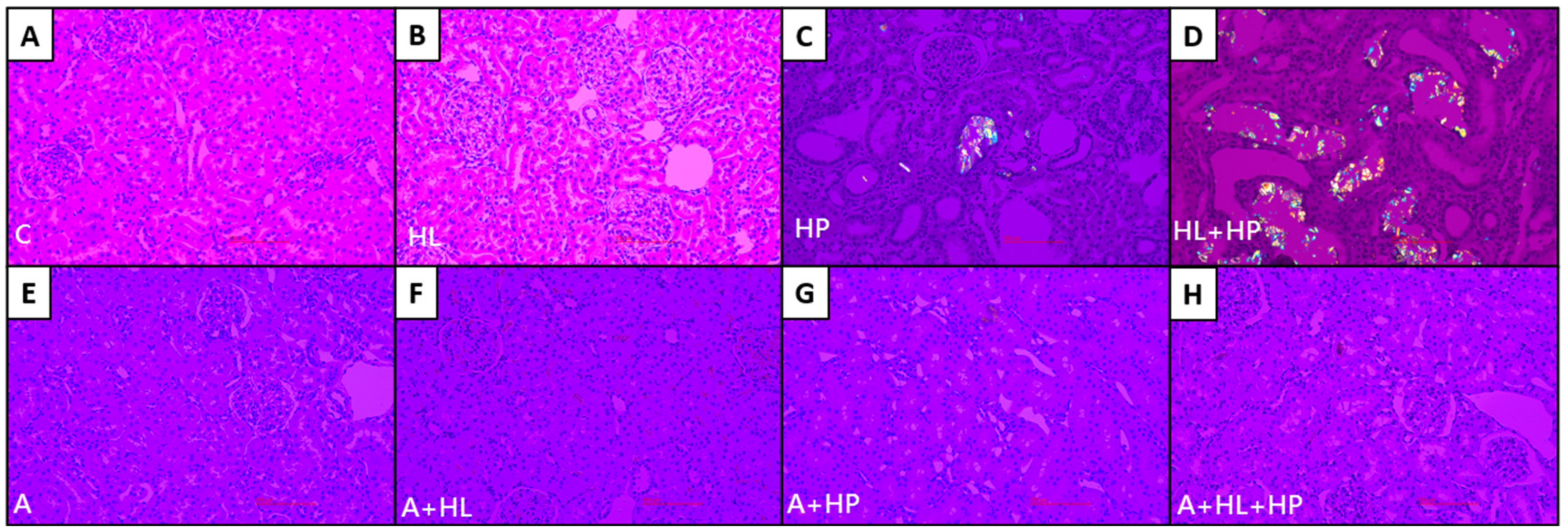
2.2. Crystal Morphology and Deposition in the Kidney Interstitial Tissues and Urinary Sediments
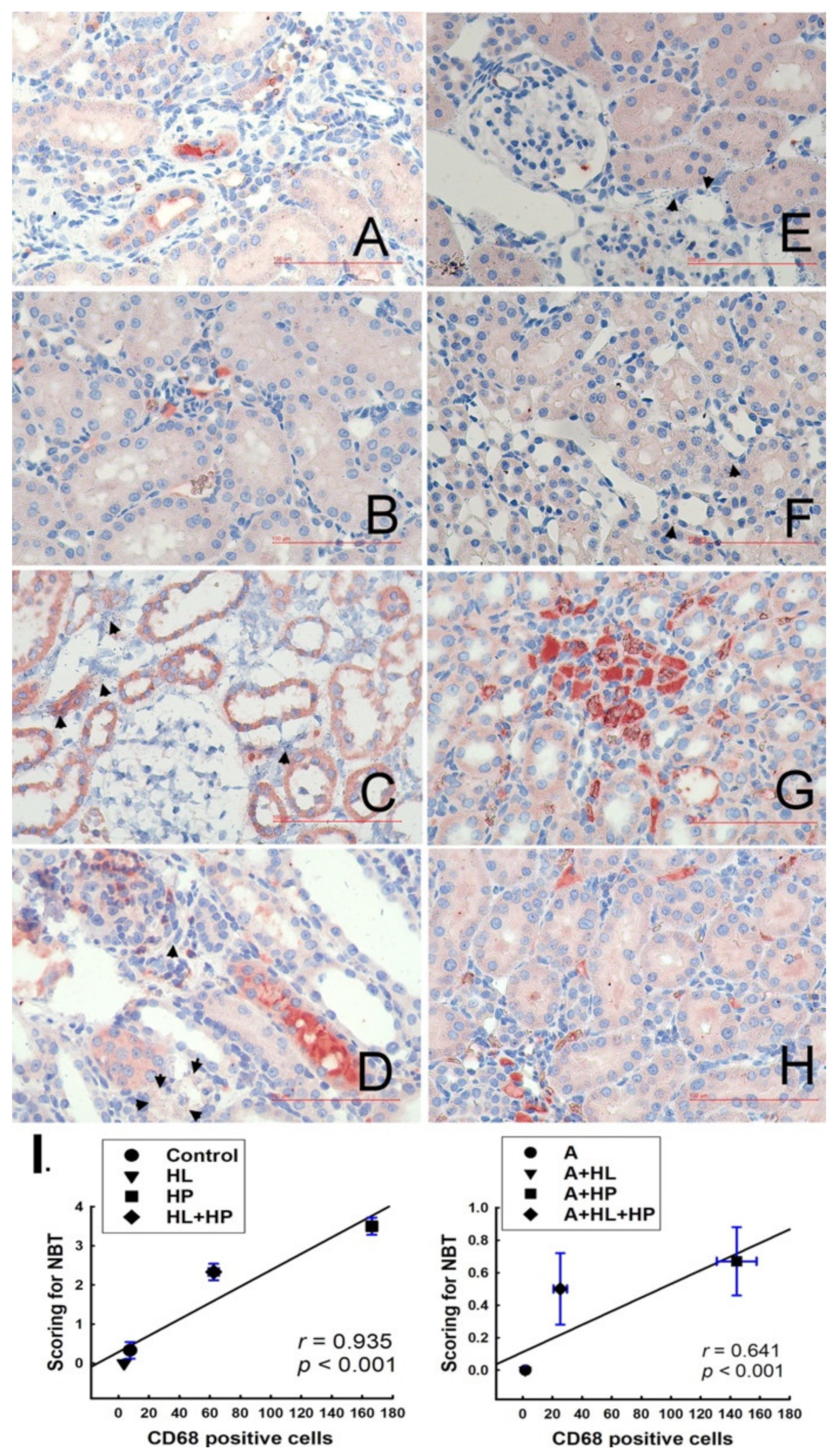
2.3. Detection of Cell Death In Situ and Its Relationship with Macrophages
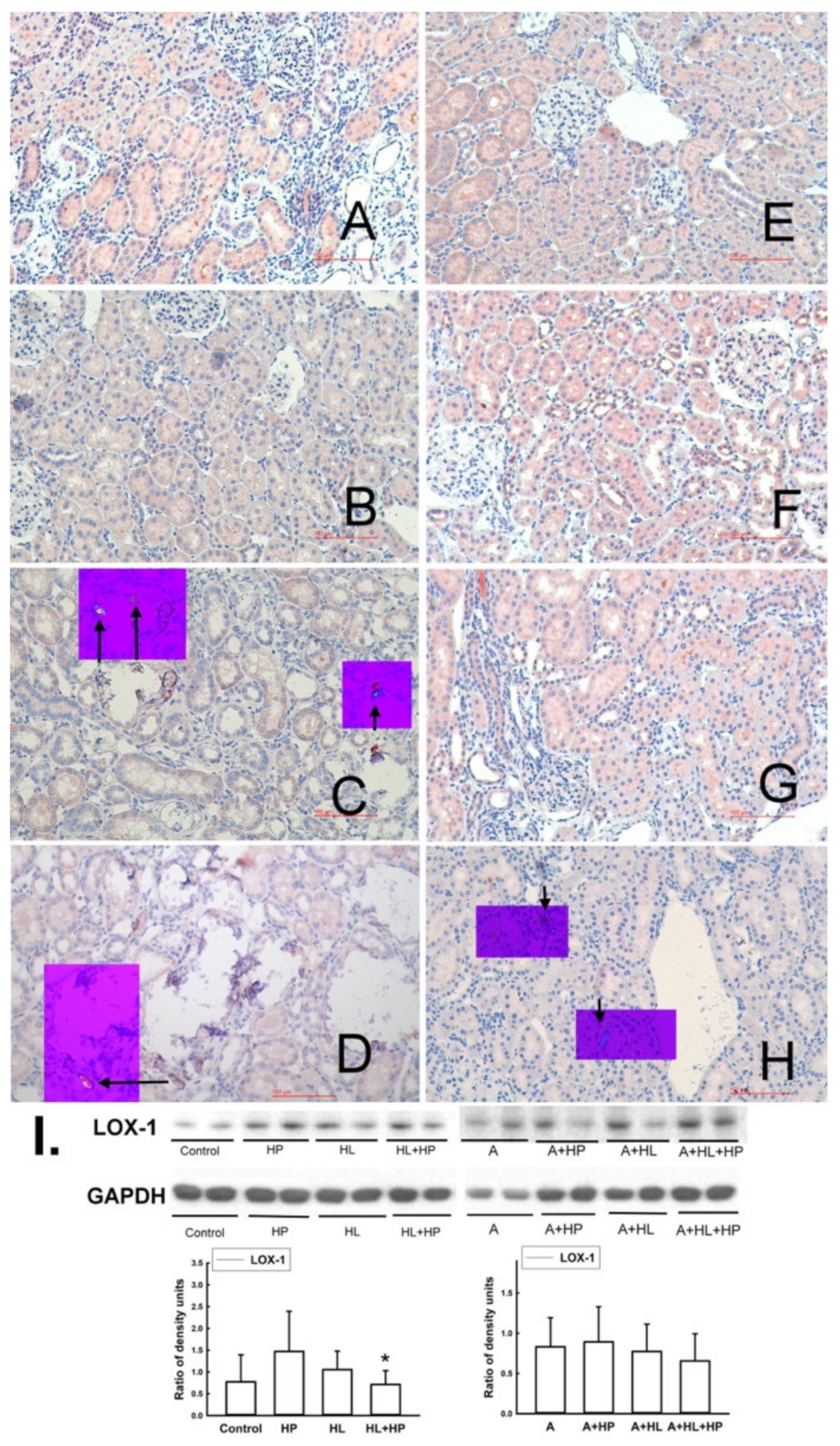
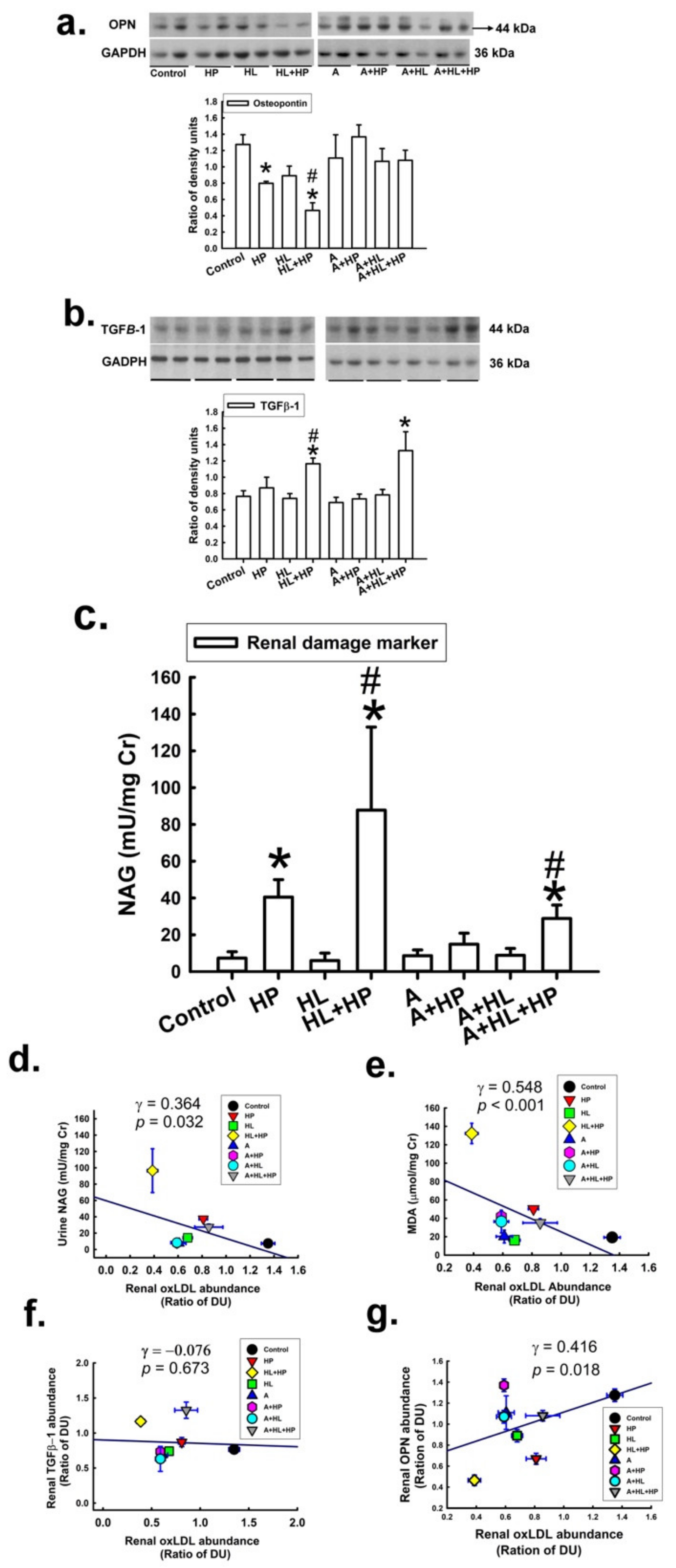
2.4. Impacts of Statin on the Expression of Lectin-like Ox-LDL Receptor-1 (LOX-1), Antilithic Proteins, and Tubule Injury Markers in the Kidney
3. Discussion
4. Materials and Methods
4.1. Experimental Induction of Renal CaOx Crystals and Atorvastatin Treatment
4.2. Immunohistochemistry and Nitro Blue Tetrazolium Staining
4.3. Detection of Calcium Crystals in Renal Sections and Urine Sediment
4.4. Determinants of Superoxide Formation and Associations with Monocytes/Macrophages
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.H.R.; Adams-Huet, B.; Poindexter, J.R.; Maalouf, N.M.; Moe, O.W.; Sakhaee, K. Temporal Changes in Kidney Stone Composition and in Risk Factors Predisposing to Stone Formation. J. Urol. 2017, 197, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wu, J.S.; Huang, H.S. Decreased Associated Risk of Gout in Diabetes Patients with Uric Acid Urolithiasis. J. Clin. Med. 2019, 8, 1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohjimoto, Y.; Sasaki, Y.; Iguchi, M.; Matsumura, N.; Inagaki, T.; Hara, I. Association of metabolic syndrome traits and severity of kidney stones: Results from a nationwide survey on urolithiasis in Japan. Am. J. Kidney Dis. 2013, 61, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Liao, P.C.; Liu, C.J. Calcium Kidney Stones are Associated with Increased Risk of Carotid Atherosclerosis: The Link between Urinary Stone Risks, Carotid Intima-Media Thickness, and Oxidative Stress Markers. J. Clin. Med. 2020, 9, 729. [Google Scholar] [CrossRef] [Green Version]
- Lieske, J.C. New insights regarding the interrelationship of obesity, diet, physical activity, and kidney stones. J. Am. Soc. Nephrol. 2014, 25, 211–212. [Google Scholar] [CrossRef] [Green Version]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Med. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef] [Green Version]
- Hartley, A.; Haskard, D.; Khamis, R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis—Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2019, 29, 22–26. [Google Scholar] [CrossRef]
- Lara-Guzman, O.J.; Gil-Izquierdo, A.; Medina, S.; Osorio, E.; Alvarez-Quintero, R.; Zuluaga, N.; Oger, C.; Galano, J.M.; Durand, T.; Munoz-Durango, K. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Khan, S.R. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: Evidence from clinical and experimental investigations. J. Urol. 2013, 189, 803–811. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, A.C.P.; Torres, L.C.; Duarte, M.; da Matta, M.C.; Casarini, D.E.; Schor, N. Is oxidized low-density lipoprotein the connection between atherosclerosis, cardiovascular risk and nephrolithiasis? Urolithiasis 2019, 47, 347–356. [Google Scholar] [CrossRef]
- McGregor, G.H.; Campbell, A.D.; Fey, S.K.; Tumanov, S.; Sumpton, D.; Blanco, G.R.; Mackay, G.; Nixon, C.; Vazquez, A.; Sansom, O.J.; et al. Targeting the Metabolic Response to Statin-Mediated Oxidative Stress Produces a Synergistic Antitumor Response. Cancer Res. 2020, 80, 175–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorensen, A.L.; Hasselbalch, H.C.; Nielsen, C.H.; Poulsen, H.E.; Ellervik, C. Statin treatment, oxidative stress and inflammation in a Danish population. Redox Biol. 2019, 21, 101088. [Google Scholar] [CrossRef] [PubMed]
- Sur, R.L.; Masterson, J.H.; Palazzi, K.L.; L’Esperance, J.O.; Auge, B.K.; Chang, D.C.; Stoller, M.L. Impact of statins on nephrolithiasis in hyperlipidemic patients: A 10-year review of an equal access health care system. Clin. Nephrol. 2013, 79, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Adamsky, M.A.; Nottingham, C.U.; Pruitt, J.; Lapin, B.; Wang, C.H.; Park, S. Impact of Statin Intake on Kidney Stone Formation. Urology 2019, 124, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Huang, H.S. Statins significantly alter urinary stone-related urine biochemistry in calcium kidney stone patients with dyslipidemia. Int. J. Urol. 2020, 27, 839–844. [Google Scholar] [CrossRef]
- Khan, S.R.; Glenton, P.A.; Byer, K.J. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: Experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 2006, 70, 914–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujihata, M.; Momohara, C.; Yoshioka, I.; Tsujimura, A.; Nonomura, N.; Okuyama, A. Atorvastatin inhibits renal crystal retention in a rat stone forming model. J. Urol. 2008, 180, 2212–2217. [Google Scholar] [CrossRef]
- Tiselius, H.G. Estimated levels of supersaturation with calcium phosphate and calcium oxalate in the distal tubule. Urol. Res. 1997, 25, 153–159. [Google Scholar] [CrossRef]
- Huang, H.S.; Ma, M.C.; Chen, J. Low-vitamin E diet exacerbates calcium oxalate crystal formation via enhanced oxidative stress in rat hyperoxaluric kidney. Am. J. Physiol. Ren. Physiol. 2009, 296, F34–F45. [Google Scholar] [CrossRef] [Green Version]
- Amos, F.F.; Dai, L.; Kumar, R.; Khan, S.R.; Gower, L.B. Mechanism of formation of concentrically laminated spherules: Implication to Randall’s plaque and stone formation. Urol. Res. 2009, 37, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Vernon, H.J.; Osborne, C.; Tzortzaki, E.G.; Yang, M.; Chen, J.; Rittling, S.R.; Denhardt, D.T.; Buyske, S.; Bledsoe, S.B.; Evan, A.P.; et al. Aprt/Opn double knockout mice: Osteopontin is a modifier of kidney stone disease severity. Kidney Int. 2005, 68, 938–947. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Chen, H.; Romeo, F.; Sawamura, T.; Saldeen, T.; Mehta, J.L. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: Role of LOX-1. J. Pharmacol. Exp. Ther. 2002, 302, 601–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Yamashita, M.; Iwai, M.; Hayashi, M. Endothelial Kruppel-Like Factor 4 Mediates the Protective Effect of Statins against Ischemic AKI. J. Am. Soc. Nephrol. 2016, 27, 1379–1388. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Chen, J.; Chen, C. Circulating adhesion molecules and neutral endopeptidase enzymuria in patients with urolithiasis and hydronephrosis. Urology 2000, 55, 961–965. [Google Scholar] [CrossRef]
- Taguchi, K.; Okada, A.; Hamamoto, S.; Unno, R.; Moritoki, Y.; Ando, R.; Mizuno, K.; Tozawa, K.; Kohri, K.; Yasui, T. M1/M2-macrophage phenotypes regulate renal calcium oxalate crystal development. Sci. Rep. 2016, 6, 35167. [Google Scholar] [CrossRef] [Green Version]
- Nancollas, G.H.; Henneman, Z.J. Calcium oxalate: Calcium phosphate transformations. Urol. Res. 2010, 38, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Wesson, J.A.; Johnson, R.J.; Mazzali, M.; Beshensky, A.M.; Stietz, S.; Giachelli, C.; Liaw, L.; Alpers, C.E.; Couser, W.G.; Kleinman, J.G.; et al. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J. Am. Soc. Nephrol. 2003, 14, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, J.H.; Coe, F.L.; Evan, A.P.; Worcester, E.M. Urine pH in renal calcium stone formers who do and do not increase stone phosphate content with time. Nephrol. Dial. Transplant. 2009, 24, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Krieger, N.S.; Asplin, J.R.; Frick, K.K.; Granja, I.; Culbertson, C.D.; Ng, A.; Grynpas, M.D.; Bushinsky, D.A. Effect of Potassium Citrate on Calcium Phosphate Stones in a Model of Hypercalciuria. J. Am. Soc. Nephrol. 2015, 26, 3001–3008. [Google Scholar] [CrossRef] [Green Version]
- Porowski, T.; Kirejczyk, J.K.; Mrozek, P.; Protas, P.; Kozerska, A.; Labieniec, L.; Szymanski, K.; Wasilewska, A. Upper metastable limit osmolality of urine as a predictor of kidney stone formation in children. Urolithiasis 2019, 47, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Kuroczycka-Saniutycz, E.; Porowski, T.; Protas, P.T.; Pszczolkowska, M.; Porowska, H.; Kirejczyk, J.K.; Wasilewska, A. Does obesity or hyperuricemia influence lithogenic risk profile in children with urolithiasis? Pediatr. Nephrol. 2015, 30, 797–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigner, P.; Bijak, M.; Saluk-Bijak, J. Probiotics in the Prevention of the Calcium Oxalate Urolithiasis. Cells 2022, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Roger, V.L.; Melton, L.J., 3rd; Bergstralh, E.J.; Li, X.; Peyser, P.A.; Krambeck, A.E.; Lieske, J.C. Kidney stones associate with increased risk for myocardial infarction. J. Am. Soc. Nephrol. 2010, 21, 1641–1644. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Kawashima, H.; Miyake, Y.; Zeniya, T.; Yamamoto, A.; Koshino, K.; Temma, T.; Fukuda, T.; Fujita, Y.; Kakino, A.; et al. (123)I-Labeled oxLDL Is Widely Distributed Throughout the Whole Body in Mice. Nucl. Med. Mol. Imaging 2018, 52, 144–153. [Google Scholar] [CrossRef]
- Bosmans, J.L.; Holvoet, P.; Dauwe, S.E.; Ysebaert, D.K.; Chapelle, T.; Jurgens, A.; Kovacic, V.; Van Marck, E.A.; De Broe, M.E.; Verpooten, G.A. Oxidative modification of low-density lipoproteins and the outcome of renal allografts at 1 years. Kidney Int. 2001, 59, 2346–2356. [Google Scholar] [CrossRef]
- Ruan, X.Z.; Varghese, Z.; Moorhead, J.F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 2009, 5, 713–721. [Google Scholar] [CrossRef]
- Wang, L.; Sun, S.; Zhou, A.; Yao, X.; Wang, Y. oxLDL-induced lipid accumulation in glomerular podocytes: Role of IFN-gamma, CXCL16, and ADAM10. Cell Biochem. Biophys. 2014, 70, 529–538. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Lin, N.; Dong, X.; Li, W.; Deng, Y.; Ma, L. Interleukin-1beta Promotes Ox-LDL Uptake by Human Glomerular Mesangial Cells via LOX-1. Int. J. Med. Sci. 2020, 17, 1056–1061. [Google Scholar] [CrossRef]
- Huang, H.S.; Chen, J.; Chen, C.F.; Ma, M.C. Vitamin E attenuates crystal formation in rat kidneys: Roles of renal tubular cell death and crystallization inhibitors. Kidney Int. 2006, 70, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Schmiedl, A.; Schwille, P.O.; Bonucci, E.; Erben, R.G.; Grayczyk, A.; Sharma, V. Nephrocalcinosis and hyperlipidemia in rats fed a cholesterol- and fat-rich diet: Association with hyperoxaluria, altered kidney and bone minerals, and renal tissue phospholipid-calcium interaction. Urol. Res. 2000, 28, 404–415. [Google Scholar] [CrossRef]

| N | HL | HP | HL + HP | A | A + HL | A + HP | A + HL + HP | |
|---|---|---|---|---|---|---|---|---|
| BW (g) | 408.2 ± 7.9 | 417.0 ±20.4 | 328.8 ± 21.4 a | 365.1 ± 12.1 | 395.5 ± 8.1 | 422.3 ± 10.7 | 334.3 ± 14.4 a,b | 407.3 ± 9.4 |
| Food intake (g/24 h) | 29.6 ± 1.0 | 28.2 ± 1.6 | 21.0 ± 1.4 a | 21.4 ± 3.0 a | 30.6 ± 0.5 | 29.9 ± 1.3 | 22.8 ± 2.4 a,b | 23.2 ± 2.6 a,b |
| Water intake (mL/24 h) | 50.6 ± 6.2 | 51.7 ± 8.0 | 36.8 ± 4.6 a | 35.0 ± 6.8 a | 51.1 ± 5.6 | 46.7 ± 2.7 | 36.1 ± 3.2 a,b | 31.7 ± 2.6 a,b |
| UO (mL/24 h) | 41.0 ± 5.3 | 27.1 ± 8.2 | 23.5 ± 3.7 | 15.6 ± 2.5 a | 25.4 ± 3.7 | 12.8 ± 0.7 | 20.2 ± 2.1 | 11.1 ± 0.9 a,b |
| Ccr (mL/min) | 5.2 ± 1.0 | 3.0 ± 0.6 a | 2.6 ± 0.5 a | 2.5 ± 0.8 a | 4.6 ± 0.9 | 1.8 ± 0.4 a,b | 2.1 ± 0.1 a,b | 1.6 ± 0.3 a,b |
| Urine pH | 7.6 ± 0.1 | 6.7 ± 0.1 | 6.9 ± 0.4 | 6.8 ± 0.6 | 7.2 ± 0.1 | 6.8 ± 0.1 | 6.8 ± 0.0 | 6.6 ± 0.4 |
| Kidney Crystal deposit (score) | 0 | 0 | I–II | II–III | 0 | 0 | 0–I | 0–I |
| N | HL | HP | HL + HP | A | A + HL | A + HP | A + HL + HP | |
|---|---|---|---|---|---|---|---|---|
| Serum | ||||||||
| TG (mg/dL) | 101.5 ± 6.3 | 78.6 ± 9.8 a | 99.5 ± 2.7 | 87.3 ± 6.1 | 86.3 ± 13.8 | 86.7 ± 10.1 | 80.0 ± 12.1 c | 94.0 ± 28.0 |
| Cholesterol (mg/dL) | 63.3 ± 6.0 | 728.7 ± 104.9 a | 67.0 ± 2.9 | 361.0 ± 60.7 a | 68.0 ± 2.5 | 602.0 ± 84.0 a,b,c | 47.0 ± 1.6 c | 488.5 ± 66.8 a,b |
| HDL (mg/dL) | 21.8 ± 2.0 | 6.3 ± 2.2 a | 20.3 ± 1.7 | 10.7 ± 2.3 a | 22.8 ± 1.3 | 5.3 ± 1.8 a,b | 18.7 ± 0.7 | 8.3 ± 2.0 a,b |
| LDL (mg/dL) | 7.0 ± 0.4 | 154.1 ± 28.9 a | 7.8 ± 0.8 | 56.5 ± 15.8 a | 7.5 ± 0.7 | 119.2 ± 21.2 a,b,c | 6.7 ± 0.4 | 90.8 ± 17.6 a,b,c |
| Atherogenic Index | 2.0 ± 0.2 | 144.5 ± 22.0 a | 2.1 ± 0.2 | 58.7 ± 23.1 a | 1.7 ± 0.1 | 193.4 ± 70.8 a,b | 1.4 ± 0.1 | 88.4 ± 23.3 a,b,c |
| Na (meq/L) | 141.5 ± 0.7 | 136.2 ± 1.7 | 138.7 ± 1.4 | 136.7 ± 2.2 | 140.3 ± 1.3 | 135.2 ± 2.2 | 137.7 ± 2.3 | 136.0 ± 2.4 |
| K (meq/L) | 5.5 ± 0.3 | 5.7 ± 0.2 | 5.5 ± 0.3 | 5.5 ± 0.2 | 5.6 ± 0.3 | 5.5 ± 0.1 | 5.8 ± 0.5 | 5.5 ± 0.2 |
| Ca (mg/dL) | 10.2 ± 0.2 | 10.5 ± 0.2 | 9.6 ± 0.2 | 10.3 ± 0.1 | 10.0 ± 0.2 | 10.6 ± 0.2 | 9.7 ± 0.2 | 10.4 ± 0.2 |
| P (mg/dL) | 9.0 ± 0.1 | 7.7 ± 0.2 | 8.1 ± 0.2 | 7.4 ± 0.2 | 8.1 ± 0.2 | 7.5 ± 0.4 | 6.9 ± 0.5 | 7.5 ± 0.2 |
| Urine | ||||||||
| Na (mmol/24 h) | 2.9 ± 0.5 | 3.1 ± 0.4 | 1.9 ± 0.5 | 1.8 ± 0.3 | 3.1 ± 0.5 | 2.7 ± 0.4 | 1.6 ± 0.2 | 1.7 ± 0.5 |
| K (mmol/24 h) | 6.6 ± 0.6 | 2.9 ± 0.4 a | 4.1 ± 1.2 | 2.1 ± 0.5 a | 6.4 ± 1.1 | 2.4 ± 0.3 a,b | 3.3 ± 0.5 | 1.7 ± 0.4 a,b |
| Ca (mg/24 h) | 0.24 ± 0.11 | 0.05 ± 0.01 a | 0.33 ± 0.26 | 0.05 ± 0.02 a | 0.29 ± 0.06 | 0.07 ± 0.01 a,b | 0.12 ± 0.04 a,b | 0.06 ± 0.01 a,b |
| P (mg/24 h) | 40.0 ± 12.8 | 46.3 ± 6.4 | 35.5 ± 6.0 | 47.6 ± 17.3 | 24.3 ± 4.6 a,c | 35.1 ± 5.7 | 17.6 ± 2.1 a,c | 30.0 ± 6.2 a,c |
| Mg (μmol/24 h) | 35.4 ± 9.4 | 15.9 ± 5.4 a | 71.1 ± 26.9 a | 14.9 ± 5.3 a | 80.1 ± 31.1 a,c | 8.3 ± 1.4 a,b,c | 60.1 ± 27.2 a,b | 10.6 ± 2.6 a,b |
| Protein (mg/24 h) | 25.0 ± 2.7 | 22.0 ± 8.3 | 12.8 ± 3.8 a | 22.7 ± 5.2 | 24.4 ± 7.4 | 11.4 ± 2.5 a,b,c | 7.3 ± 1.2 a,b,c | 11.7 ± 3.3 a,b,c |
| MDA (μmol/mg Cr) | 19.2 ± 2.0 | 26.6 ± 11.3 | 52.2 ± 2.0 a | 141.3 ± 15.1 a | 24.1 ± 6.3 | 29.2 ± 9.6 | 48.6 ± 4.7 a,b | 62.3 ± 21.0 a,b,c |
| Cit (mg/mg Cr) | 2.4 ± 0.7 | 4.6 ± 2.0 a | 1.0 ± 0.2 a | 2.0 ± 0.5 a | 3.7 ± 0.7 a,c | 5.8 ± 2.7 a,b | 4.7 ± 1.0 a,c | 5.8 ± 2.6 a,b,c |
| Ox (mg/24 h) | 4.0 ± 0.7 | 4.6 ± 0.9 | 12.1 ± 1.6 a | 7.3 ± 1.7 a | 10.3 ± 2.0 a,c | 5.2 ± 1.2 b | 10.4 ± 1.2 a | 4.9 ± 0.8 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.J.; Tsai, Y.S.; Huang, H.S. Atorvastatin Decreases Renal Calcium Oxalate Stone Deposits by Enhancing Renal Osteopontin Expression in Hyperoxaluric Stone-Forming Rats Fed a High-Fat Diet. Int. J. Mol. Sci. 2022, 23, 3048. https://doi.org/10.3390/ijms23063048
Liu CJ, Tsai YS, Huang HS. Atorvastatin Decreases Renal Calcium Oxalate Stone Deposits by Enhancing Renal Osteopontin Expression in Hyperoxaluric Stone-Forming Rats Fed a High-Fat Diet. International Journal of Molecular Sciences. 2022; 23(6):3048. https://doi.org/10.3390/ijms23063048
Chicago/Turabian StyleLiu, Chan Jung, Yau Sheng Tsai, and Ho Shiang Huang. 2022. "Atorvastatin Decreases Renal Calcium Oxalate Stone Deposits by Enhancing Renal Osteopontin Expression in Hyperoxaluric Stone-Forming Rats Fed a High-Fat Diet" International Journal of Molecular Sciences 23, no. 6: 3048. https://doi.org/10.3390/ijms23063048
APA StyleLiu, C. J., Tsai, Y. S., & Huang, H. S. (2022). Atorvastatin Decreases Renal Calcium Oxalate Stone Deposits by Enhancing Renal Osteopontin Expression in Hyperoxaluric Stone-Forming Rats Fed a High-Fat Diet. International Journal of Molecular Sciences, 23(6), 3048. https://doi.org/10.3390/ijms23063048





