Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications
Abstract
:1. Introduction
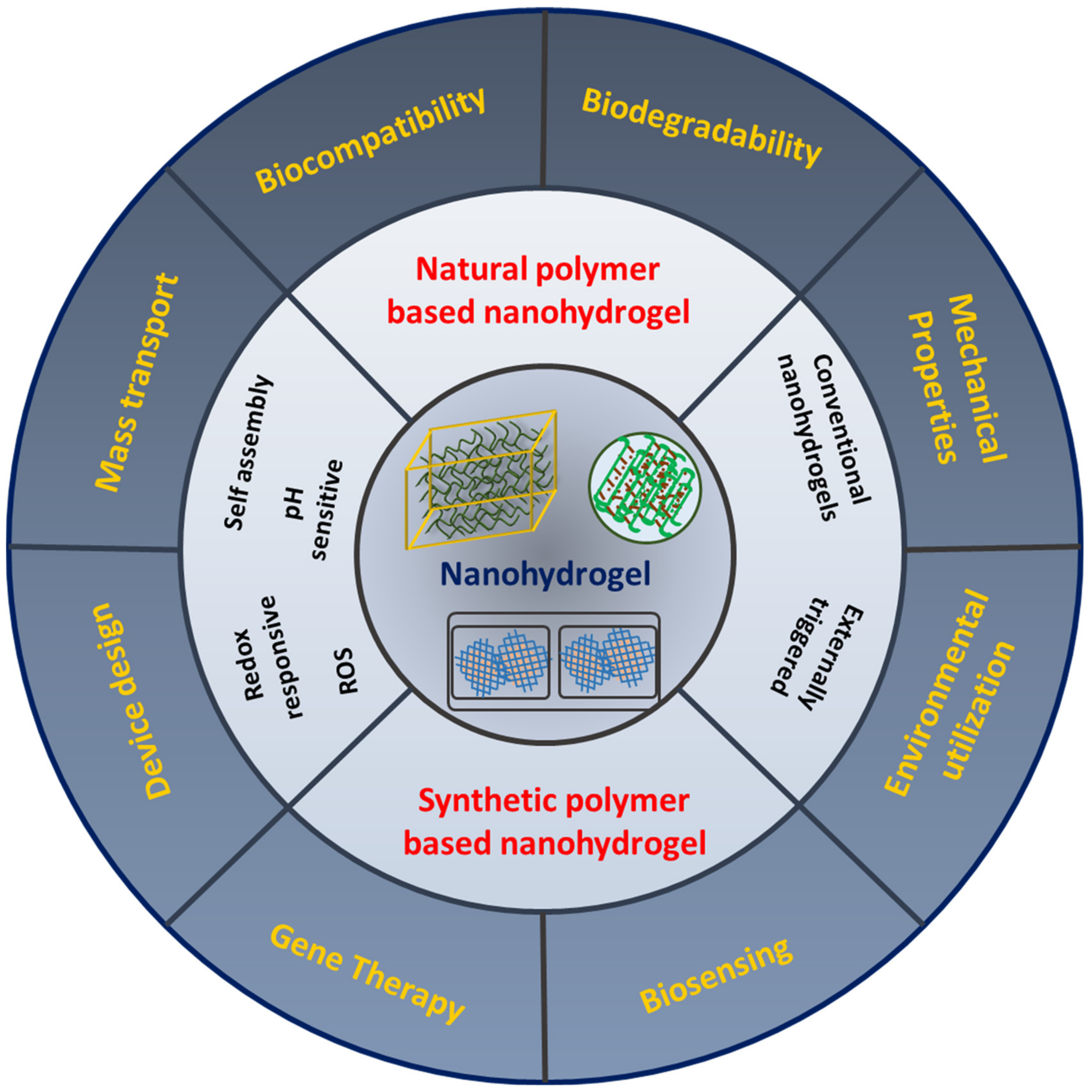
2. Nanohydrogels
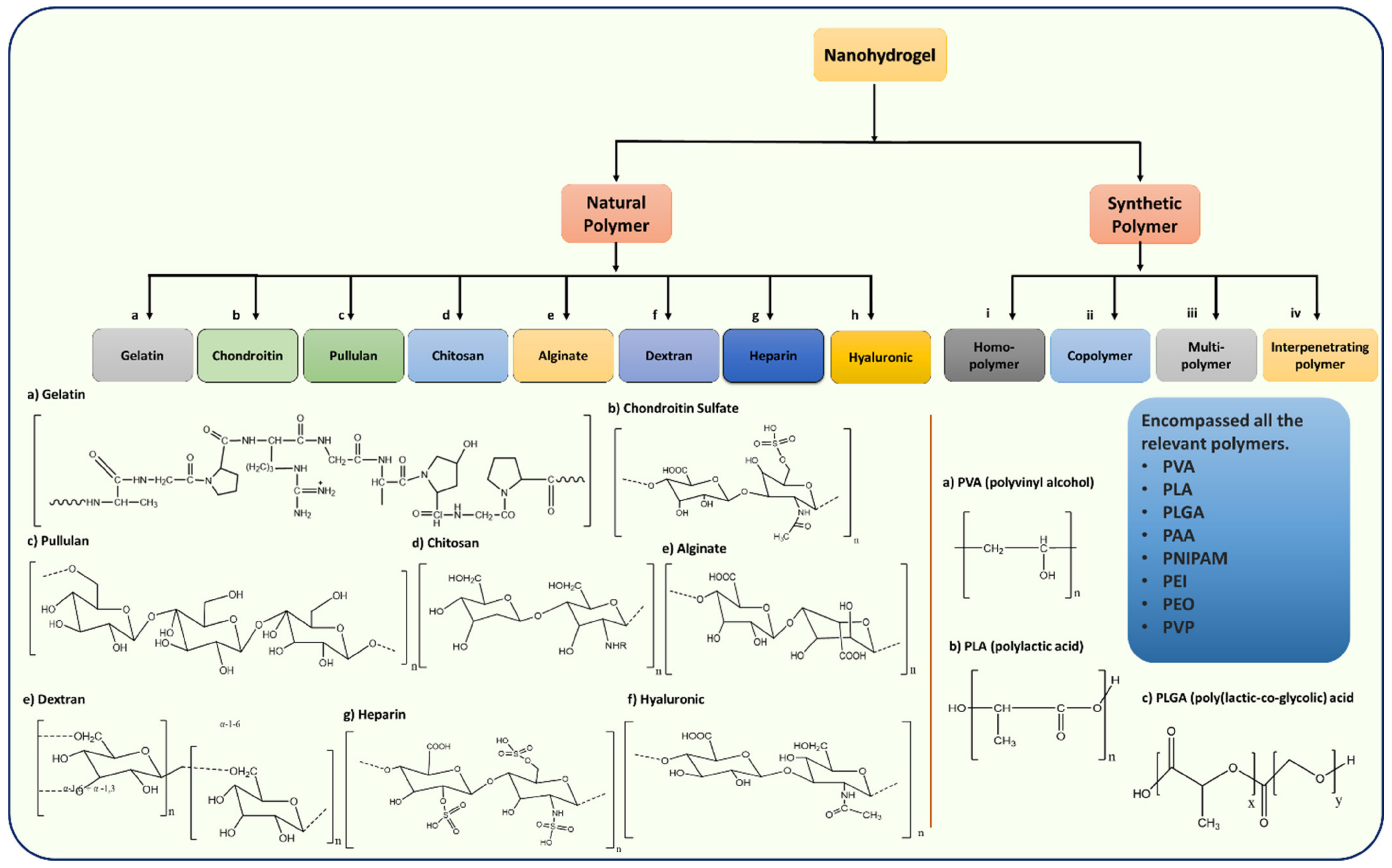
3. Classification of Nanohydrogels Based on Natural and Synthetic Polymers
3.1. Natural Polymers
3.1.1. Gelatin-Based Nanohydrogels
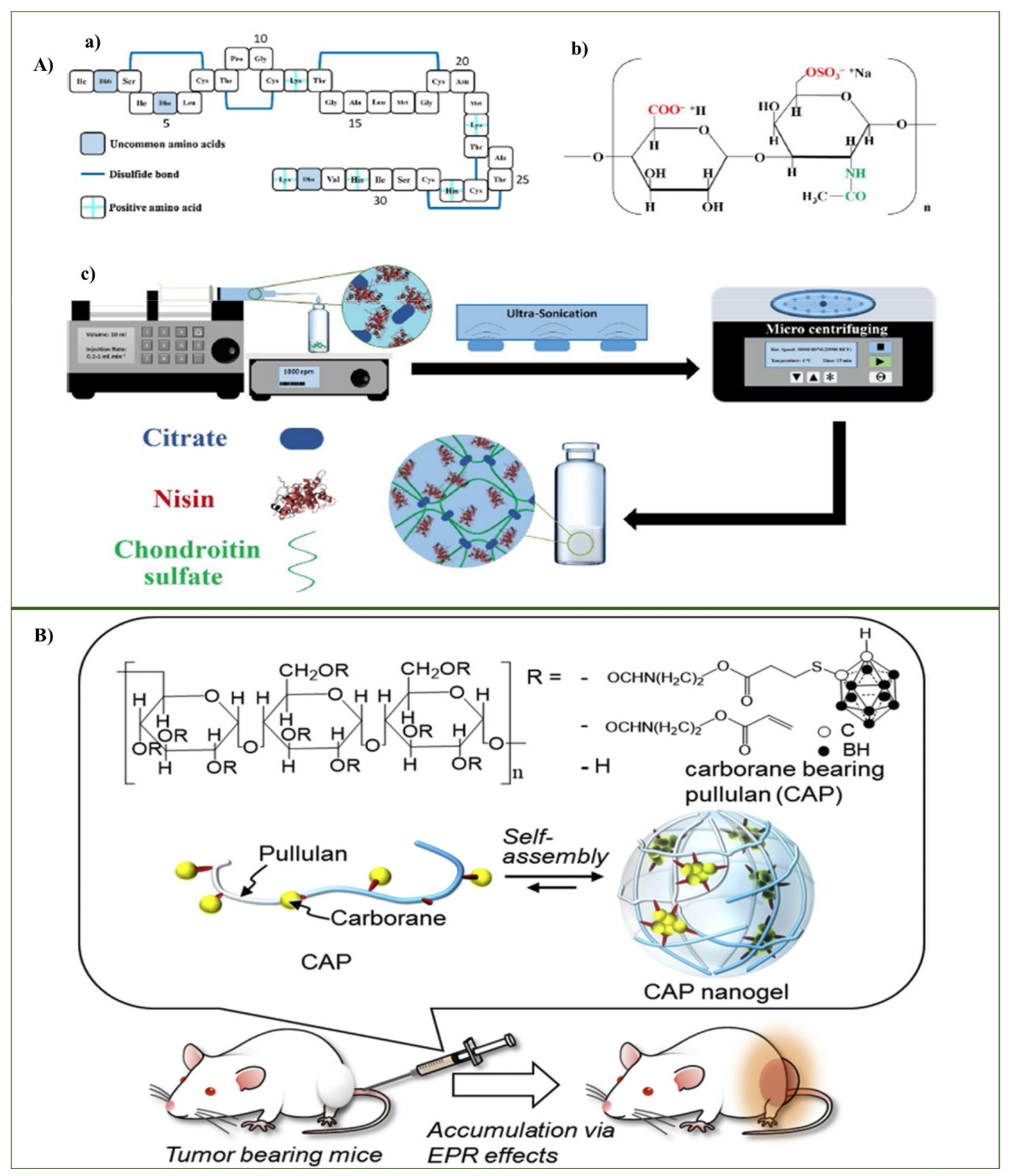
3.1.2. Chondroitin-Based Nanohydrogel
3.1.3. Pullulan-Based Nanohydrogel
3.1.4. Chitosan-Based Nanohydrogel
3.1.5. Alginate-Based Nanohydrogel
3.1.6. Dextran-Based Nanohydrogel
3.1.7. Heparin-Based Nanohydrogel
3.1.8. Hyaluronic-Based Nanohydrogel
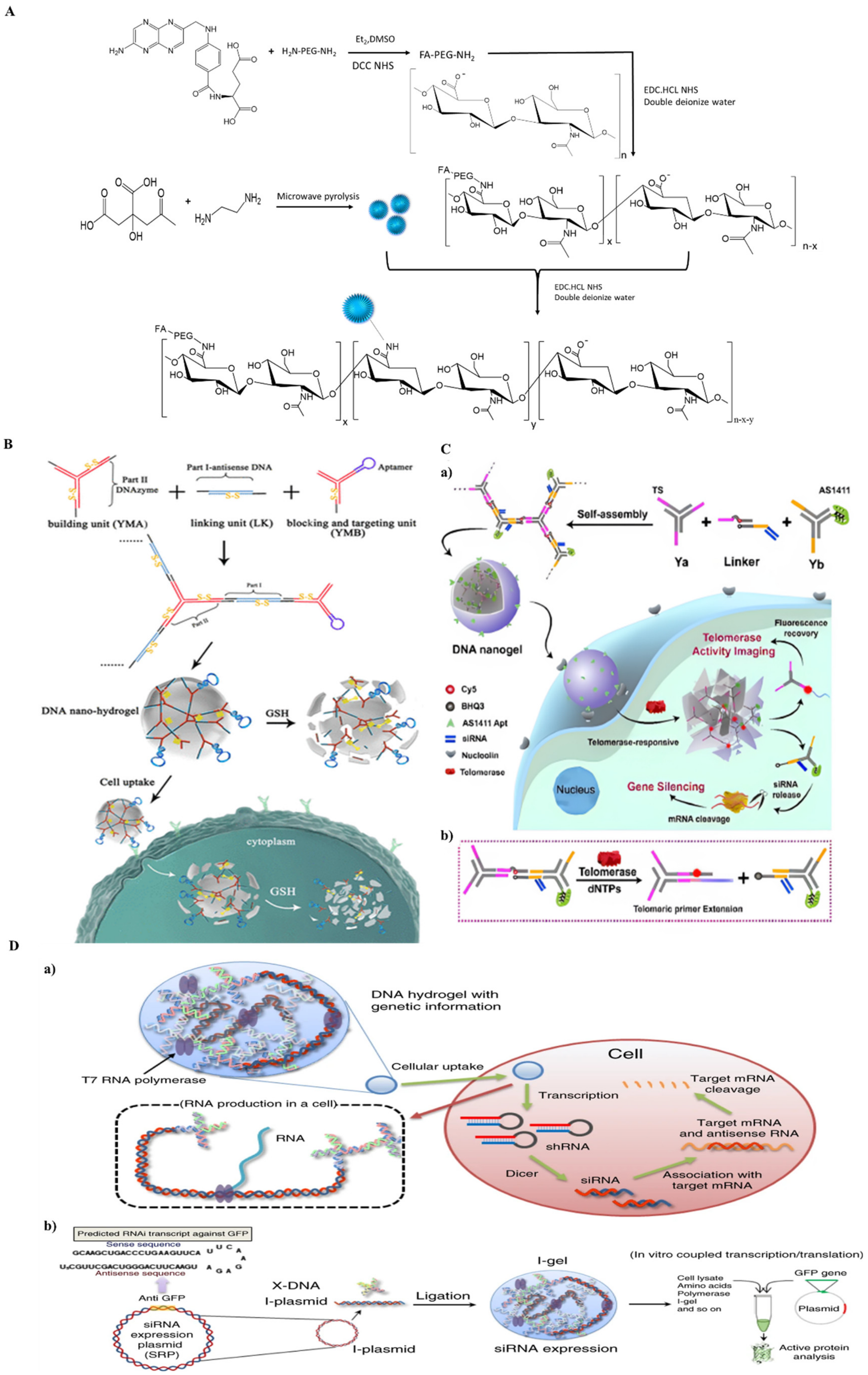
3.1.9. DNA-Based Nanohydrogel
3.2. Synthetic Polymer Based Nanohydrogels
4. Functionalized Role of Nanohydrogels
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Ai, L.; Cui, C.; Fu, T.; Cheng, X.; Qu, F.; Tan, W. Functional Aptamer-Embedded Nanomaterials for Diagnostics and Therapeutics. ACS Appl. Mater. Interfaces 2021, 13, 9542–9560. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Zhu, M.; Yang, Z.; He, P.; Wei, J.; Gao, X.; Song, J. Dual-Functionalized Apatite Nanocomposites with Enhanced Cytocompatibility and Osteogenesis for Periodontal Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Zhou, B.; Dong, Y.; Liu, D. Recent Progress in DNA Motor-Based Functional Systems. ACS Appl. Bio Mater. 2021, 4, 2251–2261. [Google Scholar] [CrossRef]
- Vigderman, L.; Zubarev, E.R. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv. Drug Deliv. Rev. 2013, 65, 663–676. [Google Scholar] [CrossRef]
- Nam, J.; La, W.; Hwang, S.; Ha, Y.S.; Park, N.; Won, N.; Jung, S.; Bhang, S.H.; Cho, Y.; Jin, M.; et al. pH-Responsive Assembly of Gold Nanoparticles and “ Spatiotemporally. ACS Nano 2013, 7, 3388–3402. [Google Scholar] [CrossRef]
- Feazell, R.P.; Nakayama-Ratchford, N.; Dai, H.; Lippard, S.J. Soluble Single-Walled Carbon Nanotubes as Longboat Delivery Systems for Platinum(IV) Anticancer Drug Design. J. Am. Chem. Soc. 2007, 129, 8438–8439. [Google Scholar] [CrossRef] [Green Version]
- Endres, T.K.; Beck-Broichsitter, M.; Samsonova, O.; Renette, T.; Kissel, T.H. Self-assembled biodegradable amphiphilic PEG–PCL–lPEI triblock copolymers at the borderline between micelles and nanoparticles designed for drug and gene delivery. Biomaterials 2011, 32, 7721–7731. [Google Scholar] [CrossRef]
- Shan, Y.; Luo, T.; Peng, C.; Sheng, R.; Cao, A.; Cao, X.; Shen, M.; Guo, R.; Tomás, H.; Shi, X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials 2012, 33, 3025–3035. [Google Scholar] [CrossRef]
- Luo, Y.; Ling, Y.; Guo, W.; Pang, J.; Liu, W.; Fang, Y.; Wen, X.; Wei, K.; Gao, X. Docetaxel loaded oleic acid-coated hydroxyapatite nanoparticles enhance the docetaxel-induced apoptosis through activation of caspase-2 in androgen independent prostate cancer cells. J. Control. Release 2010, 147, 278–288. [Google Scholar] [CrossRef]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation. Biochim. Biophys. Acta BBA Gen. Subj. 2011, 1810, 361–373. [Google Scholar] [CrossRef]
- Yan, J.; He, W.; Yan, S.; Niu, F.; Liu, T.; Ma, B.; Shao, Y.; Yan, Y.; Yang, G.; Lu, W.; et al. Self-Assembled Peptide–Lanthanide Nanoclusters for Safe Tumor Therapy: Overcoming and Utilizing Biological Barriers to Peptide Drug Delivery. ACS Nano 2018, 12, 2017–2026. [Google Scholar] [CrossRef]
- Juliano, R.L.; Carver, K. Cellular uptake and intracellular trafficking of oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Bourzac, K. Nanotechnology: Carrying drugs. Nature 2012, 491, S58–S60. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Parlea, L.; Puri, A.; Kasprzak, W.; Bindewald, E.; Zakrevsky, P.; Satterwhite, E.; Joseph, K.; Afonin, K.A.; Shapiro, B.A. Cellular Delivery of RNA Nanoparticles. ACS Comb. Sci. 2016, 18, 527–547. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. 2020, 120, 1350–1396. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, H.; Cao, T.; Liu, D. Supramolecular Hydrogels Based on DNA Self-Assembly. Acc. Chem. Res. 2017, 50, 659–668. [Google Scholar] [CrossRef]
- Eslahi, N.; Abdorahim, M.; Simchi, A. Smart Polymeric Hydrogels for Cartilage Tissue Engineering: A Review on the Chemistry and Biological Functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef]
- Dalwadi, C.; Patel, G. Application of Nanohydrogels in Drug Delivery Systems: Recent Patents Review. Recent Pat. Nanotechnol. 2015, 9, 17–25. [Google Scholar] [CrossRef]
- Qian, Z.-Y.; Fu, S.-Z.; Feng, S.-S. Nanohydrogels as a prospective member of the nanomedicine family. Nanomedicine 2013, 8, 161–164. [Google Scholar] [CrossRef]
- Oh, J.K.; Siegwart, D.J.; Lee, H.; Sherwood, G.; Peteanu, L.; Hollinger, J.O.; Kataoka, K.; Matyjaszewski, K. Biodegradable Nanogels Prepared by Atom Transfer Radical Polymerization as Potential Drug Delivery Carriers: Synthesis, Biodegradation, in Vitro Release, and Bioconjugation. J. Am. Chem. Soc. 2007, 129, 5939–5945. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Zhao, Q.; Wang, Z.; Xu, Z.; Jia, X. An Enzyme-Responsive Nanogel Carrier Based on PAMAM Dendrimers for Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 19899–19906. [Google Scholar] [CrossRef]
- Wu, H.-Q.; Wang, C.-C. Biodegradable Smart Nanogels: A New Platform for Targeting Drug Delivery and Biomedical Diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef]
- Rani, D.T. Liposomes as a potential drug delivery system: A review. Int. Res. J. Pharm. 2013, 4, 6–12. [Google Scholar]
- Chatterjee, S.; Hui, P.C.L. Review of applications and future prospects of stimuli-responsive hydrogel based on thermo-responsive biopolymers in drug delivery systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Krissanaprasit, A.; Key, C.M.; Pontula, S.; Labean, T.H. Self-Assembling Nucleic Acid Nanostructures Functionalized with Aptamers. Chem. Rev. 2021, 121, 13797–13868. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yao, C.; Zhu, Y.; Yang, L.; Luo, D.; Yang, D. Dna functional materials assembled from branched dna: Design, synthesis, and applications. Chem. Rev. 2020, 120, 9420–9481. [Google Scholar] [CrossRef] [PubMed]
- Dzamukova, M.R.; Naumenko, E.A.; Lvov, Y.M.; Fakhrullin, R.F. Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep. 2015, 5, 10560. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Chacko, R.T.; Jiwpanich, S.; Bickerton, S.; Babu, R.P.; Thayumanavan, S. Self-Cross-Linked Polymer Nanogels: A Versatile Nanoscopic Drug Delivery Platform. J. Am. Chem. Soc. 2010, 132, 17227–17235. [Google Scholar] [CrossRef]
- Li, F.; Tang, J.; Geng, J.; Luo, D.; Yang, D. Polymeric DNA hydrogel: Design, synthesis and applications. Prog. Polym. Sci. 2019, 98, 101163. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Álvarez-Bautista, A.; Duarte, C.M.M.; Mendizábal, E.; Katime, I. Controlled delivery of drugs through smart pH-sensitive nanohydrogels for anti-cancer therapies: Synthesis, drug release and cellular studies. Des. Monomers Polym. 2016, 19, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.K.; Xu, X.; Duncan, R.L.; Jia, X. Controlling the adhesion and differentiation of mesenchymal stem cells using hyaluronic acid-based, doubly crosslinked networks. Biomaterials 2011, 32, 2466–2478. [Google Scholar] [CrossRef] [Green Version]
- Asadi, H.; Rostamizadeh, K.; Salari, D.; Hamidi, M. Preparation and characterization of tri-block poly(lactide)-poly(ethylene glycol)-poly(lactide) nanogels for controlled release of naltrexone. Int. J. Pharm. 2011, 416, 356–364. [Google Scholar] [CrossRef]
- Trimaille, T.; Pertici, V.; Gigmes, D. Hydrogels à base de polymères synthétiques pour la réparation médullaire. Comptes Rendus Chim. 2016, 19, 157–166. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Lu, S.; Fan, X.; Fan, X.; Wang, H.; Wang, H.; Zhao, Y.; Zhao, Y.; Zhao, W.; Zhao, W.; et al. Synthesis of Gelatin-Based Dual-Targeted Nanoparticles of Betulinic Acid for Antitumor Therapy. ACS Appl. Bio Mater. 2020, 3, 3518–3525. [Google Scholar] [CrossRef]
- Elsaeed, S.M.; Farag, R.K.; Maysour, N.S. Synthesis and characterization of pH-sensitive crosslinked (NIPA-co-AAC) nanohydrogels copolymer. J. Appl. Polym. Sci. 2012, 124, 1947–1955. [Google Scholar] [CrossRef]
- An, J.C. Synthesis of the combined inter- and intra-crosslinked nanohydrogels by e-beam ionizing radiation. J. Ind. Eng. Chem. 2010, 16, 657–661. [Google Scholar] [CrossRef]
- Saraogi, G.K.; Gupta, P.; Gupta, U.D.; Jain, N.K.; Agrawal, G.P. Gelatin nanocarriers as potential vectors for effective management of tuberculosis. Int. J. Pharm. 2010, 385, 143–149. [Google Scholar] [CrossRef]
- Said, M.I. Role and function of gelatin in the development of the food and non-food industry: A review. In Earth and Environmental Science, Proceedings of the 2nd International Conference of Animal Science and Technology (ICAST), Makassar, Indonesia, 5–6 November 2019; IOP Conference Series; IOP Publishing: Bristol, UK, 2020; Volume 492. [Google Scholar] [CrossRef]
- Mimi, H.; Ho, K.M.; Siu, Y.S.; Wu, A.; Li, P. Polyethyleneimine-Based Core-Shell Nanogels: A Promising siRNA Carrier for Argininosuccinate Synthetase mRNA Knockdown in HeLa Cells. J. Control. Release 2012, 158, 123–130. [Google Scholar] [CrossRef]
- Jatariu, A.N.; Holban, M.N.; Peptu, C.A.; Sava, A.; Costuleanu, M.; Popa, M. Double crosslinked interpenetrated network in nanoparticle form for drug targeting—Preparation, characterization and biodistribution studies. Int. J. Pharm. 2012, 436, 66–74. [Google Scholar] [CrossRef]
- Tseng, C.-L.; Su, W.-Y.; Yen, K.-C.; Yang, K.-C.; Lin, F.-H. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef]
- Akiyama, Y.; Fujiwara, T.; Takeda, S.I.; Izumi, Y.; Nishijima, S. Preparation of stimuli-responsive protein nanogel by quantum-ray irradiation. Colloid Polym. Sci. 2007, 285, 801–807. [Google Scholar] [CrossRef]
- Gan, Z.; Ju, J.; Zhang, T.; Wu, D. Preparation of rhodamine B fluorescent poly(methacrylic acid) coated gelatin nanoparticles. J. Nanomater. 2011, 2011, 753705. [Google Scholar] [CrossRef]
- Tran, D.H.N.; Nguyen, T.H.; Vo, T.N.N.; Pham, L.P.T.; Vo, D.M.H.; Nguyen, C.K.; Bach, L.G.; Nguyen, D.H. Self-assembled poly(ethylene glycol) methyl ether-grafted gelatin nanogels for efficient delivery of curcumin in cancer treatment. J. Appl. Polym. Sci. 2019, 136, 47544. [Google Scholar] [CrossRef] [Green Version]
- Setayesh, A.; Bagheri, F.; Boddohi, S. Self-assembled formation of chondroitin sulfate-based micellar nanogel for curcumin delivery to breast cancer cells. Int. J. Biol. Macromol. 2020, 161, 771–778. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int. J. Biol. Macromol. 2020, 143, 297–304. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chung, S.-J.; Cho, H.-J.; Kim, D.-D. Bile acid-conjugated chondroitin sulfate A-based nanoparticles for tumor-targeted anticancer drug delivery. Eur. J. Pharm. Biopharm. 2015, 94, 532–541. [Google Scholar] [CrossRef]
- Mohtashamian, S.; Boddohi, S.; Hosseinkhani, S. Preparation and optimization of self-assembled chondroitin sulfate-nisin nanogel based on quality by design concept. Int. J. Biol. Macromol. 2018, 107, 2730–2739. [Google Scholar] [CrossRef]
- Cao, J.F.; Xu, W.; Zhang, Y.Y.; Shu, Y.; Wang, J.H. A Salt Stimulus-Responsive Nanohydrogel for Controlled Fishing Low-Density Lipoprotein with Superior Adsorption Capacity. ACS Appl. Mater. Interfaces 2021, 13, 4583–4592. [Google Scholar] [CrossRef]
- Kawasaki, R.; Sasaki, Y.; Akiyoshi, K. Intracellular delivery and passive tumor targeting of a self-assembled nanogel containing carborane clusters for boron neutron capture therapy. Biochem. Biophys. Res. Commun. 2017, 483, 147–152. [Google Scholar] [CrossRef]
- Gaur, R.; Singh, R.; Gupta, M.; Gaur, M.K. Aureobasidium pullulans, an economically important polymorphic yeast with special reference to pullulan. Afr. J. Biotechnol. 2010, 9, 7989–7997. [Google Scholar] [CrossRef]
- Alhaique, F.; Matricardi, P.; Di Meo, C.; Coviello, T.; Montanari, E. Polysaccharide-based self-assembling nanohydrogels: An overview on 25-years research on pullulan. J. Drug Deliv. Sci. Technol. 2015, 30, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Yang, R.; Yang, S.; Guan, J.; Zhang, D.; Ma, Y.; Liu, H. Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives. Drug Deliv. 2018, 25, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi-Ouchida, R.; Yuki, Y.; Kiyono, H. Development of a nanogel-based nasal vaccine as a novel antigen delivery system. Expert Rev. Vaccines 2017, 16, 1231–1240. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Gogoi, M.; Dev Sarma, H.; Banerjee, R.; Bahadur, D. Biocompatibility, biodistribution and efficacy of magnetic nanohydrogels in inhibiting growth of tumors in experimental mice models. Biomater. Sci. 2014, 2, 370–380. [Google Scholar] [CrossRef]
- Luckanagul, J.A.; Pitakchatwong, C.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.; Kim, T.H.; Lee, E.S.; Shin, B.S.; Chi, S.-C.; Park, E.-S.; Lee, K.C.; Youn, Y.S. Self-assembled glycol chitosan nanogels containing palmityl-acylated exendin-4 peptide as a long-acting anti-diabetic inhalation system. J. Control. Release 2012, 161, 728–734. [Google Scholar] [CrossRef]
- Ta, H.T.; Dass, C.R.; Dunstan, D.E. Injectable chitosan hydrogels for localised cancer therapy. J. Control. Release 2008, 126, 205–216. [Google Scholar] [CrossRef]
- De Souza, R.; Zahedi, P.; Allen, C.J.; Piquette-Miller, M. Biocompatibility of injectable chitosan–phospholipid implant systems. Biomaterials 2009, 30, 3818–3824. [Google Scholar] [CrossRef]
- Chan, A.W.; Neufeld, R.J. Tuneable semi-synthetic network alginate for absorptive encapsulation and controlled release of protein therapeutics. Biomaterials 2010, 31, 9040–9047. [Google Scholar] [CrossRef]
- Dong, L.; Xia, S.; Wu, K.; Huang, Z.; Chen, H.; Chen, J.; Zhang, J. A pH/Enzyme-responsive tumor-specific delivery system for doxorubicin. Biomaterials 2010, 31, 6309–6316. [Google Scholar] [CrossRef]
- Chopra, M.; Bernela, M.; Kaur, P.; Manuja, A.; Kumar, B.; Thakur, R. Alginate/gum acacia bipolymeric nanohydrogels--promising carrier for zinc oxide nanoparticles. Int. J. Biol. Macromol. 2015, 72, 827–833. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Su, C.H.; Cheng, F.Y. In vitro and in vivo applications of alginate/iron oxide nanocomposites for theranostic molecular imaging in a brain tumor model. RSC Adv. 2015, 5, 90061–90064. [Google Scholar] [CrossRef]
- Podgórna, K.; Szczepanowicz, K.; Piotrowski, M.; Gajdošová, M.; Štěpánek, F.; Warszyński, P. Gadolinium alginate nanogels for theranostic applications. Colloids Surf. B Biointerfaces 2017, 153, 183–189. [Google Scholar] [CrossRef]
- Pei, M.; Jia, X.; Zhao, X.; Li, J.; Liu, P. Alginate-based cancer-associated, stimuli-driven and turn-on theranostic prodrug nanogel for cancer detection and treatment. Carbohydr. Polym. 2018, 183, 131–139. [Google Scholar] [CrossRef]
- Malzahn, K.; Jamieson, W.D.; Dröge, M.; Mailänder, V.; Jenkins, A.T.A.; Weiss, C.K.; Landfester, K. Advanced dextran based nanogels for fighting Staphylococcus aureus infections by sustained zinc release. J. Mater. Chem. B 2014, 2, 2175–2183. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Dou, H.; Zhang, Z.; Sun, K.; Jin, Y.; Dai, T.; Zhou, G.; Shen, Z. Fluorescent dextran-based nanogels: Efficient imaging nanoprobes for adipose-derived stem cells. Polym. Chem. 2013, 4, 4103–4112. [Google Scholar] [CrossRef]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef]
- Baldwin, A.D.; Robinson, K.G.; Militar, J.L.; Derby, C.D.; Kiick, K.L.; Akins, R.E., Jr. In Situ-crosslinkable heparin-containing poly(ethylene glycol) hydrogels for sustained anticoagulant release. J. Biomed. Mater. Res. 2014, 100, 2106–2118. [Google Scholar] [CrossRef] [Green Version]
- Bae, K.H.; Mok, H.; Park, T.G. Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials 2008, 29, 3376–3383. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Shriver, Z.; Venkataraman, G.; Narayanasami, U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat. Rev. Cancer 2002, 2, 521–528. [Google Scholar] [CrossRef]
- Park, W.; Kim, K.s.; Bae, B.-c.; Kim, Y.H.; Na, K. Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. Eur. J. Pharm. Sci. 2010, 40, 367–375. [Google Scholar] [CrossRef]
- Wei, X.; Senanayake, T.H.; Warren, G.; Vinogradov, S.V. Hyaluronic Acid-Based Nanogel–Drug Conjugates with Enhanced Anticancer Activity Designed for the Targeting of CD44-Positive and Drug-Resistant Tumors. Bioconjug. Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Luan, S.; Zhu, Y.; Wu, X.; Wang, Y.; Liang, F.; Song, S. Hyaluronic-Acid-Based pH-Sensitive Nanogels for Tumor-Targeted Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 2410–2419. [Google Scholar] [CrossRef]
- Jia, X.; Han, Y.; Pei, M.; Zhao, X.; Tian, K.; Zhou, T.; Liu, P. Multi-functionalized hyaluronic acid nanogels crosslinked with carbon dots as dual receptor-mediated targeting tumor theranostics. Carbohydr. Polym. 2016, 152, 391–397. [Google Scholar] [CrossRef]
- Li, J.; Zheng, C.; Cansiz, S.; Wu, C.; Xu, J.; Cui, C.; Liu, Y.; Hou, W.; Wang, Y.; Zhang, L.; et al. Self-assembly of DNA Nanohydrogels with Controllable Size and Stimuli-Responsive Property for Targeted Gene Regulation Therapy. J. Am. Chem. Soc. 2015, 137, 1412–1415. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Y.; Yang, Y.; Jiang, L.; Xing, C.; Li, J.; Lu, C.; Yang, H. Functional Self-Assembled DNA Nanohydrogels for Specific Telomerase Activity Imaging and Telomerase-Activated Antitumor Gene Therapy. Anal. Chem. 2020, 92, 15179–15186. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, M.; Kim, T.; Na, J.; Jung, Y.; Jung, G.Y.; Kim, S.; Park, N. A RNA producing DNA hydrogel as a platform for a high performance RNA interference system. Nat. Commun. 2018, 9, 4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater. 2020, 32, 1806538. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Peng, S.; Yang, D.; Roh, Y.H.; Funabashi, H.; Park, N.; Rice, E.J.; Chen, L.; Long, R.; Wu, M.; et al. A mechanical metamaterial made from a DNA hydrogel. Nat. Nanotechnol. 2012, 7, 816–820. [Google Scholar] [CrossRef]
- Li, J.; Mo, L.; Lu, C.H.; Fu, T.; Yang, H.H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, M.A.; Bauleth-Ramos, T.; Santos, H.A. DNA Hydrogel Assemblies: Bridging Synthesis Principles to Biomedical Applications. Adv. Ther. 2018, 1, 1800042. [Google Scholar] [CrossRef]
- Deshpande, S.R.; Hammink, R.; Das, R.K.; Nelissen, F.H.T.; Blank, K.G.; Rowan, A.E.; Heus, H.A. DNA-Responsive Polyisocyanopeptide Hydrogels with Stress-Stiffening Capacity. Adv. Funct. Mater. 2016, 26, 9075–9082. [Google Scholar] [CrossRef]
- Li, C.; Chen, P.; Shao, Y.; Zhou, X.; Wu, Y.; Yang, Z.; Li, Z.; Weil, T.; Liu, D. A writable polypeptide-DNA hydrogel with rationally designed multi-modification sites. Small 2015, 11, 1138–1143. [Google Scholar] [CrossRef]
- Shin, M.; Ryu, J.H.; Park, J.P.; Kim, K.; Yang, J.W.; Lee, H. DNA/tannic acid hybrid gel exhibiting biodegradability, extensibility, tissue adhesiveness, and hemostatic ability. Adv. Funct. Mater. 2015, 25, 1270–1278. [Google Scholar] [CrossRef]
- Park, N.; Um, S.H.; Funabashi, H.; Xu, J.; Luo, D. A cell-free protein-producing gel. Nat. Mater. 2009, 8, 432–437. [Google Scholar] [CrossRef]
- Cheng, E.; Xing, Y.; Chen, P.; Yang, Y.; Sun, Y.; Zhou, D.; Xu, T.; Fan, Q.; Liu, D. A pH-triggered, fast-responding DNA hydrogel. Angew. Chem. Int. Ed. 2009, 48, 7660–7663. [Google Scholar] [CrossRef]
- Mao, X.; Chen, G.; Wang, Z.; Zhang, Y.; Zhu, X.; Li, G. Surface-immobilized and self-shaped DNA hydrogels and their application in biosensing. Chem. Sci. 2018, 9, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Hwang, S.; Im, K.; Hur, J.; Nam, J.; Hwang, S.; Ahn, G.O.; Kim, S.; Park, N. DNA hydrogel delivery vehicle for light triggered and synergistic cancer therapy (Communication). Nanoscale 2015, 7, 9433–9437. [Google Scholar] [CrossRef] [Green Version]
- Topuz, F.; Singh, S.; Albrecht, K.; Möller, M.; Groll, J. DNA Nanogels To Snare Carcinogens: A Bioinspired Generic Approach with High Efficiency. Angew. Chem. Int. Ed. 2016, 55, 12210–12213. [Google Scholar] [CrossRef]
- Hur, J.; Im, K.; Kim, S.W.; Kim, U.J.; Lee, J.; Hwang, S.; Song, J.; Kim, S.; Hwang, S.; Park, N. DNA hydrogel templated carbon nanotube and polyaniline assembly and its applications for electrochemical energy storage devices. J. Mater. Chem. A 2013, 1, 14460–14466. [Google Scholar] [CrossRef]
- Song, J.; Hwang, S.; Im, K.; Hur, J.; Nam, J.; Hwang, S.; Ahn, G.-O.; Kim, S.; Park, N. Light-responsible DNA hydrogel–gold nanoparticle assembly for synergistic cancer therapy. J. Mater. Chem. B 2015, 3, 1537–1543. [Google Scholar] [CrossRef]
- Pitakchatwong, C.; Chirachanchai, S. Thermo-Magnetoresponsive Dual Function Nanoparticles: An Approach for Magnetic Entrapable-Releasable Chitosan. ACS Appl. Mater. Interfaces 2017, 9, 10398–10407. [Google Scholar] [CrossRef]
- Qiao, Z.Y.; Zhang, R.; Du, F.S.; Liang, D.H.; Li, Z.C. Multi-responsive nanogels containing motifs of ortho ester, oligo(ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. J. Control. Release 2011, 152, 57–66. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Li, Y.; Wan, J.; Zhang, Z.; Guo, J.; Wang, C. Targeted Soft Biodegradable Glycine/PEG/RGD-Modified Poly(methacrylic acid) Nanobubbles as Intelligent Theranostic Vehicles for Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 35604–35612. [Google Scholar] [CrossRef]
- Hamada, S.; Yancey, K.G.; Pardo, Y.; Gan, M.; Vanatta, M.; An, D.; Hu, Y.; Derrien, T.L.; Ruiz, R.; Liu, P.; et al. Dynamic DNA material with emergent locomotion behavior powered by artificial metabolism. Sci. Robot. 2019, 4, eaaw3512. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, D.; Willner, I.; Tian, Y.; Jiang, L. Smart DNA Hydrogel Integrated Nanochannels with High Ion Flux and Adjustable Selective Ionic Transport. Angew. Chem. Int. Ed. 2018, 57, 7790–7794. [Google Scholar] [CrossRef]
- Kettel, M.J.; Schaefer, K.; Pich, A.; Moeller, M. Functional PMMA nanogels by cross-linking with cyclodextrin methacrylate. Polymer 2016, 86, 176–188. [Google Scholar] [CrossRef]
- Hang, Z.; Koens, L.; Lauga, E.; Mourran, A.; Moller, M. A Light-Driven Microgel Rotor. Small 2019, 15, 1903379. [Google Scholar] [CrossRef]
- Zhou, T.; Li, J.; Jia, X.; Zhao, X.; Liu, P. PH/Reduction Dual-Responsive Oxidized Alginate-Doxorubicin (mPEG-OAL-DOX/Cys) Prodrug Nanohydrogels: Effect of Complexation with Cyclodextrins. Langmuir 2018, 34, 416–424. [Google Scholar] [CrossRef]
- Park, S.; Lee, W.J.; Park, S.; Choi, D.; Kim, S.; Park, N. Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy. Sci. Rep. 2019, 9, 20180. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, J.; Sun, L.; Li, Y.; Guo, J.; Wang, C. Zinc finger-inspired nanohydrogels with glutathione/pH triggered degradation based on coordination substitution for highly efficient delivery of anti-cancer drugs. J. Control. Release 2016, 225, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Wu, D.; Su, T.; Bao, S.; Liao, C.; Wang, Q. Magnetic nanocomposite hydrogel prepared by ZnO-initiated photopolymerization for La (III) adsorption. ACS Appl. Mater. Interfaces 2014, 6, 19840–19849. [Google Scholar] [CrossRef]
- Belali, S.; Savoie, H.; O’Brien, J.M.; Cafolla, A.A.; O’Connell, B.; Karimi, A.R.; Boyle, R.W.; Senge, M.O. Synthesis and Characterization of Temperature-Sensitive and Chemically Cross-Linked Poly(N-isopropylacrylamide)/Photosensitizer Hydrogels for Applications in Photodynamic Therapy. Biomacromolecules 2018, 19, 1592–1601. [Google Scholar] [CrossRef]
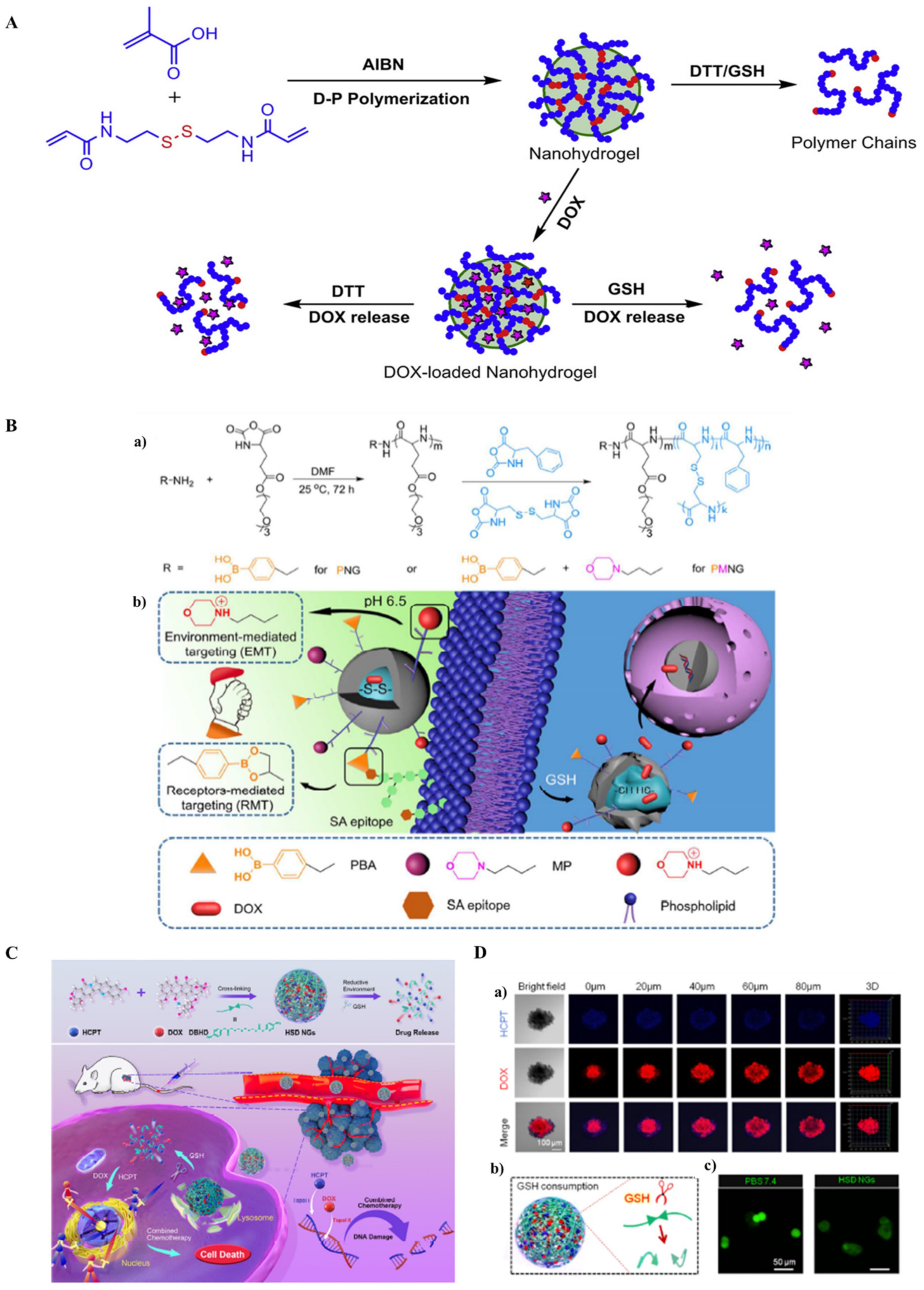
- Pan, Y.J.; Chen, Y.Y.; Wang, D.R.; Wei, C.; Guo, J.; Lu, D.R.; Chu, C.C.; Wang, C.C. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 2012, 33, 6570–6579. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Xu, W.; Sun, T.; Xiao, H.; Zhuang, X.; Chen, X. Receptor and Microenvironment Dual-Recognizable Nanogel for Targeted Chemotherapy of Highly Metastatic Malignancy. Nano Lett. 2017, 17, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jia, D.; Ma, X.; Liang, M.; Hou, S.; Qiu, W.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. Reduction-responsive chemo-capsule-based prodrug nanogel for synergistic treatment of tumor chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 8940–8951. [Google Scholar] [CrossRef] [PubMed]
- Nagar, A.; Pradeep, T. Clean Water through Nanotechnology: Needs, Gaps, and Fulfillment. ACS Nano 2020, 14, 6420–6435. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Sahoo, G.; Das, R.; Swain, S.K. Three-Dimensional Rice Straw-Structured Magnetic Nanoclay-Decorated Tripolymeric Nanohydrogels as Superadsorbent of Dye Pollutants. ACS Appl. Nano Mater. 2018, 1, 1188–1203. [Google Scholar] [CrossRef]
- Geng, J.; Pu, J.; Wang, L.; Bai, B. Surface charge effect of nanogel on emulsification of oil in water for fossil energy recovery. Fuel 2018, 223, 140–148. [Google Scholar] [CrossRef]
- Bhagat, D.; Samanta, S.K.; Bhattacharya, S. Efficient management of fruit pests by pheromone nanogels. Sci. Rep. 2013, 3, 1294. [Google Scholar] [CrossRef]
- Vundavalli, R.; Vundavalli, S.; Nakka, M.; Rao, D.S. Biodegradable Nano-Hydrogels in Agricultural Farming—Alternative Source For Water Resources. Procedia Mater. Sci. 2015, 10, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Meurer, R.A.; Kemper, S.; Knopp, S.; Eichert, T.; Jakob, F.; Goldbach, H.E.; Schwaneberg, U.; Pich, A. Biofunctional Microgel-Based Fertilizers for Controlled Foliar Delivery of Nutrients to Plants. Angew. Chem. Int. Ed. 2017, 56, 7380–7386. [Google Scholar] [CrossRef]
- Fu, L.; Ma, Q.; Liao, K.; An, J.; Bai, J.; He, Y. Application of Pickering emulsion in oil drilling and production. Nanotechnol. Rev. 2021, 11, 26–39. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Wang, A.; Lu, Y.; Li, J.; Zhu, Y.; Jin, J. Zwitterionic Nanofibrous Membranes with a Superior Antifouling Property for Gravity-Driven Crude Oil-in-Water Emulsion Separation. Langmuir 2019, 35, 1682–1689. [Google Scholar] [CrossRef]
- Zang, L.; Zheng, S.; Wang, L.; Ma, J.; Sun, L. Zwitterionic nanogels modified nanofibrous membrane for efficient oil/water separation. J. Membr. Sci. 2020, 612, 118379. [Google Scholar] [CrossRef]
- Zhang, L.; Abbaspourrad, A.; Parsa, S.; Tang, J.; Cassiola, F.; Zhang, M.; Tian, S.; Dai, C.; Xiao, L.; Weitz, D.A. Core-Shell Nanohydrogels with Programmable Swelling for Conformance Control in Porous Media. ACS Appl. Mater. Interfaces 2020, 12, 34217–34225. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quazi, M.Z.; Park, N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. Int. J. Mol. Sci. 2022, 23, 1943. https://doi.org/10.3390/ijms23041943
Quazi MZ, Park N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. International Journal of Molecular Sciences. 2022; 23(4):1943. https://doi.org/10.3390/ijms23041943
Chicago/Turabian StyleQuazi, Mohzibudin Z., and Nokyoung Park. 2022. "Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications" International Journal of Molecular Sciences 23, no. 4: 1943. https://doi.org/10.3390/ijms23041943
APA StyleQuazi, M. Z., & Park, N. (2022). Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. International Journal of Molecular Sciences, 23(4), 1943. https://doi.org/10.3390/ijms23041943





