Recent Advances in Polymer Additive Engineering for Diagnostic and Therapeutic Hydrogels
Abstract
:1. Introduction
2. Nucleic Acid Additives
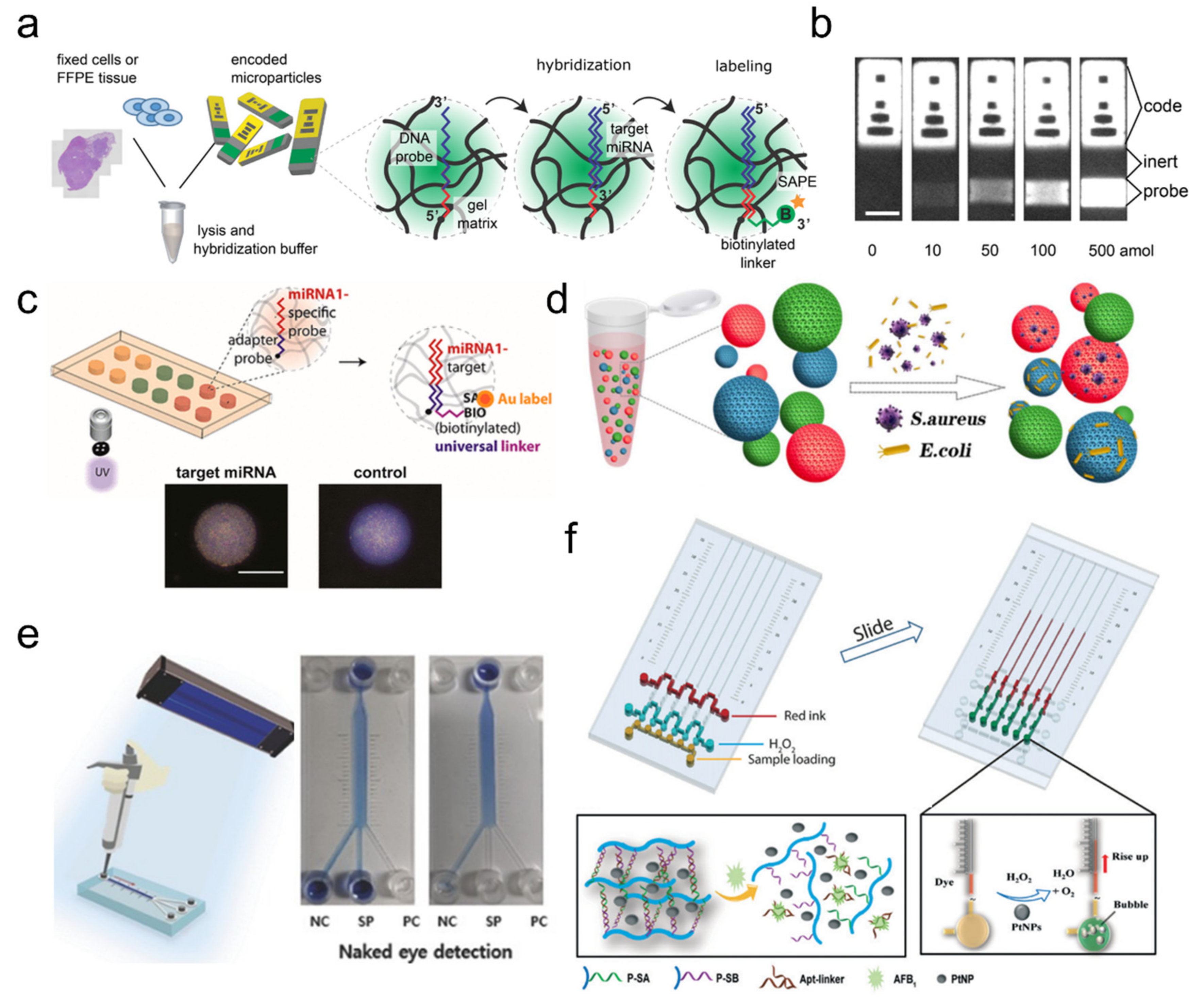
2.1. Nucleic Acid-Based Hydrogel Biosensors
2.2. DNA Hydrogels
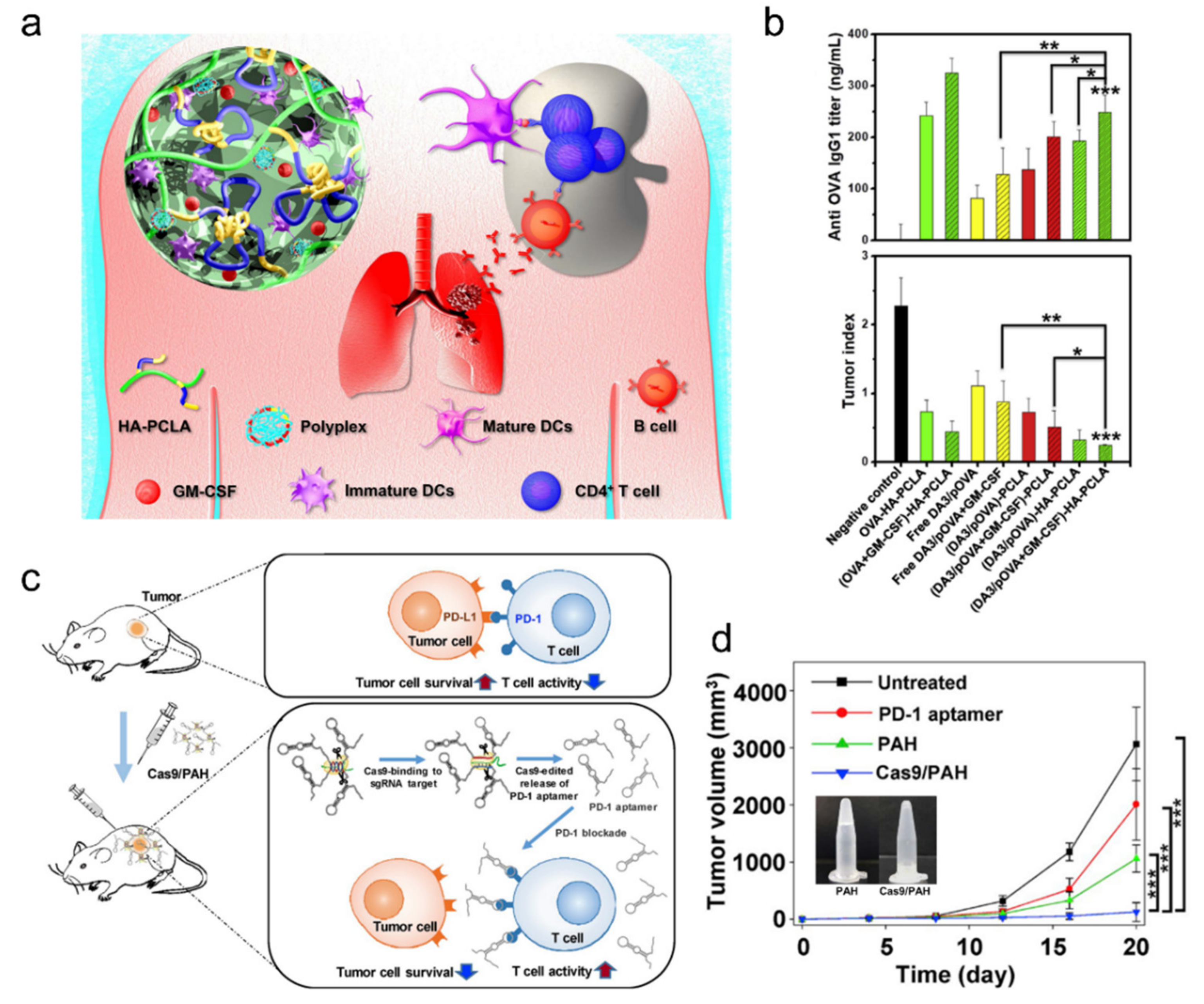
2.3. Injectable Cancer Immunotherapy Drugs
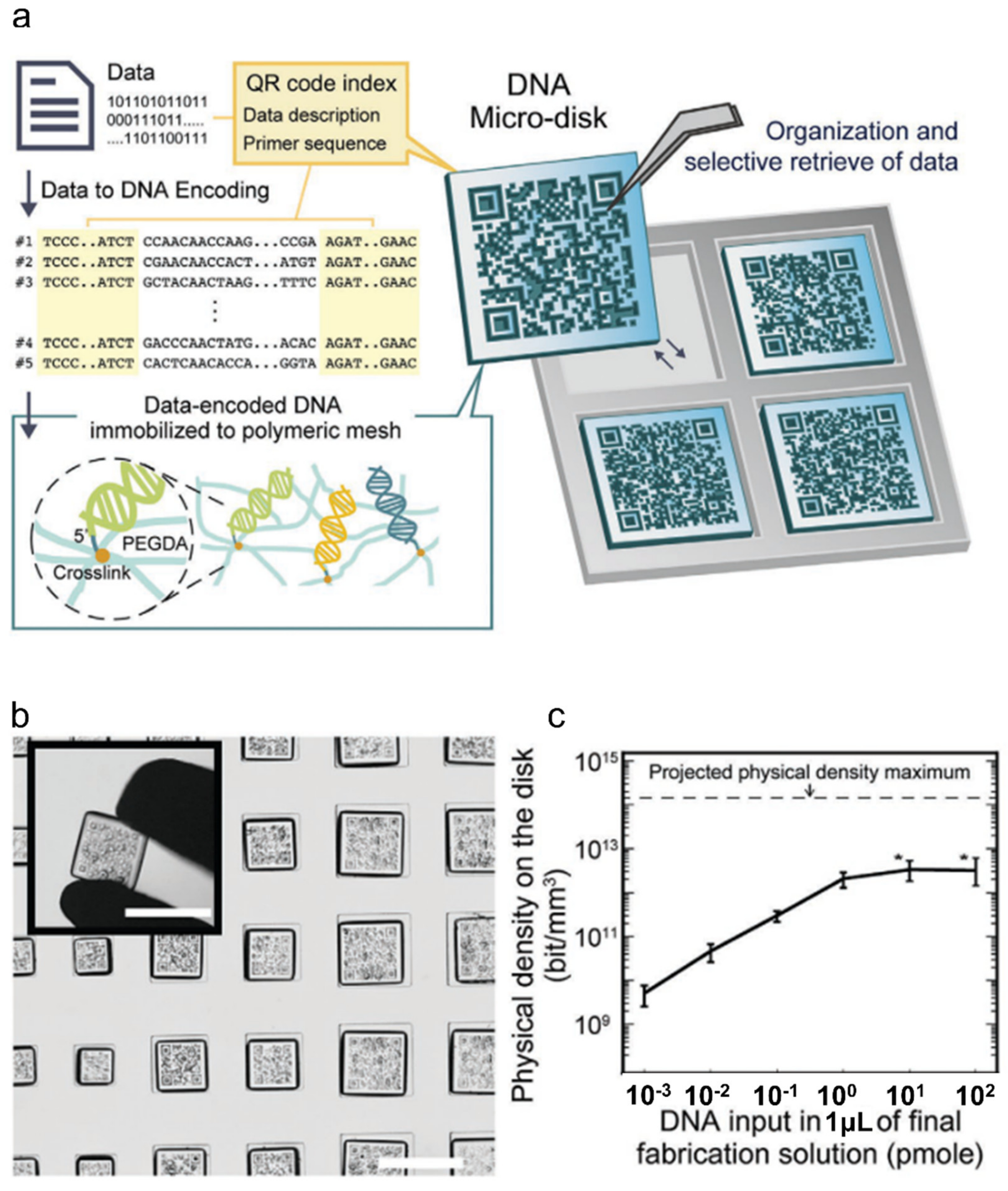
2.4. Recent Highlight: DNA Data Storage
3. Protein Additives
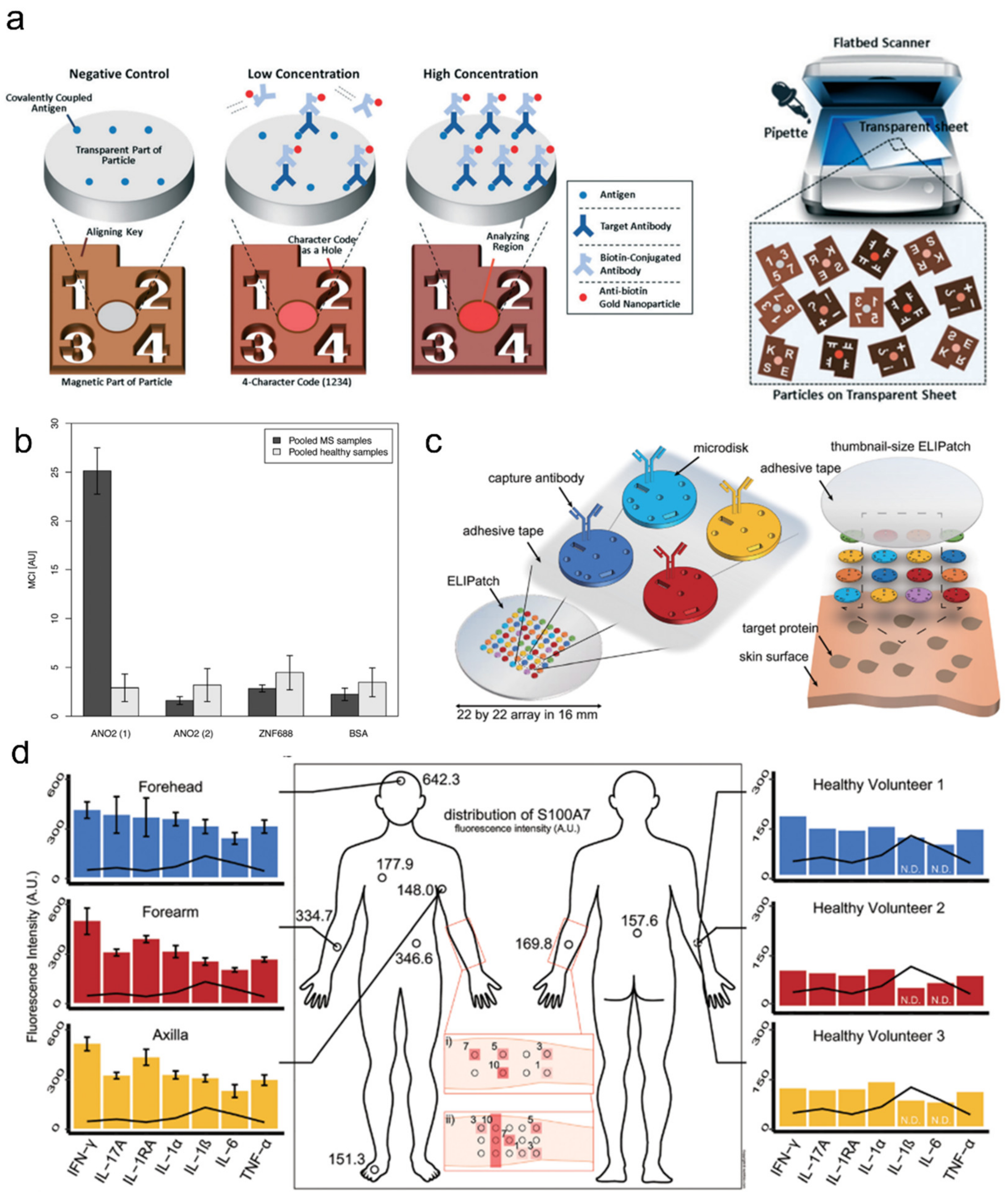
3.1. Antibody-Laden Hydrogels for Multiplexed Immunoassays
3.2. Peptide/Enzyme-Laden Hydrogels for Bioactive Implants
3.3. Hydrogel-Based Artificial Cells
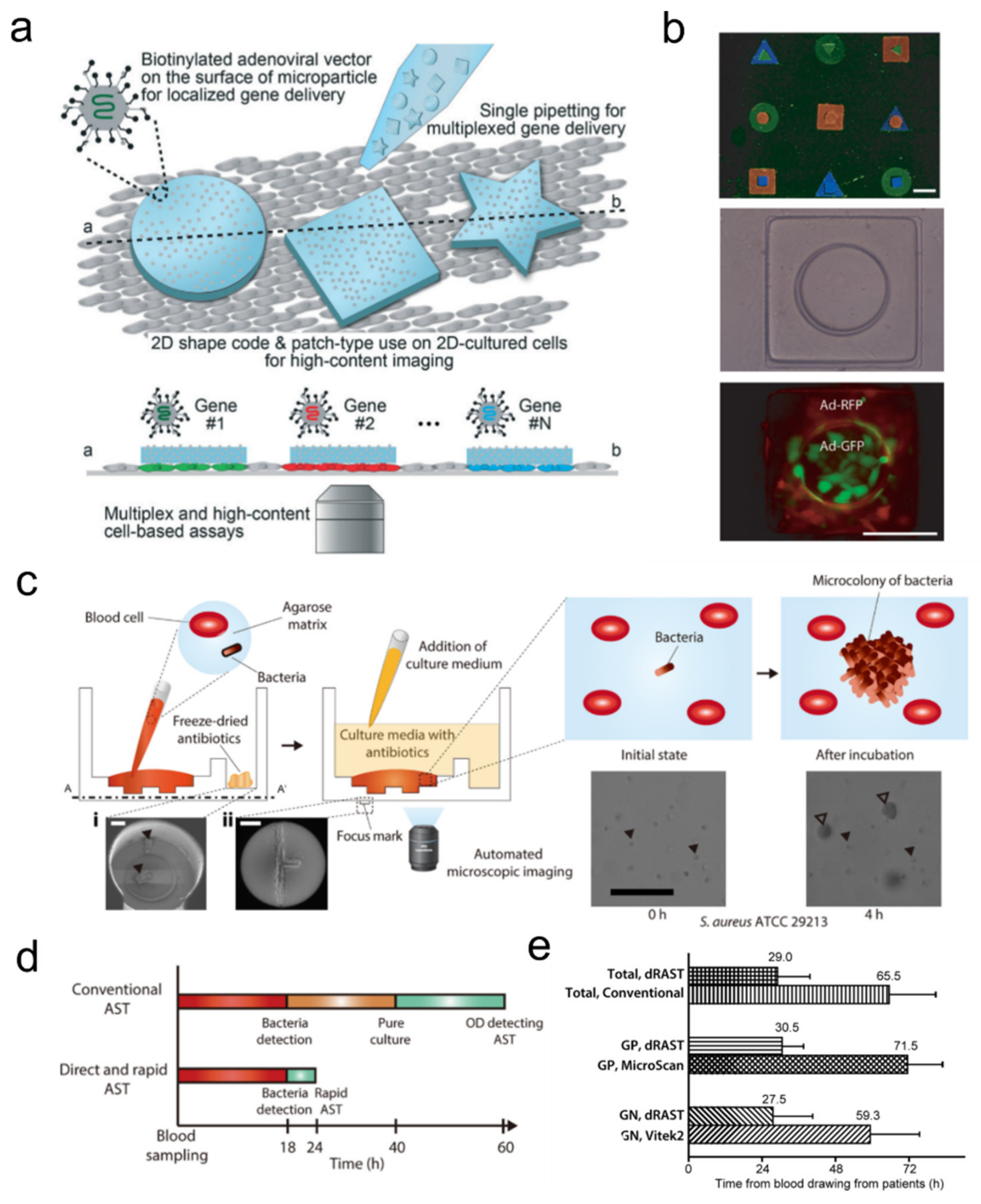
3.4. Systems for Detecting Pathogenic Cells and Molecular Diagnostics
4. Cell Additives
4.1. Mammalian-Cell Laden Hydrogels for Tissue Engineering
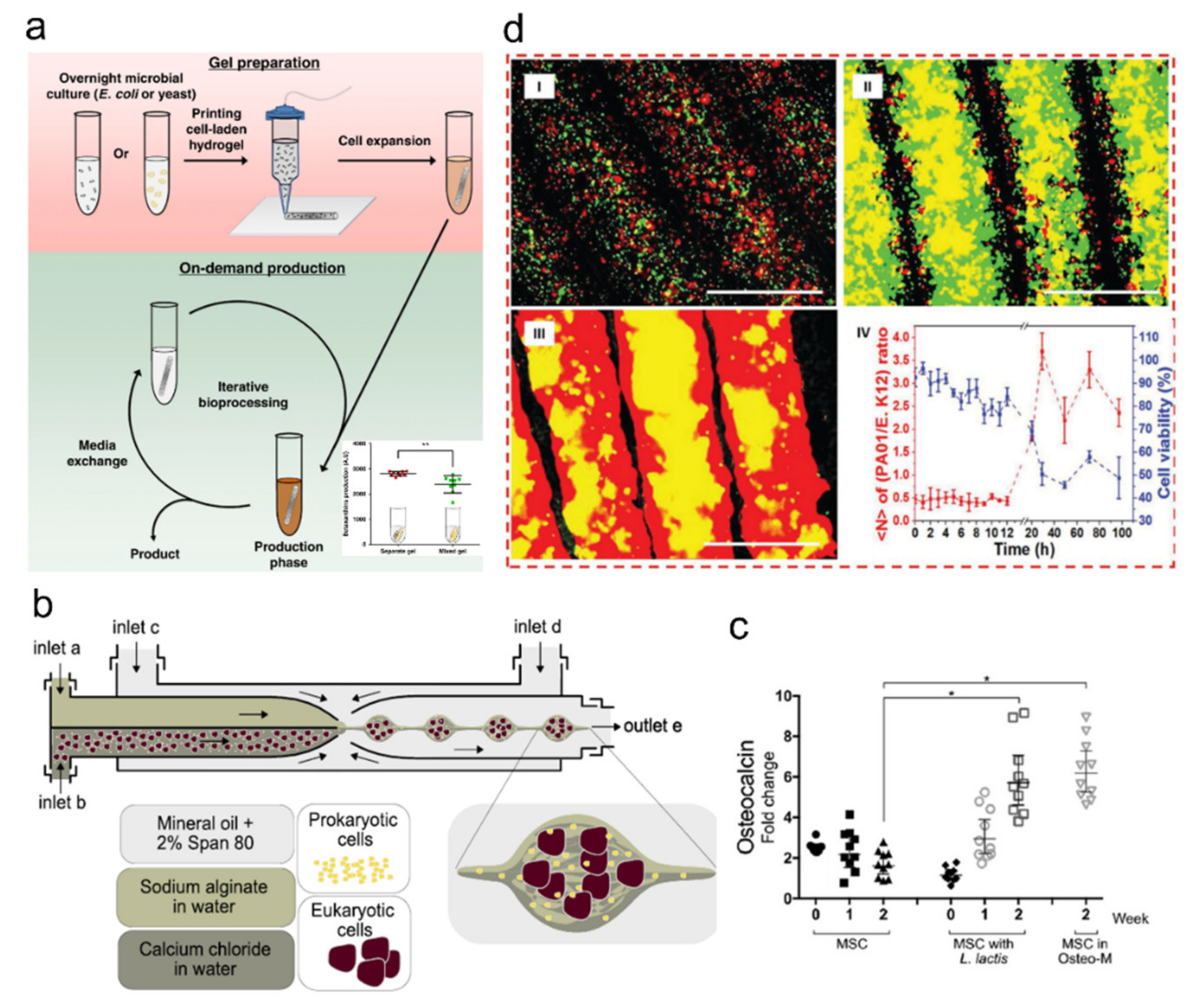
4.2. Microorganism-Laden Hydrogel-Based Synthetic Microbiota
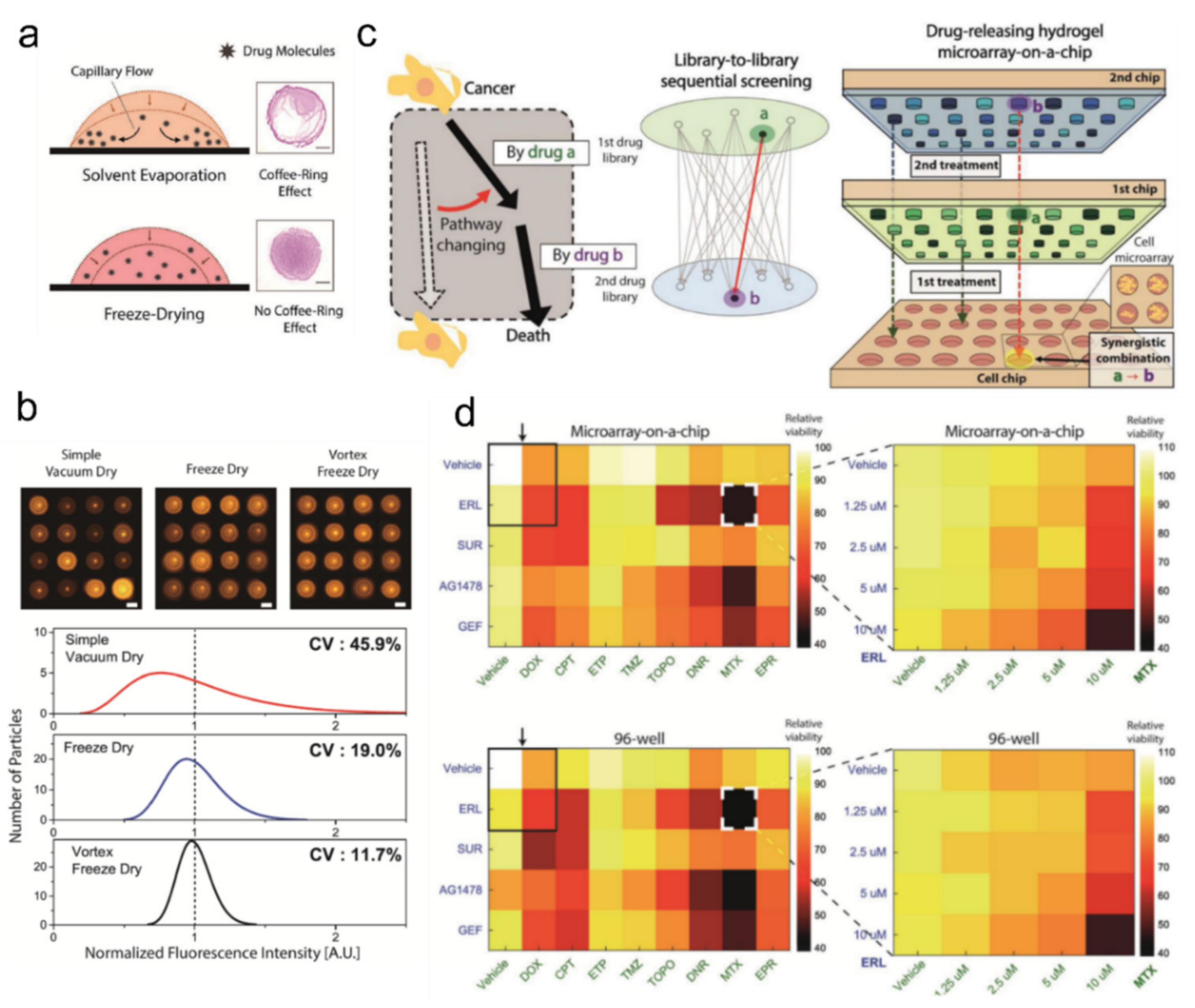
4.3. Microorganism-Laden Hydrogels for Protein and Antibiotic Drug Screening Platforms
5. Inorganic Additives
5.1. Chemical Reaction-Screening Platform
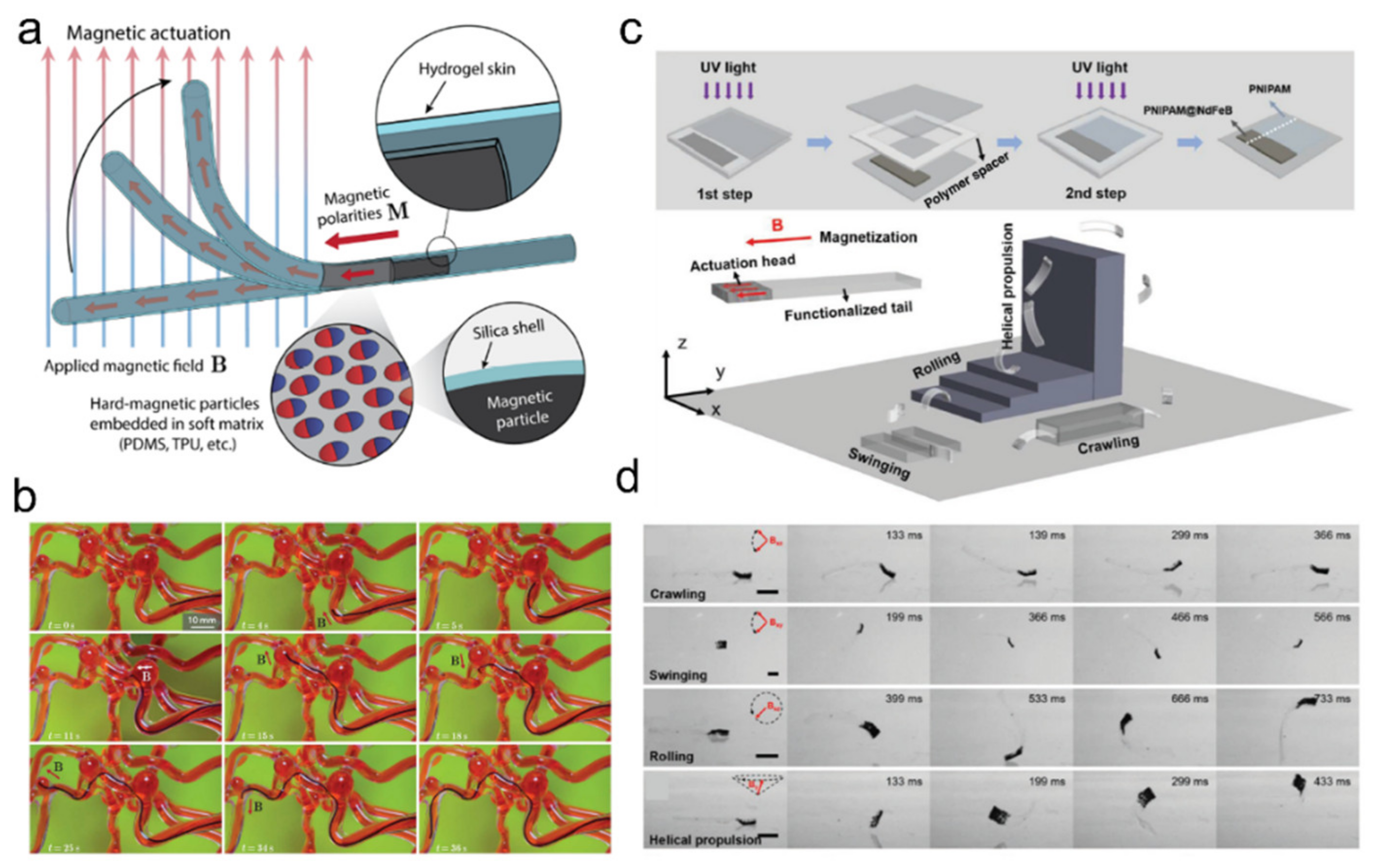
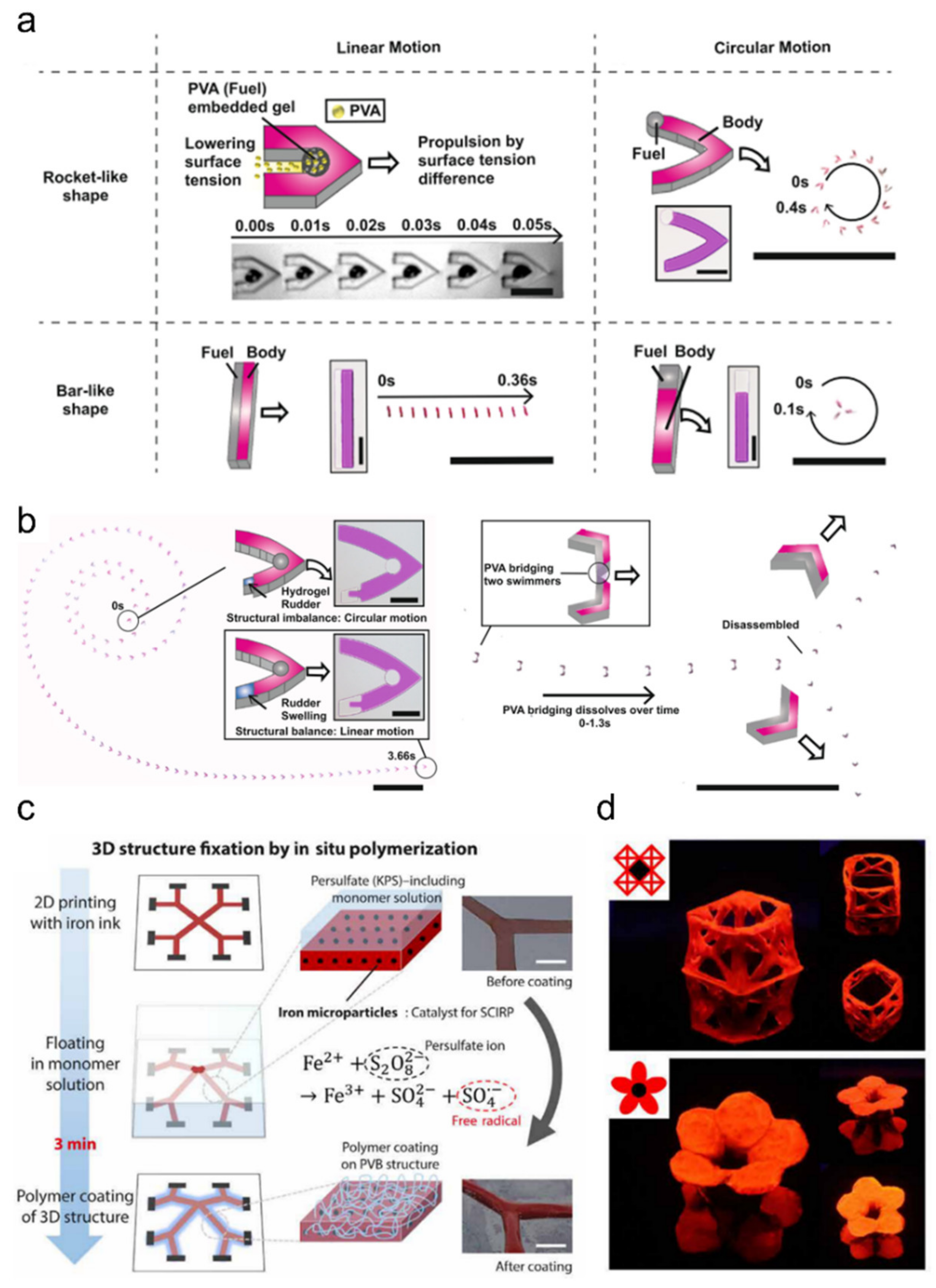
5.2. Soft Robotics
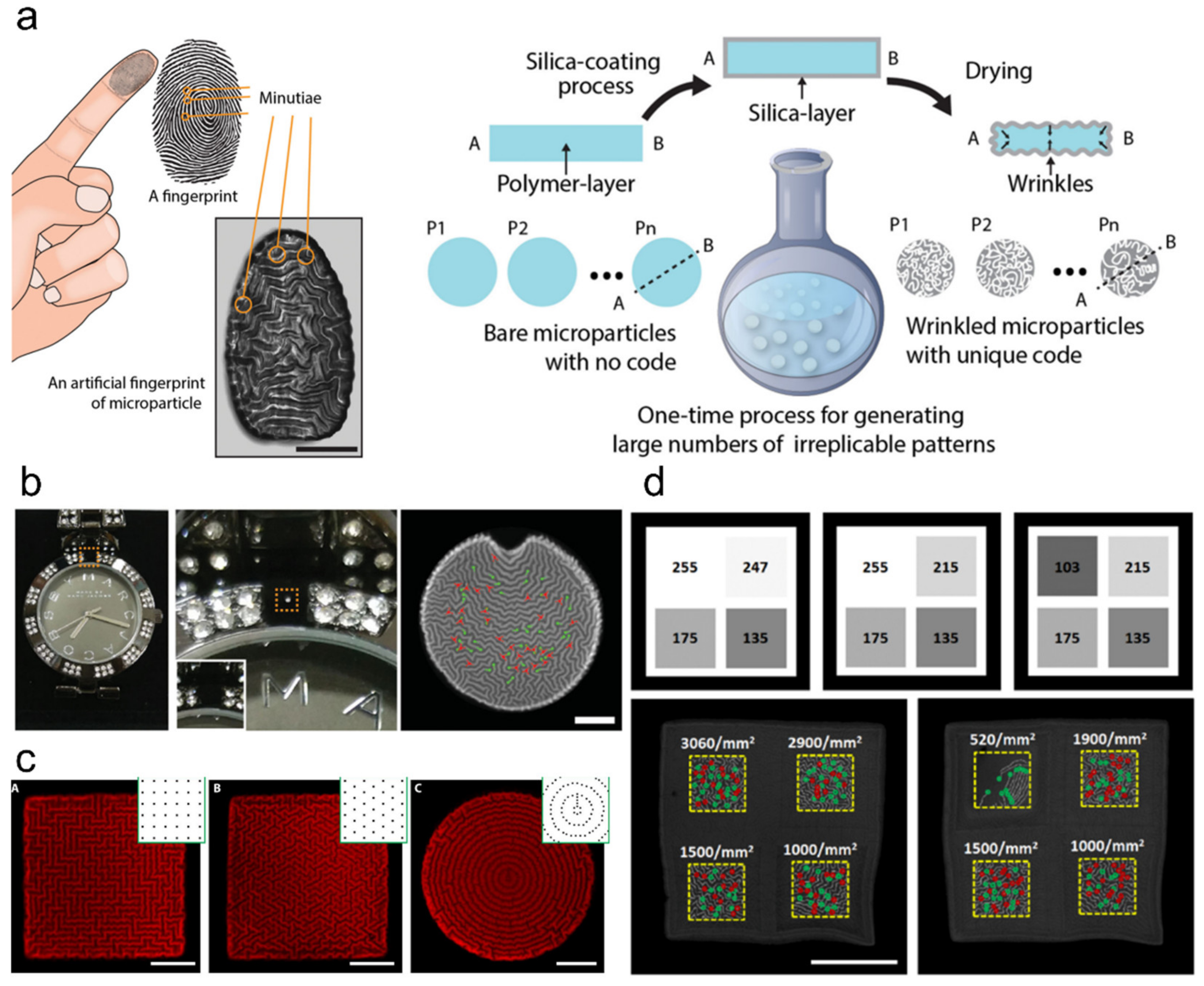
5.3. Encryption and Anti-Counterfeiting
6. Perspective Design and Development
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Mithieux, S.M.; Camci-Unal, G.; Dokmeci, M.R.; Weiss, A.S.; Khademhosseini, A. Elastomeric recombinant protein-based biomaterials. Biochem. Eng. J. 2013, 77, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A. Bioactive Nanoengineered Hydrogels for Bone Tissue Engineering: A Growth-Factor-Free Approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Annabi, N.; Shin, S.R.; Tamayol, A.; Miscuglio, M.; Bakooshli, M.A.; Assmann, A.; Mostafalu, P.; Sun, J.-Y.; Mithieux, S.; Cheung, L.; et al. Highly Elastic and Conductive Human-Based Protein Hybrid Hydrogels. Adv. Mater. 2016, 28, 40–49. [Google Scholar] [CrossRef]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.-C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid self-healing hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [Green Version]
- Gaharwar, A.K.; Avery, R.K.; Assmann, A.; Paul, A.; McKinley, G.H.; Khademhosseini, A.; Olsen, B.D. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano 2014, 8, 9833–9842. [Google Scholar] [CrossRef] [Green Version]
- Pau, A.; Hasan, A.; Al Kindi, H.; Gaharwar, A.K.; Rao, V.T.S.; Nikkhah, M.; Shin, S.R.; Krafft, D.; Dokmeci, M.R.; Shum-Tim, D.; et al. Injectable Graphene Oxide/Hydrogel-Based Angiogenic Gene Delivery System for Vasculogenesis and Cardiac Repair. ACS Nano 2014, 8, 8050–8062. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, I.Y.; You, J.B.; Choi, B.R.; Kim, J.S.; Lee, H.K.; Jang, B.; Jeong, H.S.; Lee, K.; Im, S.G.; Lee, H. A Highly Sensitive Molecular Detection Platform for Robust and Facile Diagnosis of Middle East Respiratory Syndrome (MERS) Corona Virus. Adv. Healthc. Mater. 2016, 5, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Hines, L.; Petersen, K.H.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2017, 29, 1603483. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.-S.; Jin, J.-P.; Wang, J.-Q.; Zhang, Z.-G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis Carmen. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, H.; Lu, H.; Wang, X.; Jin, H. MicroRNAs as Potential Biomarkers in Cancer: Opportunities and Challenges. BioMed Res. Int. 2015, 2015, 125094. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.L.; Choi, C.-H.; Lin, Y.; Lee, C.-S.; Yi, H. Fabrication of Uniform DNA-Conjugated Hydrogel Microparticles via Replica Molding for Facile Nucleic Acid Hybridization Assays. Anal. Chem. 2010, 82, 5851–5858. [Google Scholar] [CrossRef] [Green Version]
- Pregibon, D.C.; Toner, M.; Doyle, P.S. Multifunctional Encoded Particles for High-Throughput Biomolecule Analysis. Science 2007, 315, 1393–1396. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, J.; Kim, H.; Kim, J.; Kwon, S. Colour-barcoded magnetic microparticles for multiplexed bioassays. Nat. Mater. 2010, 9, 745–749. [Google Scholar] [CrossRef]
- Le Goff, G.C.; Srinivas, R.L.; Hill, W.A.; Doyle, P.S. Hydrogel microparticles for biosensing. Eur. Polym. J. 2015, 72, 386–412. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, M.B.; Tentori, A.M.; Zhang, W.C.; Slack, F.J.; Doyle, P.S. Nonfouling, Encoded Hydrogel Microparticles for Multiplex MicroRNA Profiling Directly from Formalin-Fixed, Paraffin-Embedded Tissue. Anal. Chem. 2018, 90, 10279–10285. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; He, R.; Zhang, Y.; He, Y.; Wang, C.; Lu, Z.; Liu, Y.; Ju, H. Fluorescence hydrogel array based on interfacial cation exchange amplification for highly sensitive microRNA detection. Anal. Chim. Acta 2019, 1080, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.; Lee, S.-G.; Doyle, P.S. Hydrogel-Based Colorimetric Assay for Multiplexed MicroRNA Detection in a Microfluidic Device. Anal. Chem. 2020, 92, 5750–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Srinivas, R.L.; Gupta, A.; Doyle, P.S. Sensitive and Multiplexed On-chip microRNA Profiling in Oil-Isolated Hydrogel Chambers. Angew. Chem. Int. Ed. 2015, 54, 2477–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, H.; Luan, C.; Liu, Y.; Chen, B.; Zhao, Y. Aptamer-based hydrogel barcodes for the capture and detection of multiple types of pathogenic bacteria. Biosens. Bioelectron. 2018, 100, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mao, Y.; Huang, D.; He, Z.; Yan, J.; Tian, T.; Shi, Y.; Song, Y.; Li, X.; Zhu, Z.; et al. Portable visual quantitative detection of aflatoxin B1 using a target-responsive hydrogel and a distance-readout microfluidic chip. Lab Chip 2016, 16, 3097–3104. [Google Scholar] [CrossRef]
- Abune, L.; Davis, B.; Wang, Y. Aptamer-functionalized hydrogels: An emerging class of biomaterials for protein delivery, cell capture, regenerative medicine, and molecular biosensing. WIREs Nanomed. Nanobiotechnology 2021, 13, e1731. [Google Scholar] [CrossRef]
- Srinivas, R.L.; Chapin, S.C.; Doyle, P.S. Aptamer-Functionalized Microgel Particles for Protein Detection. Anal. Chem. 2011, 83, 9138–9145. [Google Scholar] [CrossRef] [Green Version]
- Shastri, A.; McGregor, L.; Liu, Y.; Harris, V.; Nan, H.; Mujica, M.; Vasquez, Y.; Bhattacharya, A.; Ma, Y.; Aizenberg, M.; et al. An aptamer-functionalized chemomechanically modulated biomolecule catch-and-release system. Nat. Chem. 2015, 7, 447–454. [Google Scholar] [CrossRef]
- Battig, M.; Huang, Y.; Chen, N.; Wang, Y. Aptamer-functionalized superporous hydrogels for sequestration and release of growth factors regulated via molecular recognition. Biomaterials 2014, 35, 8040–8048. [Google Scholar] [CrossRef] [PubMed]
- Khajouei, S.; Ravan, H.; Ebrahimi, A. DNA hydrogel-empowered biosensing. Adv. Colloid Interface Sci. 2020, 275, 102060. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Nam, D.; Lee, H.; Shin, S. Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation. Biosens. Bioelectron. 2018, 108, 9–13. [Google Scholar] [CrossRef]
- Kim H soo Abbas, N.; Shin, S. A rapid diagnosis of SARS-CoV-2 using DNA hydrogel formation on microfluidic pores. Biosens. Bioelectron. 2021, 177, 113005. [Google Scholar]
- Cheng, E.; Xing, Y.; Chen, P.; Yang, Y.; Sun, Y.; Zhou, D.; Xu, L.; Fan, Q.; Liu, D. A pH-Triggered, Fast-Responding DNA Hydrogel. Angew. Chem. Int. Ed. 2009, 48, 7660–7663. [Google Scholar] [CrossRef]
- Gawel, K.; Stokke, B.T. Logic swelling response of DNA–polymer hybrid hydrogel. Soft Matter 2011, 7, 4615–4618. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Y.; Ma, Y.; Jia, S.; Gao, M.; Li, J.; Zhang, H.; Xu, D.; Wu, M.; Chen, Y.; et al. Design and Synthesis of Target-Responsive Aptamer-Cross-linked Hydrogel for Visual Quantitative Detection of Ochratoxin A. ACS Appl. Mater. Interfaces 2015, 7, 6982–6990. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.R.; Hammink, R.; Nelissen, F.H.T.; Rowan, A.; Heus, H.A. Biomimetic Stress Sensitive Hydrogel Controlled by DNA Nanoswitches. Biomacromolecules 2017, 18, 3310–3317. [Google Scholar] [CrossRef]
- Fedotova, T.A.; Kolpashchikov, D.M. Liquid-to-gel transition for visual and tactile detection of biological analytes. Chem. Commun. 2017, 53, 12622–12625. [Google Scholar] [CrossRef]
- Liu, S.; Su, W.; Li, Y.; Zhang, L.; Ding, X. Biosensors and Bioelectronics Manufacturing of an electrochemical biosensing platform based on hybrid DNA hydrogel: Taking lung cancer-specific miR-21 as an example. Biosens. Bioelectron. 2018, 103, 1–5. [Google Scholar] [CrossRef]
- Fozooni, T.; Ravan, H.; Sasan, H. Signal Amplification Technologies for the Detection of Nucleic Acids: From Cell-Free Analysis to Live-Cell Imaging. Appl. Biochem. Biotechnol. 2017, 183, 1224–1253. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mao, Y.; An, Y.; Tian, T.; Zhang, H.; Yan, J.; Zhu, Z.; Yang, C.J. Target-responsive DNA hydrogel for non-enzymatic and visual detection of glucose. Anal. 2018, 143, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, R.; Kelso, L.C.; Ying, Y.; Li, Y. A target-responsive and size-dependent hydrogel aptasensor embedded with QD fluorescent reporters for rapid detection of avian influenza virus H5N. Sensors Actuators B Chem. 2016, 234, 98–108. [Google Scholar] [CrossRef]
- Yang, Y. Cancer Immunotherapy: Harnessing the Immune System to Battle Cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [Green Version]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Kwong, B.; Gai, S.A.; Elkhader, J.; Wittrup, K.D.; Irvine, D.J. Localized Immunotherapy via Liposome-Anchored Anti-CD137 + IL-2 Prevents Lethal Toxicity and Elicits Local and Systemic Antitumor Immunity. Cancer Res. 2013, 73, 1547–1558. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, Z.; Lai, W.-F.; Cui, L.; Zhu, X. How to overcome the side effects of tumor immunotherapy. Biomed. Pharmacother. 2020, 130, 110639. [Google Scholar] [CrossRef]
- Lei, K.; Tang, L. Surgery-free injectable macroscale biomaterials for local cancer immunotherapy. Biomater. Sci. 2019, 7, 733–749. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Thambi, T.; Yin, Y.; Kim, S.H.; Nguyen, T.L.; Phan, V.G.; Kim, J.; Jeong, J.H.; Lee, D.S. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials 2020, 230, 119599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202, Erratum: Nat. Rev. Drug Discov. 2017, 16, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Le, Q.-V.; Yang, G.; Oh, Y.-K. Cas9-edited immune checkpoint blockade PD-1 DNA polyaptamer hydrogel for cancer immunotherapy. Biomaterials 2019, 218, 119359. [Google Scholar] [CrossRef] [PubMed]
- Zhirnov, V.; Zadegan, R.; Sandhu, G.S.; Church, G.; Hughes, W.L. Nucleic acid memory. Nat. Mater. 2016, 15, 366–370. [Google Scholar] [CrossRef]
- Choi, Y.; Ryu, T.; Lee, A.C.; Choi, H.; Lee, H.; Park, J.; Song, S.-H.; Kim, S.; Kim, H.; Park, W.; et al. High information capacity DNA-based data storage with augmented encoding characters using degenerate bases. Sci. Rep. 2019, 9, 6582. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Choi, Y.; Choi, J.; Lee, A.C.; Yeom, H.; Hyun, J.; Ryu, T.; Kwon, S. Purification of multiplex oligonucleotide libraries by synthesis and selection. Nat. Biotechnol. 2021, 40, 47–53. [Google Scholar] [CrossRef]
- Choi, Y.; Bae, H.J.; Lee, A.C.; Choi, H.; Lee, D.; Ryu, T.; Hyun, J.; Kim, S.; Kim, H.; Song, S.-H.; et al. DNA Micro-Disks for the Management of DNA-Based Data Storage with Index and Write-Once–Read-Many (WORM) Memory Features. Adv. Mater. 2020, 32, 2001249. [Google Scholar] [CrossRef]
- Elshal, M.F.; McCoy, J.P. Multiplex Bead Array Assays: Performance Evaluation and Comparison of Sensitivity to ELISA. Methods 2006, 38, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Appleyard, D.C.; Chapin, S.C.; Doyle, P.S. Multiplexed Protein Quantification with Barcoded Hydrogel Microparticles. Anal. Chem. 2011, 83, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, H.J.; Koh, W.-G. Multiplex Immunoassay Platforms Based on Shape-Coded Poly(ethylene glycol) Hydrogel Microparticles Incorporating Acrylic Acid. Sensors 2012, 12, 8426–8436. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Roh, Y.H.; Kim, H.U.; Kim, S.M.; Bong, K.W. Multiplexed immunoassay using post-synthesis functionalized hydrogel microparticles. Lab Chip 2019, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2019, 5, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.N.; Kim, M.; Jung, K.; Bae, H.J.; Jang, J.; Jung, Y.; Kim, J.; Kwon, S. Shape-encoded silica microparticles for multiplexed bioassays. Chem. Commun. 2015, 51, 12130–12133. [Google Scholar] [CrossRef]
- Appleyard, D.C.; Chapin, S.C.; Srinivas, R.L.; Doyle, P.S. Bar-coded hydrogel microparticles for protein detection: Synthesis, assay and scanning. Nat. Protoc. 2011, 6, 1761–1774. [Google Scholar] [CrossRef]
- Cook, D.B.; McLucas, B.C.; Montoya, L.A.; Brotski, C.M.; Das, S.; Miholits, M.; Sebata, T.H. Multiplexing protein and gene level measurements on a single Luminex platform. Methods 2019, 158, 27–32. [Google Scholar] [CrossRef]
- Svedberg, G.; Jeong, Y.; Na, H.; Jang, J.; Nilsson, P.; Kwon, S.; Gantelius, J.; Svahn, H.A. Towards encoded particles for highly multiplexed colorimetric point of care autoantibody detection. Lab Chip 2017, 17, 549–556. [Google Scholar] [CrossRef]
- Oh, D.Y.; Na, H.; Song, S.W.; Kim, J.; In, H.; Lee, A.C.; Jeong, Y.; Lee, D.; Jang, J.; Kwon, S. ELIPatch, a thumbnail-size patch with immunospot array for multiplexed protein detection from human skin surface. Biomicrofluidics 2018, 12, 031101. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q. Enzyme-Laden Bioactive Hydrogel for Biocatalytic Monitoring and Regulation. Accounts Chem. Res. 2021, 54, 1274–1287. [Google Scholar] [CrossRef]
- Wang, Y.; Shah, V.; Lu, A.; Pachler, E.; Cheng, B.; Di Carlo, D. Counting of enzymatically amplified affinity reactions in hydrogel particle-templated drops. Lab Chip 2021, 21, 3438–3448. [Google Scholar] [CrossRef]
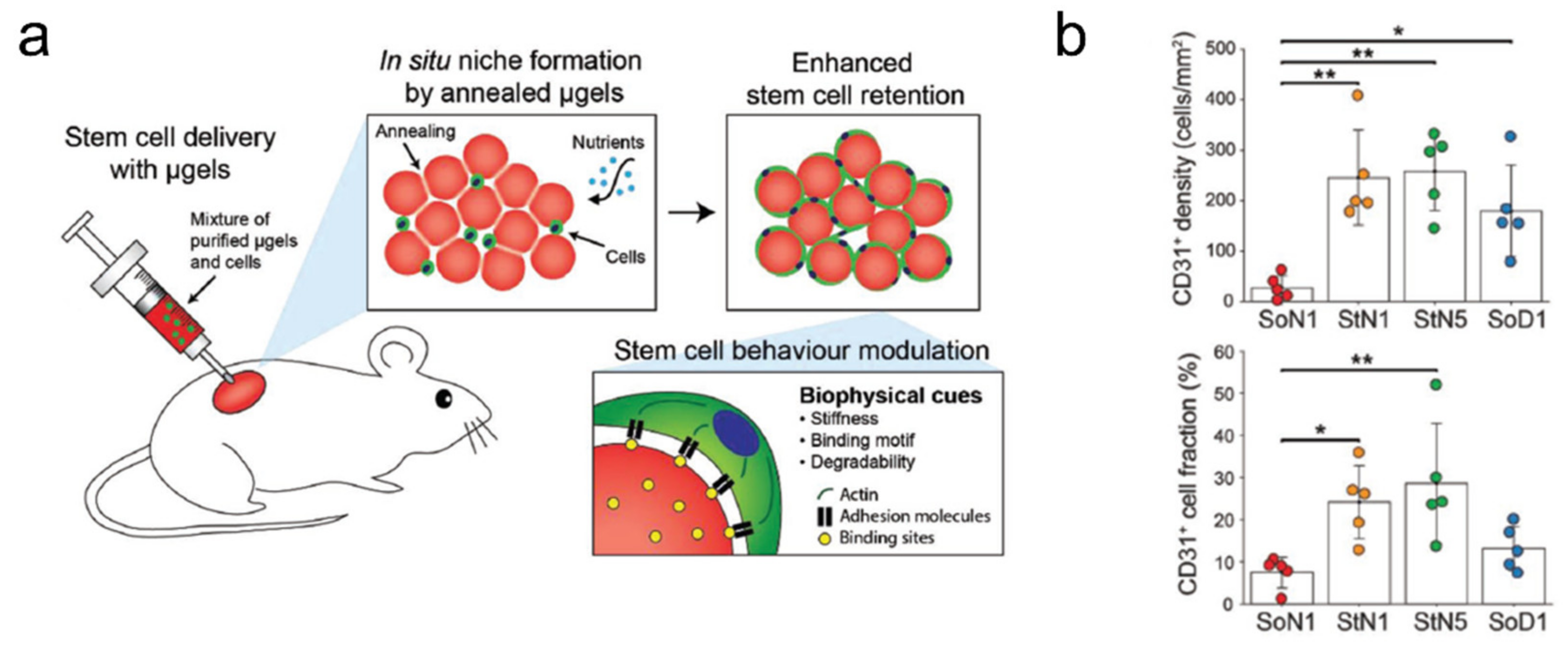
- Koh, J.; Griffin, D.R.; Archang, M.M.; Feng, A.-C.; Horn, T.; Margolis, M.; Zalazar, D.; Segura, T.; Scumpia, P.O.; Di Carlo, D. Enhanced In Vivo Delivery of Stem Cells using Microporous Annealed Particle Scaffolds. Small 2019, 15, 1903147. [Google Scholar] [CrossRef]
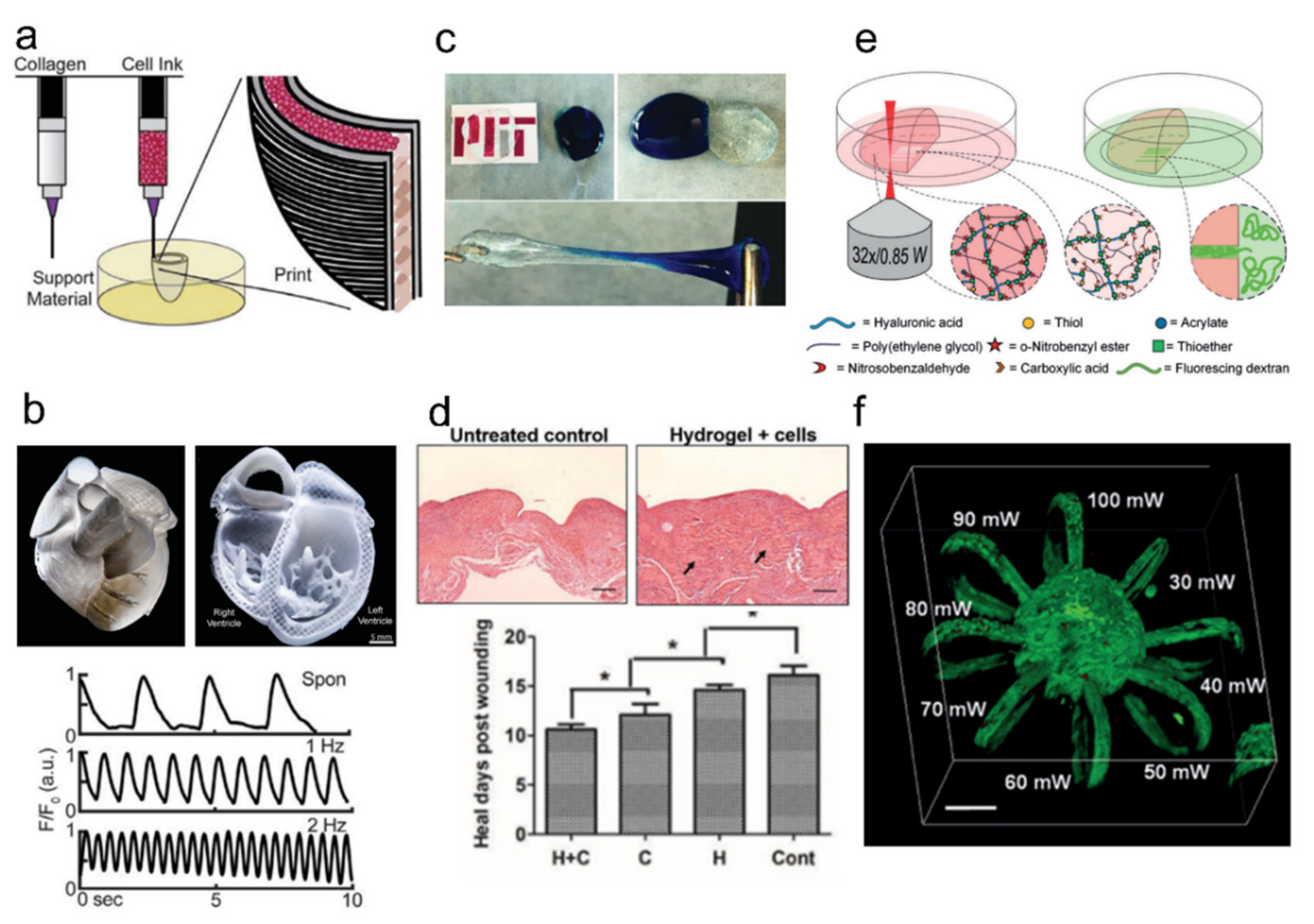
- Griffin, D.R.; Archang, M.M.; Kuan, C.-H.; Weaver, W.M.; Weinstein, J.S.; Feng, A.C.; Ruccia, A.; Sideris, E.; Ragkousis, V.; Koh, J.; et al. Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat. Mater. 2020, 20, 560–569. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, B.; Shen, H.; Qi, M.; Shang, Y.; Wu, C.; Zhu, R.; Cheng, L.; Wang, Q. Strength-tunable printing of xanthan gum hydrogel via enzymatic polymerization and amide bioconjugation. Chem. Commun. 2020, 56, 3457–3460. [Google Scholar] [CrossRef]
- Wei, Q.; Chang, Y.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. One-pot preparation of double network hydrogels via enzyme-mediated polymerization and post-self-assembly for wound healing. J. Mater. Chem. B 2019, 7, 6195–6201. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, W.; Liu, M.; Wu, Q.; Cheng, L.; Wang, Q. Viscosity-controlled printing of supramolecular-polymeric hydrogels via dual-enzyme catalysis. J. Mater. Chem. B 2016, 4, 6302–6306. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, M.; Liao, C.; Wu, Q.; Liu, M.; Zhang, Y.; Wu, C.; Cheng, L.; Wang, Q. Printable hybrid hydrogel by dual enzymatic polymerization with superactivity. Chem. Sci. 2016, 7, 2748–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Duan, J.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. Enzymatic crosslinking to fabricate antioxidant peptide-based supramolecular hydrogel for improving cutaneous wound healing. J. Mater. Chem. B 2019, 7, 2220–2225. [Google Scholar] [CrossRef]
- Ceylan, H.; Urel, M.; Erkal, T.S.; Tekinay, A.B.; Dana, A.; Guler, M.O. Mussel Inspired Dynamic Cross-Linking of Self-Healing Peptide Nanofiber Network. Adv. Funct. Mater. 2013, 23, 2081–2090. [Google Scholar] [CrossRef]
- Zhang, Q.; Lv, Y.; Liu, M.; Wang, X.; Mi, Y.; Wang, Q. Nanoinitiator for enzymatic anaerobic polymerization and graft enhancement of gelatin–PAAM hydrogel. J. Mater. Chem. B 2018, 6, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Tang, Z.; He, H.; Li, W.; Wang, X.; Liao, C.; Sun, Y.; Wang, Q. Glucose oxidase triggers gelation of N-hydroxyimide–heparin conjugates to form enzyme-responsive hydrogels for cell-specific drug delivery. Chem. Sci. 2014, 5, 4204–4209. [Google Scholar] [CrossRef]
- Wu, Q.; He, Z.; Wang, X.; Zhang, Q.; Wei, Q.; Ma, S.; Ma, C.; Li, J.; Wang, Q. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 2019, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Pan, H.; Shen, H.; Xia, X.; Wu, C.; Han, X.; He, X.; Tong, W.; Wang, X.; Wang, Q. Nanogel Multienzyme Mimics Synthesized by Biocatalytic ATRP and Metal Coordination for Bioresponsive Fluorescence Imaging. Angew. Chem. 2020, 132, 11846–11851. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Z.; Zhang, H.; Zhu, R.; Wang, S.; Wang, Q. Fe3O4@nanogel via UOx/HRP initiated surface polymerization for pH sensitive drug delivery. RSC Adv. 2016, 6, 53170–53174. [Google Scholar] [CrossRef]
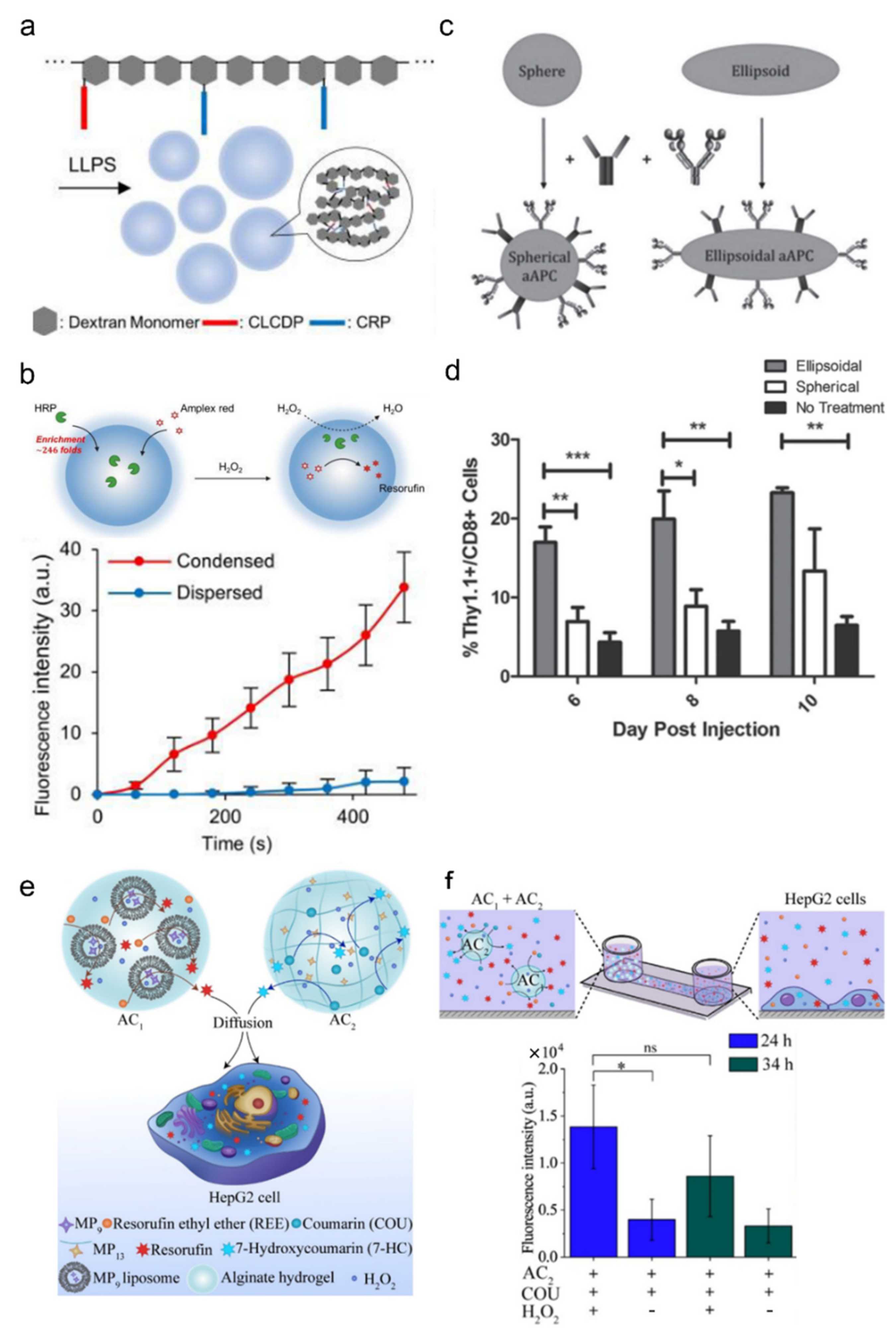
- Cho, E.; Lu, Y. Compartmentalizing Cell-Free Systems: Toward Creating Life-Like Artificial Cells and Beyond. ACS Synth. Biol. 2020, 9, 2881–2901. [Google Scholar] [CrossRef]
- Matsuura, T.; Tanimura, N.; Hosoda, K.; Yomo, T.; Shimizu, Y. Reaction dynamics analysis of a reconstituted Escherichia coli protein translation system by computational modeling. Proc. Natl. Acad. Sci. USA 2017, 114, E1336–E1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, R.J.; Hyman, A.A. Controlling compartmentalization by non-membrane-bound organelles. Philos. Trans. R. Soc. B: Biol. Sci. 2018, 373, 20170193. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Julicher, F.; Hyman, A.A. Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Han, T.W.; Kato, M.; Xie, S.; Wu, L.C.; Mirzaei, H.; Pei, J.; Chen, M.; Xie, Y.; Allen, J.; Xiao, G.; et al. Cell-free Formation of RNA Granules: Bound RNAs Identify Features and Components of Cellular Assemblies. Cell 2012, 149, 768–779. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Banjade, S.; Cheng, H.-C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nat. 2012, 483, 336–340. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Nakamura, H.; Lee, A.A.; Afshar, A.S.; Watanabe, S.; Rho, E.; Razavi, S.; Suarez, A.; Lin, Y.-C.; Tanigawa, M.; Huang, B.; et al. Intracellular production of hydrogels and synthetic RNA granules by multivalent molecular interactions. Nat. Mater. 2017, 17, 79–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, M.; Yoshii, T.; Ikuta, M.; Tsukiji, S. Synthetic Protein Condensates That Inducibly Recruit and Release Protein Activity in Living Cells. J. Am. Chem. Soc. 2021, 143, 6434–6446. [Google Scholar] [CrossRef] [PubMed]
- Reinkemeier, C.D.; Lemke, E.A. Synthetic biomolecular condensates to engineer eukaryotic cells. Curr. Opin. Chem. Biol. 2021, 64, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhorabek, F.; Dai, X.; Huang, J.; Chau, Y. Minimalist design of polymer-oligopeptide hybrid as intrinsically disordered protein-mimicking scaffold for artificial membraneless organelle. arXiv 2021, arXiv:2103.15541. [Google Scholar]
- Meyer, R.A.; Sunshine, J.; Perica, K.; Kosmides, A.K.; Aje, K.; Schneck, J.P.; Green, J.J. Biodegradable Nanoellipsoidal Artificial Antigen Presenting Cells for Antigen Specific T-Cell Activation. Small 2015, 11, 1519–1525. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Westensee, I.N.; Fernandes, C.C.; Städler, B. Enzyme Mimic Facilitated Artificial Cell to Mammalian Cell Signal Transfer. Angew. Chem. Int. Ed. 2021, 60, 18704–18711. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.E.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
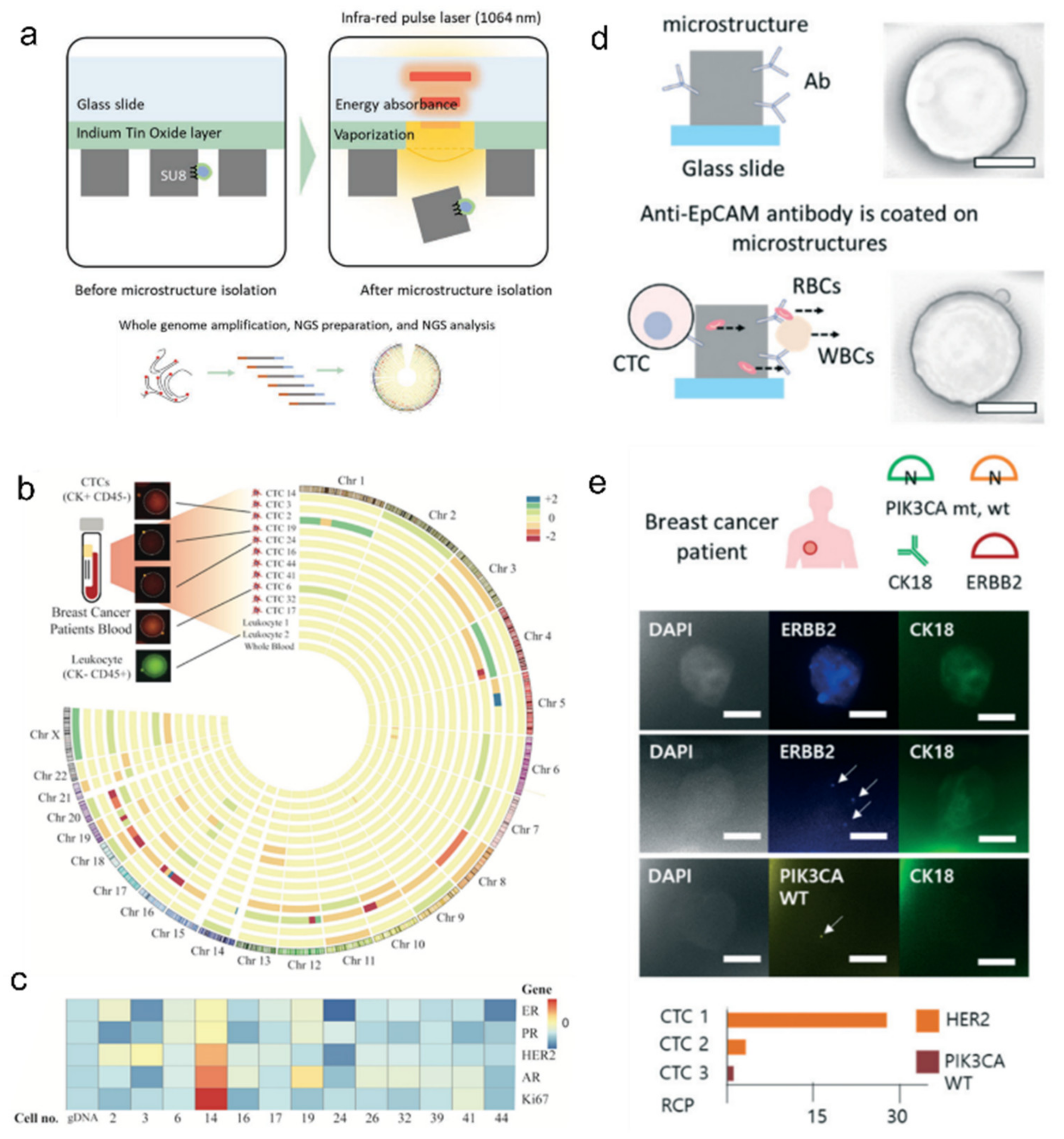
- Kim, O.; Lee, D.; Lee, A.C.; Lee, Y.; Bae, H.J.; Lee, H.-B.; Kim, R.N.; Han, W.; Kwon, S. Whole Genome Sequencing of Single Circulating Tumor Cells Isolated by Applying a Pulsed Laser to Cell-Capturing Microstructures. Small 2019, 15, e1902607. [Google Scholar] [CrossRef]
- Lee, A.C.; Svedlund, J.; Darai, E.; Lee, Y.; Lee, D.; Lee, H.-B.; Kim, S.-M.; Kim, O.; Bae, H.J.; Choi, A.; et al. OPENchip: An on-chip in situ molecular profiling platform for gene expression analysis and oncogenic mutation detection in single circulating tumour cells. Lab Chip 2020, 20, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Kim, S.; Han, J.; Ha, S.; Lee, H.; Song, S.W.; Lee, D.; Kwon, S.; Chung, J.; Kim, J. A High-Throughput Single-Clone Phage Fluorescence Microwell Immunoassay and Laser-Driven Clonal Retrieval System. Biomolecules 2020, 10, 517. [Google Scholar] [CrossRef] [Green Version]
- Shafagati, N.; Patanarut, A.; Luchini, A.; Lundberg, L.; Bailey, C.; Petricoin, E.; Liotta, L.; Narayanan, A.; Lepene, B.; Kehn-Hall, K. The use of Nanotrap particles for biodefense and emerging infectious disease diagnostics. Pathog. Dis. 2014, 71, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, R.A.; Akhrymuk, I.; Patnaik, A.; Callahan, V.; Lehman, C.; Andersen, P.; Barbero, R.; Barksdale, S.; Dunlap, R.; Goldfarb, D.; et al. Hydrogel particles improve detection of SARS-CoV-2 RNA from multiple sample types. Sci. Rep. 2020, 10, 22425. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Saroia, J.; Yanen, W.; Wei, Q.; Zhang, K.; Lu, T.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef]
- Rimann, M.; Bono, E.; Annaheim, H.; Bleisch, M.; Graf-Hausner, U. Standardized 3D Bioprinting of Soft Tissue Models with Human Primary Cells. J. Lab. Autom. 2016, 21, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef] [PubMed]
- Tijore, A.; Irvine, S.; Sarig, U.; Mhaisalkar, P.; Baisane, V.; Venkatraman, S.S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 2017, 10, 025003. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Health Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Willerth, S.M. 3-D Bioprinting of Neural Tissue for Applications in Cell Therapy and Drug Screening. Front. Bioeng. Biotechnol. 2017, 5, 69. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Rodrigues, M.; Li, X.; Kwon, S.H.; Kosaric, N.; Khong, S.; Gao, Y.; Wang, W.; Gurtner, G.C. Injectable and Tunable Gelatin Hydrogels Enhance Stem Cell Retention and Improve Cutaneous Wound Healing. Adv. Funct. Mater. 2017, 27, 1606619. [Google Scholar] [CrossRef]
- Lunzer, M.; Shi, L.; Andriotis, O.G.; Gruber, P.; Markovic, M.; Thurner, P.J.; Ossipov, D.; Liska, R.; Ovsianikov, A. A Modular Approach to Sensitized Two-Photon Patterning of Photodegradable Hydrogels. Angew. Chem. Int. Ed. 2018, 57, 15122–15127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornwell, D.J.; Smith, D.K. Expanding the scope of gels—Combining polymers with low-molecular-weight gelators to yield modified self-assembling smart materials with high-tech applications. Mater. Horizons 2015, 2, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, H.; Kinoshita, T.; Zhang, S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005, 102, 8414–8419. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.J.; Parkins, C.C.; McAbee, J.H.; Kolb, A.K.; Hein, R.; Loh, X.J.; Watts, C.; Scherman, O.A. An adherent tissue-inspired hydrogel delivery vehicle utilised in primary human glioma models. Biomaterials 2018, 179, 199–208. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Ko, D.Y.; Patel, M.; Lee, H.J.; Jeong, B. Coordinating Thermogel for Stem Cell Spheroids and Their Cyto-Effectiveness. Adv. Funct. Mater. 2018, 28, 1706286. [Google Scholar] [CrossRef]
- Madl, C.M.; LeSavage, B.L.; Dewi, R.E.; Dinh, C.B.; Stowers, R.S.; Khariton, M.; Lampe, K.J.; Nguyen, D.; Chaudhuri, O.; Enejder, A.; et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017, 16, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Tam, R.Y.; Smith, L.J.; Shoichet, M.S. Engineering Cellular Microenvironments with Photo- and Enzymatically Responsive Hydrogels: Toward Biomimetic 3D Cell Culture Models. Accounts Chem. Res. 2017, 50, 703–713. [Google Scholar] [CrossRef]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef]
- Lee, H.-P.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017, 16, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Mauck, R.L.; Burdick, J.A. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 2019, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.; Courchesne, N.-M.D.; Duraj-Thatte, A.; Praveschotinunt, P.; Joshi, N.S. Engineered Living Materials: Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials. Adv. Mater. 2018, 30, 1704847. [Google Scholar] [CrossRef] [PubMed]
- Santos-Lopez, A.; Marshall, C.W.; Scribner, M.R.; Snyder, D.J.; Cooper, V.S. Evolutionary Pathways to Antibiotic Resistance Are Dependent upon Environmental Structure and Bacterial Lifestyle. eLife 2019, 8, e47612. [Google Scholar] [CrossRef] [PubMed]
- Wondraczek, L.; Pohnert, G.; Schacher, F.H.; Köhler, A.; Gottschaldt, M.; Schubert, U.S.; Küsel, K.; Brakhage, A.A. Artificial Microbial Arenas: Materials for Observing and Manipulating Microbial Consortia. Adv. Mater. 2019, 31, e1900284. [Google Scholar] [CrossRef] [Green Version]
- Connell, J.L.; Kim, J.; Shear, J.B.; Bard, A.J.; Whiteley, M. Real-time monitoring of quorum sensing in 3D-printed bacterial aggregates using scanning electrochemical microscopy. Proc. Natl. Acad. Sci. USA 2014, 111, 18255–18260. [Google Scholar] [CrossRef] [Green Version]
- Johnston, T.G.; Yuan, S.-F.; Wagner, J.M.; Yi, X.; Saha, A.; Smith, P.; Nelson, A.; Alper, H.S. Compartmentalized microbes and co-cultures in hydrogels for on-demand bioproduction and preservation. Nat. Commun. 2020, 11, 563. [Google Scholar] [CrossRef]
- Witte, K.; Rodrigo-Navarro, A.; Salmeron-Sanchez, M. Bacteria-laden microgels as autonomous three-dimensional environments for stem cell engineering. Mater. Today Bio 2019, 2, 100011. [Google Scholar] [CrossRef]
- Pajoumshariati, S.R.; Azizi, M.; Zhang, S.; Dogan, B.; Simpson, K.W.; Abbaspourrad, A. A Microfluidic-Based Model for Spatially Constrained Culture of Intestinal Microbiota. Adv. Funct. Mater. 2018, 28, 1805568. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bae, H.J.; Kim, S.D.; Park, W.; Kwon, S. An encoded viral micropatch for multiplex cell-based assays through localized gene delivery. Lab Chip 2017, 17, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, A.C.; Han, S.; Bae, H.J.; Song, S.W.; Jeong, Y.; Oh, D.Y.; Cho, S.; Kim, J.; Park, W.; et al. Hierarchical shape-by-shape assembly of microparticles for micrometer-scale viral delivery of two different genes. Biomicrofluidics 2018, 12, 031102. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Kim, O.; Jung, Y.; Han, H.; Kim, J.-E.; Kim, S.; Lee, S.; Park, J.; Jung, R.H.; Kim, S.I.; et al. High-throughput retrieval of physical DNA for NGS-identifiable clones in phage display library. mAbs 2019, 11, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jung, Y.-G.; Kim, J.; Kim, S.; Jung, Y.; Na, H.; Kwon, S. Rapid antibiotic susceptibility testing by tracking single cell growth in a microfluidic agarose channel system. Lab Chip 2013, 13, 280–287. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, J.; Lee, M.; Kim, E.-G.; Lee, J.S.; Lee, S.; Joo, S.; Song, S.H.; Kim, E.-C.; Lee, J.C.; et al. A rapid antimicrobial susceptibility test based on single-cell morphological analysis. Sci. Transl. Med. 2014, 6, 267ra174. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, J.; Kim, K.-J.; Kim, E.-G.; Park, K.O.; Kim, H.; Kim, H.; Jung, H.; Kim, T.; Choi, M.; et al. Rapid drug susceptibility test of Mycobacterium tuberculosis using microscopic time-lapse imaging in an agarose matrix. Appl. Microbiol. Biotechnol. 2016, 100, 2355–2365. [Google Scholar] [CrossRef]
- Mehrdad, K.; Mohammad, S.; Muhammad, H.Z. PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar]
- Desai, P.N.; Yuan, Q.; Yang, H. Synthesis and Characterization of Photocurable Polyamidoamine Dendrimer Hydrogels as a Versatile Platform for Tissue Engineering and Drug Delivery. Biomacromolecules 2010, 11, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery carrier systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Pardo-Yissar, V.; Gabai, R.; Shipway, A.N.; Bourenko, T.; Willner, I. Gold Nanoparticle/Hydrogel Composites with Solvent-Switchable Electronic Properties. Adv. Mater. 2001, 13, 1320–1323. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Lee, K.; Premkumar, T.; Geckeler, K.E. Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications. Polymer 2007, 48, 158–164. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.W.; Bae, H.J.; Kim, S.; Oh, D.Y.; Kim, O.; Jeong, Y.; Kwon, S. Uniform Drug Loading into Prefabricated Microparticles by Freeze-Drying. Part. Part. Syst. Charact. 2017, 34, 1600427. [Google Scholar] [CrossRef]
- Song, S.W.; Kim, S.D.; Oh, D.Y.; Lee, Y.; Lee, A.C.; Jeong, Y.; Bae, H.J.; Lee, D.; Lee, S.; Kim, J.; et al. One-Step Generation of a Drug-Releasing Hydrogel Microarray-On-A-Chip for Large-Scale Sequential Drug Combination Screening. Adv. Sci. 2019, 6, 1801380. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.E.; Kim, J.; Oh, D.Y.; Song, Y.; Lee, S.H.; Min, S.; Kwon, S. One-step pipetting and assembly of encoded chemical-laden microparticles for high-throughput multiplexed bioassays. Nat. Commun. 2014, 5, 3468. [Google Scholar] [CrossRef] [Green Version]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Genchi, G.G.; Marino, A.; Tapeinos, C.; Ciofani, G. Smart Materials Meet Multifunctional Biomedical Devices: Current and Prospective Implications for Nanomedicine. Front. Bioeng. Biotechnol. 2017, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Pint, C.L.; Lee, M.H.; Schubert, B.E.; Jamshidi, A.; Takei, K.; Ko, H.; Gillies, A.; Bardhan, R.; Urban, J.J.; et al. Optically- and Thermally-Responsive Programmable Materials Based on Carbon Nanotube-Hydrogel Polymer Composites. Nano Lett. 2011, 11, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Liu, M.; Ishida, Y.; Ebina, Y.; Osada, M.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. Thermoresponsive actuation enabled by permittivity switching in an electrostatically anisotropic hydrogel. Nat. Mater. 2015, 14, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhou, Y.; Wu, P. Simultaneous Exfoliation and Functionalization of MoSe2Nanosheets to Prepare “Smart” Nanocomposite Hydrogels with Tunable Dual Stimuli-Responsive Behavior. Small 2016, 12, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, Z.-X.; Zhan, B.; Luo, H.; Li, Y.; Zhu, D. Actuator Based on MWNT/PVA Hydrogels. J. Phys. Chem. B 2005, 109, 14789–14791. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Z.; Chen, C.; Shi, K.; Zhang, L.; Ju, X.-J.; Wang, W.; Xie, R.; Chu, L.-Y. Reduced Graphene Oxide-Containing Smart Hydrogels with Excellent Electro-Response and Mechanical Properties for Soft Actuators. ACS Appl. Mater. Interfaces 2017, 9, 15758–15767. [Google Scholar] [CrossRef]
- Haider, H.; Yang, C.H.; Zheng, W.J.; Yang, J.H.; Wang, M.X.; Yang, S.; Zrínyi, M.; Osada, Y.; Suo, Z.; Zhang, Q.; et al. Exceptionally tough and notch-insensitive magnetic hydrogels. Soft Matter 2015, 11, 8253–8261. [Google Scholar] [CrossRef]
- Kim, J.; Chung, S.E.; Choi, S.E.; Lee, H.; Kim, J.; Kwon, S. Programming magnetic anisotropy in polymeric microactuators. Nat. Mater. 2011, 10, 747–752. [Google Scholar] [CrossRef]
- Kim, Y.; Parada, G.A.; Liu, S.; Zhao, X. Ferromagnetic soft continuum robots. Sci. Robot. 2019, 4, eaax7329. [Google Scholar] [CrossRef]
- Du, X.; Cui, H.; Xu, T.; Huang, C.; Wang, Y.; Zhao, Q.; Xu, Y.; Wu, X. Reconfiguration, Camouflage, and Color-Shifting for Bioinspired Adaptive Hydrogel-Based Millirobots. Adv. Funct. Mater. 2020, 30, 1909202. [Google Scholar] [CrossRef]
- Shen, T.; Font, M.G.; Jung, S.; Gabriel, M.L.; Stoykovich, M.P.; Vernerey, F.J. Remotely Triggered Locomotion of Hydrogel Mag-bots in Confined Spaces. Sci. Rep. 2017, 7, 16178. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Jiang, S.; Sun, Y.; Agarwal, S. Giving Direction to Motion and Surface with Ultra-Fast Speed Using Oriented Hydrogel Fibers. Adv. Funct. Mater. 2016, 26, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Yamauchi, Y.; Araoka, F.; Kim, Y.S.; Bergueiro, J.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hikima, T.; Aida, T. An Anisotropic Hydrogel Actuator Enabling Earthworm-Like Directed Peristaltic Crawling. Angew. Chem. Int. Ed. 2018, 57, 15772–15776. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Park, C.; Lee, A.C.; Bae, J.; Kim, H.; Choi, H.; Song, S.W.; Jeong, Y.; Choi, J.; Lee, H.; et al. Photopatterned microswimmers with programmable motion without external stimuli. Nat. Commun. 2021, 12, 4724. [Google Scholar] [CrossRef] [PubMed]
- Song, S.W.; Lee, S.; Choe, J.K.; Kim, N.-H.; Kang, J.; Lee, A.C.; Choi, Y.; Choi, A.; Jeong, Y.; Lee, W.; et al. Direct 2D-to-3D transformation of pen drawings. Sci. Adv. 2021, 7, eabf3804. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Baek, D.; Lee, T.K.; Kang, D.; Hwang, H.; Go, E.M.; Jeon, I.; You, Y.; Son, C.; Kim, D.; et al. Dynamic multimodal holograms of conjugated organogels via dithering mask lithography. Nat. Mater. 2021, 20, 385–394. [Google Scholar] [CrossRef]
- Han, S.; Bae, H.J.; Kim, J.; Shin, S.; Choi, S.-E.; Lee, S.H.; Kwon, S.; Park, W. Lithographically Encoded Polymer Microtaggant Using High-Capacity and Error-Correctable QR Code for Anti-Counterfeiting of Drugs. Adv. Mater. 2012, 24, 5924–5929. [Google Scholar] [CrossRef]
- Miller, H.I.; Winegarden, W. Fraud in Your Pill Bottle; Pacific Research Institute Publication: Pasadena, CA, USA, 2020; Volume 1, pp. 1–19. [Google Scholar]
- Bae, H.J.; Bae, S.; Park, C.; Han, S.; Kim, J.; Kim, L.N.; Kim, K.; Song, S.-H.; Park, W.; Kwon, S. Biomimetic Microfingerprints for Anti-Counterfeiting Strategies. Adv. Mater. 2015, 27, 2083–2089. [Google Scholar] [CrossRef]
- Bae, H.J.; Bae, S.; Yoon, J.; Park, C.; Kim, K.; Kwon, S.; Park, W. Self-organization of maze-like structures via guided wrinkling. Sci. Adv. 2017, 3, e1700071. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Bae, H.J.; Yoon, J.; Song, S.W.; Jeong, Y.; Kim, K.; Kwon, S.; Park, W. Gradient-Wrinkled Microparticle with Grayscale Lithography Controlling the Cross-Linking Densities for High Security Level Anti-Counterfeiting Strategies. ACS Omega 2021, 6, 2121–2126. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.-W.; Kim, J.; Kwon, S. Recent Advances in Polymer Additive Engineering for Diagnostic and Therapeutic Hydrogels. Int. J. Mol. Sci. 2022, 23, 2955. https://doi.org/10.3390/ijms23062955
Bae S-W, Kim J, Kwon S. Recent Advances in Polymer Additive Engineering for Diagnostic and Therapeutic Hydrogels. International Journal of Molecular Sciences. 2022; 23(6):2955. https://doi.org/10.3390/ijms23062955
Chicago/Turabian StyleBae, Sang-Wook, Jiyun Kim, and Sunghoon Kwon. 2022. "Recent Advances in Polymer Additive Engineering for Diagnostic and Therapeutic Hydrogels" International Journal of Molecular Sciences 23, no. 6: 2955. https://doi.org/10.3390/ijms23062955
APA StyleBae, S.-W., Kim, J., & Kwon, S. (2022). Recent Advances in Polymer Additive Engineering for Diagnostic and Therapeutic Hydrogels. International Journal of Molecular Sciences, 23(6), 2955. https://doi.org/10.3390/ijms23062955





