Human Amnion Epithelial Cells: A Potential Cell Source for Pulp Regeneration?
Abstract
1. Introduction
2. Results
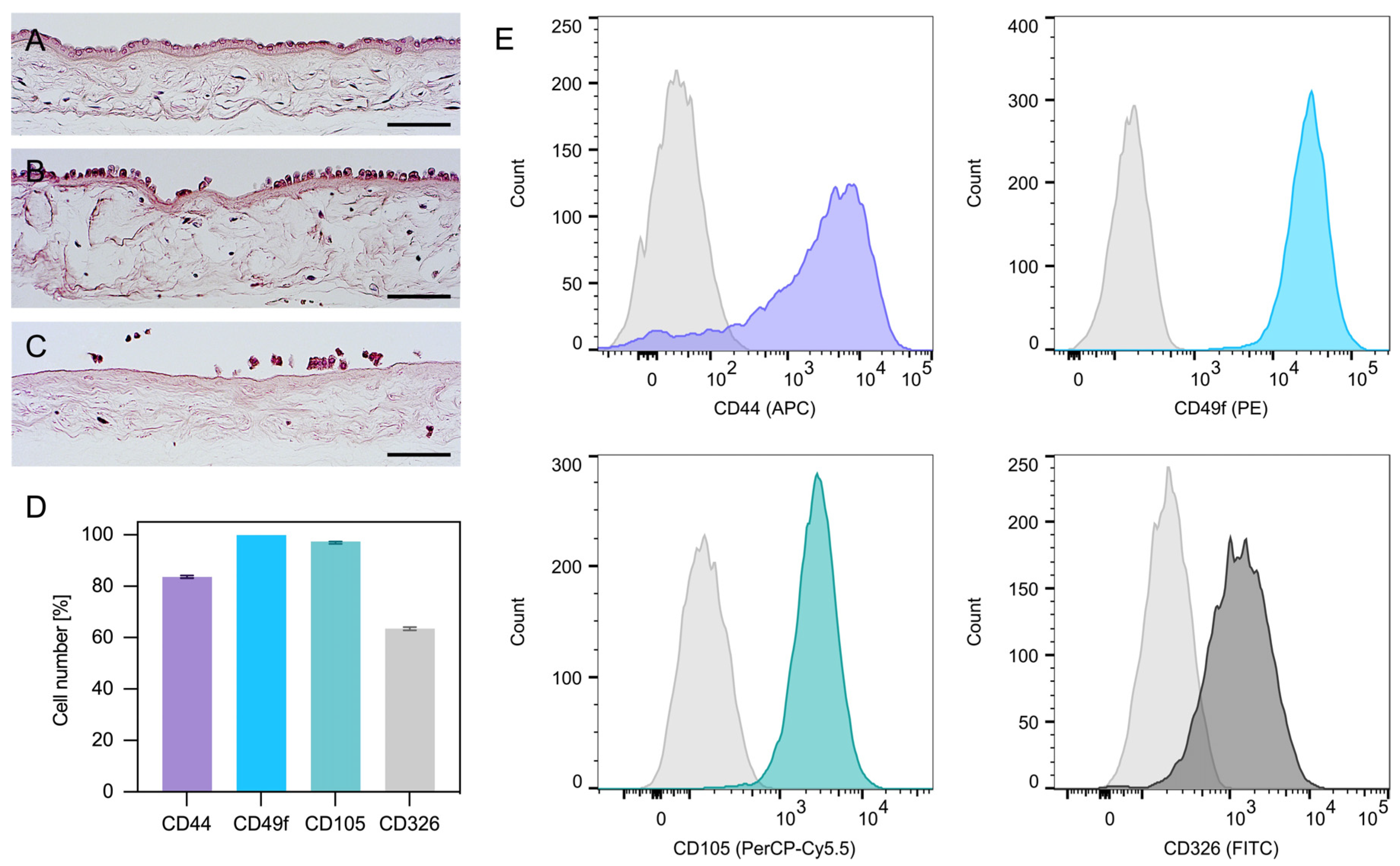
2.1. Isolation and Characterization of hAECs
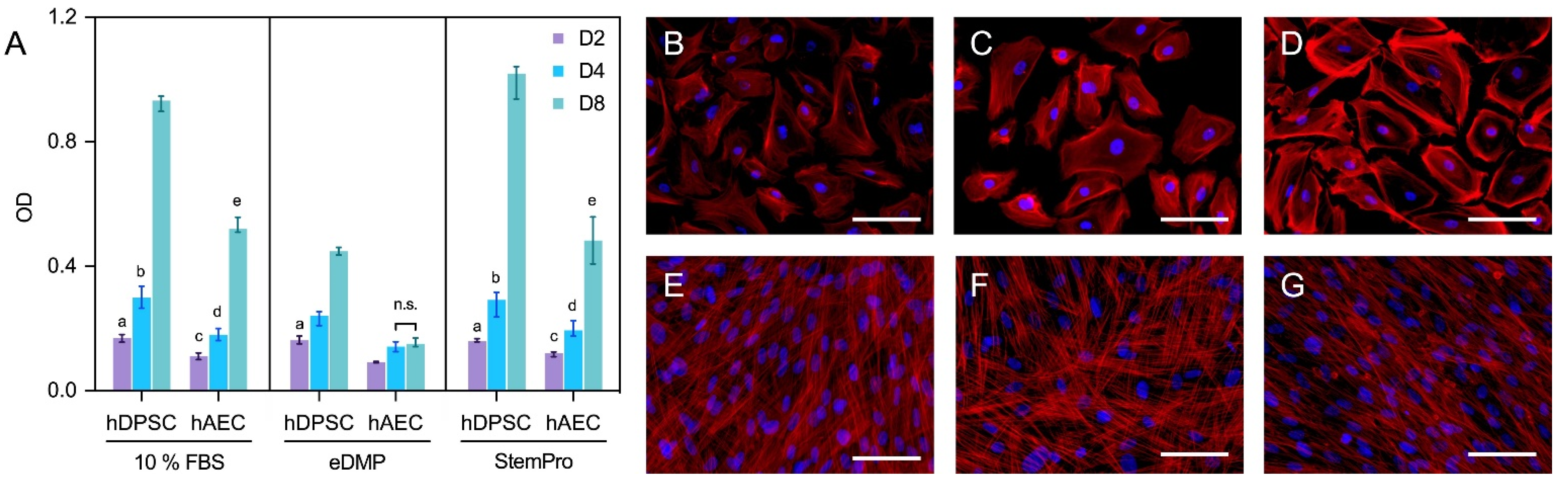
2.2. Cell Viability
2.3. Fluorescence Microscopy
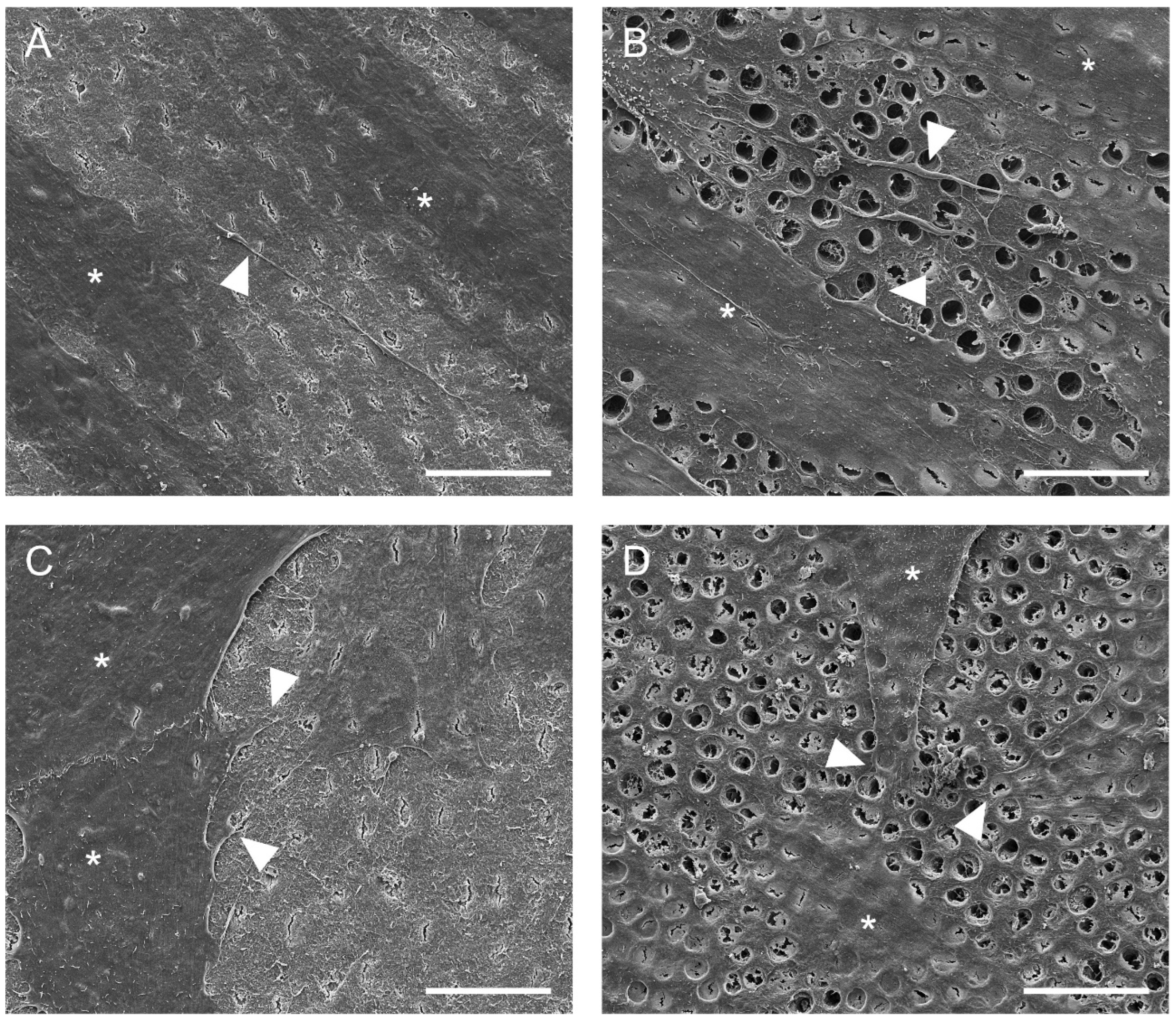
2.4. Cell Adhesion to Dentin
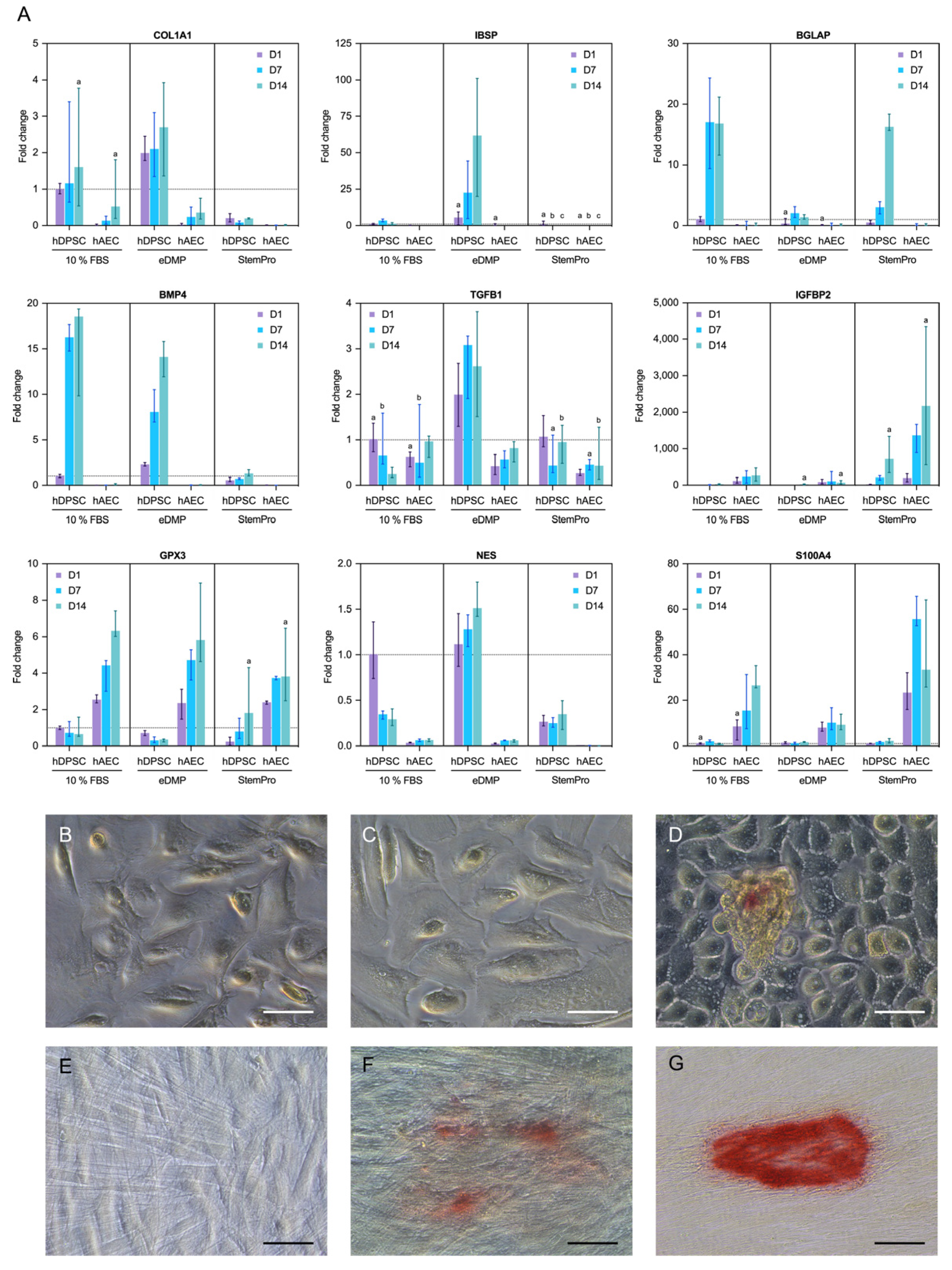
2.5. Gene Expression
2.6. Mineralization
3. Discussion
3.1. Cell Adhesion to Dentin
3.2. Mineralization
3.3. Cell Differentiation
3.4. Epithelial–Mesenchymal Transition
3.5. Impact of Culture Conditions
4. Materials and Methods
4.1. Isolation and Characterization of hAECs
4.2. Isolation and Characterization of hDPSCs
4.3. Extraction of Dentin Matrix Proteins (eDMPs)
4.4. Cell Viability
4.5. Fluorescence Microscopy
4.6. Cell Adhesion to Dentin
4.7. Gene Expression
4.8. Mineralization
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, P.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Dent. Traumatol. 1992, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.J.; Rajan, S.; Bhujel, N.; Kang, J.; Duggal, M.; Nazzal, H. Regenerative Endodontic Therapy in the Management of Nonvital Immature Permanent Teeth: A Systematic Review—Outcome Evaluation and Meta-analysis. J. Endod. 2017, 43, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zeng, Q.; Wei, X.; Zhao, W.; Cui, M.; Gu, J.; Lu, J.; Yang, M.; Ling, J. Regenerative Endodontics Versus Apexification in Immature Permanent Teeth with Apical Periodontitis: A Prospective Randomized Controlled Study. J. Endod. 2017, 43, 1821–1827. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, H.; Peng, C. Clinical and Radiographic Assessment of the Efficacy of a Collagen Membrane in Regenerative Endodontics: A Randomized, Controlled Clinical Trial. J. Endod. 2017, 43, 1465–1471. [Google Scholar] [CrossRef]
- Nazzal, H.; Kenny, K.; Altimimi, A.; Kang, J.; Duggal, M.S. A prospective clinical study of regenerative endodontic treatment of traumatized immature teeth with necrotic pulps using bi-antibiotic paste. Int. Endod. J. 2018, 51, e204–e215. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, X.; Huang, G.T.-J.; Cheung, G.S.P.; Dissanayaka, W.; Zhang, C. Regeneration of dental pulp tissue in immature teeth with apical periodontitis using platelet-rich plasma and dental pulp cells. Int. Endod. J. 2013, 46, 962–970. [Google Scholar] [CrossRef]
- Meschi, N.; Hilkens, P.; Lambrichts, I.; Van den Eynde, K.; Mavridou, A.; Strijbos, O.; De Ketelaere, M.; Van Gorp, G.; Lambrechts, P. Regenerative endodontic procedure of an infected immature permanent human tooth: An immunohistological study. Clin. Oral Investig. 2015, 20, 807–814. [Google Scholar] [CrossRef]
- Wang, X.; Thibodeau, B.; Trope, M.; Lin, L.M.; Huang, G.T.-J. Histologic Characterization of Regenerated Tissues in Canal Space after the Revitalization/Revascularization Procedure of Immature Dog Teeth with Apical Periodontitis. J. Endod. 2010, 36, 56–63. [Google Scholar] [CrossRef]
- Bucchi, C.; Marcé-Nogué, J.; Galler, K.M.; Widbiller, M. Biomechanical performance of an immature maxillary central incisor after revitalization: A finite element analysis. Int. Endod. J. 2019, 52, 1508–1518. [Google Scholar] [CrossRef]
- Orti, V.; Collart-Dutilleul, P.-Y.; Piglionico, S.; Pall, O.; Cuisinier, F.; Panayotov, I. Pulp Regeneration Concepts for Nonvital Teeth: From Tissue Engineering to Clinical Approaches. Tissue Eng. Part B Rev. 2018, 24, 419–442. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Widbiller, M. Perspectives for Cell-homing Approaches to Engineer Dental Pulp. J. Endod. 2017, 43, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, J.; Gong, Q.; Cheng, B.; Kim, S.G.; Ling, J.; Mao, J.J. Regenerative Endodontics by Cell Homing. Dent. Clin. 2017, 61, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef]
- Ducret, M.; Fabre, H.; Celle, A.; Mallein-Gerin, F.; Perrier-Groult, E.; Alliot-Licht, B.; Farges, J.-C. Current challenges in human tooth revitalization. Bio.-Med. Mater. Eng. 2017, 28, S159–S168. [Google Scholar] [CrossRef]
- Huang, G.T.-J. Dental pulp and dentin tissue engineering and regeneration advancement and challenge. Front. Biosci. 2011, 3, 788–800. [Google Scholar] [CrossRef]
- Couve, E.; Osorio, R.; Schmachtenberg, O. Reactionary Dentinogenesis and Neuroimmune Response in Dental Caries. J. Dent. Res. 2014, 93, 788–793. [Google Scholar] [CrossRef]
- Couve, E.; Osorio, R.; Schmachtenberg, O. The Amazing Odontoblast. J. Dent. Res. 2013, 92, 765–772. [Google Scholar] [CrossRef]
- Farges, J.-C.; Keller, J.-F.; Carrouel, F.; Durand, S.H.; Romeas, A.; Bleicher, F.; Lebecque, S.; Staquet, M.-J. Odontoblasts in the dental pulp immune response. J. Exp. Zoöl. Part B Mol. Dev. Evol. 2009, 312, 425–436. [Google Scholar] [CrossRef]
- Farges, J.-C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediat. Inflamm. 2015, 2015, 230251. [Google Scholar] [CrossRef]
- Farges, J.-C.; Bellanger, A.; Ducret, M.; Aubert-Foucher, E.; Richard, B.; Alliot-Licht, B.; Bleicher, F.; Carrouel, F. Human odontoblast-like cells produce nitric oxide with antibacterial activity upon TLR2 activation. Front. Physiol. 2015, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Farges, J.-C.; Carrouel, F.; Keller, J.-F.; Baudouin, C.; Msika, P.; Bleicher, F.; Staquet, M.-J. Cytokine production by human odontoblast-like cells upon Toll-like receptor-2 engagement. Immunobiology 2011, 216, 513–517. [Google Scholar] [CrossRef]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete Pulp Regeneration after Pulpectomy by Transplantation of CD105+ Stem Cells with Stromal Cell-Derived Factor-1. Tissue Eng. Part A 2011, 17, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Eidt, A.; Lindner, S.R.; Hiller, K.-A.; Schweikl, H.; Buchalla, W.; Galler, K.M. Dentine matrix proteins: Isolation and effects on human pulp cells. Int. Endod. J. 2018, 51, e278–e290. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.; Federlin, M.; Cavender, A.C.; Hartgerink, J.; Hecker, S.; Schmalz, G. Dentin Conditioning Codetermines Cell Fate in Regenerative Endodontics. J. Endod. 2011, 37, 1536–1541. [Google Scholar] [CrossRef]
- Miki, T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am. J. Reprod. Immunol. 2018, 80, e13003. [Google Scholar] [CrossRef]
- Gramignoli, R.; Srinivasan, R.C.; Kannisto, K.; Strom, S.C. Isolation of Human Amnion Epithelial Cells According to Current Good Manufacturing Procedures. Curr. Protoc. Stem Cell Biol. 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Ilancheran, S.; Michalska, A.; Peh, G.; Wallace, E.M.; Pera, M.; Manuelpillai, U. Stem Cells Derived from Human Fetal Membranes Display Multilineage Differentiation Potential. Biol. Reprod. 2007, 77, 577–588. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-J.; Yuan, W.-X.; Liu, J.; Li, J.-Y.; Tan, B.; Qiu, C.; Zhu, X.-L.; Qiu, C.; Lai, D.-M.; Guo, L.-H.; et al. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol. Sin. 2018, 39, 1305–1316. [Google Scholar] [CrossRef]
- Nemr, W.; Bashandy, M.; Araby, E.; Khamiss, O. Molecular displaying of differential immunoresponse to various infections of amniotic epithelia. Am. J. Reprod. Immunol. 2017, 77, e12662. [Google Scholar] [CrossRef]
- Motedayyen, H.; Fathi, F.; Fasihi-Ramandi, M.; Taheri, R.A. The effect of lipopolysaccharide on anti-inflammatory and pro-inflammatory cytokines production of human amniotic epithelial cells. Reprod. Biol. 2018, 18, 404–409. [Google Scholar] [CrossRef]
- Zhu, D.; Muljadi, R.; Chan, S.T.; Vosdoganes, P.; Lo, C.; Mockler, J.C.; Wallace, E.; Lim, R. Evaluating the Impact of Human Amnion Epithelial Cells on Angiogenesis. Stem Cells Int. 2015, 2016, 4565612. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem Cell Characteristics of Amniotic Epithelial Cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Hodge, A.; Moore, G.; Wallace, E.M.; Sievert, W. A Pilot Study Evaluating the Safety of Intravenously Administered Human Amnion Epithelial Cells for the Treatment of Hepatic Fibrosis. Front. Pharmacol. 2017, 8, 549. [Google Scholar] [CrossRef]
- Miki, T.; Strom, S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. Rep. 2006, 2, 133–141. [Google Scholar] [CrossRef]
- Bajaj, M.; Soni, A.J. Revascularization of a Nonvital, Immature Permanent Tooth Using Amniotic Membrane: A Novel Approach. Int. J. Clin. Pediatr. Dent. 2019, 12, 150–152. [Google Scholar] [CrossRef]
- Chrepa, V.; Joon, R.; Austah, O.; Diogenes, A.; Hargreaves, K.M.; Ezeldeen, M.; Ruparel, N.B. Clinical Outcomes of Immature Teeth Treated with Regenerative Endodontic Procedures—A San Antonio Study. J. Endod. 2020, 46, 1074–1084. [Google Scholar] [CrossRef]
- Becerra, P.; Ricucci, D.; Loghin, S.; Gibbs, J.L.; Lin, L.M. Histologic Study of a Human Immature Permanent Premolar with Chronic Apical Abscess after Revascularization/Revitalization. J. Endod. 2014, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Zhou, L.; Sagayaraj, A.; Jumat, N.H.B.; Choolani, M.; Chan, J.K.Y.; Biswas, A.; Wong, P.C.; Lim, S.G.; Dan, Y.Y. Hepatic differentiation of human amniotic epithelial cells and in vivo therapeutic effect on animal model of cirrhosis. J. Gastroenterol. Hepatol. 2015, 30, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, M.; Gloria, A.; Turriani, M.; Mauro, A.; Curini, V.; Russo, V.; Tetè, S.; Marchisio, M.; Pierdomenico, L.; Berardinelli, P.; et al. Stemness characteristics and osteogenic potential of sheep amniotic epithelial cells. Cell Biol. Int. 2011, 36, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-H.; Jin, J.; Joe, J.-H.; Song, Y.-S.; So, B.-I.; Lim, S.M.; Cheon, G.J.; Woo, S.-K.; Ra, J.-C.; Lee, Y.-Y.; et al. In Vivo Differentiation of Human Amniotic Epithelial Cells into Cardiomyocyte-Like Cells and Cell Transplantation Effect on Myocardial Infarction in Rats: Comparison with Cord Blood and Adipose Tissue-Derived Mesenchymal Stem Cells. Cell Transplant. 2012, 21, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.-A.; Hoffer, P.C.; Schmalz, G.H. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int. Endod. J. 2016, 49, 581–590. [Google Scholar] [CrossRef]
- Morago, A.; Ruiz-Linares, M.; Ferrer-Luque, C.M.; Baca, P.; Archilla, A.R.; Arias-Moliz, M.T. Dentine tubule disinfection by different irrigation protocols. Microsc. Res. Tech. 2019, 82, 558–563. [Google Scholar] [CrossRef]
- Galler, K.M.; Buchalla, W.; Hiller, K.-A.; Federlin, M.; Eidt, A.; Schiefersteiner, M.; Schmalz, G. Influence of Root Canal Disinfectants on Growth Factor Release from Dentin. J. Endod. 2015, 41, 363–368. [Google Scholar] [CrossRef]
- Martin, D.E.; de Almeida, J.F.A.; Henry, M.A.; Khaing, Z.; Schmidt, C.E.; Teixeira, F.B.; Diogenes, A. Concentration-dependent Effect of Sodium Hypochlorite on Stem Cells of Apical Papilla Survival and Differentiation. J. Endod. 2014, 40, 51–55. [Google Scholar] [CrossRef]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Widbiller, M.; Eidt, A.; Wölflick, M.; Lindner, S.R.; Schweikl, H.; Hiller, K.-A.; Buchalla, W.; Galler, K.M. Interactive effects of LPS and dentine matrix proteins on human dental pulp stem cells. Int. Endod. J. 2018, 51, 877–888. [Google Scholar] [CrossRef]
- Si, J.; Zhang, J.; Dai, J.; Yu, D.; Yu, H.; Shi, J.; Wang, X.; Shen, S.G.F.; Guo, L. Osteogenic Differentiation of Human Amniotic Epithelial Cells and Its Application in Alveolar Defect Restoration. Stem Cells Transl. Med. 2014, 3, 1504–1513. [Google Scholar] [CrossRef]
- Fatimah, S.S.; Ng, S.L.; Chua, K.H.; Hayati, A.R.; Tan, A.E.; Tan, G.C. Value of human amniotic epithelial cells in tissue engineering for cornea. Hum. Cell 2010, 23, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Shagramanova, K.; Chan, S.W. Formation of Odontoblast-Like Cells from Cultured Human Dental Pulp Cells on Dentin In Vitro. J. Endod. 2006, 32, 1066–1073. [Google Scholar] [CrossRef]
- Liu, G.; Xu, G.; Gao, Z.; Liu, Z.; Xu, J.; Wang, J.; Zhang, C.; Wang, S. Demineralized Dentin Matrix Induces Odontoblastic Differentiation of Dental Pulp Stem Cells. Cells Tissues Organs 2016, 201, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Braut, A.; Kollar, E.J.; Mina, M. Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a Col1a1-2.3-GFP transgene. Int. J. Dev. Biol. 2003, 47, 281–292. [Google Scholar] [PubMed]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]
- Kaneto, C.M.; Lima, P.S.P.; Zanette, D.L.; Oliveira, T.Y.K.; Pereira, F.D.A.; Lorenzi, J.C.C.; Dos Santos, J.L.; Prata, K.L.; Neto, J.M.P.; De Paula, F.J.A.; et al. Osteoblastic differentiation of bone marrow mesenchymal stromal cells in Bruck Syndrome. BMC Med Genet. 2016, 17, 38. [Google Scholar] [CrossRef]
- Simon, S.; Smith, A.; Lumley, P.; Berdal, A.; Smith, G.; Finney, S.; Cooper, P. Molecular characterization of young and mature odontoblasts. Bone 2009, 45, 693–703. [Google Scholar] [CrossRef]
- Smith, A.J.; Scheven, B.A.; Takahashi, Y.; Ferracane, J.L.; Shelton, R.M.; Cooper, P.R. Dentine as a bioactive extracellular matrix. Arch. Oral Biol. 2012, 57, 109–121. [Google Scholar] [CrossRef]
- Chen, L.; Jacquet, R.; Lowder, E.; Landis, W.J. Refinement of collagen–mineral interaction: A possible role for osteocalcin in apatite crystal nucleation, growth and development. Bone 2015, 71, 7–16. [Google Scholar] [CrossRef]
- Dacic, S.; Kalajzic, I.; Visnjic, D.; Lichtler, A.C.; Rowe, D.W. Col1a1-Driven Transgenic Markers of Osteoblast Lineage Progression. J. Bone Miner. Res. 2001, 16, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Nagasawa, H.; Yamada, Y.; Reddi, A.H. Regulatory Role of Transforming Growth Factor-β, Bone Morphogenetic Protein-2, and Protein-4 on Gene Expression of Extracellular Matrix Proteins and Differentiation of Dental Pulp Cells. Dev. Biol. 1994, 162, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Nazarian, H.; Khojasteh, A.; Shokouhinejad, N. Gene Expression and Cytokine Release during Odontogenic Differentiation of Human Dental Pulp Stem Cells Induced by 2 Endodontic Biomaterials. J. Endod. 2014, 40, 387–392. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, M.J.; Javed, A. Dentin and Bone: Similar Collagenous Mineralized Tissues. In Bone and Development; Bronner, F., Farach-Carson, M.C., Roach, H.T., Eds.; Springer London: London, UK, 2010; pp. 183–200. [Google Scholar] [CrossRef]
- Niwa, T.; Yamakoshi, Y.; Yamazaki, H.; Karakida, T.; Chiba, R.; Hu, J.C.C.; Nagano, T.; Yamamoto, R.; Simmer, J.P.; Margo-lis, H.C.; et al. The dynamics of TGF-β in dental pulp, odontoblasts and dentin. Sci. Rep. 2018, 8, 4450. [Google Scholar] [CrossRef] [PubMed]
- About, I.; Laurent-Maquin, D.; Lendahl, U.; Mitsiadis, T.A. Nestin Expression in Embryonic and Adult Human Teeth under Normal and Pathological Conditions. Am. J. Pathol. 2000, 157, 287–295. [Google Scholar] [CrossRef]
- Dds, M.W.; Bucchi, C.; Rosendahl, A.; Spanier, G.; Buchalla, W.; Galler, K.M. Isolation of primary odontoblasts: Expectations and limitations. Aust. Endod. J. 2019, 45, 378–387. [Google Scholar] [CrossRef]
- Stadler, G.; Hennerbichler, S.; Lindenmair, A.; Peterbauer, A.; Hofer, K.; van Griensven, M.; Gabriel, C.; Redl, H.; Wolbank, S. Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy 2008, 10, 743–752. [Google Scholar] [CrossRef]
- Okada, H.; Danoff, T.M.; Kalluri, R.; Neilson, E.G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol. Physiol. 1997, 273, F563–F574. [Google Scholar] [CrossRef]
- Hay, E.D. An Overview of Epithelio-Mesenchymal Transformation. Cells Tissues Organs 1995, 154, 8–20. [Google Scholar] [CrossRef]
- Pratama, G.; Vaghjiani, V.; Tee, J.Y.; Liu, Y.H.; Chan, J.; Tan, C.; Murthi, P.; Gargett, C.; Manuelpillai, U. Changes in Culture Expanded Human Amniotic Epithelial Cells: Implications for Potential Therapeutic Applications. PLoS ONE 2011, 6, e26136. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Mosaffa, N.; Nikoo, S.; Bozorgmehr, M.; Ghods, R.; Kazemnejad, S.; Rezania, S.; Keshavarzi, B.; Arefi, S.; Ramezani-Tehrani, F.; et al. Isolation and partial characterization of human amniotic epithelial cells: The effect of tryp-sin. Avicenna. J. Med. Biotechnol. 2014, 6, 10–20. [Google Scholar] [PubMed]
- Murphy, S.V.; Kidyoor, A.; Reid, T.; Atala, A.; Wallace, E.M.; Lim, R. Isolation, Cryopreservation and Culture of Human Amnion Epithelial Cells for Clinical Applications. J. Vis. Exp. 2014, 94, e52085. [Google Scholar] [CrossRef] [PubMed]
- De Coppi, P.; Atala, A. Stem Cells from the Amnion. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–148. [Google Scholar] [CrossRef]
- Portmann-Lanz, C.B.; Schoeberlein, A.; Huber, A.; Sager, R.; Malek, A.; Holzgreve, W.; Surbek, D.V. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am. J. Obstet. Gynecol. 2006, 194, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wolbank, S.; Peterbauer, A.; Fahrner, M.; Hennerbichler, S.; van Griensven, M.; Stadler, G.; Redl, H.; Gabriel, C. Dose-Dependent Immunomodulatory Effect of Human Stem Cells from Amniotic Membrane: A Comparison with Human Mesenchymal Stem Cells from Adipose Tissue. Tissue Eng. 2007, 13, 1173–1183. [Google Scholar] [CrossRef]
- Toda, A.; Okabe, M.; Yoshida, T.; Nikaido, T. The Potential of Amniotic Membrane/Amnion-Derived Cells for Regeneration of Various Tissues. J. Pharmacol. Sci. 2007, 105, 215–228. [Google Scholar] [CrossRef]
- Ambartsumian, N.; Klingelhöfer, J.; Grigorian, M. The Multifaceted S100A4 Protein in Cancer and Inflammation. In Calcium Binding Proteins of the EF-Hand Superfamily; Heizmann, C.W., Ed.; Springer: New York, NY, USA, 2019; Volume 1929, pp. 339–365. [Google Scholar] [CrossRef]
- Li, T.; Forbes, M.E.; Fuller, G.N.; Li, J.; Yang, X.; Zhang, W. IGFBP2: Integrative hub of developmental and oncogenic signaling network. Oncogene 2020, 39, 2243–2257. [Google Scholar] [CrossRef]
- Zavadil, J.; Böttinger, E.P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 2005, 24, 5764–5774. [Google Scholar] [CrossRef]
- Alcaraz, A.; Mrowiec, A.; Insausti, C.L.; García-Vizcaíno, E.M.; Ruiz-Canada, C.; López-Martínez, C.; Moraleda, J.M.; Nicolás, F.J. Autocrine TGF-β Induces Epithelial to Mesenchymal Transition in Human Amniotic Epithelial Cells. Cell Transplant. 2013, 22, 1351–1367. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett. 2003, 540, 3–6. [Google Scholar] [CrossRef]
- Stolzing, A.; Sethe, S.; Scutt, A.M. Stressed Stem Cells: Temperature Response in Aged Mesenchymal Stem Cells. Stem Cells Dev. 2006, 15, 478–487. [Google Scholar] [CrossRef]
- Terada, S.; Matsuura, K.; Enosawa, S.; Miki, M.; Hoshika, A.; Suzuki, S.; Sakuragawa, N. Inducing Proliferation of Human Amniotic Epithelial (HAE) Cells for Cell Therapy. Cell Transplant. 2000, 9, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Rothmaier, C.; Saliter, D.; Wölflick, M.; Rosendahl, A.; Buchalla, W.; Schmalz, G.; Spruss, T.; Galler, K.M. Histology of human teeth: Standard and specific staining methods revisited. Arch. Oral Biol. 2021, 127, 105136. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Schweikl, H.; Thonemann, B.; D’Souza, R.N.; Schmalz, G. Human pulp-derived cells immortalized with Simian Virus 40 T-antigen. Eur. J. Oral Sci. 2006, 114, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucchi, C.; Ohlsson, E.; Anta, J.M.d.; Woelflick, M.; Galler, K.; Manzanares-Cespedes, M.C.; Widbiller, M. Human Amnion Epithelial Cells: A Potential Cell Source for Pulp Regeneration? Int. J. Mol. Sci. 2022, 23, 2830. https://doi.org/10.3390/ijms23052830
Bucchi C, Ohlsson E, Anta JMd, Woelflick M, Galler K, Manzanares-Cespedes MC, Widbiller M. Human Amnion Epithelial Cells: A Potential Cell Source for Pulp Regeneration? International Journal of Molecular Sciences. 2022; 23(5):2830. https://doi.org/10.3390/ijms23052830
Chicago/Turabian StyleBucchi, Cristina, Ella Ohlsson, Josep Maria de Anta, Melanie Woelflick, Kerstin Galler, María Cristina Manzanares-Cespedes, and Matthias Widbiller. 2022. "Human Amnion Epithelial Cells: A Potential Cell Source for Pulp Regeneration?" International Journal of Molecular Sciences 23, no. 5: 2830. https://doi.org/10.3390/ijms23052830
APA StyleBucchi, C., Ohlsson, E., Anta, J. M. d., Woelflick, M., Galler, K., Manzanares-Cespedes, M. C., & Widbiller, M. (2022). Human Amnion Epithelial Cells: A Potential Cell Source for Pulp Regeneration? International Journal of Molecular Sciences, 23(5), 2830. https://doi.org/10.3390/ijms23052830






