Antibody Responses to Transglutaminase 3 in Dermatitis Herpetiformis: Lessons from Celiac Disease
Abstract
1. Introduction
2. Transglutaminase 3-The Epidermal Transglutaminase
3. Systemic Responses against TG3 in DH
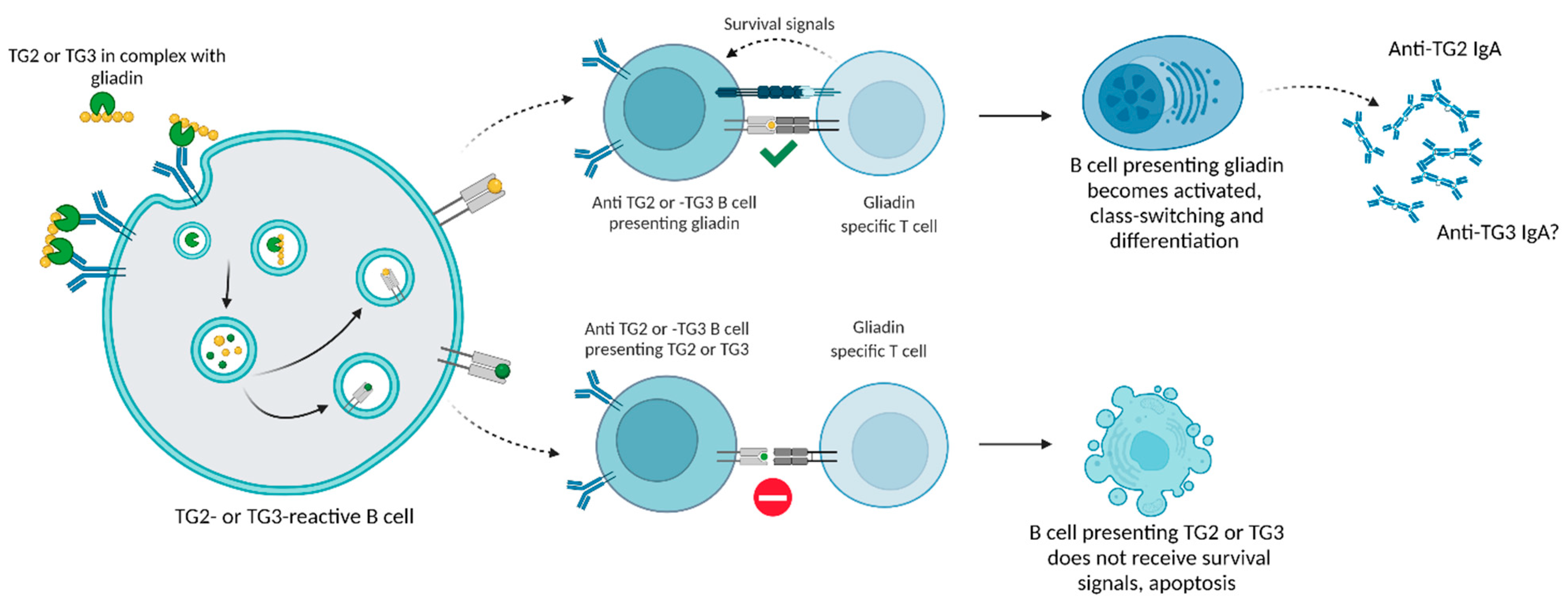
3.1. Mechanisms of Anti-TG3 Antibody Development
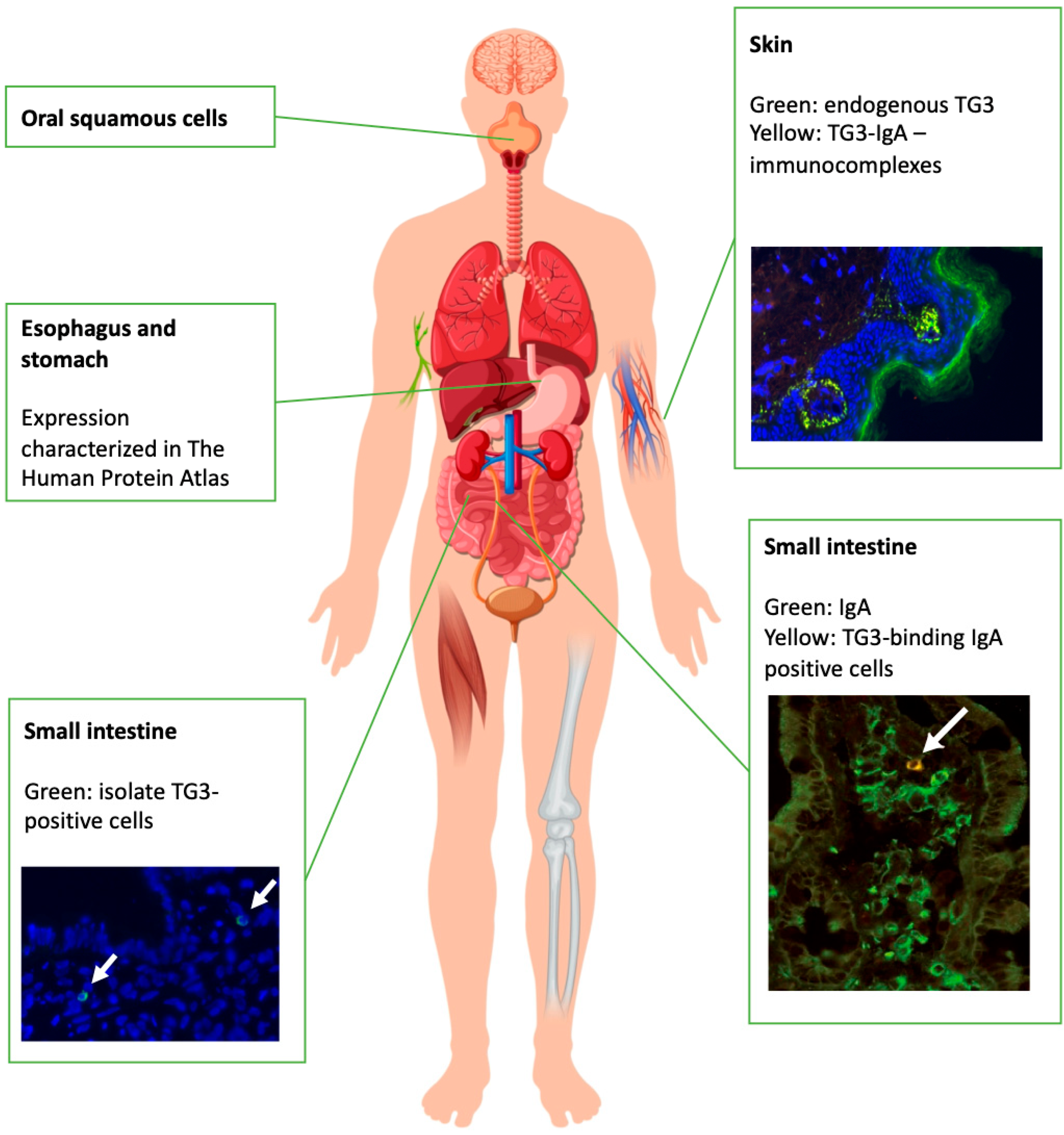
3.2. Origins of Serum and Skin Antibodies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reunala, T.; Salmi, T.T.; Hervonen, K. Dermatitis Herpetiformis: Pathognomonic Transglutaminase IgA Deposits in the Skin and Excellent Prognosis on a Gluten-Free Diet. Acta Derm. Venereol. 2015, 95, 917–922. [Google Scholar] [CrossRef]
- Spurkland, A.; Ingvarsson, G.; Falk, E.S.; Knutsen, I.; Sollid, L.M.; Thorsby, E. Dermatitis Herpetiformis and Celiac Disease Are Both Primarily Associated with the HLA-DQ (Alpha 1*0501, Beta 1*02) or the HLA-DQ (Alpha 1*03, Beta 1*0302) Heterodimers. Tissue Antigens 1997, 49, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Ehnis, T.; Bauer, M.; Donner, P.; Volta, U.; Riecken, E.O.; Schuppan, D. Identification of Tissue Transglutaminase as the Autoantigen of Celiac Disease. Nat. Med. 1997, 3, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schuppan, D.; Laag, E.; Bruckner-Tuderman, L.; Reunala, T.; Kárpáti, S.; Zágoni, T.; Riecken, E.O. Antibodies to Tissue Transglutaminase as Serologic Markers in Patients with Dermatitis Herpetiformis. J. Investig. Dermatol. 1999, 113, 133–136. [Google Scholar] [CrossRef]
- Sárdy, M.; Kárpáti, S.; Merkl, B.; Paulsson, M.; Smyth, N. Epidermal Transglutaminase (TGase 3) Is the Autoantigen of Dermatitis Herpetiformis. J. Exp. Med. 2002, 195, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.R.; Zone, J.J.; Schmidt, L.A.; Taylor, T.B.; Neuhausen, S.L.; Hull, C.M.; Meyer, L.J. Epidermal Transglutaminase Deposits in Perilesional and Uninvolved Skin in Patients with Dermatitis Herpetiformis. J. Investig. Dermatol. 2007, 127, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Gorman, J.J.; Park, S.C.; Chung, S.I.; Steinert, P.M. The Deduced Sequence of the Novel Protransglutaminase E (TGase3) of Human and Mouse. J. Biol. Chem. 1993, 268, 12682–12690. [Google Scholar] [CrossRef]
- Hitomi, K.; Kanehiro, S.; Ikura, K.; Maki, M. Characterization of Recombinant Mouse Epidermal-Type Transglutaminase (TGase 3): Regulation of Its Activity by Proteolysis and Guanine Nucleotides. J. Biochem. 1999, 125, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Hitomi, K.; van Vlijmen-Willems, I.M.J.J.; de Jongh, G.J.; Yamamoto, K.; Nishi, K.; Watts, C.; Reinheckel, T.; Schalkwijk, J.; Zeeuwen, P.L.J.M. Cystatin M/E Is a High Affinity Inhibitor of Cathepsin V and Cathepsin L by a Reactive Site That Is Distinct from the Legumain-Binding Site. J. Biol. Chem. 2006, 281, 15893–15899. [Google Scholar] [CrossRef]
- Kim, H.C.; Lewis, M.S.; Gorman, J.J.; Park, S.C.; Girard, J.E.; Folk, J.E.; Chung, S.I. Protransglutaminase E from Guinea Pig Skin. Isolation and Partial Characterization. J. Biol. Chem. 1990, 265, 21971–21978. [Google Scholar] [CrossRef]
- Ogawa, H.; Goldsmith, L.A. Human Epidermal Transglutaminase. Preparation and Properties. J. Biol. Chem. 1976, 251, 7281–7288. [Google Scholar] [CrossRef]
- Buxman, M.M.; Wuepper, K.D. Isolation, Purification and Characterization of Bovine Epidermal Transglutaminase. Biochim. Biophys. Acta BBA Enzymol. 1976, 452, 356–369. [Google Scholar] [CrossRef]
- Hitomi, K.; Horio, Y.; Ikura, K.; Yamanishi, K.; Maki, M. Analysis of Epidermal-Type Transglutaminase (TGase 3) Expression in Mouse Tissues and Cell Lines. Int. J. Biochem. Cell Biol. 2001, 33, 491–498. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Grant, P.; Lee, J.-H.; Pant, H.C.; Steinert, P.M. Differential Expression of Multiple Transglutaminases in Human Brain. J. Biol. Chem. 1999, 274, 30715–30721. [Google Scholar] [CrossRef] [PubMed]
- Stamnaes, J.; Dorum, S.; Fleckenstein, B.; Aeschlimann, D.; Sollid, L.M. Gluten T Cell Epitope Targeting by TG3 and TG6; Implications for Dermatitis Herpetiformis and Gluten Ataxia. Amino Acids 2010, 39, 1183–1191. [Google Scholar] [CrossRef]
- Vanderlugt, C.J.; Miller, S.D. Epitope Spreading. Curr. Opin. Immunol. 1996, 8, 831–836. [Google Scholar] [CrossRef]
- Monneaux, F.; Muller, S. Epitope Spreading in Systemic Lupus Erythematosus: Identification of Triggering Peptide Sequences. Arthritis Rheum. 2002, 46, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Salmi, T.T.; Kurppa, K.; Hervonen, K.; Laurila, K.; Collin, P.; Huhtala, H.; Saavalainen, P.; Sievänen, H.; Reunala, T.; Kaukinen, K. Serum Transglutaminase 3 Antibodies Correlate with Age at Celiac Disease Diagnosis. Dig. Liver Dis. 2016, 48, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Finamore, A.; Mengheri, E.; Millimaggi, D.; Esslinger, B.; Dieterich, W.; Papola, F.; Colangeli, S.; Tombolino, V.; Schuppan, D.; et al. Isolation and Characterization of Circulating Tissue Transglutaminase-Specific T Cells in Coeliac Disease. Int. J. Immunopathol. Pharmacol. 2010, 23, 179–191. [Google Scholar] [CrossRef]
- Comerford, R.; Coates, C.; Byrne, G.; Lynch, S.; Dunne, P.; Dunne, M.; Kelly, J.; Feighery, C. Characterisation of Tissue Transglutaminase-Reactive T Cells from Patients with Coeliac Disease and Healthy Controls. Clin. Immunol. 2014, 154, 155–163. [Google Scholar] [CrossRef]
- Jabri, B.; Sollid, L.M. T Cells in Celiac Disease. J. Immunol. 2017, 198, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- di Niro, R.; Mesin, L.; Zheng, N.-Y.; Stamnaes, J.; Morrissey, M.; Lee, J.-H.; Huang, M.; Iversen, R.; du Pré, M.F.; Qiao, S.-W.; et al. High Abundance of Plasma Cells Secreting Transglutaminase 2-Specific IgA Autoantibodies with Limited Somatic Hypermutation in Celiac Disease Intestinal Lesions. Nat. Med. 2012, 18, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, I.; Zhou, C.; Eggesbø, L.M.; Miao, Z.; Polak, J.; Lundin, K.E.A.; Jahnsen, J.; Qiao, S.-W.; Iversen, R.; Sollid, L.M. Longevity, clonal relationship, and transcriptional program of celiac disease-specific plasma cells. J. Exp. Med. 2021, 218, e20200852. [Google Scholar] [CrossRef]
- Snir, O.; Mesin, L.; Gidoni, M.; Lundin, K.E.A.; Yaari, G.; Sollid, L.M. Analysis of Celiac Disease Autoreactive Gut Plasma Cells and Their Corresponding Memory Compartment in Peripheral Blood Using High-Throughput Sequencing. J. Immunol. 2015, 194, 5703–5712. [Google Scholar] [CrossRef] [PubMed]
- Iversen, R.; Niro, R.D.; Stamnaes, J.; Lundin, K.E.A.; Wilson, P.C.; Sollid, L.M. Transglutaminase 2–Specific Autoantibodies in Celiac Disease Target Clustered, N-Terminal Epitopes Not Displayed on the Surface of Cells. J. Immunol. 2013, 190, 5981–5991. [Google Scholar] [CrossRef] [PubMed]
- Iversen, R.; Roy, B.; Stamnaes, J.; Høydahl, L.S.; Hnida, K.; Neumann, R.S.; Korponay-Szabó, I.R.; Lundin, K.E.A.; Sollid, L.M. Efficient T Cell–B Cell Collaboration Guides Autoantibody Epitope Bias and Onset of Celiac Disease. Proc. Natl. Acad. Sci. USA 2019, 116, 15134–15139. [Google Scholar] [CrossRef]
- Lindstad, C.B.; Dewan, A.E.; Stamnaes, J.; Sollid, L.M.; Pré, M.F. du TG2-Gluten Complexes as Antigens for Gluten-Specific and Transglutaminase-2 Specific B Cells in Celiac Disease. PLoS ONE 2021, 16, e0259082. [Google Scholar] [CrossRef] [PubMed]
- Stamnaes, J.; Iversen, R.; du Pré, M.F.; Chen, X.; Sollid, L.M. Enhanced B-Cell Receptor Recognition of the Autoantigen Transglutaminase 2 by Efficient Catalytic Self-Multimerization. PLoS ONE 2015, 10, e0134922. [Google Scholar] [CrossRef]
- Nemazee, D.A.; Bürki, K. Clonal Deletion of B Lymphocytes in a Transgenic Mouse Bearing Anti-MHC Class I Antibody Genes. Nature 1989, 337, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Antiga, E.; Maglie, R.; Quintarelli, L.; Verdelli, A.; Bonciani, D.; Bonciolini, V.; Caproni, M. Dermatitis Herpetiformis: Novel Perspectives. Front. Immunol. 2019, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- du Pré, M.F.; Blazevski, J.; Dewan, A.E.; Stamnaes, J.; Kanduri, C.; Sandve, G.K.; Johannesen, M.K.; Lindstad, C.B.; Hnida, K.; Fugger, L.; et al. B Cell Tolerance and Antibody Production to the Celiac Disease Autoantigen Transglutaminase 2. J. Exp. Med. 2019, 217, e20190860. [Google Scholar] [CrossRef] [PubMed]
- Iversen, R.; Snir, O.; Stensland, M.; Kroll, J.E.; Steinsbø, Ø.; Korponay-Szabó, I.R.; Lundin, K.E.A.; de Souza, G.A.; Sollid, L.M. Strong Clonal Relatedness between Serum and Gut IgA despite Different Plasma Cell Origins. Cell Rep. 2017, 20, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Hietikko, M.; Koskinen, O.; Kurppa, K.; Laurila, K.; Saavalainen, P.; Salmi, T.; Ilus, T.; Huhtala, H.; Kaukinen, K.; Lindfors, K. Small-Intestinal TG2-Specific Plasma Cells at Different Stages of Coeliac Disease. BMC Immunol. 2018, 19, 36. [Google Scholar] [CrossRef]
- Fleckenstein, B.; Qiao, S.-W.; Larsen, M.R.; Jung, G.; Roepstorff, P.; Sollid, L.M. Molecular Characterization of Covalent Complexes between Tissue Transglutaminase and Gliadin Peptides. J. Biol. Chem. 2004, 279, 17607–17616. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M.; Molberg, Ø.; McAdam, S.; Lundin, K.E.A. Autoantibodies in Coeliac Disease: Tissue Transglutaminase—Guilt by Association? Gut 1997, 41, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Shreder, K. Synthetic Haptens as Probes of Antibody Response and Immunorecognition. Methods 2000, 20, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Reunala, T.; Hervonen, K.; Aeschlimann, P.; Aeschlimann, D. TG6 Auto-Antibodies in Dermatitis Herpetiformis. Nutrients 2020, 12, 2884. [Google Scholar] [CrossRef] [PubMed]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking Enzymes with Pleiotropic Functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Hietikko, M.; Hervonen, K.; Ilus, T.; Salmi, T.; Huhtala, H.; Laurila, K.; Rauhavirta, T.; Reunala, T.; Kaukinen, K.; Lindfors, K. Ex Vivo Culture of Duodenal Biopsies from Patients with Dermatitis Herpetiformis Indicates That Transglutaminase 3 Antibody Production Occurs in the Gut. Acta Derm. Venereol. 2018, 98, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Sankari, H.; Hietikko, M.; Kurppa, K.; Kaukinen, K.; Mansikka, E.; Huhtala, H.; Laurila, K.; Reunala, T.; Hervonen, K.; Salmi, T.; et al. Intestinal TG3- and TG2-Specific Plasma Cell Responses in Dermatitis Herpetiformis Patients Undergoing a Gluten Challenge. Nutrients 2020, 12, E467. [Google Scholar] [CrossRef] [PubMed]
- Iversen, R.; Amundsen, S.F.; Kleppa, L.; du Pré, M.F.; Stamnaes, J.; Sollid, L.M. Evidence That Pathogenic Transglutaminase 2 in Celiac Disease Derives From Enterocytes. Gastroenterology 2020, 159, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Heil, P.M.; Volc-Platzer, B.; Karlhofer, F.; Gebhart, W.; Huber, W.-D.; Benesch, T.; Vogelsang, H.; Stingl, G. Transglutaminases as Diagnostically Relevant Autoantigens in Patients with Gluten Sensitivity. JDDG J. Dtsch. Dermatol. Ges. 2005, 3, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Jaskowski, T.D.; Hamblin, T.; Wilson, A.R.; Hill, H.R.; Book, L.S.; Meyer, L.J.; Zone, J.J.; Hull, C.M. IgA Anti-Epidermal Transglutaminase Antibodies in Dermatitis Herpetiformis and Pediatric Celiac Disease. J. Investig. Dermatol. 2009, 129, 2728–2730. [Google Scholar] [CrossRef] [PubMed]
- Marietta, E.V.; Camilleri, M.J.; Castro, L.A.; Krause, P.K.; Pittelkow, M.R.; Murray, J.A. Transglutaminase Autoantibodies in Dermatitis Herpetiformis and Celiac Sprue. J. Investig. Dermatol. 2008, 128, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Reunala, T.; Salmi, T.T.; Hervonen, K.; Laurila, K.; Kautiainen, H.; Collin, P.; Kaukinen, K. IgA Antiepidermal Transglutaminase Antibodies in Dermatitis Herpetiformis: A Significant but Not Complete Response to a Gluten-Free Diet Treatment. Br. J. Dermatol. 2015, 172, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Llamazares, J.; Gibson, L.E.; Rogers, R.S. Clinical, Pathologic, and Immunopathologic Features of Dermatitis Herpetiformis: Review of the Mayo Clinic Experience. Int. J. Dermatol. 2007, 46, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Mansikka, E.; Salmi, T.; Kaukinen, K.; Collin, P.; Huhtala, H.; Reunala, T.; Hervonen, K. Diagnostic Delay in Dermatitis Herpetiformis in a High-Prevalence Area. Acta Derm. Venereol. 2018, 98, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Görög, A.; Németh, K.; Kolev, K.; Zone, J.J.; Mayer, B.; Silló, P.; Bognár, P.; Kárpáti, S. Circulating Transglutaminase 3-Immunoglobulin A Immune Complexes in Dermatitis Herpetiformis. J. Investig. Dermatol. 2016, 136, 1729–1731. [Google Scholar] [CrossRef][Green Version]
- Zone, J.J.; Schmidt, L.A.; Taylor, T.B.; Hull, C.M.; Sotiriou, M.C.; Jaskowski, T.D.; Hill, H.R.; Meyer, L.J. Dermatitis Herpetiformis Sera or Goat Anti–Transglutaminase-3 Transferred to Human Skin-Grafted Mice Mimics Dermatitis Herpetiformis Immunopathology. J. Immunol. 2011, 186, 4474–4480. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Bhatt, M.L.B.; Goel, M.M.; Gupta, S.; Mahdi, A.A.; Mishra, A.; Mehrotra, D. Tissue and Serum Expression of TGM-3 May Be Prognostic Marker in Patients of Oral Squamous Cell Carcinoma Undergoing Chemo-Radiotherapy. PLoS ONE 2018, 13, e0199665. [Google Scholar] [CrossRef]
- Kemppainen, E.; Salmi, T.; Lindfors, K. Missing Insight Into T and B Cell Responses in Dermatitis Herpetiformis. Front. Immunol. 2021, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Hietikko, M.; Hervonen, K.; Salmi, T.; Ilus, T.; Zone, J.J.; Kaukinen, K.; Reunala, T.; Lindfors, K. Disappearance of Epidermal Transglutaminase and IgA Deposits from the Papillary Dermis of Patients with Dermatitis Herpetiformis after a Long-Term Gluten-Free Diet. Br. J. Dermatol. 2018, 178, e198–e201. [Google Scholar] [CrossRef] [PubMed]
- Mansikka, E.; Hervonen, K.; Kaukinen, K.; Ilus, T.; Oksanen, P.; Lindfors, K.; Laurila, K.; Hietikko, M.; Taavela, J.; Jernman, J.; et al. Gluten Challenge Induces Skin and Small Bowel Relapse in Long-Term Gluten-Free Diet-Treated Dermatitis Herpetiformis. J. Investig. Dermatol. 2019, 139, 2108–2114. [Google Scholar] [CrossRef]
- Antiga, E.; Maglie, R.; Lami, G.; Tozzi, A.; Bonciolini, V.; Calella, F.; Bianchi, B.; del Bianco, E.; Renzi, D.; Mazzarese, E.; et al. Granular Deposits of IgA in the Skin of Coeliac Patients Without Dermatitis Herpetiformis: A Prospective Multicentric Analysis. Acta Derm. Venereol. 2021, 101, adv00382. [Google Scholar] [CrossRef] [PubMed]
- Bonciolini, V.; Antiga, E.; Bianchi, B.; Del Bianco, E.; Ninci, A.; Maio, V.; Pimpinelli, N.; Caproni, M. Granular IgA Deposits in the Skin of Patients with Coeliac Disease: Is It Always Dermatitis Herpetiformis? Acta Derm. Venereol. 2019, 99, 78–83. [Google Scholar] [CrossRef]
- Cannistraci, C.; Lesnoni La Parola, I.; Cardinali, G.; Bolasco, G.; Aspite, N.; Stigliano, V.; Picardo, M. Co-Localization of IgA and TG3 on Healthy Skin of Coeliac Patients. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 509–514. [Google Scholar] [CrossRef]
- Unsworth, D.J.; Leonard, J.N.; Payne, A.W.; Fry, L.; Holborow, E.J. Iga in Dermatitis-Herpetiformis Skin is Dimeric. Lancet 1982, 319, 478–480. [Google Scholar] [CrossRef]
- Hall, R.P.; Lawley, T.J. Characterization of Circulating and Cutaneous IgA Immune Complexes in Patients with Dermatitis Herpetiformis. J. Immunol. 1985, 135, 1760–1765. [Google Scholar]
- Wojnarowska, F.; Delacroix, D.; Gengoux, P. Cutaneous IgA Subclasses in Dermatitis Herpetiformis and Linear IgA Disease. J. Cutan. Pathol. 1988, 15, 272–275. [Google Scholar] [CrossRef]
- Lemke, A.; Kraft, M.; Roth, K.; Riedel, R.; Lammerding, D.; Hauser, A.E. Long-Lived Plasma Cells Are Generated in Mucosal Immune Responses and Contribute to the Bone Marrow Plasma Cell Pool in Mice. Mucosal Immunol. 2016, 9, 83–97. [Google Scholar] [CrossRef]
- Mei, H.E.; Wirries, I.; Frölich, D.; Brisslert, M.; Giesecke, C.; Grün, J.R.; Alexander, T.; Schmidt, S.; Luda, K.; Kühl, A.A.; et al. A Unique Population of IgG-Expressing Plasma Cells Lacking CD19 Is Enriched in Human Bone Marrow. Blood 2015, 125, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.J. The Clinical Response to Gluten Challenge: A Review of the Literature. Nutrients 2013, 5, 4614–4641. [Google Scholar] [CrossRef] [PubMed]
- Vossenkämper, A.; Blair, P.A.; Safinia, N.; Fraser, L.D.; Das, L.; Sanders, T.J.; Stagg, A.J.; Sanderson, J.D.; Taylor, K.; Chang, F.; et al. A Role for Gut-Associated Lymphoid Tissue in Shaping the Human B Cell Repertoire. J. Exp. Med. 2013, 210, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaunisto, H.; Salmi, T.; Lindfors, K.; Kemppainen, E. Antibody Responses to Transglutaminase 3 in Dermatitis Herpetiformis: Lessons from Celiac Disease. Int. J. Mol. Sci. 2022, 23, 2910. https://doi.org/10.3390/ijms23062910
Kaunisto H, Salmi T, Lindfors K, Kemppainen E. Antibody Responses to Transglutaminase 3 in Dermatitis Herpetiformis: Lessons from Celiac Disease. International Journal of Molecular Sciences. 2022; 23(6):2910. https://doi.org/10.3390/ijms23062910
Chicago/Turabian StyleKaunisto, Helka, Teea Salmi, Katri Lindfors, and Esko Kemppainen. 2022. "Antibody Responses to Transglutaminase 3 in Dermatitis Herpetiformis: Lessons from Celiac Disease" International Journal of Molecular Sciences 23, no. 6: 2910. https://doi.org/10.3390/ijms23062910
APA StyleKaunisto, H., Salmi, T., Lindfors, K., & Kemppainen, E. (2022). Antibody Responses to Transglutaminase 3 in Dermatitis Herpetiformis: Lessons from Celiac Disease. International Journal of Molecular Sciences, 23(6), 2910. https://doi.org/10.3390/ijms23062910





