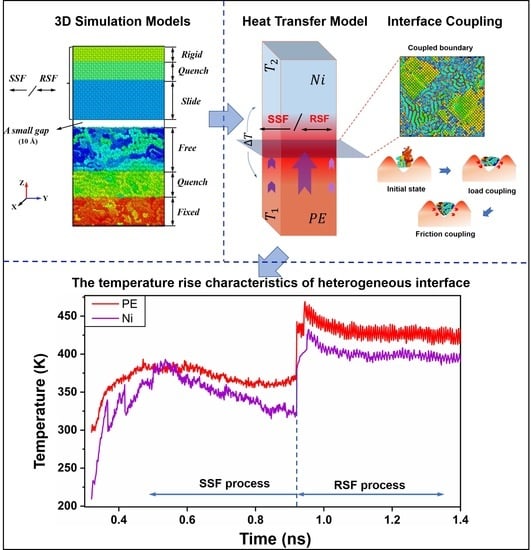
Polymer–Metal Interfacial Friction Characteristics under Ultrasonic Plasticizing Conditions: A United-Atom Molecular Dynamics Study
Abstract
1. Introduction
2. Results and Discussion
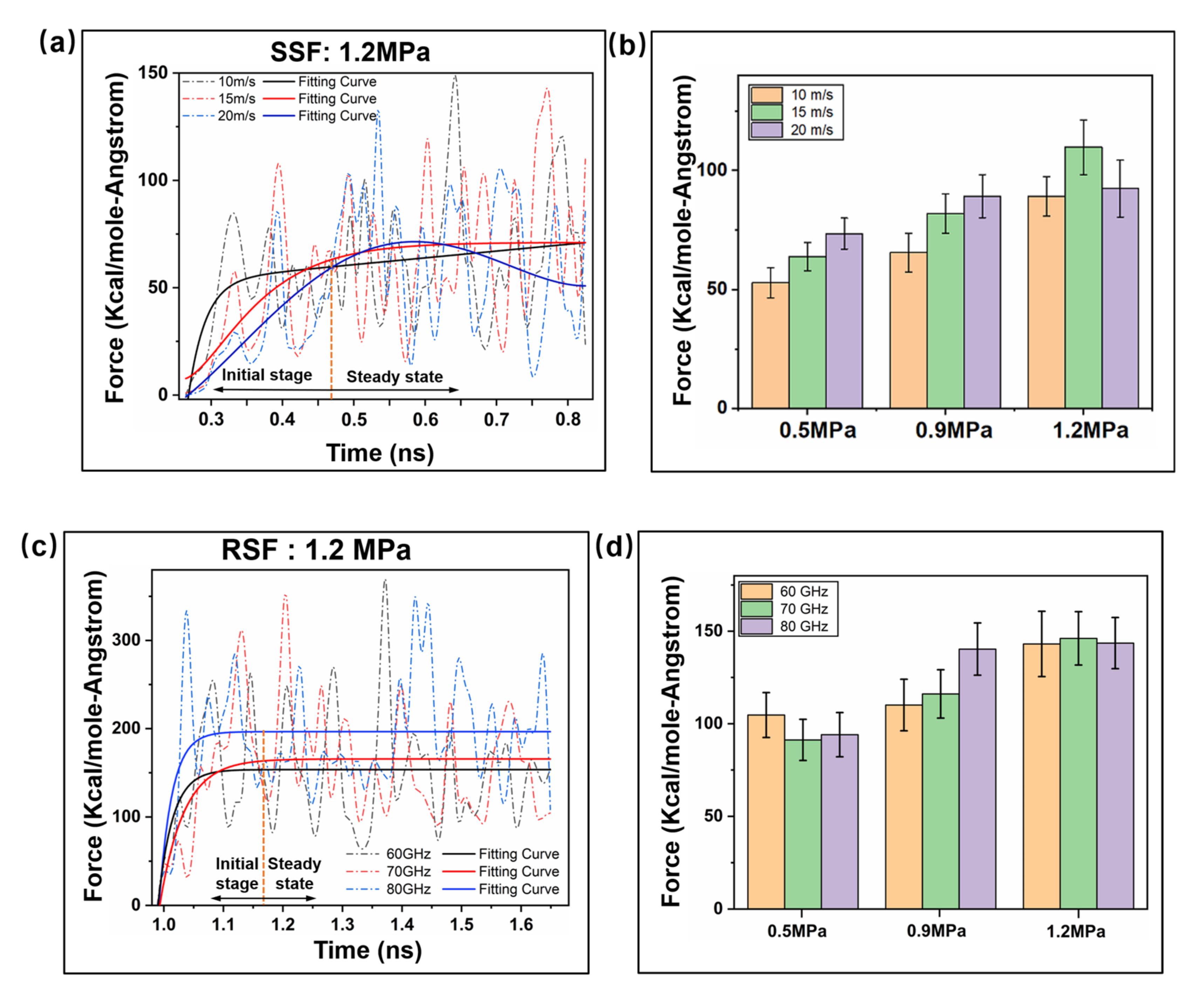
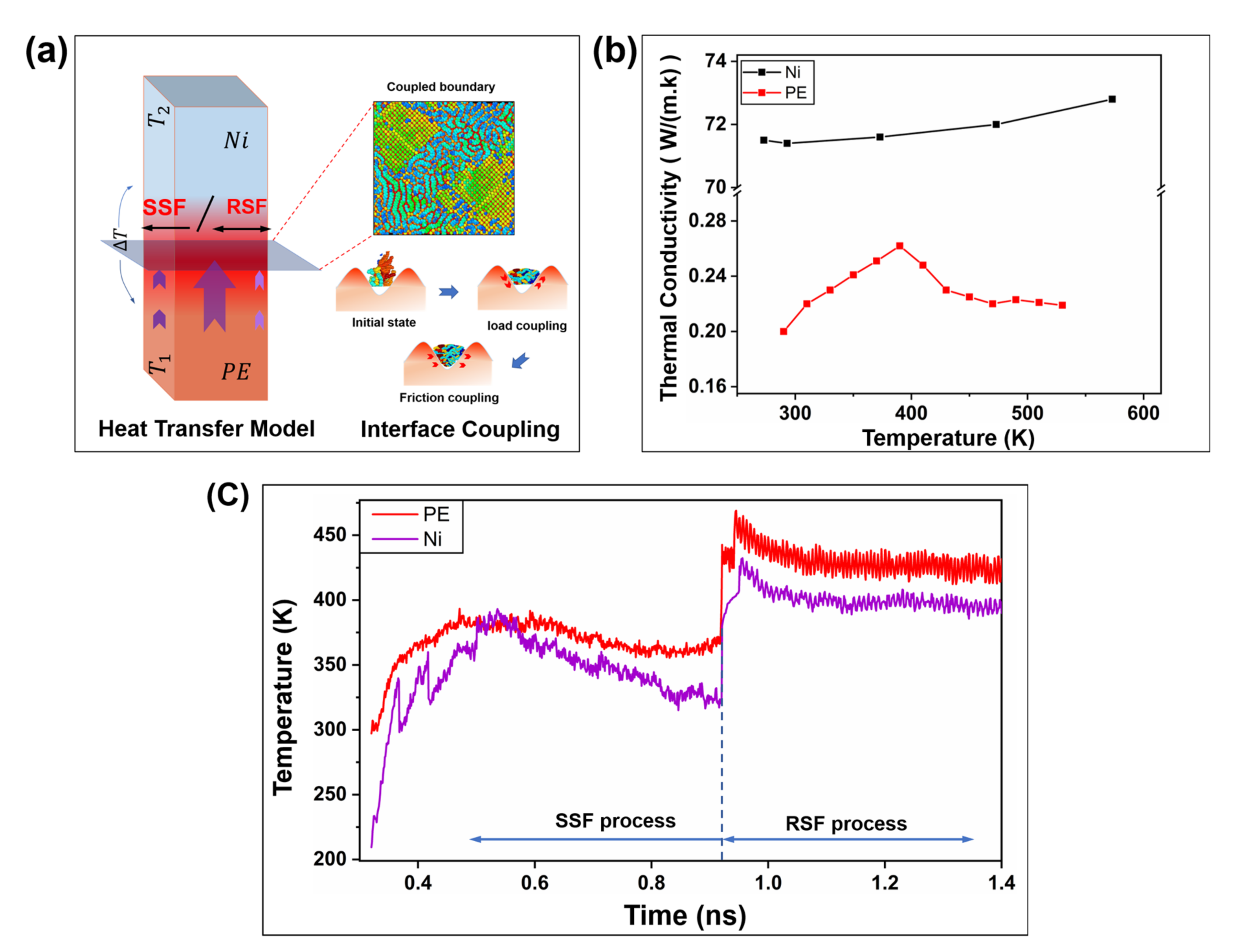
2.1. Interfacial Loading Characteristics under Precompaction
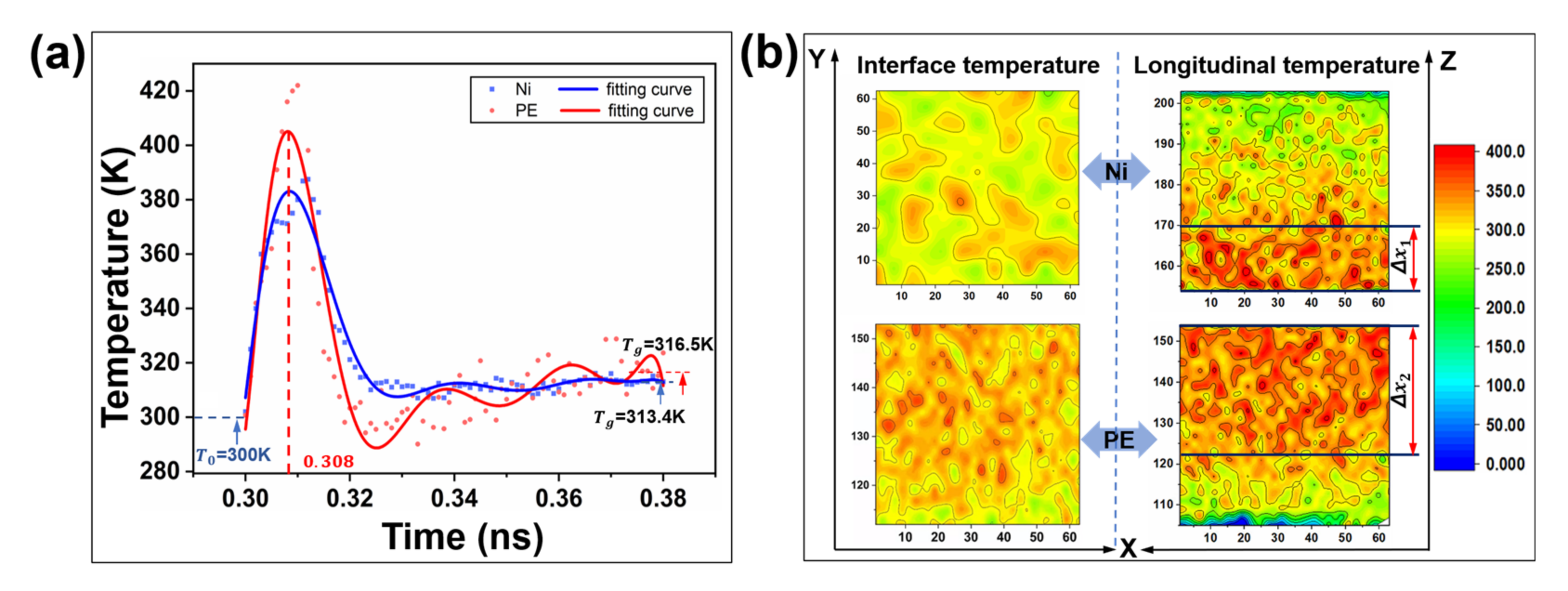
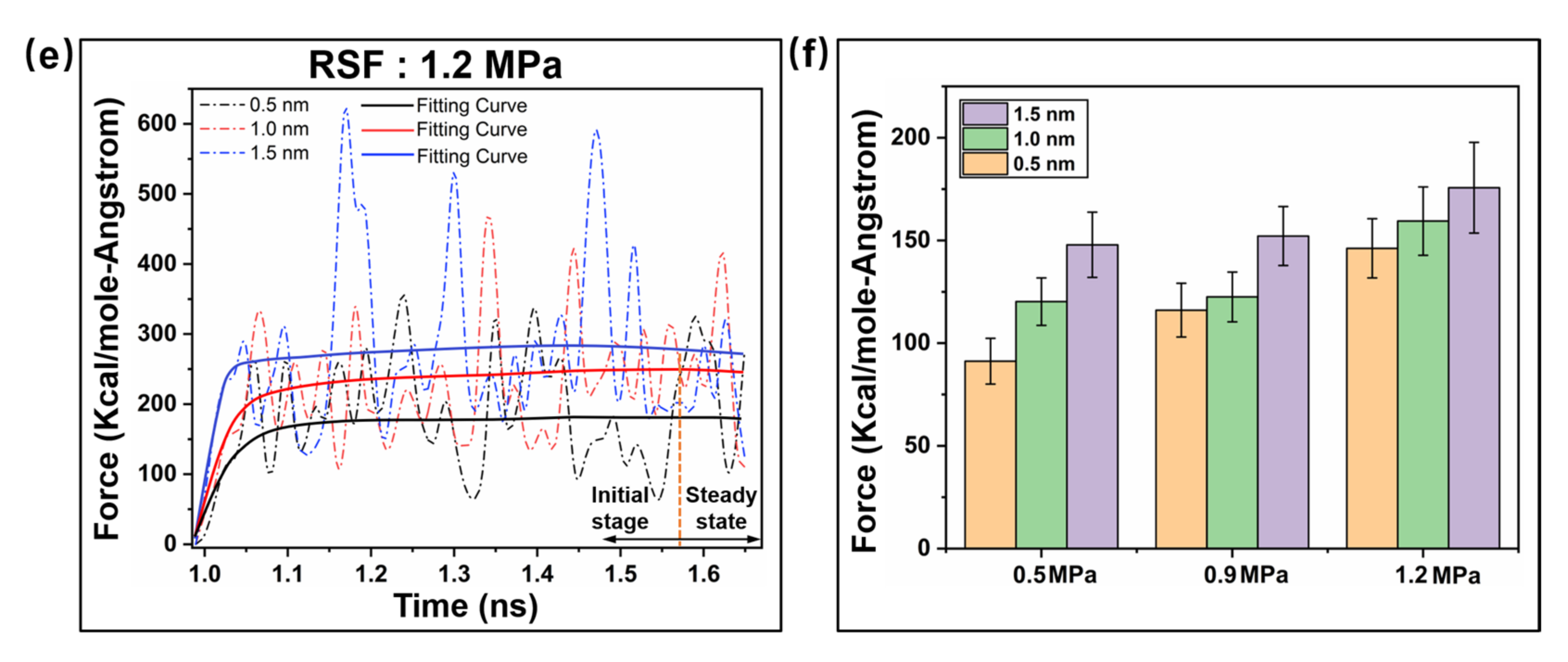
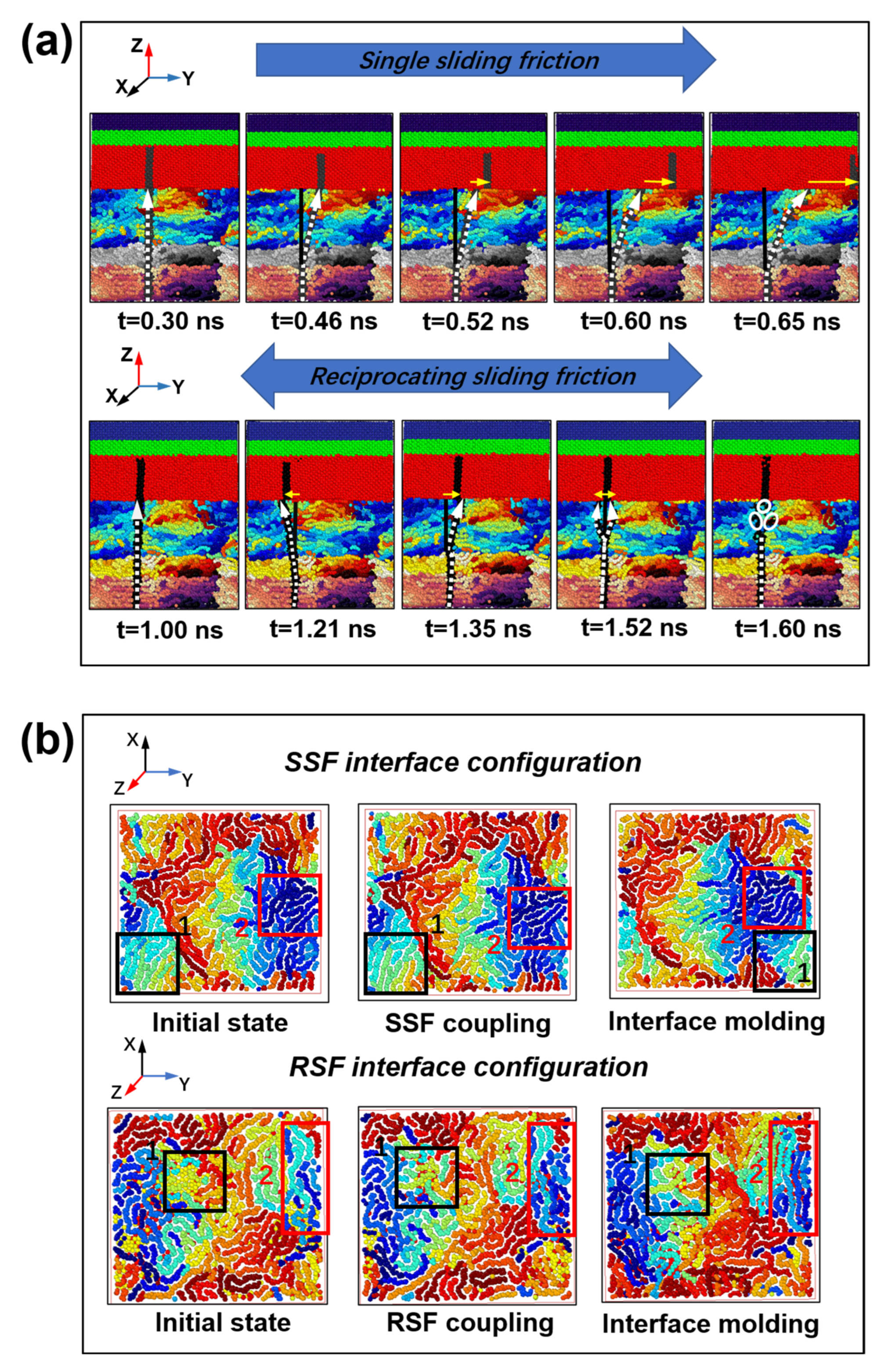
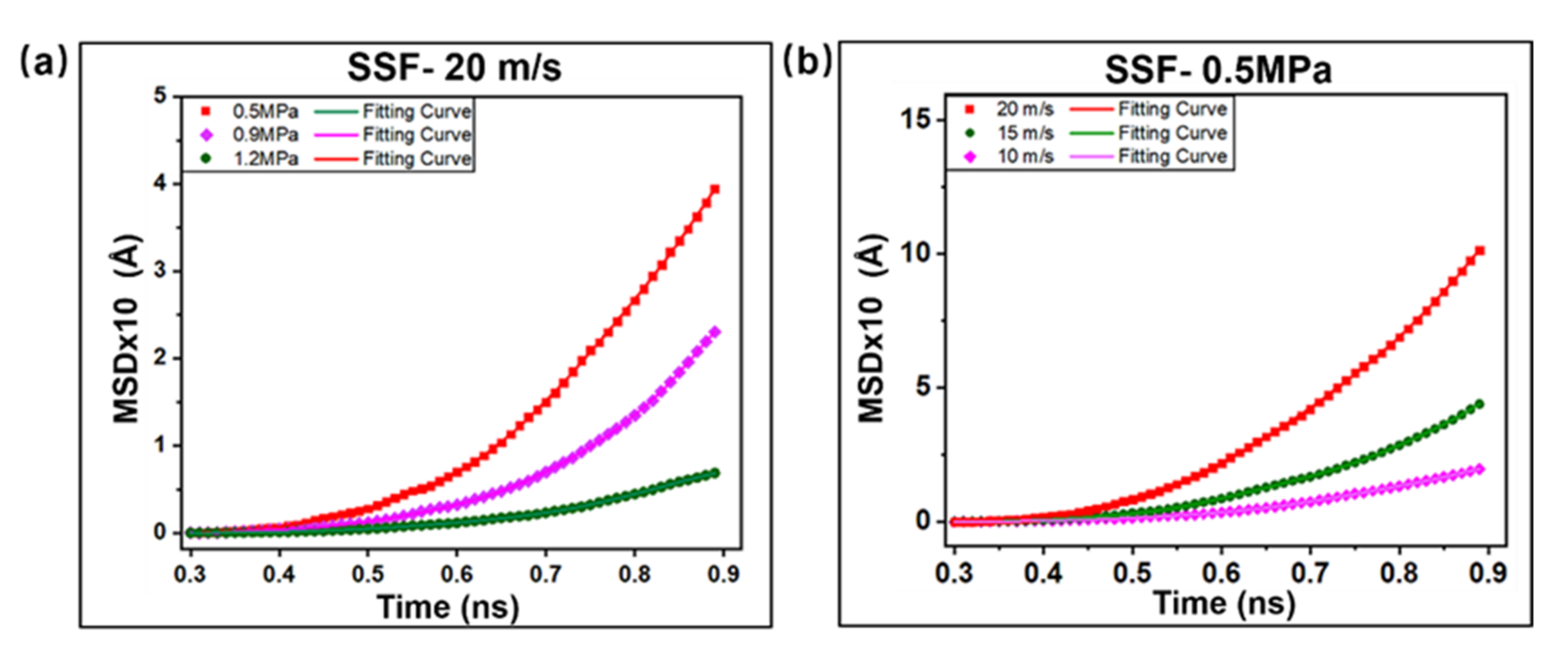
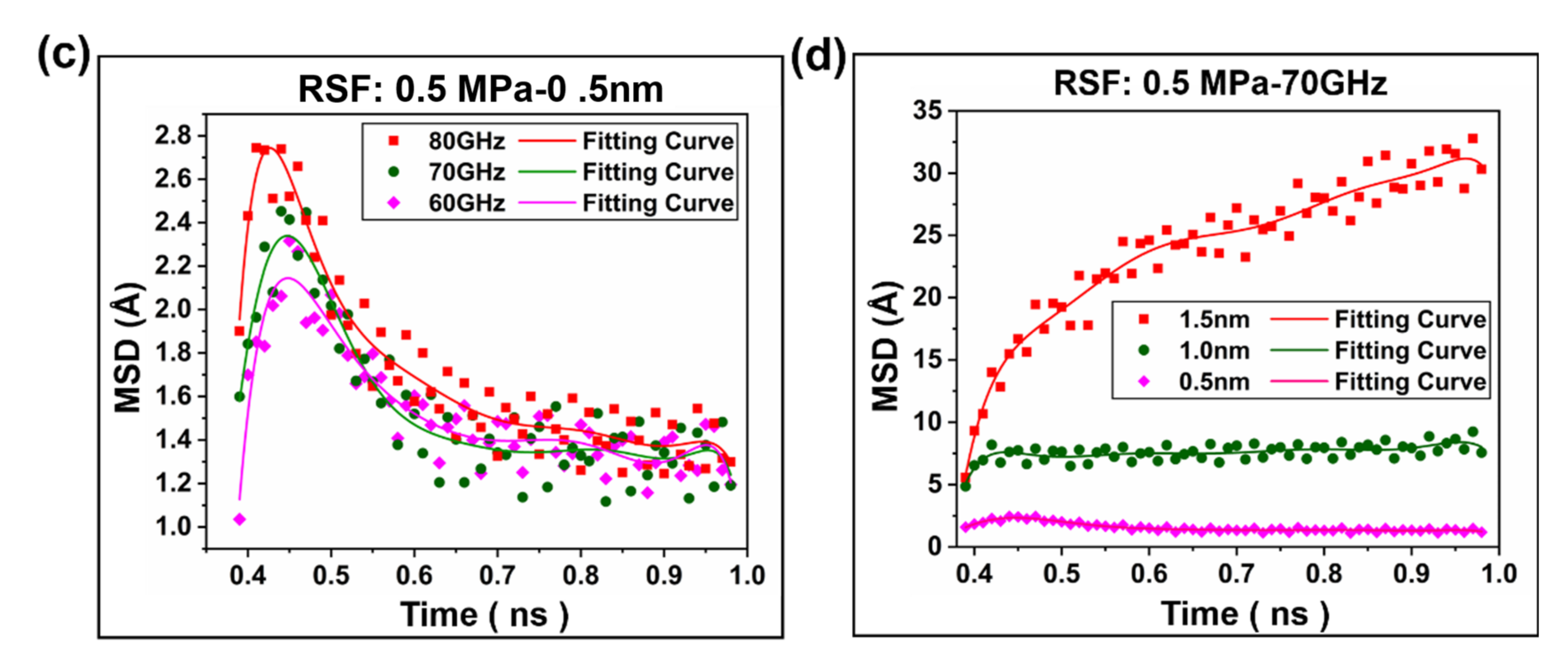
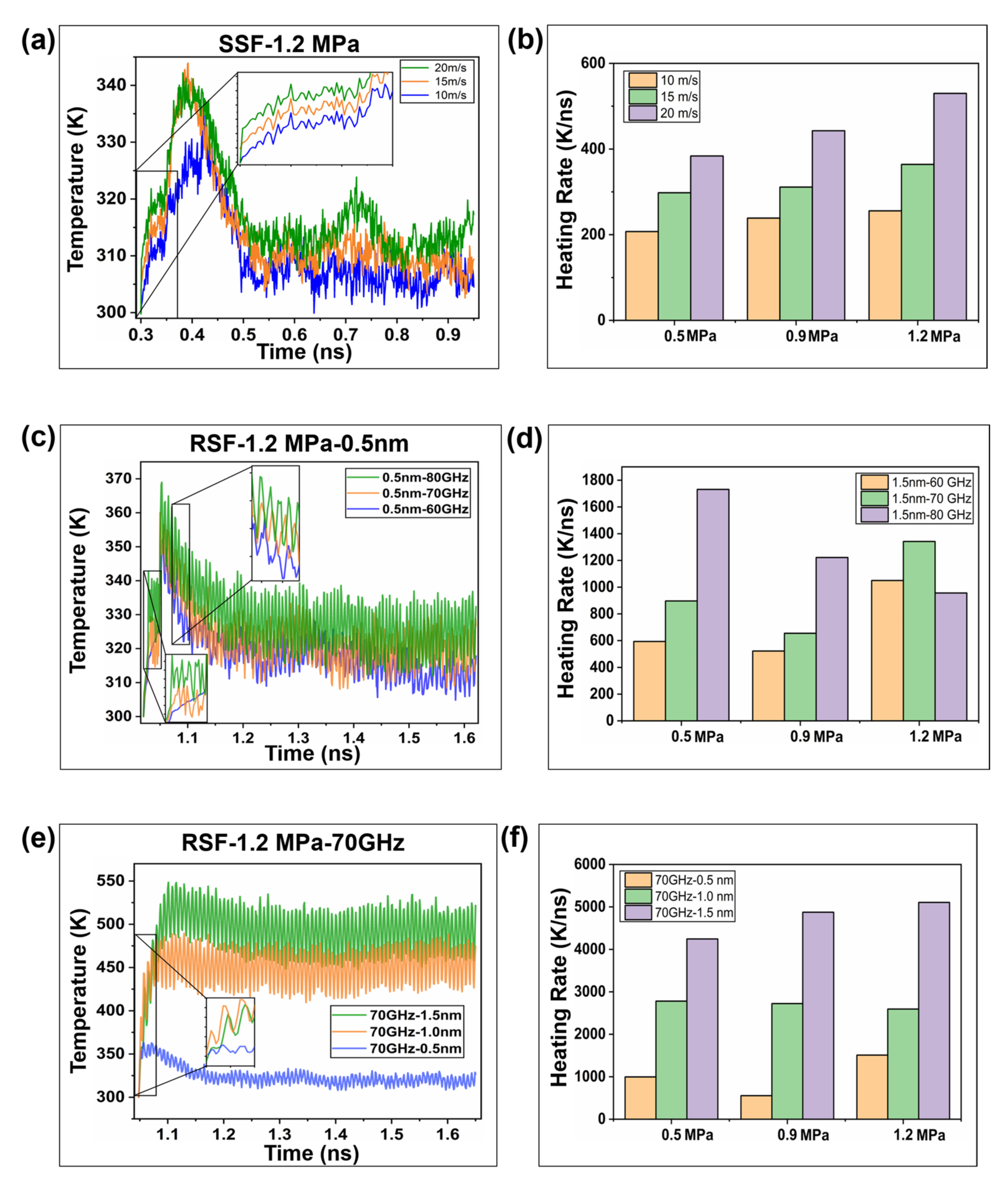
2.2. Interfacial Friction Characteristics under Sliding Friction
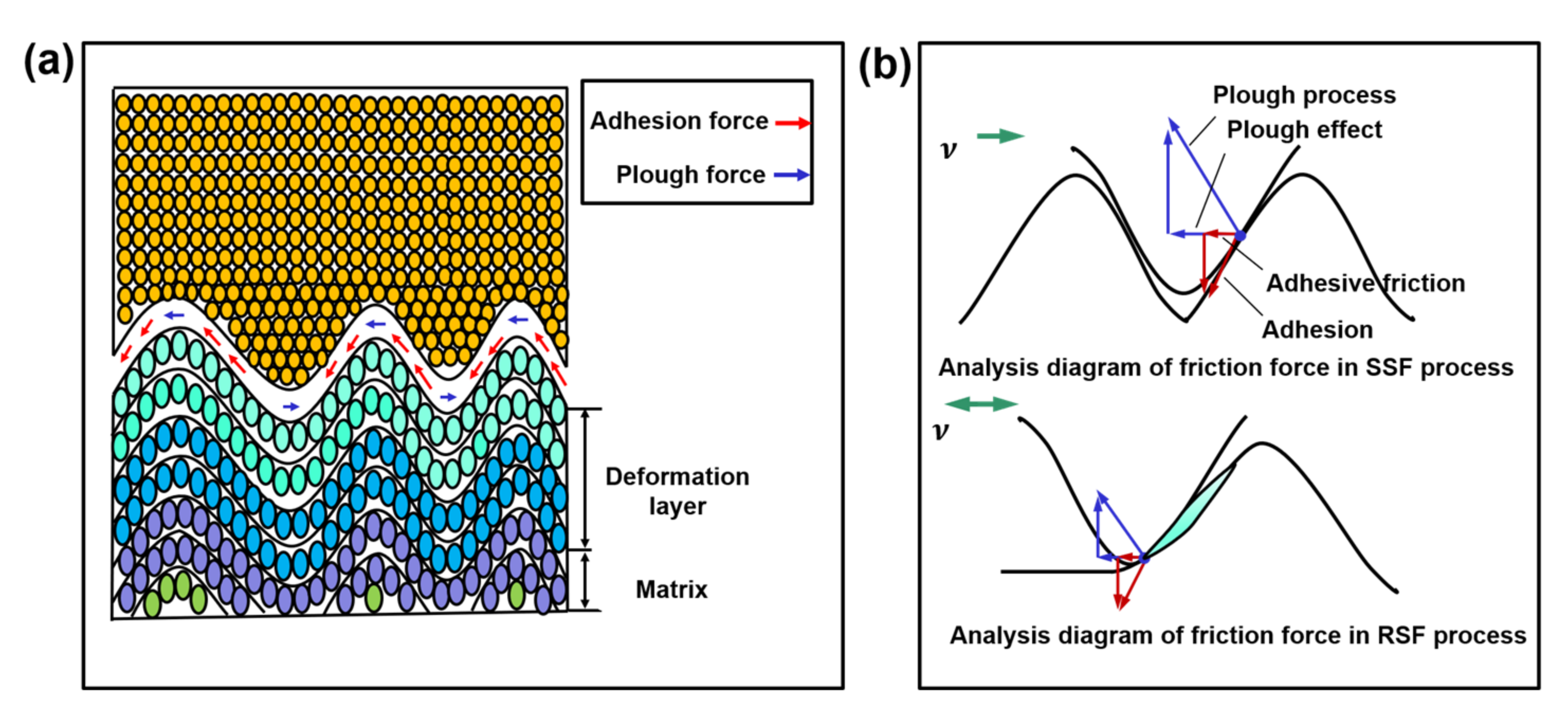
2.2.1. Interfacial Contact Mechanics
2.2.2. Polymer Segment Rearrangement
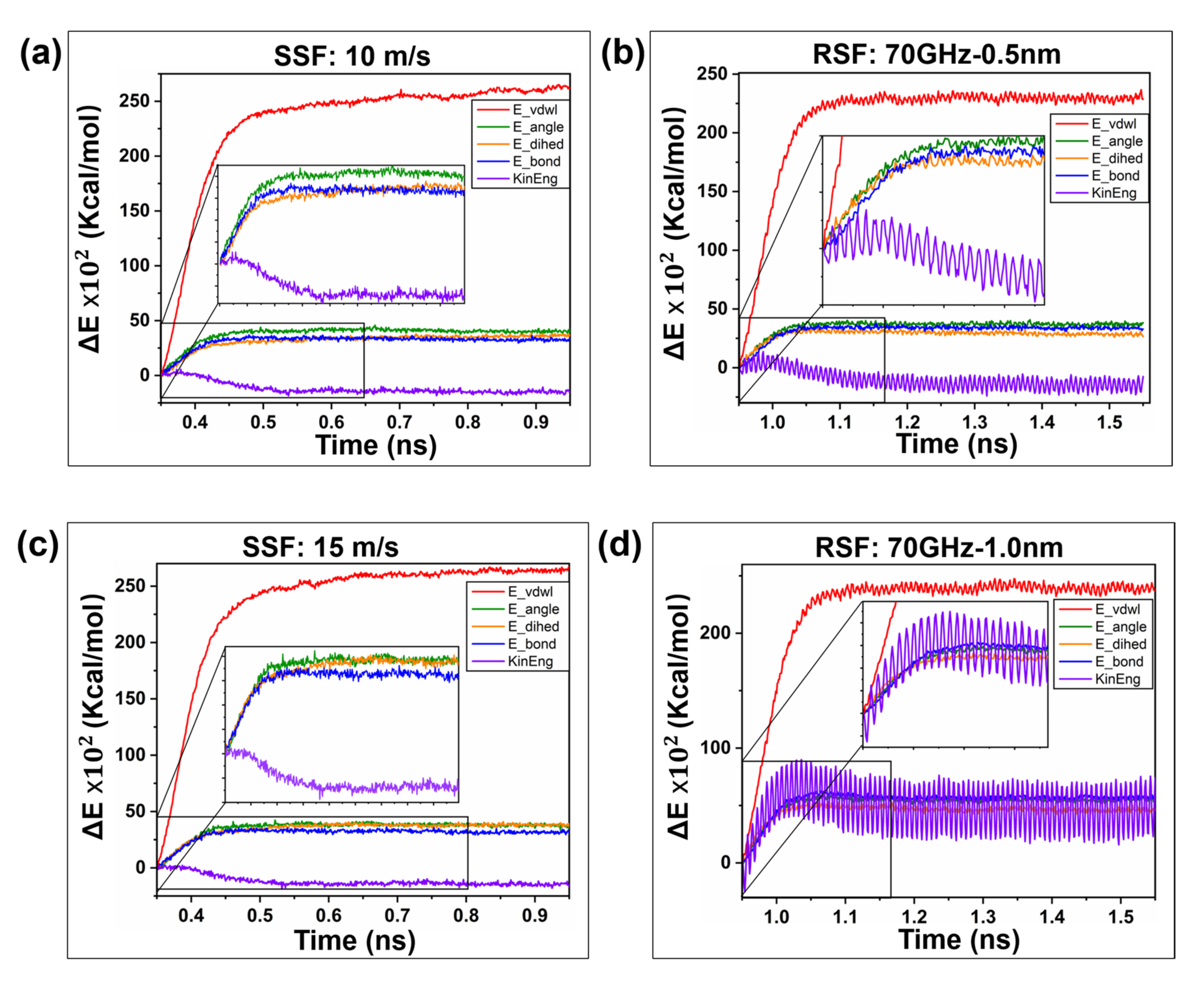
2.2.3. Frictional Energy Transfer
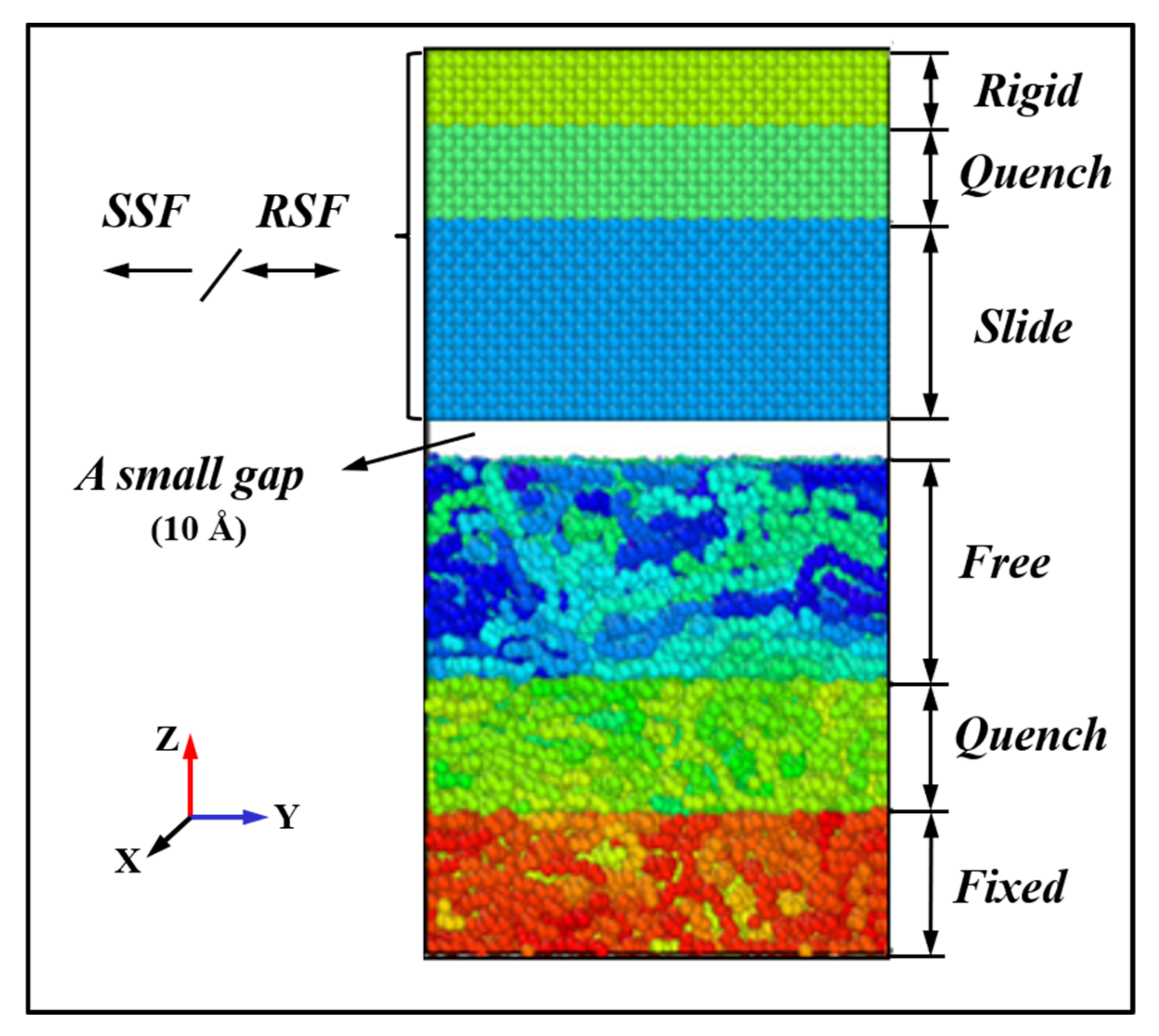
3. Materials and Methods
3.1. Material
3.2. Methods
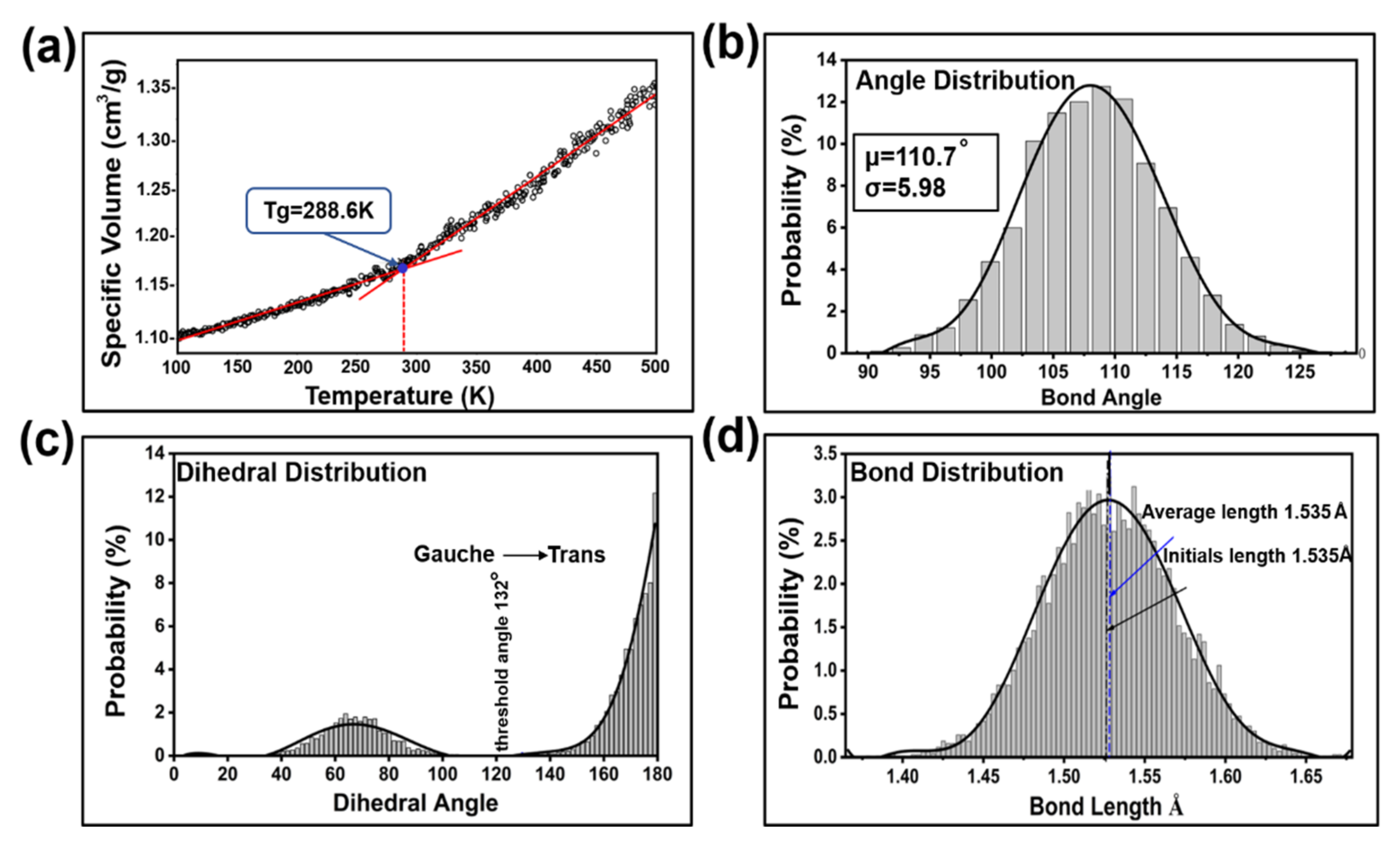
3.2.1. Initial Model Analysis
3.2.2. Calculation of the Interaction Potentials and Kinetic Energy
3.2.3. Calculation Scheme
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | Acceleration |
| Δx1, Δx2 | Displacement |
| () | Friction (direction vectors) |
| Energy change | |
| Glass transition temperature | |
| Bond angle | |
| Bond length | |
| Dihedral angle | |
| Bond stretching force constant | |
| Bond angle bending force constant | |
| Dihedral angle polyharmony coefficient | |
| The collision diameter | |
| The minimum of the potential for the interaction | |
| Bond length, bond angle, dihedral angle energy | |
| Interaction energy | |
| Energy displacement coefficient | |
| Total kinetic energy | |
| Centroid kinetic energy | |
| Atomic mass | |
| Average speed | |
| Atomic kinetic energy | |
| Temperature |
References
- Jin, Z.; Gao, F.; Zhu, F. An experimental study of solid-bed break-up in plasticization of a reciprocating-screw injection molding. Polym. Eng. Sci. 2004, 44, 1313–1318. [Google Scholar] [CrossRef]
- Park, S.-Y.; Cho, H.-S.; Rhee, G.-H.; Nam, Y.-H.; Lyu, M.-Y. Effect of Barrier Screw Design in Injection Molding Machines on the Plasticization Efficiency of Polypropylene Resin. Polym. Korea 2016, 40, 751–758. [Google Scholar] [CrossRef]
- Sato, A.; Sakaguchi, H.; Ito, H.; Koyama, K. Evaluation of replication properties on moulded surface by ultrasonic injection moulding system. Plast. Rubber Compos. 2010, 39, 315–320. [Google Scholar] [CrossRef]
- Qiu, Z.; Yang, X.; Zheng, H.; Gao, S.; Fang, F. Investigation of micro-injection molding based on longitudinal ultrasonic vibration core. Appl. Opt. 2015, 54, 8399–8405. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, W.; Zhou, X.; Wei, G.; Jiang, B. A novel method for the quantitative characterization of the simultaneous plasticizing and filling performance in ultrasonic plasticization micro injection molding. Mater. Des. 2021, 204, 109680. [Google Scholar] [CrossRef]
- Li, J.; Xu, K.; Lin, X.; Wu, X.; Gao, G. Research on the Flow Characteristics of Polymer Injection Molding Under Ultrasonic Vibration and Plastics’ Mechanical Strength. In Advances in Engineering Design and Optimization, Pts 1 and 2; Deng, Y.M., Yu, A., Li, W.H., Zheng, D., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2011; Volume 37–38, p. 1092. [Google Scholar]
- Luo, F.; Li, K.-H.; Zhong, J.-M.; Gong, F.; Wu, X.-Y.; Ruan, S.-C. An ultrasonic microforming process for thin sheet metals and its replication abilities. J. Mater. Process. Technol. 2015, 216, 10–18. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Park, K. Direct patterning of micro-features on a polymer substrate using ultrasonic vibration. Microsyst. Technol. 2012, 18, 2053–2061. [Google Scholar] [CrossRef]
- Grabalosa, J.; Ferrer, I.; Elías-Zúñiga, A.; Ciurana, J. Influence of processing conditions on manufacturing polyamide parts by ultrasonic molding. Mater. Des. 2016, 98, 20–30. [Google Scholar] [CrossRef]
- Michaeli, W.; Spennemann, A.; Gärtner, R. New plastification concepts for micro injection moulding. Microsyst. Technol. 2002, 8, 55–57. [Google Scholar] [CrossRef]
- Praher, B.; Straka, K.; Usanovic, J.; Steinbichler, G. Ultrasound based monitoring of the injection moulding process—Methods, applications and limitations. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; Volume 1593, pp. 159–162. [Google Scholar] [CrossRef]
- Jiang, B.; Peng, H.; Wu, W.; Jia, Y.; Zhang, Y. Numerical Simulation and Experimental Investigation of the Viscoelastic Heating Mechanism in Ultrasonic Plasticizing of Amorphous Polymers for Micro Injection Molding. Polymers 2016, 8, 199. [Google Scholar] [CrossRef]
- Jiang, B.; Zou, Y.; Wei, G.; Wu, W. Evolution of Interfacial Friction Angle and Contact Area of Polymer Pellets during the Initial Stage of Ultrasonic Plasticization. Polymers 2019, 11, 2103. [Google Scholar] [CrossRef]
- Jiang, B.; Hu, J.; Wu, W.; Pan, S. Research on the Polymer Ultrasonic Plastification. In Advanced Polymer Processing; Ma, L., Wang, C., Yang, W., Eds.; Trans Tech Publications Ltd.: Bäch, Switzerland, 2010; Volume 87–88, pp. 542–549. [Google Scholar]
- Michaeli, W.; Kamps, T.; Hopmann, C. Manufacturing of polymer micro parts by ultrasonic plasticization and direct injection. Microsyst. Technol. 2011, 17, 243–249. [Google Scholar] [CrossRef]
- Li, X.; Tian, H.; Dong, Y. Simulation Study on Interfacial Friction Heat Generation of Ultrasonically Plasticized Amorphous Polymer Particles. China Plast. 2017, 31, 88–93. [Google Scholar]
- Wu, W.; Peng, H.; Jia, Y.; Jiang, B. Characteristics and mechanisms of polymer interfacial friction heating in ultrasonic plasticization for micro injection molding. Microsyst. Technol. 2016, 23, 1385–1392. [Google Scholar] [CrossRef]
- Progressive Molecular Rearrangement and Heat Generation of Amorphous Polyethene under Sliding Friction: Insight from the United-Atom Molecular Dynamics Simulations. Langmuir. Available online: https://pubs.acs.org/doi/10.1021/acs.langmuir.0c01949?fig=tgr1&ref=pdf (accessed on 31 December 2021).
- Kulkarni, M.V.; Elangovan, K.; Reddy, K.H.; Basappa, S.J. Tribological Behaviours of Abs and Pa6 Polymer-Metal Sliding Combinations Under Dry Friction, Water Absorbed and Electroplated Conditions. J. Eng. Sci. Technol. 2016, 11, 68–84. [Google Scholar]
- Nosko, A.L.; Belyakov, N.S. Application of the generalized boundary condition to solving thermal friction problems. J. Frict. Wear 2009, 30, 455–462. [Google Scholar] [CrossRef]
- Krishna, K.G.; Divakar, C.; Venkatesh, K.; Mohan, C.; Lohith, K.M. Tribological studies of polymer based ceramic–metal composites processed at ambient temperature. Wear 2009, 266, 878–883. [Google Scholar] [CrossRef]
- Al-Obaidi, A.; Majewski, C. Ultrasonic Welding of Polymer–Metal Hybrid Joints. In Transactions on Intelligent Welding Manufacturing; Chen, S., Zhang, Y., Feng, Z., Eds.; Springer: Singapore, 2019; pp. 21–38. ISBN 9789811336508. [Google Scholar]
- Dai, L.; Sorkin, V.; Sha, Z.D.; Pei, Q.-X.; Branicio, P.; Zhang, Y.W. Molecular Dynamics Simulations on the Frictional Behavior of a Perfluoropolyether Film Sandwiched between Diamond-like-Carbon Coatings. Langmuir 2014, 30, 1573–1579. [Google Scholar] [CrossRef]
- Chepchurov, M.S.; Lubimyi, N.S.; Voronenko, V.P.; Adeniyi, D.R. Determination of the Thermal Conductivity of Metal-Polymers. Mater. Sci. Forum 2019, 973, 9–14. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Electrical4U. Thermal Conductivity of Metals. Available online: https://www.electrical4u.com/thermal-conductivity-of-metals/ (accessed on 20 January 2022).
- Katiyar, P. Effect of Weak Adhesion Interface on Mechanical and Dielectric Properties of Composite Materials. Ph.D. Thesis, University of South Carolina, Columbia, SC, USA, 2018. [Google Scholar]
- Chang, J.; Ho, A.; Chin, W.-K. Behaviors of the positive temperature coefficient of resistance of poly(styrene-co-n-butylacrylate) filled with Ni-plated core-shell polymeric particles. J. Polym. Sci. Part B Polym. Phys. 2006, 45, 322–329. [Google Scholar] [CrossRef]
- Kausar, A. Thermally conducting polymer/nanocarbon and polymer/inorganic nanoparticle nanocomposite: A review. Polym. Technol. Mater. 2020, 59, 895–909. [Google Scholar] [CrossRef]
- Philippon, S.; Sutter, G.; Molinari, A. An experimental study of friction at high sliding velocities. Wear 2004, 257, 777–784. [Google Scholar] [CrossRef]
- Graf, M.; Ostermeyer, G.-P. Friction-induced vibration and dynamic friction laws: Instability at positive friction-velocity-characteristic. Tribol. Int. 2015, 92, 255–258. [Google Scholar] [CrossRef]
- Tambe, N.; Bhushan, B. Friction model for the velocity dependence of nanoscale friction. Nanotechnology 2005, 16, 2309–2324. [Google Scholar] [CrossRef] [PubMed]
- Bowden, F.P.; Moore, A.J.W.; Tabor, D. The Ploughing and Adhesion of Sliding Metals. J. Appl. Phys. 1943, 14, 80–91. [Google Scholar] [CrossRef]
- Uehara, T. Molecular Dynamics Simulation of Stick-Slip Friction on a Metal Surface. Appl. Mech. Mater. 2013, 459, 26–33. [Google Scholar] [CrossRef]
- Chen, N.; Maeda, N.; Tirrell, A.M.; Israelachvili, J. Adhesion and Friction of Polymer Surfaces: The Effect of Chain Ends. Macromolecules 2005, 38, 3491–3503. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Helali, M. The effect of amplitude of vibration on the coefficient of friction for different materials. Tribol. Int. 2008, 41, 307–314. [Google Scholar] [CrossRef]
- Singhal, A.; Sahu, S.A.; Chaudhary, S.; Baroi, J. Initial and couple stress influence on the surface waves transmission in material layers with imperfect interface. Mater. Res. Express 2019, 6, 105713. [Google Scholar] [CrossRef]
- Zou, Z.; Hameed, M. Combining interface damage and friction in cohesive interface models using an energy based approach. Compos. Part A Appl. Sci. Manuf. 2018, 112, 290–298. [Google Scholar] [CrossRef]
- Guiamatsia, I.; Nguyen, G. A thermodynamics-based cohesive model for interface debonding and friction. Int. J. Solids Struct. 2014, 51, 647–659. [Google Scholar] [CrossRef]
- Harris, K.R.; Kanakubo, M. Self-diffusion, velocity cross-correlation, distinct diffusion and resistance coefficients of the ionic liquid [BMIM][Tf2N] at high pressure. Phys. Chem. Chem. Phys. 2015, 17, 23977–23993. [Google Scholar] [CrossRef]
- Guzowski, J.; Cichocki, B.; Wajnryb, E.; Abade, G.C. The short-time self-diffusion coefficient of a sphere in a suspension of rigid rods. J. Chem. Phys. 2008, 128, 94502. [Google Scholar] [CrossRef]
- Khakhin, L.A.; Kulik, A.V.; Arutyunov, I.A.; Potapova, S.N.; Korolev, E.V.; Svetikov, D.V.; Joint Research and Development Center. Review Production Technology of Ultra High Modulus Polyethylene. Oil Gas Technol. 2020, 130, 3–10. [Google Scholar] [CrossRef]
- Feldshtein, E.E.; Vitjaz, P.A.; Leksycki, K. Tribotechnical Characteristics of Ti6Al4V Titanium Alloy–Ultra-High-Molecular-Weight Polyethylene Friction Pairs. J. Frict. Wear 2020, 41, 399–404. [Google Scholar] [CrossRef]
- Shimada, S.; Toyoda, T.; Tsunemi, K. Speciation of Nickel Compounds in Air Particulate Matter. Bunseki Kagaku 2009, 58, 159–165. [Google Scholar] [CrossRef][Green Version]
- Thoe, T.; Aspinwall, D.; Killey, N. Combined ultrasonic and electrical discharge machining of ceramic coated nickel alloy. J. Mater. Process. Technol. 1999, 92–93, 323–328. [Google Scholar] [CrossRef]
- Bao, Q.; Yang, Z.; Lu, Z. Molecular dynamics simulation of amorphous polyethylene (PE) under cyclic tensile-compressive loading below the glass transition temperature. Polymer 2020, 186, 121968. [Google Scholar] [CrossRef]
- Hossain, D.; Tschopp, M.A.; Ward, D.K.; Bouvard, J.L.; Wang, P.; Horstemeyer, M.F. Molecular dynamics simulations of deformation mechanisms of amorphous polyethylene. Polymer 2010, 51, 6071–6083. [Google Scholar] [CrossRef]
- Jakse, N.; Pasturel, A. Molecular-dynamics study of liquid nickel above and below the melting point. J. Chem. Phys. 2005, 123, 244512. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ding, N.; Lu, J.W.Z.; Leung, A.Y.T.; Iu, V.P.; Mok, K.M. Mechanical Behavior of Nanometer Ni by MD Simulation. In Iscm Ii and Epmesc Xii, Pts 1 and 2; Lu, J.W.Z., Leung, A.Y.T., Iu, V.P., Mok, K.M., Eds.; Amer Inst Physics: Melville, Australia, 2010; Volume 1233, p. 261. [Google Scholar] [CrossRef]
- Sivebaek, I.M.; Samoilov, V.; Persson, B.N.J. Frictional properties of confined polymers. Eur. Phys. J. E 2008, 27, 37–46. Available online: https://link.springer.com/article/10.1140/epje/i2008-10349-8 (accessed on 20 January 2022). [CrossRef] [PubMed]
- Molecular Dynamics Simulation of Microstructure Evolution and Heat Dissipation of Nanoscale Friction—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0017931016333488 (accessed on 20 January 2022).
- Mostafavi, S.; Markert, B. Molecular Dynamics Simulation of Ultrasonic Metal Welding of Aluminum Alloys—2019. PAMM; Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/pamm.201900304 (accessed on 20 January 2022).




| Interaction | Parameter | |
|---|---|---|
| Bond length | Å | |
| Bond angle | (1.911 rad) | |
| Dihedral angle | ||
| Lennard–Jones | Å | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; He, C.; Qiang, Y.; Peng, H.; Zhou, M. Polymer–Metal Interfacial Friction Characteristics under Ultrasonic Plasticizing Conditions: A United-Atom Molecular Dynamics Study. Int. J. Mol. Sci. 2022, 23, 2829. https://doi.org/10.3390/ijms23052829
Wu W, He C, Qiang Y, Peng H, Zhou M. Polymer–Metal Interfacial Friction Characteristics under Ultrasonic Plasticizing Conditions: A United-Atom Molecular Dynamics Study. International Journal of Molecular Sciences. 2022; 23(5):2829. https://doi.org/10.3390/ijms23052829
Chicago/Turabian StyleWu, Wangqing, Changsheng He, Yuanbao Qiang, Huajian Peng, and Mingyong Zhou. 2022. "Polymer–Metal Interfacial Friction Characteristics under Ultrasonic Plasticizing Conditions: A United-Atom Molecular Dynamics Study" International Journal of Molecular Sciences 23, no. 5: 2829. https://doi.org/10.3390/ijms23052829
APA StyleWu, W., He, C., Qiang, Y., Peng, H., & Zhou, M. (2022). Polymer–Metal Interfacial Friction Characteristics under Ultrasonic Plasticizing Conditions: A United-Atom Molecular Dynamics Study. International Journal of Molecular Sciences, 23(5), 2829. https://doi.org/10.3390/ijms23052829