On the Efficacy of ZnO Nanostructures against SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of ZnONPs
2.3. SARS-CoV-2 Nucleocapsid Protein Antigen Quantification
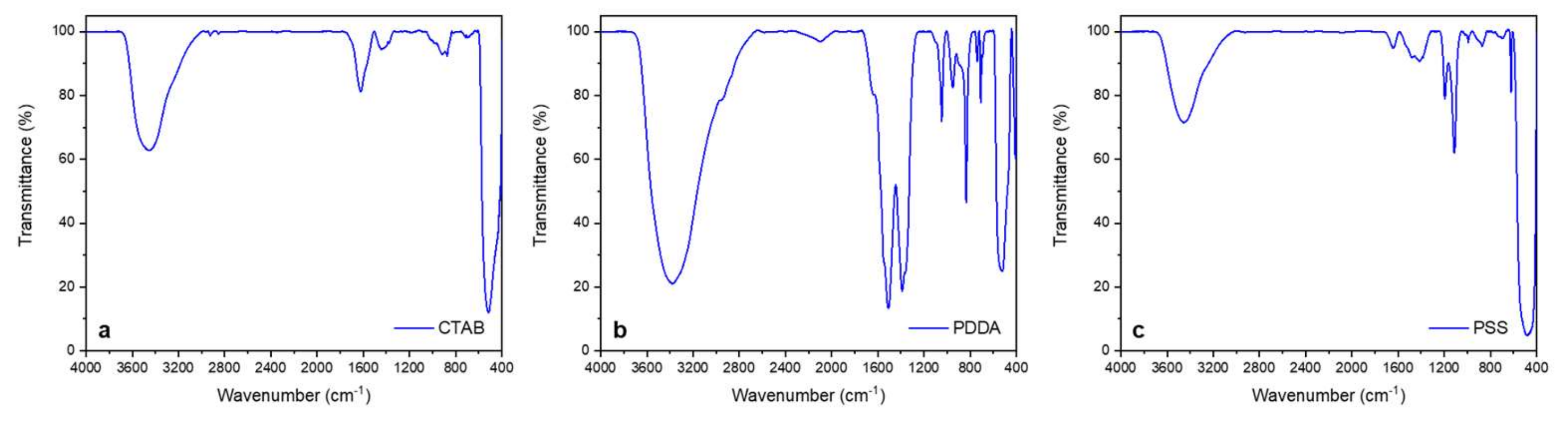
2.4. Preparation of ZnO-Based Coatings for Touching Surfaces
2.5. Surface Chemical Composition of ZnO-Based Coatings by X-ray Photoelectron Spectroscopy
2.6. Colorimetric Quantification of Zn2+ Ionic Release from ZnO-Based Coatings
3. Results
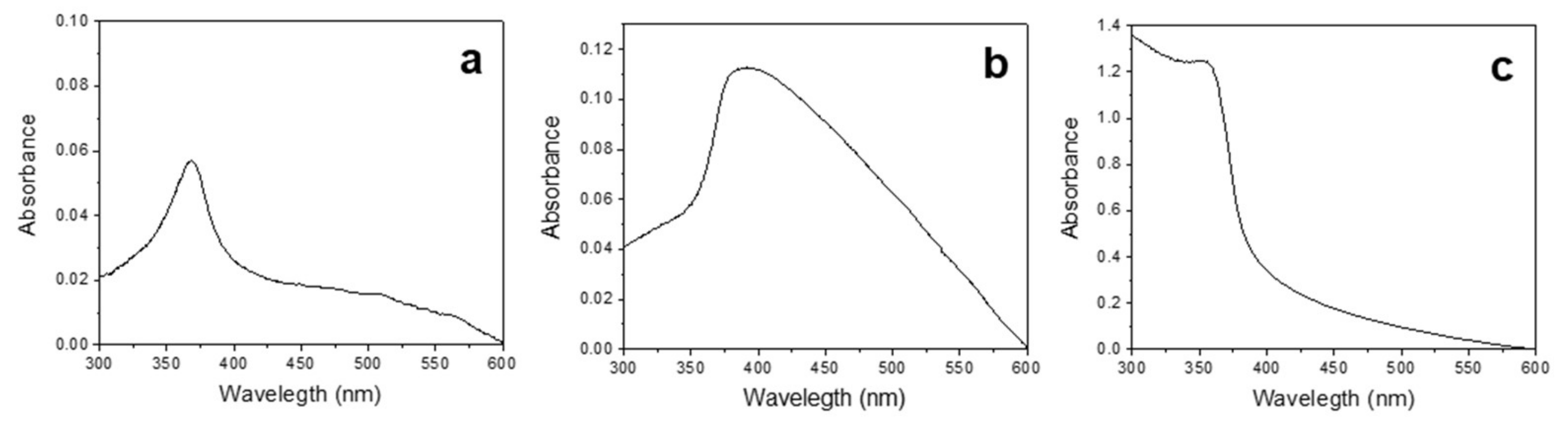
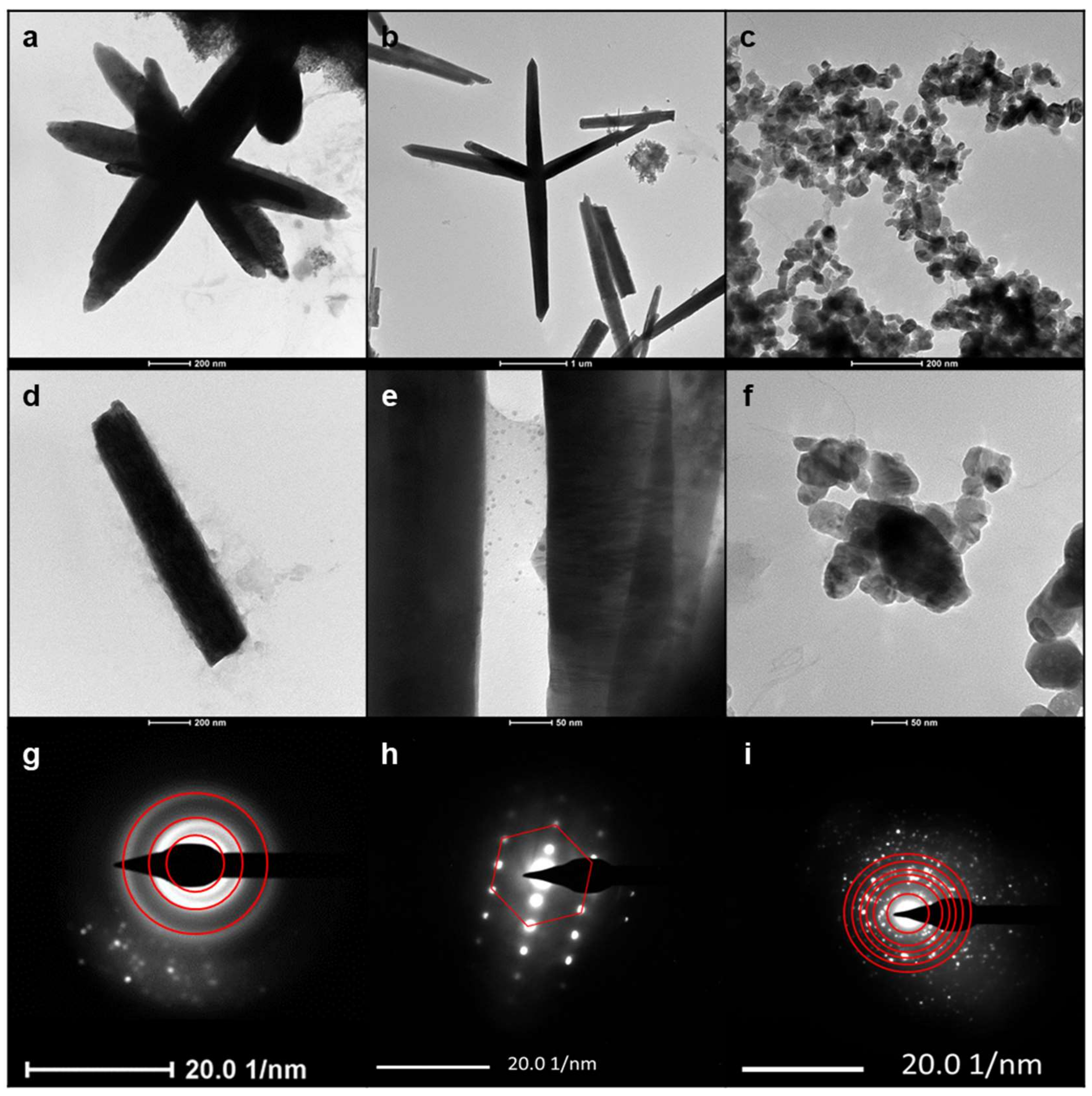
3.1. Synthesis and Characterization of ZnONPs
3.2. Antiviral Activity of ZnONPs against SARS-CoV-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review). Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, J. Nanoparticles as a Powerful Tool to Fight with COVID-19. Soc. Sci. Res. Netw. 2020, 2020. [Google Scholar] [CrossRef]
- Hosseini, M.; Behzadinasab, S.; Chin, A.W.H.; Poon, L.L.M.; Ducker, W.A. Reduction of Infectivity of SARS-CoV-2 by Zinc Oxide Coatings. ACS Biomater. Sci. Eng. 2021, 7, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef]
- Tavakoli, A.; Ataei-Pirkooh, A.; Sadeghi, G.M.M.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Polyethylene glycol-coated zinc oxide nanoparticle: An efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine 2018, 13, 2675–2690. [Google Scholar] [CrossRef]
- Pormohammad, A.; Monych, N.K.; Turner, R.J. Zinc and SARS-CoV-2: A molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int. J. Mol. Med. 2021, 47, 326–334. [Google Scholar] [CrossRef]
- McPherson, S.W.; Keunen, J.E.; Bird, A.C.; Chew, E.Y.; van Kuijk, F.J. Investigate Oral Zinc as a Prophylactic Treatment for Those at Risk for COVID-19. Am. J. Ophthalmol. 2020, 216, A5–A6. [Google Scholar] [CrossRef]
- Rahman, M.T.; Idid, S.Z. Can Zn Be a Critical Element in COVID-19 Treatment? Biol. Trace Elem. Res. 2021, 199, 550–558. [Google Scholar] [CrossRef]
- Speth, R.; Carrera, E.; Jean-Baptiste, M.; Joachim, A.; Linares, A. Concentration-dependent effects of zinc on angiotensin-converting enzyme-2 activity (1067.4). FASEB J. 2014, 28, 1067.4. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martín-Sampedro, R.; del Real, G.; Aranda, P. Nanotechnology Responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979. [Google Scholar] [CrossRef] [PubMed]
- Abo-zeid, Y.; Ismail, N.S.M.; McLean, G.R.; Hamdy, N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef] [PubMed]
- Picca, R.A.; Sportelli, M.C.; Hötger, D.; Manoli, K.; Kranz, C.; Mizaikoff, B.; Torsi, L.; Cioffi, N. Electrosynthesis and characterization of ZnO nanoparticles as inorganic component in organic thin-film transistor active layers. Electrochim. Acta 2015, 178, 45–54. [Google Scholar] [CrossRef]
- Picca, R.A.; Sportelli, M.C.; Lopetuso, R.; Cioffi, N. Electrosynthesis of ZnO nanomaterials in aqueous medium with CTAB cationic stabilizer. J. Sol-Gel Sci. Technol. 2016, 81, 338–345. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Izzi, M.; Palazzo, G.; Gristina, R.; Innocenti, M.; Torsi, L.; Cioffi, N. ZnO Nanostructures with Antibacterial Properties Prepared by a Green Electrochemical-Thermal Approach. Nanomaterials 2020, 10, 473. [Google Scholar] [CrossRef] [Green Version]
- Säbel, C.E.; Neureuther, J.M.; Siemann, S. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 2010, 397, 218–226. [Google Scholar] [CrossRef]
- Rush, R.M.; Yoe, J.H. Colorimetric Determination of Zinc and Copper with 2-Carboxy-2′-hydroxy-5’-sulfoformazylbenzene. Anal. Chem. 1954, 26, 1345–1347. [Google Scholar] [CrossRef]
- Moussodia, R.-O.; Balan, L.; Merlin, C.; Mustin, C.; Schneider, R. Biocompatible and stable ZnO quantum dots generated by functionalization with siloxane-core PAMAM dendrons. J. Mater. Chem. 2010, 20, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Kasamechonchung, P.; Horprathum, M.; Boonpavanitchakul, K.; Supaka, N.; Prompinit, P.; Kangwansupamonkon, W.; Somboonkaew, A.; Wetcharungsri, J.; Pratontep, S.; Porntheeraphat, S.; et al. Morphology-controlled seed-assisted hydrothermal ZnO nanowires via critical concentration for nucleation and their photoluminescence properties. Phys. Status Solidi A 2015, 212, 394–400. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Cioffi, N. Recent advances in the synthesis and characterization of nano-antimicrobials. TrAC Trends Anal. Chem. 2016, 84 Pt A, 131–138. [Google Scholar] [CrossRef]
- Kang, S.; Yang, M.; Hong, Z.; Zhang, L.; Huang, Z.; Chen, X.; He, S.; Zhou, Z.; Zhou, Z.; Chen, Q.; et al. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B 2020, 10, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Cubuk, J.; Alston, J.J.; Incicco, J.J.; Singh, S.; Stuchell-Brereton, M.D.; Ward, M.D.; Zimmerman, M.I.; Vithani, N.; Griffith, D.; Wagoner, J.A.; et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun. 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Liu, G.; Ma, H.; Zhao, D.; Yang, Y.; Liu, M.; Mohammed, A.; Zhao, C.; Yang, Y.; Xie, J.; et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem. Biophys. Res. Commun. 2020, 527, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.J.; Dal Sasso, G.; Bertolotti, F.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Pedersen, J.S.; Masciocchi, N.; Guagliardi, A. The role of nanoparticle structure and morphology in the dissolution kinetics and nutrient release of nitrate-doped calcium phosphate nanofertilizers. Sci. Rep. 2020, 10, 12396. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Usmani, Z.; Thakur, V.K.; Gupta, V.K.; Mishra, Y.K. Tackling COVID-19 pandemic through nanocoatings: Confront and exactitude. Curr. Res. Green Sustain. Chem. 2020, 3, 100011. [Google Scholar] [CrossRef]
- Hamdi, M.; Abdel-Bar, H.M.; Elmowafy, E.; El-khouly, A.; Mansour, M.; Awad, G.A.S. Investigating the Internalization and COVID-19 Antiviral Computational Analysis of Optimized Nanoscale Zinc Oxide. ACS Omega 2021, 6, 6848–6860. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Heppt, W.; Cryer, A.; Wussow, A.; Peiser, C.; Zweng, M.; Thai Dinh, Q.; Witt, C.; Fischer, A. Toxic Rhinitis-Induced Changes of Human Nasal Mucosa Innervation. Toxicol. Pathol. 2003, 31, 326–331. [Google Scholar] [CrossRef] [Green Version]
- LaForce, F.M.; Boose, D.S. Effect of zinc and phosphate on an antibacterial peptide isolated from lung lavage. Infect. Immun. 1984, 45, 692–696. [Google Scholar] [CrossRef] [Green Version]
- Novick, S.G.; Godfrey, J.C.; Pollack, R.L.; Wilder, H.R. Zinc-induced suppression of inflammation in the respiratory tract, caused by infection with human rhinovirus and other irritants. Med. Hypotheses 1997, 49, 347–357. [Google Scholar] [CrossRef]
- Monette, A.; Mouland, A.J. Zinc and Copper Ions Differentially Regulate Prion-Like Phase Separation Dynamics of Pan-Virus Nucleocapsid Biomolecular Condensates. Viruses 2020, 12, 1179. [Google Scholar] [CrossRef]
- Zoghi, S.; Khamirani, H.J.; Dastgheib, S.A.; Dianatpour, M.; Ghaffarieh, A. An analysis of inhibition of the severe acute respiratory syndrome coronavirus 2 RNA-dependent RNA polymerase by zinc ion: An in silico approach. Future Virol. 2021, 16, 331–339. [Google Scholar] [CrossRef]
- Gutiérrez Rodelo, C.; Salinas, R.A.; Armenta Jaime, E.; Armenta, S.; Galdámez-Martínez, A.; Castillo-Blum, S.E.; Astudillo-de la Vega, H.; Nirmala Grace, A.; Aguilar-Salinas, C.A.; Gutiérrez Rodelo, J.; et al. Zinc associated nanomaterials and their intervention in emerging respiratory viruses: Journey to the field of biomedicine and biomaterials. Coord. Chem. Rev. 2022, 457, 214402. [Google Scholar] [CrossRef] [PubMed]
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The antimicrobial activity of free and immobilized poly (diallyldimethylammonium) chloride in nanoparticles of poly (methylmethacrylate). J. Nanobiotechnol. 2015, 13, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef] [PubMed]
- Kelner, A.; Schacht, E.H. Tailor-made polymers for local drug delivery: Release of macromolecular model drugs from biodegradable hydrogels based on poly(ethylene oxide). J. Control. Release 2005, 101, 13–20. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; Ditaranto, N.; Cioffi, N. Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apicella, A.; Cappello, B.; Del Nobile, M.A.; La Rotonda, M.I.; Mensitieri, G.; Nicolais, L. Poly(Ethylene oxide) (PEO) and different molecular weight PEO blends monolithic devices for drug release. Biomaterials 1993, 14, 83–90. [Google Scholar] [CrossRef]
- Health and Consumers European Commission—Opinions on Zinc Oxide (Nano Form). Available online: https://ec.europa.eu/health/scientific_committees/opinions_layman/zinc-oxide/en/l-3/6.htm (accessed on 9 February 2022).
- Agency for Toxic Substances and Disease Registry ATSDR—PUBLIC HEALTH STATEMENT—Zinc—CAS#: 7440-66-6. Available online: https://www.atsdr.cdc.gov/index.html (accessed on 9 February 2022).

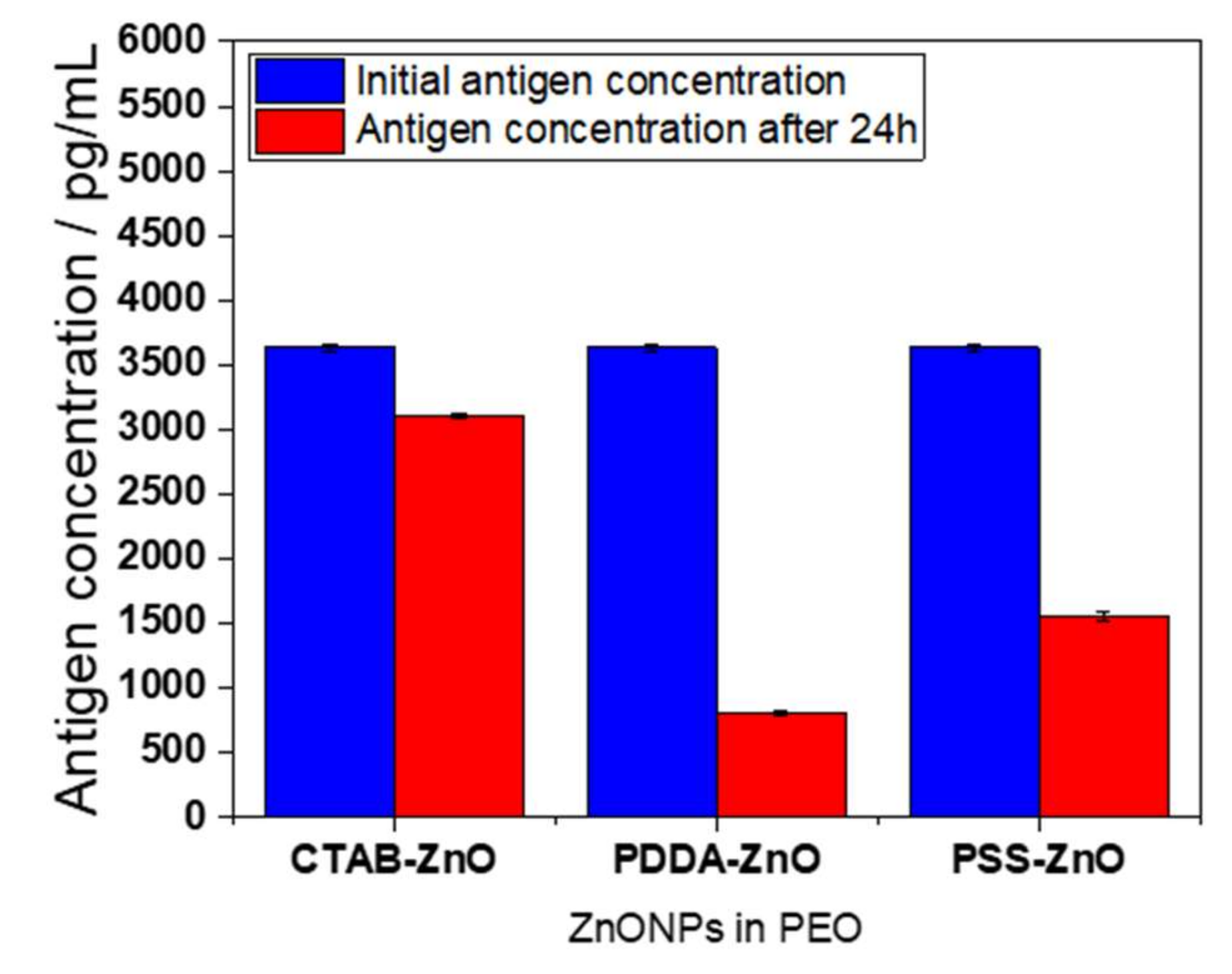
| Sample Type | Dilution Factor | Initial (pg/mL) | Final (pg/mL) | Decreasing % | Mean (pg/mL) | Decr. Mean % |
|---|---|---|---|---|---|---|
| ZnO/PSS 10 g/L | 1:10 | 3634.9 ± 0.5 | 2363.3 ± 0.5 | 35.0 | 2436 ± 72 | 33 ± 2 |
| 2507.8 ± 0.5 | 31.0 | |||||
| ZnO/PSS 1 g/L | 2831.5 ± 0.5 | 22.1 | 2695 ± 136 | 26 ± 4 | ||
| 2559.1 ± 0.5 | 29.6 | |||||
| ZnO/PDDA 10 g/L | 1:10 | 3634.9 ± 0.5 | 385.5 ± 0.5 | 89.4 | 375 ± 11 | 89.7 ± 0.3 |
| 363.9 ± 0.5 | 90.0 | |||||
| ZnO/PDDA 1 g/L | 1187.3 ± 0.5 | 67.3 | 1257 ± 70 | 65.4 ± 1.9 | ||
| 1327.6 ± 0.5 | 63.5 | |||||
| ZnO/CTAB 10 g/L | 1:10 | 3634.9 ± 0.5 | 2649.7 ± 0.5 | 27.1 | 2651.5 ± 1.8 | 27.0 ± 0.1 |
| 2653.3 ± 0.5 | 27.0 | |||||
| ZnO/CTAB 1 g/L | 2713.4 ± 0.5 | 25.4 | 2895 ± 319 | 20 ± 5 | ||
| 3075.7 ± 0.5 | 15.4 |
| Sample | [Zn2+]/mg/L (in 24 h) | Zn% | Inhibition % |
|---|---|---|---|
| CTAB-ZnO in PEO | 4.2 ± 0.6 | 1.7 ± 0.2 | 14 ± 3 |
| PDDA-ZnO in PEO | 45 ± 4 | 0.5 ± 0.2 | 78 ± 2 |
| PSS-ZnO in PEO | 13 ± 1 | 6.6 ± 0.7 | 57 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sportelli, M.C.; Izzi, M.; Loconsole, D.; Sallustio, A.; Picca, R.A.; Felici, R.; Chironna, M.; Cioffi, N. On the Efficacy of ZnO Nanostructures against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 3040. https://doi.org/10.3390/ijms23063040
Sportelli MC, Izzi M, Loconsole D, Sallustio A, Picca RA, Felici R, Chironna M, Cioffi N. On the Efficacy of ZnO Nanostructures against SARS-CoV-2. International Journal of Molecular Sciences. 2022; 23(6):3040. https://doi.org/10.3390/ijms23063040
Chicago/Turabian StyleSportelli, Maria Chiara, Margherita Izzi, Daniela Loconsole, Anna Sallustio, Rosaria Anna Picca, Roberto Felici, Maria Chironna, and Nicola Cioffi. 2022. "On the Efficacy of ZnO Nanostructures against SARS-CoV-2" International Journal of Molecular Sciences 23, no. 6: 3040. https://doi.org/10.3390/ijms23063040
APA StyleSportelli, M. C., Izzi, M., Loconsole, D., Sallustio, A., Picca, R. A., Felici, R., Chironna, M., & Cioffi, N. (2022). On the Efficacy of ZnO Nanostructures against SARS-CoV-2. International Journal of Molecular Sciences, 23(6), 3040. https://doi.org/10.3390/ijms23063040












