1. Introduction
Blood flow to different organs is regulated by changes in artery diameter. Factors released from the endothelium are among the most potent regulators of vessel diameter. Nitric oxide (NO) is a powerful endothelium-derived vasodilator in different vascular beds. For this reason, NO-donors are widely used in clinical practice.
It has been shown that the vasodilating effect of NO is mediated by several mechanisms. Early data suggested that NO and NO-donors activate vascular smooth muscle high-conductance, calcium-activated potassium channels (BK channels), e.g., [
1,
2,
3]. Activation of BK channels is thought to produce hyperpolarization of vascular smooth muscle cells, leading to closure of voltage-dependent calcium channels, a subsequent decrease in intracellular calcium concentration, and vasodilation.
Recently, we have demonstrated that NO-donors evoke the deactivation of BK channels at intermediate and low levels of vessel contractility [
4]. This effect, particularly that induced by the NO-donor sodium nitroprusside (SNP), was suggested to be due to an NO-induced reduction in calcium influx via
l-type calcium channels. Thus, BK channels have been shown to be able to limit NO-induced vasodilation. In contrast, we have also shown that activation of BK channels facilitates NO-induced vasodilation at higher levels of contractility. Of note, the latter effect induced by SNP was only partially blocked by Rp-8-Br-PET-cGMPS, a selective protein kinase G (PKG) inhibitor [
5]. This partial block might be due to incomplete inhibition of PKG by Rp-8-Br-PET-cGMPS at the concentration used [
4]. Unfortunately, higher concentrations of Rp-8-Br-PET-cGMPS could not be employed because, at such concentrations, this substance may stimulate basal PKG activity [
6,
7]. Thus, our data obtained on intact vessel preparations could not clarify the extent to which PKG contributes to the SNP-induced activation of the BK channel.
Indeed, there exist two hypotheses explaining the effect of NO and NO-donors on vascular smooth muscle BK channels. One states that these agents activate soluble guanylate cyclase, resulting in an increased formation of cGMP and subsequent activation of PKG. PKG then increases the activity of BK channels. This has been shown in excised membrane patches or isolated smooth muscle cells from, for example, porcine coronary artery [
8], rabbit cerebral artery [
2], human pulmonary artery [
9], mouse aorta [
10], and rat cerebral artery [
11] smooth muscle cells as well as for cloned smooth muscle BK channels [
12]. The other hypothesis claims that NO activates BK channels independently of PKG, and a direct action of NO on the channel has been suggested. Thus, BK channels were activated in excised membrane patches after application of NO to the intracellular side of the channel in rabbit aorta [
13] and of NO and the NO-donors SIN-1 and SNP in rat mesenteric artery [
14] smooth muscle cells. Hence, even when taking into account the data of our recent study together with previously published results, we were unable to clarify the degree of contribution of PKG to the SNP-induced activation of the BK channel.
Therefore, this study tested the hypothesis that the SNP-induced activation of vascular smooth muscle BK channels is fully mediated by PKG. In order to obtain direct evidence, BK channel activity was measured in excised membrane patches and BK channel currents were recorded in freshly isolated vascular smooth muscle cells.
3. Discussion
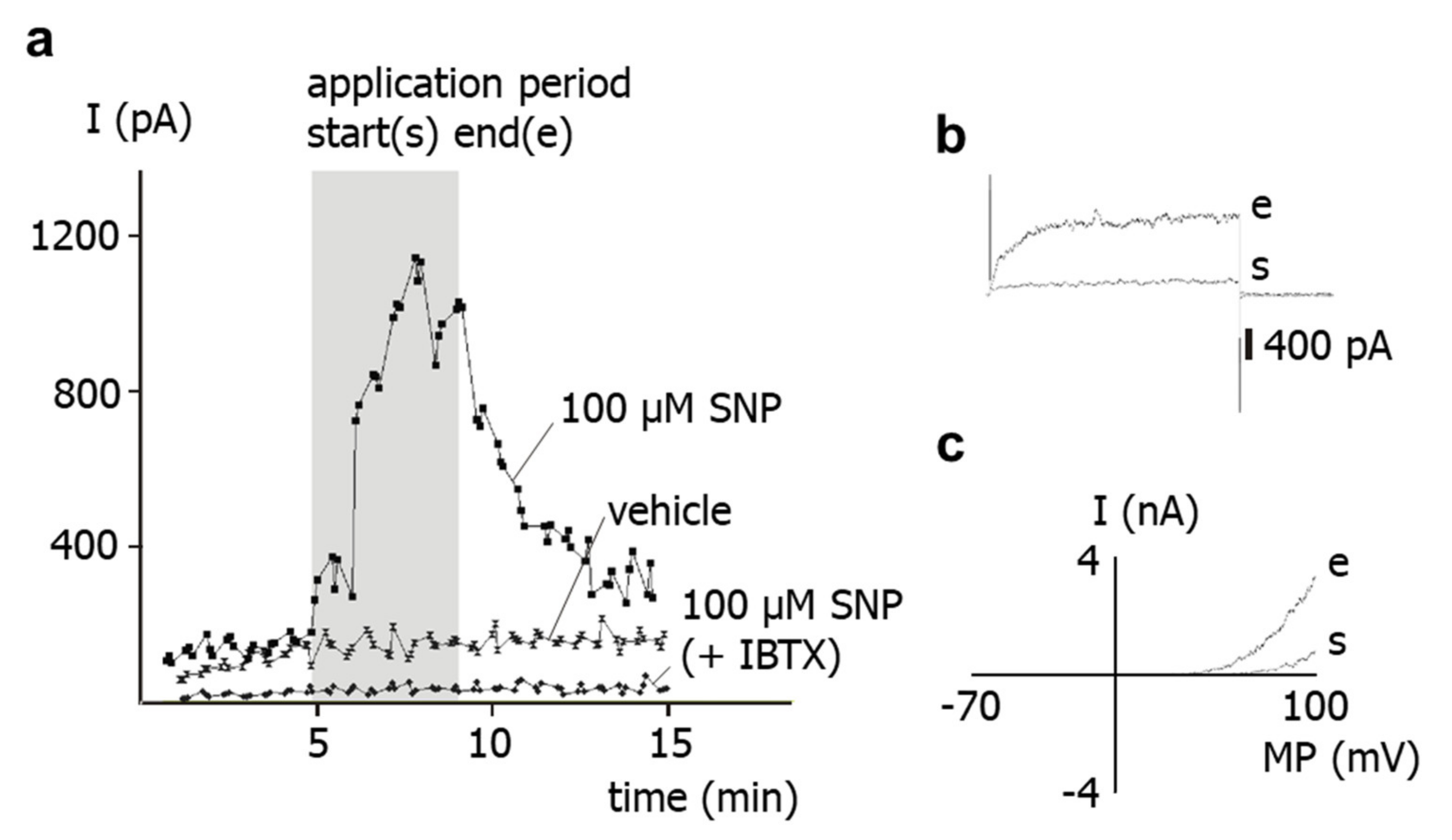
The results of the present study show that application of the NO-donor SNP to freshly isolated rat tail artery smooth muscle cells increases their outward current considerably. Previously, it has been shown that the outward current of these cells consists mainly of an iberiotoxin-sensitive current, i.e., represents a BK current [
15]. This finding suggests that the SNP-induced increase in the outward current observed in the present study is due to a stimulation of the BK current by SNP. Indeed, the present study also demonstrated that SNP was unable to affect the residual outward current after pre-treatment of the cells with iberiotoxin, i.e., under conditions where BK channels are blocked. Therefore, it is concluded that the increase in the outward current of rat tail artery smooth muscle cells after SNP application is due to an SNP-induced stimulation of a BK current.
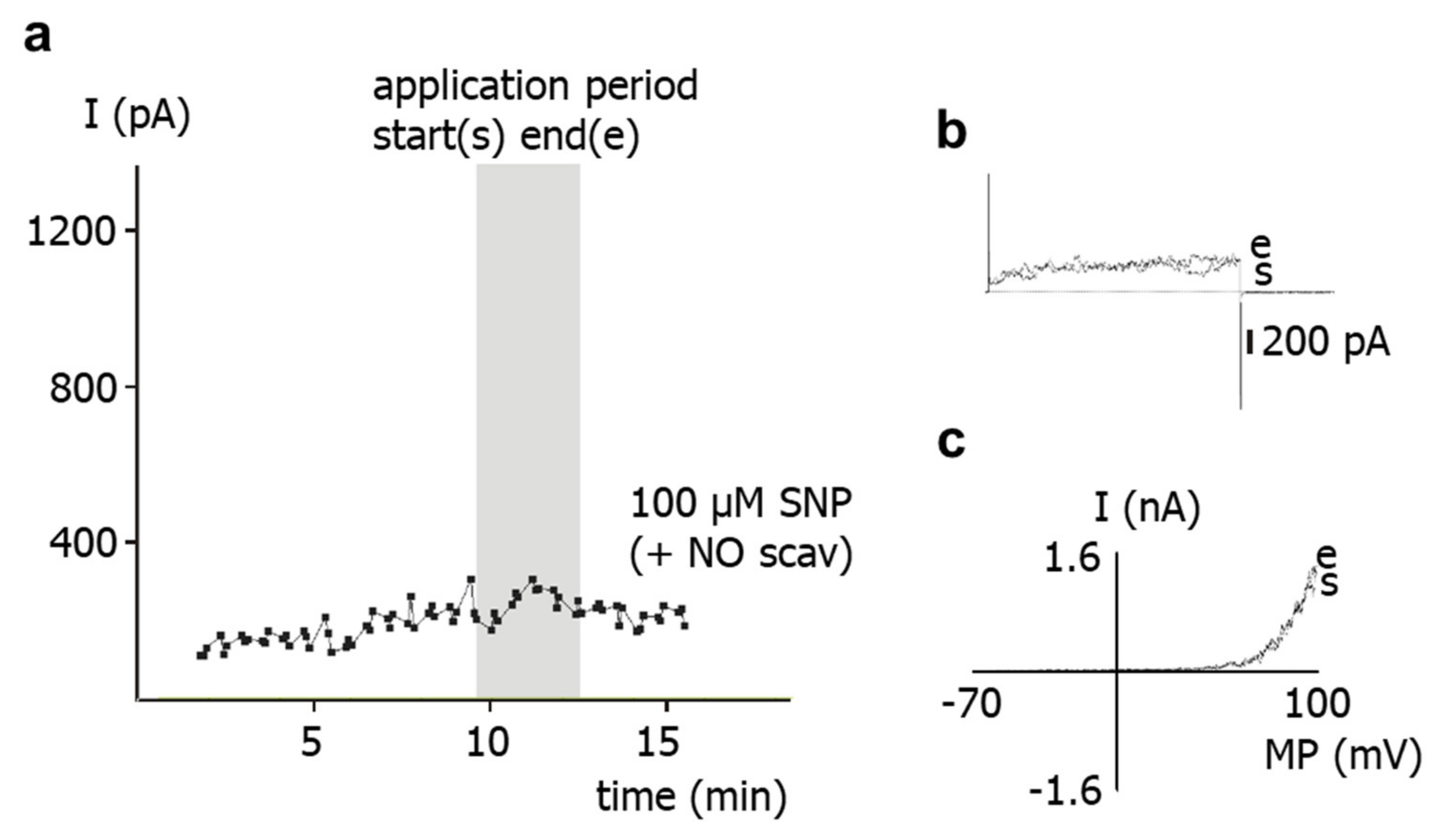
SNP is a well-known NO-donor. The SNP-induced stimulation of the BK current observed in the present study is most likely due to NO. Indeed, we demonstrated that application of SNP did not increase the BK current in the presence of the NO-scavenger hydroxocobalamin [
18]. Furthermore, hydroxocobalamin itself had no effect on the BK current and on excised BK channels. In addition, as mentioned in methods, hydroxocobalamin removed NO from solutions. Consequently, an interaction between NO and hydroxocobalamin may explain the lack of effect of SNP on the BK current in the presence of hydroxocobalamin. Importantly, strong support for this notion was provided in our recent study on intact rat tail arteries, i.e., the vessel from which the cells investigated in the present study were isolated [
4]. In the previous study, the vasodilatory effect of SNP was abolished by hydroxocobalamin and was not mediated by either cyanide, which could also be released by SNP [
20], or nitroxyl (HNO), which may be generated by the direct interaction of H
2S with SNP [
21]. Thus, data from excised BK channels, freshly isolated single smooth muscle cells and intact vessel preparations from rat tail artery consistently demonstrate that the stimulation of the BK current after SNP application is solely due to NO released from SNP.
In contrast to the coherent data on the contribution of NO in mediating the effect of SNP on BK currents in rat tail arteries, the role of PKG, the canonical mediator of NO-induced effects [
22,
23], is still unclear. Thus, in our recent study on intact rat tail arteries, the vasodilatory effect of SNP was only partially blocked by Rp-8-Br-PET-cGMPS, a selective PKG inhibitor [
4,
5]. Of note, it has been suggested that the effects of NO and NO-donors could be explained by a direct action of NO on the BK channel [
13,
14,
24]. However, a direct, PKG-independent effect of NO on rat tail artery BK channels seems unlikely based on the following findings of the present study.
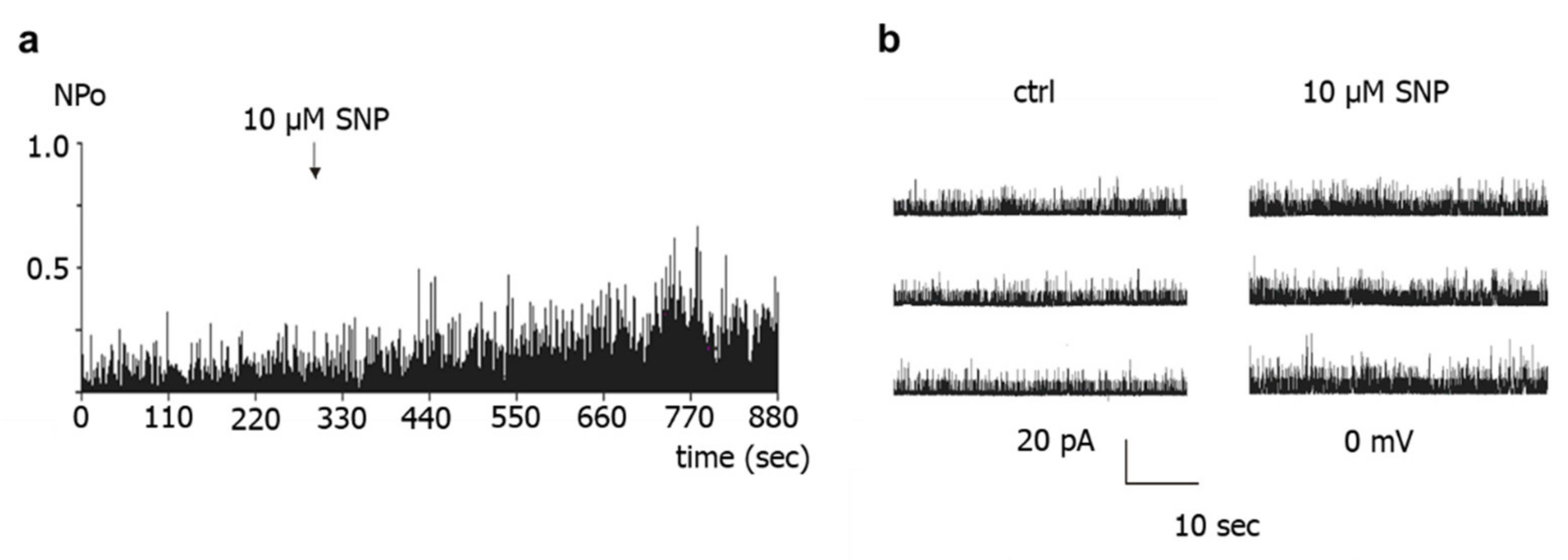
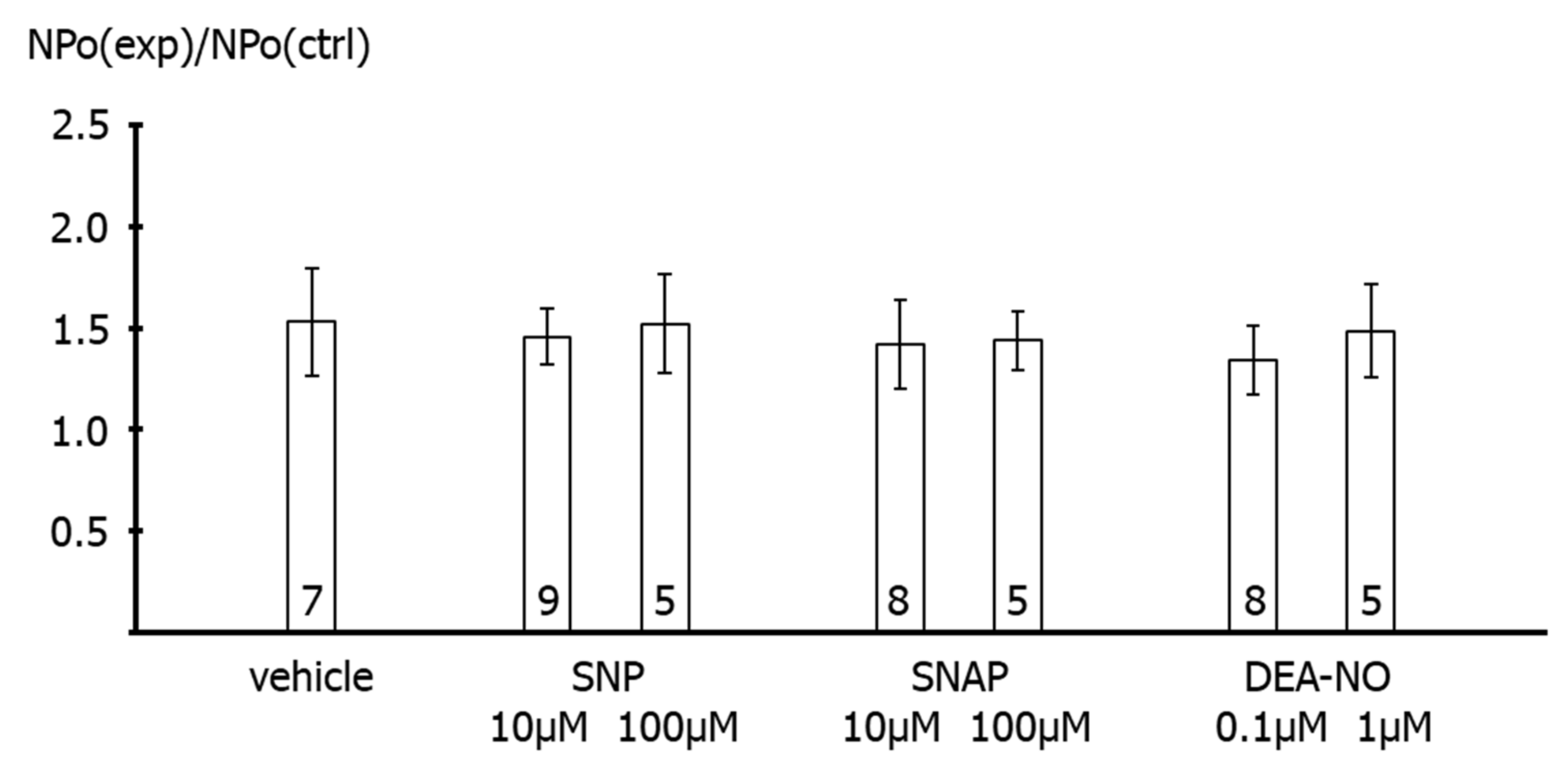
First, application of the NO-donors SNP, SNAP and DEA-NO to the intracellular side of excised BK channels from rat tail artery smooth muscle cells increased the activity of the channel by about 50–60%, independent of the NO-donor concentration and the NO release mechanism of the respective NO-donor. A similar elevation of the activity of excised BK channels has been reported for NO, SIN-1 and SNP in rat mesenteric arteries [
14] and for NO in rabbit aorta [
13]. In contrast, no change in channel activity was observed with the NO-donors SNP and SNAP on the BK channel from rat mesenteric arteries [
25] and on the cloned BK channel from human aorta [
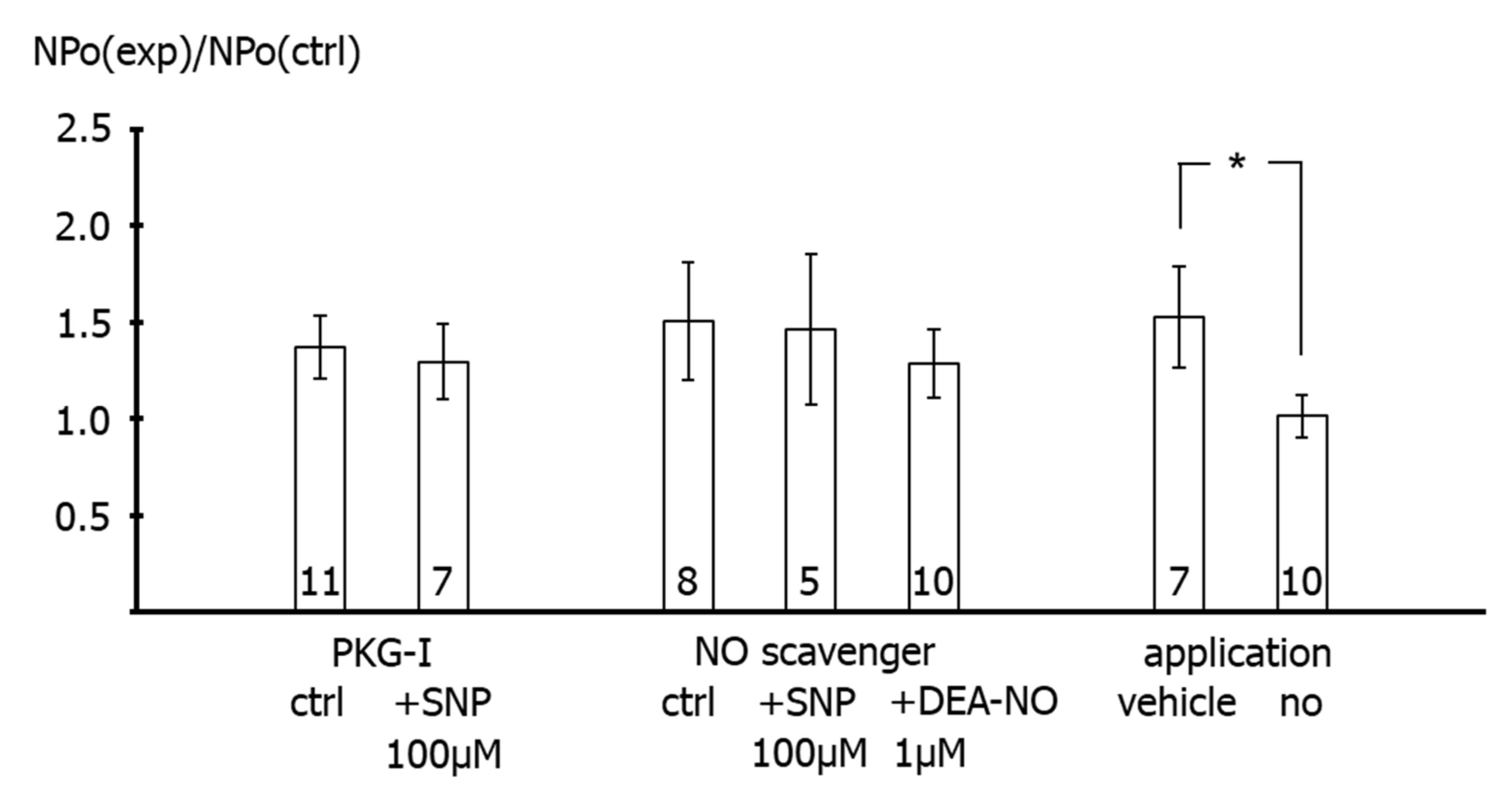
26]. Importantly, extending the findings of previous reports, we observed in the present study that the increase in BK channel activity was not different from the increase in single channel activity observed after application of the solvent of the NO-donors in time control experiments. Moreover, additional experiments on excised BK channels designed to interact with either NO released from the NO-donors or with PKG, which mediates the effect of NO on BK channels, all produced a similar effect. Thus, the increase in single BK channel activity induced by Rp-8-Br-PET-cGMPS did not differ from the effect of Rp-8-Br-PET-cGMPS together with SNP, the effect of hydroxocobalamin did not differ from the effect of hydroxocobalamin together with SNP as well as with DEA-NO, and the effects of Rp-8-Br-PET-cGMPS and hydroxocobalamin did not differ from the effect of the solvent alone.
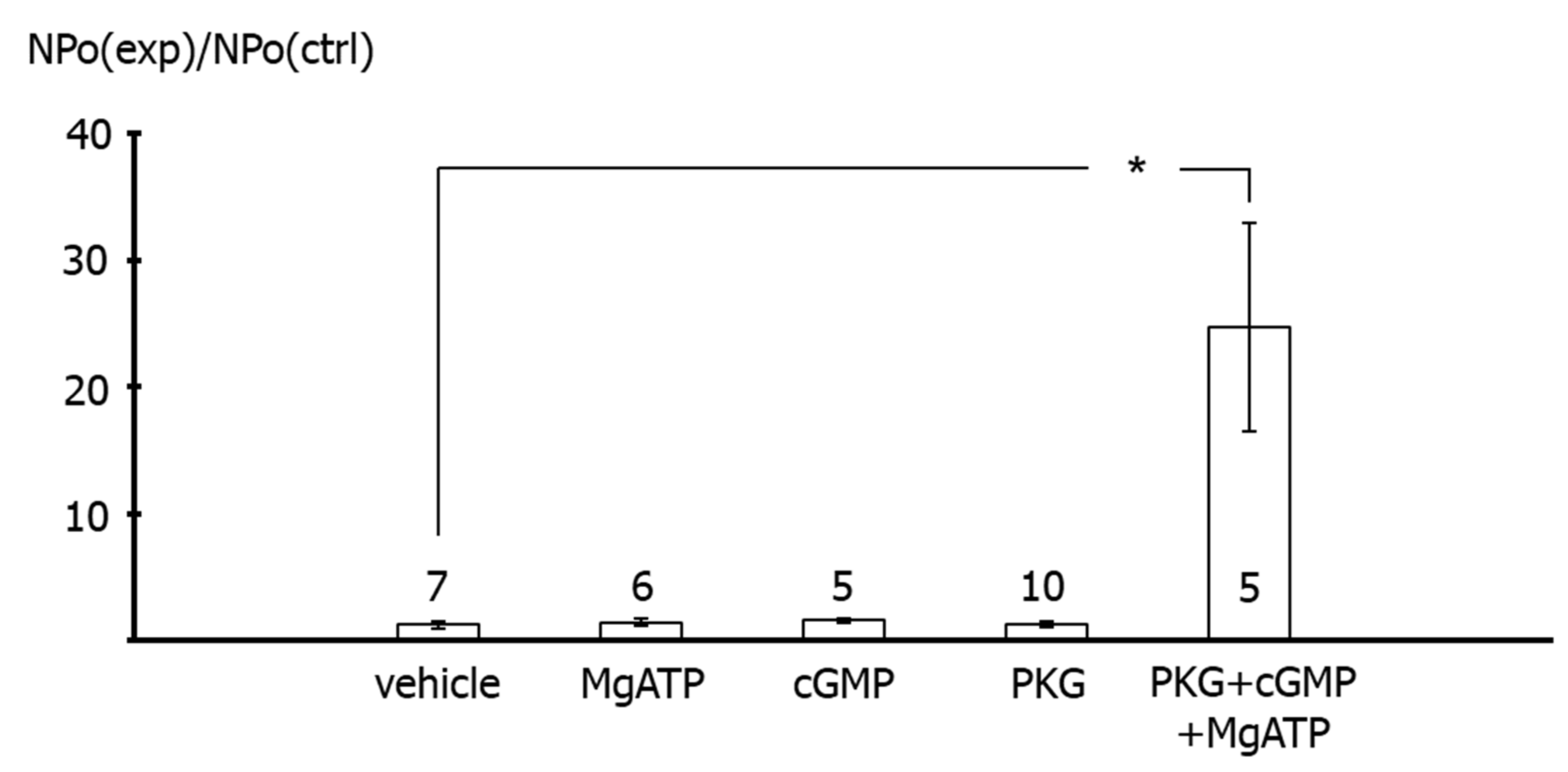
In contrast, application of the catalytic subunit of PKG together with MgATP and cGMP to the intracellular side of excised BK channels from rat tail artery smooth muscle cells increased the activity of the channel considerably. This effect of PKG most likely indicates phosphorylation of the BK channel by PKG, because MgATP, cGMP and the catalytic subunit of PKG alone were not able to affect BK channel activity. The combined effect of MgATP, cGMP and the catalytic subunit of PKG was required to observe an increase in BK channel activity. Of note, these findings are consistent with numerous similar observations, e.g., [
2] and reviewed in [
27]. Importantly, using the same method, we had previously shown that excised BK channels from rat tail artery smooth muscle cells can be activated by application of protein kinase A (PKA) [
19] and inhibited by application of protein kinase C (PKC) [
15]. Thus, the data shown in the present study, as well as previously published data, provide strong evidence that excised BK channels can be activated under the experimental conditions used in the present study.
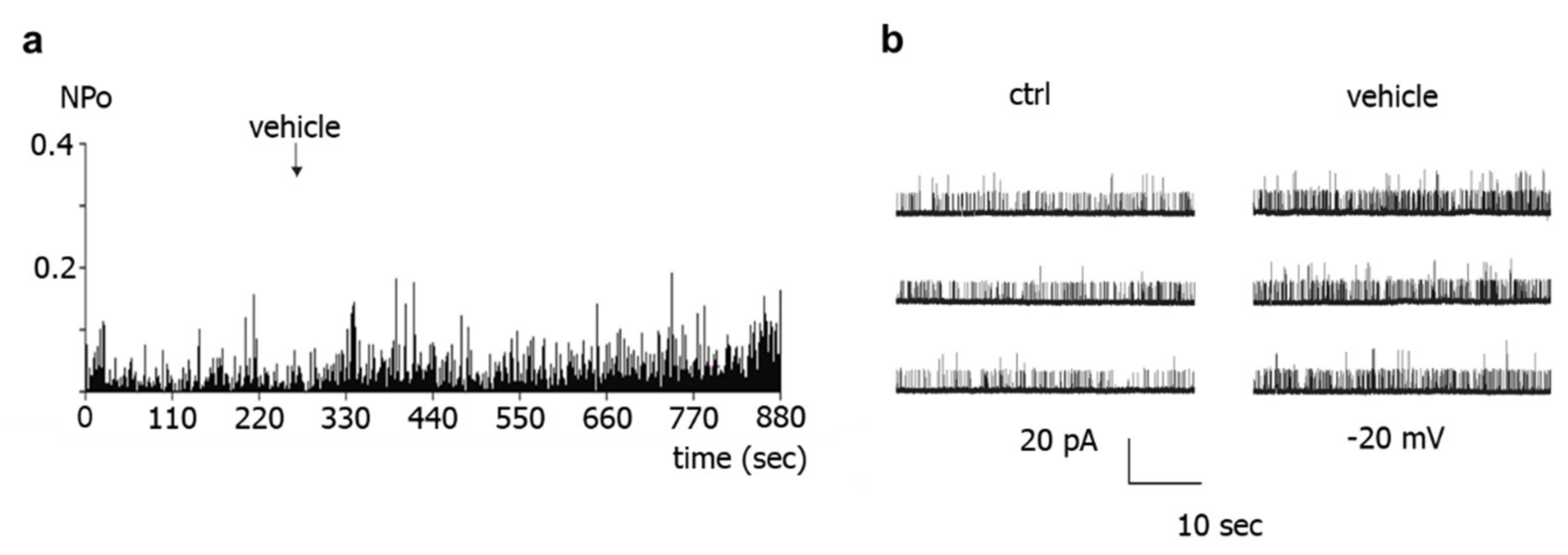
Further, the present study shows that BK channel activity was higher during vehicle application to excised BK channels than in the absence of such application. This effect cannot be explained by an alteration in calcium concentration, because the calcium concentration was the same on both sides of the patch and was buffered with EGTA. However, similar changes in BK channel activity have been reported previously during longer recordings and referred to as Wanderlust kinetics [
28]. Thus, mechanical stimulation of the BK channel due to the application maneuver or application-related changes in the microenvironment of the channel or slow fluctuations in open probability with time, which underlie Wanderlust kinetics, could explain the increase in the activity of excised BK channels during vehicle application. Of note, the search for the reason of this phenomenon was not the focus of the present study. Regardless of the cause of this phenomenon, the occurrence of similar alterations in channel activity after application of different substances, substance combinations, and of the experimental bath solution indicates that the observed effect is not due to a specific direct interaction with the BK channel, in particular not to an interaction induced by NO. In conclusion, we did not obtain any evidence that the NO-donor-induced activation of excised BK channels observed in our study could be due to a direct action of NO on the BK channel.
Second, in freshly isolated single smooth muscle cells, application of SNP did not affect the BK current at all in the presence of the PKG-inhibitor Rp-8-Br-PET-cGMPS. It was also shown that Rp-8-Br-PET-cGMPS itself had no effect on the BK current and on excised BK channels. As mentioned in the methods, Rp-8-Br-PET-cGMPS produced only a small, if any, reduction in NO concentration, which cannot explain the lack of effect of SNP in the presence of Rp-8-Br-PET-cGMPS. Thus, since, in the presence of Rp-8-Br-PET-cGMPS, NO is still present and the channel is still functioning, the lack of effect of SNP should be caused by an action of Rp-8-Br-PET-cGMPS on components of signal transduction pathways that mediate the effect of NO on the BK channel, most likely PKG [
5]. Consistent with this interpretation of our data, a complete inhibition of the effect of NO or NO-donors on vascular smooth muscle BK currents has been reported previously. Thus, BK currents were not affected after application of a PKG-inhibitor and SNP in rat mesenteric arteries [
25], of a guanylate cyclase inhibitor and SNP in porcine coronary arteries [
29], of a PKG-inhibitor and NO in human pulmonary arteries [
9], of a PKG-inhibitor and SNAP in rabbit coronary arteries [
30] and of DEA-NO in PKG-knock-out mouse aorta [
10]. Therefore, the stimulation of the BK current after application of SNP observed in our study is most likely mediated only by PKG.
Of note, the data of the present study, obtained on excised BK channels and BK currents of freshly isolated smooth muscle cells, are consistent with and strongly support our suggestion that the partial inhibition of the vasodilatory effect of SNP on intact rat tail arteries induced by Rp-8-Br-PET-cGMPS [
4] is due to an incomplete inhibition of PKG by Rp-8-Br-PET-cGMPS at the concentrations used. Higher concentrations of Rp-8-Br-PET-cGMPS could not be employed because they might stimulate basal PKG activity [
6,
7].
In summary, the data of the present study show that the increase in the outward current of freshly isolated rat tail artery smooth muscle cells after application of SNP is due to an SNP-induced stimulation of a BK current. Further, data from excised BK channels, freshly isolated single smooth muscle cells and intact vessel preparations from rat tail artery consistently show that stimulation of the BK current after SNP application is solely due to NO released from SNP. Moreover, we did not obtain any evidence that the NO-donor-induced activation of excised BK channels observed in our study could be due to a direct action of NO on the BK channel. Thus, the stimulation of the BK current observed in our study after application of SNP is most likely mediated only by PKG. These data fill a gap, at the molecular level, in our recent extensive study [
4] addressing the role of vascular smooth muscle BK channels in NO-induced vasodilation. In addition, they add to the already existing data, collected by several groups, on the mechanisms of NO-induced activation of vascular smooth muscle BK channels and, thus, contribute to a better understanding of the extent to which these mechanisms contribute to the NO-dependent regulation of BK channels.
4. Materials and Methods
The investigation is in accordance with the US Guide for the Care and Use of Laboratory Animals (Eighth edition, National Academy of Sciences, 2011). Approval for the use of laboratory animals in this study was granted by a governmental committee on animal welfare.
4.1. Cell Isolation
Cell isolation was performed using a method that has long been established in our laboratory and has been successfully used in a number of patch-clamp studies. This procedure provides single smooth muscle cells from rat tail arteries with well-preserved intracellular signaling pathways, in particular PKC- [
15], PKG- [
31] and PKA- [
19,
31,
32] dependent signaling. Moreover, the activity of BK channels is long-lasting and stable, as observed in whole-cell and single-channel recordings [
15,
19,
31,
32]. Briefly, male Wistar-Kyoto rats, 16–25 weeks old, were sacrificed under CO
2 narcosis by decapitation. The rat tail was removed and placed into a physiological salt solution containing (in mM): 145 NaCl, 4.5 KCl, 1.2 NaH
2PO
4, 1.0 MgSO
4, 0.1 CaCl
2, 0.025 EDTA, 5 HEPES at pH 7.3 at 4 °C. Tail arteries were dissected free and cleaned of connective tissue. A 5 to 10 mm-long piece was then transferred into a microtube containing 1 mL of an enzyme solution and stored there overnight at 4 °C. The enzyme solution consisted of (in mM): 110 NaCl, 5 KCl, 0.16 CaCl
2, 2 MgCl
2, 10 NaHEPES, 10 NaHCO
3, 0.5 KH
2PO
4, 0.5 NaH
2PO
4, 10 glucose, 0.49 EDTA, and 10 taurine at pH 7.0, as well as 1.5 mg/mL papain, 1.6 mg/mL albumin and 0.4 mg/mL DL-dithiothreitol. The next day, the microtube with the vessel was incubated for 5–20 min at 37 °C. Single cells were released by trituration with a polyethylene pipette into the experimental bath solution. Only spindle-shaped cells with a voluminous appearance in phase contrast were used for further experiments. The experimental bath solution contained: in whole-cell experiments (in mM), 135 NaCl, 6 KCl, 1 MgCl
2, 0.1 CaCl
2, 3 EGTA (purity 96%) and 10 HEPES at pH 7.4 having a free calcium concentration <100 nM; and in inside-out experiments (in mM), 140 KCl, 1 MgCl
2, 3 EGTA (purity 96%), 10 HEPES, and an appropriate amount of CaCl
2 to obtain a free calcium concentration of 300 nM at pH 7.4. The free calcium concentrations were calculated with the following apparent reaction constants at pH 7.4: log K
CaEGTA = 7.17, log K
MgEGTA = 1.93 [
33]. The pipette solution contained: in whole-cell experiments (in mM) 102 KCl, 10 NaCl, 1 MgCl
2, 1 CaCl
2, 0.1 MgATP, 10 EGTA (purity 96%), 10 HEPES giving a free calcium concentration <10 nM at pH 7.4; and in inside-out experiments (in mM), 130 NaCl, 15 KCl, 1 MgCl
2, 3 EGTA (purity 96%), 10 HEPES and an appropriate amount of CaCl
2 to obtain a free calcium concentration of 300 nM at pH 7.4.
4.2. Patch-Clamp Recording
All experiments were performed at room temperature (22–24 °C). Patch pipettes were prepared from borosilicate glass (WP Instruments, Berlin, Germany) and had a resistance in the range of 2–5 MOhm. Recordings were made with an Axopatch 200 amplifier (Axon Instruments, Burlingame, CA, USA) with the whole-cell and the inside-out patch-clamp configurations. In the whole-cell experiments, stimulation of currents with pulse and ramp protocols, data sampling at a rate of 1 kHz, and data analysis were all performed with the software package ISO2 (MFK, Frankfurt, Germany). Stability of the access resistance was tested regularly during the course of the experiment. Whole-cell current amplitudes were measured during the last 200 ms of the current traces and determined after calculation of the mean of three consecutive traces. Single channel data were stored on a DTR-1800 data recorder (Biologic, Seyssinet-Pariset, France) and later replayed for analysis, where they were filtered at 1 kHz with use of an eight-pole Bessel filter (model 902, Frequency Devices, Ottawa, IL, USA) and digitized at 5 kHz. Thereafter, they were analyzed off-line with the software package ASCD (G. Droogmans, Lab. Fysiologie, KU Leuven, Belgium). The single channel amplitudes were determined by fitting Gaussian distributions to the amplitude histograms of the closed and the open state, respectively. The activity of the channel in a patch was determined as NPo, where Po is the open probability of one channel and N is the number of channels in the patch, which could not be determined in most cases because of the low level of activity. NPo was calculated as the sum of the times spent at current levels corresponding to 1, 2, …, N channels open multiplied by the number of open channels, and this sum was then divided by the total registration time. The registration time was 6–12 min. All potentials are expressed as membrane potentials.
In whole-cell investigations, experimental agents were applied to cells with use of a pipette placed at a distance from the cell and filled with the agent dissolved in the whole-cell bath solution. Application lasted 3–4 min, a period sufficient to obtain a new steady state of the whole-cell current. In the same manner, the whole-cell bath solution without agent was applied to obtain an appropriate control for the statistical evaluation of the results. In inside-out investigations, experimental agents were applied to patches by adding the agent dissolved in the inside-out bath solution directly to the bath. Thus, after addition, the agent was continuously present throughout the experiment. In preliminary experiments, other methods of agent application to inside-out patches such as bath exchange or constant superfusion had been tested by application of solutions with different calcium concentrations. It was found that, only with the direct application method, the occurrence of technical drawbacks, especially loss of patches, vesicle formation and run-down of channel activity, was sufficiently infrequent to obtain the necessary number of reproducible experiments for statistical analysis of the results.
The pharmacological tools used—the NO-scavenger hydroxocobalamin and the PKG-inhibitor Rp-8-Br-PET-cGMPS—have been tested in pilot experiments for their interaction with NO. NO concentration was measured with a Malinski type porphyrinic NO sensor (Biologic, Seyssinet-Pariset, France). In the presence of hydroxocobalamin, NO was undetectable upon addition of NO up to a concentration of 800 nM. In the presence of Rp-8-Br-PET-cGMPS, NO concentrations tested up to 800 nM were evidently reduced by 40%. However, calibration curves of the electrode obtained in Rp-8-Br-PET-cGMPS-free solutions before and after testing Rp-8-Br-PET-cGMPS were considerably different, demonstrating an interaction of Rp-8-Br-PET-cGMPS with the electrode. Thus, a large part of the apparent reduction in NO concentration is caused by a change in electrode properties and it was concluded that there is only a small, if any, reduction in NO concentration in Rp-8-Br-PET-cGMPS-containing solutions.
4.3. Drugs and Chemicals
Albumin, DL-dithiothreitol, hydroxocobalamin, MgATP, cGMP, sodium nitroprusside, and the salts for the solutions were obtained from Sigma (Deisenhofen, Germany). Papain was purchased from Ferak (Berlin, Germany). SNAP (S-nitroso-N-acetyl-penicillamine) was from Calbiochem (Bad Soden, Germany), DEA/NO from ALEXIS (Grünberg, Germany) and iberiotoxin from RBI (Cologne, Germany). Rp-8-Br-PET-cGMPS was obtained from Biolog (Bremen, Germany). The catalytic subunit of PKG was from Promega (Mannheim, Germany).
4.4. Statistics
All data are presented as means ± SE; n is the number of cells. Statistical analysis was performed with use of a one-sample t-test, independent sample t-test, Mann–Whitney test, or Kruskal–Wallis test with Dunn’s post hoc test, as appropriate (SPSS 9.0 for Windows, IBM, Ehningen, Germany).