Addressing the Needs of the Rapidly Aging Society through the Development of Multifunctional Bioactive Coatings for Orthopedic Applications
Abstract
1. Introduction
2. Statistics on the Number of Orthopedic Procedures and the Rationale for Developing Enhanced
3. Biocompatible Metal Materials
3D-Printed Biocompatible Metal Materials
4. Coating Material Selection
4.1. Carrier Materials
4.1.1. Naturally Occurring Polymers
4.1.2. Synthetic Polymers
4.1.3. Inorganic Coating Materials
4.2. Active Ingredients
4.2.1. The Selection of an Antimicrobial Agent
4.2.2. The Choice of Pain Medications
| Drug Class | Examples | Contraindications and Cautions in Systemic Delivery | Total Hip Replacement | Total Knee Replacement | Spinal Fusion |
|---|---|---|---|---|---|
| NSAID | Ketorolac, ibuprofen, meloxicam, diclofenac | Gastrointestinal bleeding and ulceration, cardiovascular events, renal dysfunction | YES | YES | NO |
| Anti-neuropathic | Gabapentin | Dizziness, sedation; reduced dose with renal dysfunction | Gabapentin OR pregabalin | Gabapentin OR pregabalin | Gabapentin OR pregabalin |
| pregabalin | |||||
| Analgesic and antipyretic | Acetaminophen, paracetamol | Hepatotoxicity | AND/OR | AND/OR | YES |
| Local anesthetic | Lignocaine, bupivacaine, ropivacaine, prilocaine [194] | Local anesthetic systemic toxicity (LAST), methemoglobinemia [195] | YES | YES | NO |
4.2.3. A Presentation of Anticoagulation Agents
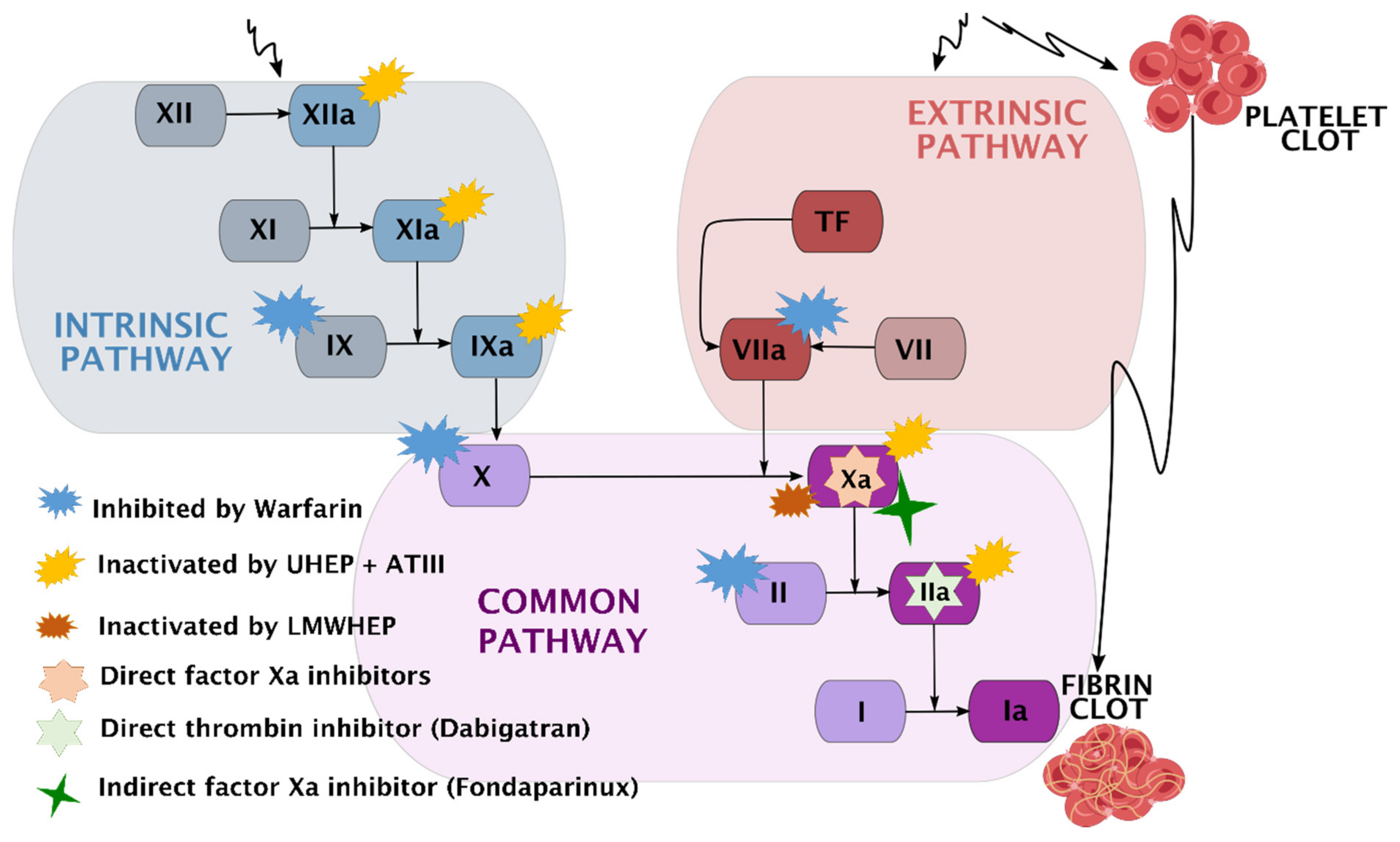
5. The Latest Strategies in the Development of Multifunctional Bioactive Coatings
6. Concluding Remarks and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Suvan, J.E.; Finer, N.; D’Aiuto, F. Periodontal complications with obesity. Periodontology 2018, 78, 98–128. [Google Scholar] [CrossRef]
- Wastesson, J.W.; Morin, L.; Tan, E.C.K.; Johnell, K. An update on the clinical consequences of polypharmacy in older adults: A narrative review. Expert Opin. Drug Saf. 2018, 17, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, A.S.; Gorczyca, J.T. Orthopedic surgery and the geriatric patient. Clin. Geriatr. Med. 2019, 35, 65–92. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.S.L.; Booth, M.A.; Rifai, A.; Fox, K.; Gelmi, A. Diamond in the rough: Toward improved materials for the bone−implant interface. Adv. Healthcare Mater. 2021, 10, 2100007. [Google Scholar] [CrossRef]
- Tobin, E.J. Recent coating developments for combination devices in orthopedic and dental applications: A literature review. Adv. Drug Deliv. Rev. 2017, 112, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Aik Khor, K. Preparation and properties of coatings and thin films on metal implants. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 203–212. [Google Scholar]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional coatings and nanotopographies: Toward cell instructive and antibacterial implants. Adv. Health Mater. 2018, 8, e1801103. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Thandapani, G.; Radha, E.; Jayashri, J.; Florence, J.A.K.; Sudha, P. Bioactive metallic surfaces for bone tissue engineering. In Fundamental Biomaterials: Metals; Balakrishnan, P., Sreekala, M.S., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 79–110. [Google Scholar]
- Romanò, C.L.; Tsuchiya, H.; Morelli, I.; Battaglia, A.G.; Drago, L. Antibacterial coating of implants: Are we missing something? Bone Jt. Res. 2019, 8, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Maver, U.; Xhanari, K.; Žižek, M.; Gradišnik, L.; Repnik, K.; Potočnik, U.; Finšgar, M. Carboxymethyl cellulose/diclofenac bioactive coatings on AISI 316LVM for controlled drug delivery, and improved osteogenic potential. Carbohydr. Polym. 2020, 230, 115612. [Google Scholar] [CrossRef] [PubMed]
- Finšgar, M.; Uzunalić, A.P.; Stergar, J.; Gradišnik, L.; Maver, U. Novel chitosan/diclofenac coatings on medical grade stainless steel for hip replacement applications. Sci. Rep. 2016, 6, 26653. [Google Scholar] [CrossRef]
- Suchý, T.; Vištejnová, L.; Šupová, M.; Klein, P.; Bartoš, M.; Kolinko, Y.; Blassová, T.; Tonar, Z.; Pokorný, M.; Sucharda, Z.; et al. Vancomycin-loaded collagen/hydroxyapatite layers electrospun on 3D printed titanium implants prevent bone destruction associated with S. epidermidis infection and enhance osseointegration. Biomedicines 2021, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.-J.; Emanuel, N.; Cohen, O.; Reichart, M.; Potapova, I.; Schmid, T.; Segal, D.; Riool, M.; Kwakman, P.H.; de Boer, L.; et al. A doxycycline-loaded polymer-lipid encapsulation matrix coating for the prevention of implant-related osteomyelitis due to doxycycline-resistant methicillin-resistant Staphylococcus aureus. J. Control. Release 2015, 209, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.J.; Lawrence, A.J. Hurdles in basic science translation. Front. Pharmacol. 2017, 8, 478. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide—Identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- World Implantable Biomaterials Market Report 2020–2030. Available online: https://www.globenewswire.com/news-release/2020/08/04/2072251/0/en/World-Implantable-Biomaterials-Market-Report-2020-2030.html (accessed on 15 October 2021).
- OECD. Hip and Knee Replacement, 2016. Available online: https://www.oecd-ilibrary.org/docserver/2fc83b9a-en.pdf?expires=1646047913&id=id&accname=guest&checksum=806BCE10094670EF576AD8124144844B (accessed on 2 September 2021).
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of total hip and knee replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Gronbeck, C.; Cusano, A.; Cardenas, J.M.; Harrington, M.A.; Halawi, M.J. Primary total hip arthroplasty in Hispanic/Latino patients: An updated nationwide analysis of length of stay, 30-day outcomes, and risk factors. Arthroplast. Today 2020, 6, 721–725. [Google Scholar] [CrossRef]
- Papin, P.; Berthonnaud, E. Incidence of osteosynthesis of members in France. Int. Orthop. 2017, 41, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Surgical Operations and Procedures Performed in Hospitals by ICD-9-CM. Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/hlth_co_proc2 (accessed on 23 September 2021).
- Rony, L.; Lancigu, R.; Hubert, L. Intraosseous metal implants in orthopedics: A review. Morphologie 2018, 102, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Patel, R. Infection associated with prosthetic joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gallo, J. Periprosthetic osteolysis: Mechanisms, prevention and treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef]
- Brochin, R.L.; Phan, K.; Poeran, J.; Zubizarreta, N.; Galatz, L.M.; Moucha, C.S. Trends in periprosthetic hip infection and associated costs: A population-based study assessing the impact of hospital factors using national data. J. Arthroplast. 2018, 33, S233–S238. [Google Scholar] [CrossRef] [PubMed]
- Haenle, M.; Skripitz, C.; Mittelmeier, W.; Skripitz, R. Economic impact of infected total knee arthroplasty. Sci. World J. 2012, 2012, 196515. [Google Scholar] [CrossRef]
- Kamath, A.F.; Ong, K.L.; Lau, E.; Chan, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J.; Bozic, K.J. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J. Arthroplast. 2015, 30, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Klouche, S.; Sariali, E.; Mamoudy, P. Total hip arthroplasty revision due to infection: A cost analysis approach. Orthop. Traumatol. Surg. Res. 2010, 96, 124–132. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65.e61. [Google Scholar] [CrossRef]
- Lieb, E.; Hanstein, T.; Schuerings, M.; Trampuz, A.; Perka, C. Eine Verkürzung der Behandlungsdauer von periprothetischen Infektionen durch ein Fast-Track-Konzept ist ökonomisch unmöglich. Z. Orthop. Unfall. 2015, 153, 618–623. [Google Scholar] [CrossRef]
- Romanò, C.L.; Romanò, D.; Logoluso, N.; Meani, E. Septic versus aseptic hip revision: How different? J. Orthop. Traumatol. 2010, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of total joint replacement in the United States: Future projections to 2020–2040 using the national inpatient sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Vanhegan, I.S.; Malik, A.K.; Jayakumar, P.; Islam, S.U.; Haddad, F.S. A financial analysis of revision hip arthroplasty: Arthroplasty: The economic burden in relation to the national tariff. J. Bone Jt. Surg. Br. 2012, 94, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Lenguerrand, E.; Whitehouse, M.R.; Beswick, A.D.; Toms, A.D.; Porter, M.L.; Blom, A.W. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: An analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open 2017, 7, e014056. [Google Scholar] [CrossRef]
- Sousa, A.; Carvalho, A.; Pereira, C.; Reis, E.; Santos, A.C.; Abreu, M.A.; Soares, D.; Fragoso, R.; Ferreira, S.; Reis, M.; et al. Economic impact of prosthetic joint infection—An evaluation within the Portuguese National Health System. J. Bone Jt. Infect. 2018, 3, 197–202. [Google Scholar] [CrossRef]
- Morin, L.; Johnell, K.; Laroche, M.-L.; Fastbom, J.; Wastesson, J.W. The epidemiology of polypharmacy in older adults: Register-based prospective cohort study. Clin. Epidemiol. 2018, 10, 289–298. [Google Scholar] [CrossRef]
- Zazzara, M.B.; Palmer, K.; Vetrano, D.L.; Carfì, A.; Graziano, O. Adverse drug reactions in older adults: A narrative review of the literature. Eur. Geriatr. Med. 2021, 12, 463–473. [Google Scholar] [CrossRef]
- Tsai, Y.; Chang, C.-H.; Lin, Y.-C.; Lee, S.-H.; Hsieh, P.-H.; Chang, Y. Different microbiological profiles between hip and knee prosthetic joint infections. J. Orthop. Surg. 2019, 27, 2309499019847768. [Google Scholar] [CrossRef]
- Du, Y.; Guo, J.L.; Wang, J.; Mikos, A.G.; Zhang, S. Hierarchically designed bone scaffolds: From internal cues to external stimuli. Biomaterials 2019, 218, 119334. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Izmir, M.; Tufan, Y.; Tan, G.; Ercan, B. Ti6Al4V foams having nanotubular surfaces for orthopaedic applications. Surf. Interface Anal. 2019, 51, 954–963. [Google Scholar] [CrossRef]
- Hoppe, V.; Szymczyk-Ziółkowska, P.; Rusińska, M.; Dybała, B.; Poradowski, D.; Janeczek, M. Assessment of mechanical, chemical, and biological properties of Ti-Nb-Zr alloy for medical applications. Materials 2020, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical–chemical and biological properties of the new Ti–30Nb–13Ta–5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Konushkin, S.V.; Ivannikov, A.Y.; Nasakina, E.O.; Shatova, L.A.; Kolmakov, A.G.; Sevostyanov, M.A. Preparation, structural and microstructural characterization of Ti–30Nb–10Ta–5Zr alloy for biomedical applications. J. Mater. Res. Technol. 2020, 9, 16018–16028. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloy. Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Brooks, E.K.; Brooks, R.P.; Ehrensberger, M.T. Effects of simulated inflammation on the corrosion of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 200–205. [Google Scholar] [CrossRef]
- Zaman, H.A.; Sharif, S.; Kim, D.-W.; Idris, M.H.; Suhaimi, M.A.; Tumurkhuyag, Z. Machinability of cobalt-based and cobalt chromium molybdenum alloys—A review. Procedia Manuf. 2017, 11, 563–570. [Google Scholar] [CrossRef]
- Ghosh, S.; Sanghavi, S.; Sancheti, P. 6—Metallic biomaterial for bone support and replacement. In Fundamental Biomaterials: Metals; Balakrishnan, P., Sreekala, M.S., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 139–165. [Google Scholar]
- Pfeifer, R.; Müller, C.W.; Hurschler, C.; Kaierle, S.; Wesling, V.; Haferkamp, H. Adaptable orthopedic shape memory implants. Procedia CIRP 2013, 5, 253–258. [Google Scholar] [CrossRef]
- Ma, C.; Andani, M.T.; Qin, H.; Moghaddam, N.S.; Ibrahim, H.; Jahadakbar, A.; Amerinatanzi, A.; Ren, Z.; Zhang, H.; Doll, G.L.; et al. Improving surface finish and wear resistance of additive manufactured nickel-titanium by ultrasonic nano-crystal surface modification. J. Mater. Process. Technol. 2017, 249, 433–440. [Google Scholar] [CrossRef]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E. Selective laser melting of titanium alloy with 50 wt% tantalum: Microstructure and mechanical properties. J. Alloy. Compd. 2016, 660, 461–470. [Google Scholar] [CrossRef]
- Han, Q.; Wang, C.; Chen, H.; Zhao, X.; Wang, J. Porous tantalum and titanium in orthopedics: A review. ACS Biomater. Sci. Eng. 2019, 5, 5798–5824. [Google Scholar] [CrossRef] [PubMed]
- Paganias, C.G.; Tsakotos, G.; Koutsostathis, S.D.; Macheras, G.A. Osseous integration in porous tantalum implants. Indian J. Orthop. 2012, 46, 505–513. [Google Scholar] [CrossRef] [PubMed]
- FDA. Biological Responses to Metal Implants; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019; p. 143.
- Majumdar, T.; Eisenstein, N.; Frith, J.E.; Cox, S.; Birbilis, N. Additive manufacturing of titanium alloys for orthopedic applications: A materials science viewpoint. Adv. Eng. Mater. 2018, 20, 1800172. [Google Scholar] [CrossRef]
- Cole, B.J.; Sayegh, E.T.; Yanke, A.B.; Chalmers, P.N.; Frank, R.M. Fixation of soft tissue to bone. J. Am. Acad. Orthop. Surg. 2016, 24, 83–95. [Google Scholar] [CrossRef]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Aksakal, B.; Yildirim, S.; Gul, H. Metallurgical failure analysis of various implant materials used in orthopedic applications. J. Fail. Anal. Prev. 2004, 4, 17–23. [Google Scholar] [CrossRef]
- White, A.A.; Best, S.M. 5—Properties and characterisation of bone repair materials. In Bone Repair Biomaterials; Planell, J.A., Best, S.M., Lacroix, D., Merolli, A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 121–153. [Google Scholar]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Ding, Y.F.; Li, R.W.; Nakai, M.; Majumdar, T.; Zhang, D.H.; Niinomi, M.; Birbilis, N.; Smith, P.N.; Chen, X.B. Osteoanabolic implant materials for orthopedic treatment. Adv. Healthcare Mater. 2016, 5, 1740–1752. [Google Scholar] [CrossRef]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio 2019, 3, 100024. [Google Scholar] [CrossRef]
- Calvo-Haro, J.A.; Pascau, J.; Mediavilla-Santos, L.; Sanz-Ruiz, P.; Sánchez-Pérez, C.; Vaquero-Martín, J.; Perez-Mañanes, R. Conceptual evolution of 3D printing in orthopedic surgery and traumatology: From “do it yourself” to “point of care manufacturing”. BMC Musculoskelet. Disord. 2021, 22, 360. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.N.; Miller, A.T.; Hollister, S.; Guldberg, R.E.; Gall, K. Design and structure-function characterization of 3D printed synthetic porous biomaterials for tissue engineering. Adv. Healthcare Mater. 2017, 7, e1701095. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wei, F.; Liu, X.; Jiang, L.; Cai, H.; Li, Z.; Yu, M.; Wu, F.; Liu, Z. Reconstruction of the upper cervical spine using a personalized 3D-Printed vertebral body in an adolescent with Ewing sarcoma. Spine 2016, 41, E50–E54. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, Z.; Liu, Z.; Liu, X.; Jiang, L.; Yu, M.; Xu, N.; Wu, F.; Dang, L.; Zhou, H.; et al. Upper cervical spine reconstruction using customized 3D-printed vertebral body in 9 patients with primary tumors involving C2. Ann. Transl. Med. 2020, 8, 332. [Google Scholar] [CrossRef]
- Wong, K.C.; Kumta, S.M.; Geel, N.V.; Demol, J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided Surg. 2015, 20, 14–23. [Google Scholar] [CrossRef]
- Hamid, K.S.; Parekh, S.G.; Adams, S.B. Salvage of severe foot and ankle trauma with a 3D printed scaffold. Foot Ankle Int. 2016, 37, 433–439. [Google Scholar] [CrossRef]
- Salmi, M.; Tuomi, J.; Paloheimo, K.; Björkstrand, R.; Paloheimo, M.; Salo, J.; Kontio, R.; Mesimäki, K.; Mäkitie, A.A. Patient-specific reconstruction with 3D modeling and DMLS additive manufacturing. Rapid Prototyp. J. 2012, 18, 209–214. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Filho, R.M.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; Löwenheim, H.; Hoffmann, J. Image data-based reconstruction of the midface using a patient-specific implant in combination with a vascularized osteomyocutaneous scapular flap. J. Cranio-Maxillofac. Surg. 2013, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Qin, Y.; Zou, Y.; Wang, C.; Bai, H.; Yu, T.; Huang, L.; Wang, J. Novel exploration of 3D printed wrist arthroplasty to solve the severe and complicated bone defect of wrist. Rapid Prototyp. J. 2017, 23, 465–473. [Google Scholar] [CrossRef]
- Mazzoni, S.; Bianchi, A.; Schiariti, G.; Badiali, G.; Marchetti, C. Computer-aided design and computer-aided manufacturing cutting guides and customized titanium plates are useful in upper maxilla waferless repositioning. J. Oral Maxillofac. Surg. 2014, 73, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Li, S.; Hao, Y. Additive manufacturing of titanium alloys by electron beam melting: A review. Adv. Eng. Mater. 2018, 20, 1700842. [Google Scholar] [CrossRef]
- Levesque, J.N.; Shah, A.; Ekhtiari, S.; Yan, J.R.; Thornley, P.; Williams, D.S. Three-dimensional printing in orthopaedic surgery: A scoping review. EFORT Open Rev. 2020, 5, 430–441. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous titanium implants: A review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Baikin, A.S.; Kolmakov, A.G.; Shatova, L.A.; Nasakina, E.O.; Sharapov, M.G.; Baymler, I.V.; Gudkov, S.V.; Sevostyanov, M.A. Polylactide-based stent coatings: Biodegradable polymeric coatings capable of maintaining sustained release of the thrombolytic enzyme prourokinase. Materials 2019, 12, 4107. [Google Scholar] [CrossRef]
- Horvat, G.; Xhanari, K.; Finšgar, M.; Gradišnik, L.; Maver, U.; Knez, Ž.; Novak, Z. Novel ethanol-induced pectin–xanthan aerogel coatings for orthopedic applications. Carbohydr. Polym. 2017, 166, 365–376. [Google Scholar] [CrossRef]
- Ajdnik, U.; Finšgar, M.; Fras Zemljič, L. Characterization of chitosan-lysine surfactant bioactive coating on silicone substrate. Carbohydr. Polym. 2019, 232, 115817. [Google Scholar] [CrossRef]
- Maver, T.; Hribernik, S.; Mohan, T.; Smrke, D.M.; Maver, U.; Stana-Kleinschek, K. Functional wound dressing materials with highly tunable drug release properties. RSC Adv. 2015, 5, 77873–77884. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Mostegel, F.; Griesser, T.; Spirk, S.; Smrke, D.M.; Kleinschek, K.S. Cellulose based thin films as a platform for drug release studies to mimick wound dressing materials. Cellulose 2014, 22, 749–761. [Google Scholar] [CrossRef]
- Maver, T.; Gradišnik, L.; Kurečič, M.; Hribernik, S.; Smrke, D.M.; Maver, U.; Kleinschek, K.S. Layering of different materials to achieve optimal conditions for treatment of painful wounds. Int. J. Pharm. 2017, 529, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Stana, J.; Stergar, J.; Gradišnik, L.; Flis, V.; Kargl, R.; Fröhlich, E.; Kleinschek, K.S.; Mohan, T.; Maver, U. Multilayered polysaccharide nanofilms for controlled delivery of pentoxifylline and possible treatment of chronic venous ulceration. Biomacromolecules 2017, 18, 2732–2746. [Google Scholar] [CrossRef] [PubMed]
- Maver, U.; Xhanari, K.; Žižek, M.; Korte, D.; Gradišnik, L.; Franko, M.; Finšgar, M. A combination of interdisciplinary analytical tools for evaluation of multi-layered coatings on medical grade stainless steel for biomedical applications. Eur. J. Pharm. Biopharm. 2018, 128, 230–246. [Google Scholar] [CrossRef]
- Maver, T.; Gradišnik, L.; Smrke, D.M.; Stana Kleinschek, K.; Maver, U. Systematic evaluation of a diclofenac-loaded carboxymethyl cellulose-based wound dressing and its release performance with changing pH and temperature. AAPS PharmSciTech 2019, 20, 29. [Google Scholar] [CrossRef]
- Maver, T.; Mohan, T.; Gradišnik, L.; Finšgar, M.; Kleinschek, K.S.; Maver, U. Polysaccharide thin solid films for analgesic drug delivery and growth of human skin cells. Front. Chem. 2019, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Maver, U.; Gradišnik, L.; Smrke, D.M.; Kleinschek, K.S.; Maver, T. Impact of growth factors on wound healing in polysaccharide blend thin films. Appl. Surf. Sci. 2019, 489, 485–493. [Google Scholar] [CrossRef]
- Kaplan, M.A.; Sergienko, K.V.; Kolmakova, A.A.; Konushkin, S.V.; Baikin, A.S.; Kolmakov, A.G.; Sevostyanov, M.A.; Kulikov, A.V.; Ivanov, V.E.; Belosludtsev, K.N.; et al. Development of a biocompatible PLGA polymers capable to release thrombolytic enzyme prourokinase. J. Biomater. Sci. Polym. Ed. 2020, 31, 1405–1420. [Google Scholar] [CrossRef]
- Mathiowitz, E. Encyclopedia of Controlled Drug Delivery; John Wiley & Sons Inc.: New York, NY, USA, 1999; p. 1061. [Google Scholar]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 220. [Google Scholar] [CrossRef]
- Chu, C.C. 21—Surface degradation and microenvironmental outcomes. In Surfaces and Interfaces for Biomaterials; Vadgama, P., Ed.; Woodhead Publishing: Sawston, UK, 2005; pp. 585–618. [Google Scholar]
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Chapter 18—Application of compatibilized polymer blends in biomedical fields. In Compatibilization of Polymer Blends; Ajitha, A.R., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537. [Google Scholar]
- Zoratto, N.; Matricardi, P. 4—Semi-IPNs and IPN-based hydrogels. In Polymeric Gels; Pal, K., Banerjee, I., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 91–124. [Google Scholar]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Chapter 2—Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar]
- Liu, H.; Yang, Q.; Zhang, L.; Zhuo, R.; Jiang, X. Synthesis of carboxymethyl chitin in aqueous solution and its thermo- and pH-sensitive behaviors. Carbohydr. Polym. 2016, 137, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Oh, S.-G. Size effect of carboxymethyl chitin nanocrystals on the properties of foams in aqueous surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125306. [Google Scholar] [CrossRef]
- Latifi, M.; Ahmad, A.; Kaddami, H.; Hassan, N.H.; Dieden, R.; Habibi, Y. Chemical modification and processing of chitin for sustainable production of biobased electrolytes. Polymers 2020, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Logith Kumar, R.; Keshav Narayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2020, 16, 280–306. [Google Scholar] [CrossRef]
- Tabasum, S.; Noreen, A.; Maqsood, M.F.; Umar, H.; Akram, N.; Nazli, Z.-I.; Chatha, S.A.S.; Zia, K.M. A review on versatile applications of blends and composites of pullulan with natural and synthetic polymers. Int. J. Biol. Macromol. 2018, 120, 603–632. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef]
- Silver, F.H.; Jaffe, M. 7—Structure and behavior of collagen fibers. In Handbook of Tensile Properties of Textile and Technical Fibres; Bunsell, A.R., Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 179–193. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Behra, J.S.; Mattsson, J.; Cayre, O.J.; Robles, E.S.J.; Tang, H.; Hunter, T.N. Characterization of sodium carboxymethyl cellulose aqueous solutions to support complex product formulation: A rheology and light scattering study. ACS Appl. Polym. Mater. 2019, 1, 344–358. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C. Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical applications of cellulose ethers and cellulose ether esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Meereboer, K.W.; Pal, A.K.; Misra, M.; Mohanty, A.K. Sustainable PHBV/cellulose acetate blends: Effect of a chain extender and a plasticizer. ACS Omega 2020, 5, 14221–14231. [Google Scholar] [CrossRef] [PubMed]
- Caceres, M.; Petit, E.; Deratani, A. Partial depolymerization of hydroxypropylmethyl cellulose for production of low molar mass polymer chains. Carbohydr. Polym. 2020, 229, 115461. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar] [CrossRef]
- Etman, S.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Fucoidan, a natural biopolymer in cancer combating: From edible algae to nanocarrier tailoring. Int. J. Biol. Macromol. 2020, 147, 799–808. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.C.; Krieghoff, J.; Mikos, A.G. Chapter 33—Synthetic polymers. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 559–590. [Google Scholar]
- Samavedi, S.; Poindexter, L.K.; Van Dyke, M.; Goldstein, A.S. Chapter 7—Synthetic biomaterials for regenerative medicine applications. In Regenerative Medicine Applications in Organ Transplantation; Orlando, G., Lerut, J., Soker, S., Stratta, R.J., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 81–99. [Google Scholar]
- Mulazzi, M.; Campodoni, E.; Bassi, G.; Montesi, M.; Panseri, S.; Bonvicini, F.; Gentilomi, G.; Tampieri, A.; Sandri, M. Medicated hydroxyapatite/collagen hybrid scaffolds for bone regeneration and local antimicrobial therapy to prevent bone infections. Pharmaceutics 2021, 13, 1090. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.; Shyshov, O.; Suraeva, O.; Lieberwirth, I.; Von Delius, M.; Wurm, F.R. Long-chain polyorthoesters as degradable polyethylene mimics. Macromolecules 2019, 52, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Ottoboni, T.; Gelder, M.S.; O’Boyle, E. Biochronomer™ technology and the development of APF530, a sustained release formulation of granisetron. J. Exp. Pharmacol. 2014, 6, 15–21. [Google Scholar] [CrossRef]
- Tschan, M.J.; Ieong, N.S.; Todd, R.; Everson, J.; Dove, A.P. Unlocking the potential of poly(ortho ester)s: A general catalytic approach to the synthesis of surface-erodible materials. Angew. Chem. Int. Ed. 2017, 56, 16664–16668. [Google Scholar] [CrossRef]
- Mastnak, T.; Lobnik, A.; Mohr, G.J.; Finšgar, M. Indicator layers based on ethylene-vinyl acetate copolymer (EVA) and dicyanovinyl azobenzene dyes for fast and selective evaluation of vaporous biogenic amines. Sensors 2018, 18, 4361. [Google Scholar] [CrossRef]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J.; Zhao, Z.; Zhang, Q.; Zhu, X.; Li, B. Polydopamine-assisted surface modification for orthopaedic implants. J. Orthop. Transl. 2019, 17, 82–95. [Google Scholar] [CrossRef]
- Xie, K.; Zhou, Z.; Guo, Y.; Wang, L.; Li, G.; Zhao, S.; Liu, X.; Li, J.; Jiang, W.; Wu, S.; et al. Long-term prevention of bacterial infection and enhanced osteoinductivity of a hybrid coating with selective silver toxicity. Adv. Healthcare Mater. 2019, 8, e1801465. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Razak, S.I.A.; Shahir, S.; Al-Moalemi, H.A.A.; Kadir, M.R.A.; Nayan, N.H.M. Development of prolonged drug delivery system using electrospun cellulose acetate/polycaprolactone nanofibers: Future subcutaneous implantation. Polym. Adv. Technol. 2021, 32, 3664–3678. [Google Scholar] [CrossRef]
- Chang, S.H.; Lee, H.J.; Park, S.; Kim, Y.; Jeong, B. Fast degradable polycaprolactone for drug delivery. Biomacromolecules 2018, 19, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Granados, A.; Fernández, E.; Pleixats, R.; Vallribera, A. Anti-inflammatory cotton fabrics and silica nanoparticles with potential topical medical applications. ACS Appl. Mater. Interfaces 2020, 12, 25658–25675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, N.; Mankoci, S.; Sahai, N. Silicates in orthopedics and bone tissue engineering materials. J. Biomed. Mater. Res. Part A 2017, 105, 2090–2102. [Google Scholar] [CrossRef]
- Givens, B.E.; Wilson, E.; Fiegel, J. The effect of salts in aqueous media on the formation of the BSA corona on SiO2 nanoparticles. Colloids Surf. B Biointerfaces 2019, 179, 374–381. [Google Scholar] [CrossRef]
- Jones, J.R. 12—Bioactive glass. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 266–283. [Google Scholar]
- Ben-Nissan, B.; Cazalbou, S.; Choi, A.H. Bioceramics. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 16–33. [Google Scholar]
- Huang, Y.-Z.; Xie, H.-Q.; Li, X. Scaffolds in bone tissue engineering: Research progress and current applications. In Encyclopedia of Bone Biology; Zaidi, M., Ed.; Academic Press: Oxford, UK, 2020; pp. 204–215. [Google Scholar]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. 7—Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 143–157. [Google Scholar]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Ren, L.; Pan, S.; Li, H.; Li, Y.; He, L.; Zhang, S.; Che, J.; Niu, Y. Effects of aspirin-loaded graphene oxide coating of a titanium surface on proliferation and osteogenic differentiation of MC3T3-E1 cells. Sci. Rep. 2018, 8, 15143. [Google Scholar] [CrossRef]
- Wright, Z.M.; Pandit, A.M.; Karpinsky, M.M.; Holt, B.D.; Zovinka, E.P.; Sydlik, S.A. Bioactive, ion-releasing PMMA bone cement filled with functional graphenic materials. Adv. Healthcare Mater. 2020, 10, e2001189. [Google Scholar] [CrossRef]
- Nasakina, E.O.; Baikin, A.S.; Sergienko, K.V.; Sevost’Yanov, M.A.; Kolmakov, A.G.; Goncharenko, B.A.; Zabolotnyi, V.T.; Fadeev, R.S.; Fadeeva, I.S.; Gudkov, S.V.; et al. Biocompatibility of nanostructured nitinol with titanium or tantalum surface composite layers formed by magnetron sputtering. Dokl. Chem. 2015, 461, 86–88. [Google Scholar] [CrossRef]
- Nasakina, E.O.; Baikin, A.S.; Sergiyenko, K.V.; Leonov, A.V.; Kaplan, M.A.; Seryogin, A.V.; Konushkin, S.V.; Myasnikova, N.V.; Sevostyanov, M.A.; Kolmakov, A.G.; et al. Formation and investigation of composite material silver–nitinol for medical purposes. Inorg. Mater. Appl. Res. 2017, 8, 112–117. [Google Scholar] [CrossRef]
- Bagherpour, I.; Naghib, S.M.; Yaghtin, A.H. Synthesis and characterisation of nanostructured hardystonite coating on stainless steel for biomedical application. IET Nanobiotechnol. 2018, 12, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Rizvi, S.A.; Abbas, N.; Sajjad, U.; Shad, M.; Badshah, M.; Malik, A. Recent developments in coatings for orthopedic metallic implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Brigham, N.C.; Ji, R.-R.; Becker, M.L. Degradable polymeric vehicles for postoperative pain management. Nat. Commun. 2021, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2014, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.F.; Kuehl, R.; Coenye, T.; Metsemakers, W.-J.; Morgenstern, M.; Schwarz, E.M.; Riool, M.; Zaat, S.A.; Khana, N.; Kates, S.L.; et al. Orthopaedic device-related infection: Current and future interventions for improved prevention and treatment. EFORT Open Rev. 2017, 1, 89–99. [Google Scholar] [CrossRef]
- Horprasertkij, K.; Dwivedi, A.; Riansuwan, K.; Kiratisin, P.; Nasongkla, N. Spray coating of dual antibiotic-loaded nanospheres on orthopedic implant for prolonged release and enhanced antibacterial activity. J. Drug Deliv. Sci. Technol. 2019, 53, 101102. [Google Scholar] [CrossRef]
- Devlin-Mullin, A.; Todd, N.M.; Golrokhi, Z.; Geng, H.; Konerding, M.A.; Ternan, N.G.; Hunt, J.A.; Potter, R.J.; Sutcliffe, C.; Jones, E.; et al. Atomic layer deposition of a silver nanolayer on advanced titanium orthopedic implants inhibits bacterial colonization and supports vascularized de novo bone ingrowth. Adv. Healthcare Mater. 2017, 6, 1700033. [Google Scholar] [CrossRef]
- Costa, B.; Martínez-De-Tejada, G.; Gomes, P.A.C.; Martins, M.C.L.; Costa, F. Antimicrobial peptides in the battle against orthopedic implant-related infections: A review. Pharmaceutics 2021, 13, 1918. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Clerici, P.; Morelli, I.; Ashok, J.; Benzakour, T.; Bozhkova, S.; Alizadeh, C.; Del Sel, H.; Sharma, H.K.; Peel, T.; et al. The World Association against Infection in Orthopaedics and Trauma (WAIOT) procedures for Microbiological Sampling and Processing for Periprosthetic Joint Infections (PJIs) and other implant-related infections. J. Clin. Med. 2019, 8, 933. [Google Scholar] [CrossRef] [PubMed]
- Shillingford, J.N.; Laratta, J.L.; Reddy, H.; Ha, A.; Lehman, R.A., Jr.; Lenke, L.G.; Fischer, C.R. Postoperative surgical site infection after spine surgery: An update from the Scoliosis Research Society (SRS) morbidity and mortality database. Spine Deform. 2018, 6, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Salib, C.G.; Rose, P.S.; Sim, F.H.; Lewallen, D.G.; Abdel, M.P. Reconstruction of the hip after resection of periacetabular oncological lesions: A systematic review. Bone Jt. J. 2018, 100, 22–30. [Google Scholar] [CrossRef]
- Lee, J.; Byun, H.; Perikamana, S.K.M.; Lee, S.; Shin, H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthcare Mater. 2018, 8, e1801106. [Google Scholar] [CrossRef]
- Zhu, M.; Fang, J.; Li, Y.; Zhong, C.; Feng, S.; Ge, X.; Ye, H.; Wang, X.; Zhu, W.; Lu, X.; et al. Metallic thin films: The synergy of Topographical Micropatterning and Ta|TaCu Bilayered Thin Film on Titanium Implants Enables Dual-Functions of Enhanced osteogenesis and anti-infection (Adv. Healthcare Mater. 9/2021). Adv. Healthcare Mater. 2021, 10, 2170045. [Google Scholar] [CrossRef]
- Corvec, S.; Portillo, M.E.; Pasticci, B.M.; Borens, O.; Trampuz, A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int. J. Artif. Organs 2012, 35, 923–934. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; Nasongkla, N. Layer-by-layer nanocoating of antibacterial niosome on orthopedic implant. Int. J. Pharm. 2018, 547, 235–243. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef]
- Brook, I. Treatment of anaerobic infection. Expert Rev. Anti-Infect. Ther. 2007, 5, 991–1006. [Google Scholar] [CrossRef]
- Sulovari, A.; Ninomiya, M.J.; Beck, C.A.; Ricciardi, B.F.; Ketonis, C.; Mesfin, A.; Kaplan, N.B.; Soin, S.P.; McDowell, S.M.; Mahmood, B.; et al. Clinical utilization of species-specific immunoassays for identification of Staphylococcus aureus and Streptococcus agalactiae in orthopedic infections. J. Orthop. Res. 2020, 39, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Janz, V.; Wassilew, G.I.; Kribus, M.; Trampuz, A.; Perka, C. Improved identification of polymicrobial infection in total knee arthroplasty through sonicate fluid cultures. Arch. Orthop. Trauma. Surg. 2015, 135, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Bryson, D.J.; Morris, D.L.J.; Shivji, F.S.; Rollins, K.; Snape, S.; Ollivere, B.J. Antibiotic prophylaxis in orthopaedic surgery. Bone Jt. J. 2016, 98, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pardo, D. Antibiotic prophylaxis in orthopaedic surgery: Clinical practice guidelines or individualized prophylaxis? Enferm. Infecc. Microbiol. Clin. 2019, 37, 557–559. [Google Scholar] [CrossRef]
- Davat, M.; Wuarin, L.; Stafylakis, D.; Abbas, M.; Harbarth, S.; Hannouche, D.; Uçkay, I. Should antibiotic prophylaxis before orthopedic implant surgery depend on the duration of pre-surgical hospital stay? Antimicrob. Resist. Infect. Control 2018, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Parvizi, J.; Shohat, N.; Gehrke, T. Prevention of periprosthetic joint infection: New guidelines. Bone Jt. J. 2017, 99, 3–10. [Google Scholar] [CrossRef]
- Van Vugt, T.A.G.; Arts, J.; Geurts, J.A.P. Antibiotic-loaded polymethylmethacrylate beads and spacers in treatment of orthopedic infections and the role of biofilm formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. Chemokines associated with pathologic responses to orthopedic implant debris. Front. Endocrinol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Giavaresi, G.; Meani, E.; Sartori, M.; Ferrari, A.; Bellini, D.; Sacchetta, A.C.; Meraner, J.; Sambri, A.; Vocale, C.; Sambri, V.; et al. Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant. Int. Orthop. 2014, 38, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.; Sandiford, N.A.; Cerbone, V.; De Araujo, L.C.T.; Kendoff, D. Defensive antibacterial coating in revision total hip arthroplasty: New concept and early experience. HIP Int. 2020, 30, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Donati, F.; Di Giacomo, G.; D’Adamio, S.; Ziranu, A.; Careri, S.; Rosa, M.; Maccauro, G. Silver-coated hip megaprosthesis in oncological limb savage surgery. BioMed Res. Int. 2016, 2016, 9079041. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Hegde, V.; Zoller, S.D.; Park, H.Y.; Hart, C.M.; Kondo, T.; Hamad, C.D.; Hu, Y.; Loftin, A.H.; Johansen, D.O.; et al. Point-of-care antimicrobial coating protects orthopaedic implants from bacterial challenge. Nat. Commun. 2021, 12, 5473. [Google Scholar] [CrossRef]
- Cyphert, E.L.; Von Recum, H.A. Emerging technologies for long-term antimicrobial device coatings: Advantages and limitations. Exp. Biol. Med. 2017, 242, 788–798. [Google Scholar] [CrossRef]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef]
- Zhao-Fleming, H.; Hand, A.; Zhang, K.; Polak, R.; Northcut, A.; Jacob, D.; Dissanaike, S.; Rumbaugh, K.P. Effect of non-steroidal anti-inflammatory drugs on post-surgical complications against the backdrop of the opioid crisis. Burn. Trauma 2018, 6, 25. [Google Scholar] [CrossRef]
- Parvizi, J.; Bloomfield, M.R. Multimodal pain management in orthopedics: Implications for joint arthroplasty surgery. Orthopedics 2013, 36, 7–14. [Google Scholar] [CrossRef]
- Kheirabadi, D.; Safavi, M.R.; Taghvaei, M.; Habibzadeh, M.R.; Honarmand, A. Comparing the prophylactic effects of oral gabapentin, pregabalin, and celecoxib on postoperative pain management in orthopedic surgery of the lower extremity: A double-blind randomized controlled trial. J. Res. Med. Sci. 2020, 25, 9. [Google Scholar] [CrossRef]
- Joshi, G.P.; Schug, S.A.; Kehlet, H. Procedure-specific pain management and outcome strategies. Best Pr. Res. Clin. Anaesthesiol. 2014, 28, 191–201. [Google Scholar] [CrossRef]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on regional anesthesia, executive committee, and administrative council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Fung, G.; Liu, S.E. Regional anaesthesia for orthopaedic procedures. Anaesth. Intensive Care Med. 2020, 22, 13–18. [Google Scholar] [CrossRef]
- Grotticelli, J. Chapter 12—Side effects of local anesthetics. In Side Effects of Drugs Annual; Ray, S.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 43, pp. 169–177. [Google Scholar]
- ZYNRELEF™ (Bupivacaine and Meloxicam) Extended-Release Solution. Available online: https://www.herontx.com/product-portfolio/zynrelef/ (accessed on 27 September 2021).
- Ottoboni, T.; Quart, B.; Pawasauskas, J.; Dasta, J.F.; Pollak, R.A.; Viscusi, E.R. Mechanism of action of HTX-011: A novel, extended-release, dual-acting local anesthetic formulation for postoperative pain. Reg. Anesth. Pain Med. 2019, 45, 117–123. [Google Scholar] [CrossRef]
- Kline, J.A.; Kabrhel, C. Emergency evaluation for pulmonary embolism, part 1: Clinical factors that increase risk. J. Emerg. Med. 2015, 48, 771–780. [Google Scholar] [CrossRef]
- Saleh, J.; El-Othmani, M.M.; Saleh, K.J. Deep vein thrombosis and pulmonary embolism considerations in orthopedic surgery. Orthop. Clin. N. Am. 2017, 48, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bilawicz, J.; Trzebicki, J.; Kostrubiec, M. Current role of heparin in thromboprophylaxis of major orthopaedic surgery. Blood Heart Circ. 2018, 2, 1000130. [Google Scholar] [CrossRef]
- Warren, J.A.; Sundaram, K.; Hampton, R.; Billow, D.; Patterson, B.; Piuzzi, N.S. Venous thromboembolism rates remained unchanged in operative lower extremity orthopaedic trauma patients from 2008 to 2016. Injury 2019, 50, 1620–1626. [Google Scholar] [CrossRef]
- Barkoudah, E.; Piazza, G.; Hecht, T.E.; Grant, P.; Deitelzweig, S.; Fang, M.C.; Fanikos, J.; Kao, C.-K.; Barnes, G.D.; Chen, T.; et al. Extended venous thromboembolism prophylaxis in medically ill patients: An NATF anticoagulation action initiative. Am. J. Med. 2020, 133, 1–27. [Google Scholar] [CrossRef]
- Haljamäe, H. Thromboprophylaxis, coagulation disorders, and regional anaesthesia. Acta Anaesthesiol. Scand. 1996, 40, 1024–1040. [Google Scholar] [CrossRef]
- Jackson, S.P.; Darbousset, R.; Schoenwaelder, S.M. Thromboinflammation: Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 2019, 133, 906–918. [Google Scholar] [CrossRef]
- LaPelusa, A.; Dave, H.D. Physiology, Hemostasis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545263/ (accessed on 28 October 2021).
- Azboy, I.; Barrack, R.; Thomas, A.M.; Haddad, F.S.; Parvizi, J. Aspirin and the prevention of venous thromboembolism following total joint arthroplasty: Commonly asked questions. Bone Jt. J. 2017, 99, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Pomin, V.H. Marine antithrombotics. Mar. Drugs 2020, 18, 514. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Levi, M.; Toh, C.-H. Disseminated intravascular coagulation. Nat. Rev. Dis. Prim. 2016, 2, 16037. [Google Scholar] [CrossRef] [PubMed]
- Jay, R.M.; Lui, P. How anticoagulants work. Tech. Reg. Anesth. Pain Manag. 2006, 10, 30–39. [Google Scholar] [CrossRef]
- Solayar, G.N.; Shannon, F.J. Thromboprophylaxis and orthopaedic surgery: Options and current guidelines. Malays. J. Med. Sci. 2014, 21, 71–77. [Google Scholar]
- Lobo, B.L. Use of newer anticoagulants in patients with chronic kidney disease. Am. J. Health Pharm. 2007, 64, 2017–2026. [Google Scholar] [CrossRef]
- King, A.E.; Szarlej, D.K.; Rincon, F. Dabigatran-associated intracranial hemorrhage: Literature review and institutional experience. Neurohospitalist 2015, 5, 234–244. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Y.; Zhang, L.; Guan, W. Dabigatran must be used carefully: Literature review and recommendations for management of adverse events. Drug Des. Dev. Ther. 2019, 13, 1527–1533. [Google Scholar] [CrossRef]
- Dong, W.-J.; Qian, H.-J.; Qian, Y.; Zhou, L.; Hu, S.-L. Fondaparinux vs. enoxaparin for the prevention of venous thromboembolism after total hip replacement: A meta-analysis. Exp. Ther. Med. 2016, 12, 969–974. [Google Scholar] [CrossRef]
- Groult, H.; Poupard, N.; Herranz, F.; Conforto, E.; Bridiau, N.; Sannier, F.; Bordenave, S.; Piot, J.-M.; Ruiz-Cabello, J.; Fruitier-Arnaudin, I.; et al. Family of bioactive heparin-coated iron oxide nanoparticles with positive contrast in magnetic resonance imaging for specific biomedical applications. Biomacromolecules 2017, 18, 3156–3167. [Google Scholar] [CrossRef]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.S.; Jaenisch, R.; Mooney, D.J. Engineered tissues and strategies to overcome challenges in drug development. Adv. Drug Deliv. Rev. 2020, 158, 116–139. [Google Scholar] [CrossRef] [PubMed]
- Engbers, M.J.; Vlieg, A.V.H.; Rosendaal, F.R. Venous thrombosis in the elderly: Incidence, risk factors and risk groups. J. Thromb. Haemost. 2010, 8, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.M.; Lessard, D.; Emery, C.; Pacifico, L.; Reed, G.; Gurwitz, J.H.; Goldberg, R.J.; Spencer, F.A. Venous thromboembolism in the elderly. Thromb. Haemost. 2008, 100, 780–788. [Google Scholar] [CrossRef]
- Hedayati, M.; Neufeld, M.J.; Reynolds, M.M.; Kipper, M.J. The quest for blood-compatible materials: Recent advances and future technologies. Mater. Sci. Eng. R Rep. 2019, 138, 118–152. [Google Scholar] [CrossRef]
- Cherng, W.-J.; Pan, Y.-H.; Wu, T.-C.; Chou, C.-C.; Yeh, C.-H.; Ho, J.-J. Hemocompatibility and adhesion of heparin/dopamine and heparin/collagen self-assembly multilayers coated on a titanium substrate. Appl. Surf. Sci. 2018, 463, 732–740. [Google Scholar] [CrossRef]
- Bozzini, B.; Barca, A.; Bogani, F.; Boniardi, M.; Carlino, P.; Mele, C.; Verri, T.; Romano, A. Electrodeposition of nanostructured bioactive hydroxyapatite-heparin composite coatings on titanium for dental implant applications. J. Mater. Sci. Mater. Electron. 2014, 25, 1425–1434. [Google Scholar] [CrossRef]
- Guarise, C.; Barbera, C.; Pavan, M.; Pluda, S.; Celestre, M.; Galesso, D. Dopamine-functionalized sulphated hyaluronic acid as a titanium implant coating enhances biofilm prevention and promotes osseointegration. Biofouling 2018, 34, 719–730. [Google Scholar] [CrossRef]
- Pan, C.-J.; Pang, L.-Q.; Gao, F.; Wang, Y.-N.; Liu, T.; Ye, W.; Hou, Y.-H. Anticoagulation and endothelial cell behaviors of heparin-loaded graphene oxide coating on titanium surface. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 333–340. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, M.; Gao, W.; Fang, D.; Liu, Z.; Wu, G.; Wan, M.; Mao, C.; Shen, J. Coronary stents decorated by heparin/nonoate nanoparticles for anticoagulant and endothelialized effects. Langmuir 2020, 36, 2901–2910. [Google Scholar] [CrossRef]
- Moon, H.-J.; Yun, Y.-P.; Han, C.-W.; Kim, M.S.; Kim, S.E.; Bae, M.S.; Kim, G.-T.; Choi, Y.-S.; Hwang, E.-H.; Lee, J.W.; et al. Effect of heparin and alendronate coating on titanium surfaces on inhibition of osteoclast and enhancement of osteoblast function. Biochem. Biophys. Res. Commun. 2011, 413, 194–200. [Google Scholar] [CrossRef]
- Yang, D.H.; Lee, D.-W.; Kwon, Y.-D.; Kim, H.J.; Chun, H.J.; Jang, J.W.; Khang, G. Surface modification of titanium with hydroxyapatite-heparin-BMP-2 enhances the efficacy of bone formation and osseointegration in vitro and in vivo. J. Tissue Eng. Regen. Med. 2015, 9, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chen, Q.; Ren, H.; Wu, X.; Liu, X.; Lu, T. Electrophoretic deposition of dexamethasone-loaded gelatin nanospheres/chitosan coating and its dual function in anti-inflammation and osteogenesis. Colloids Surf. B Biointerfaces 2018, 169, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yao, Y.; Cheng, X.; Luo, J.; Luo, P.; Wang, Q.; Yang, F.; Wei, Q.; Zhang, Z. Electrophoretic deposition of gentamicin-loaded silk fibroin coatings on 3D-printed porous cobalt–chromium–molybdenum bone substitutes to prevent orthopedic implant infections. Biomacromolecules 2017, 18, 3776–3787. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Liu, Y.; Jin, X.; Fan, C.; Ye, H.; Ou, M.; Lv, L.; Wu, G.; Zhou, Y. Bi-functionalization of a calcium phosphate-coated titanium surface with slow-release simvastatin and metronidazole to provide antibacterial activities and pro-osteodifferentiation capabilities. PLoS ONE 2014, 9, e97741. [Google Scholar] [CrossRef][Green Version]
- Pishbin, F.; Mouriño, V.; Flor, S.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition of gentamicin-loaded bioactive glass/chitosan composite coatings for orthopaedic implants. ACS Appl. Mater. Interfaces 2014, 6, 8796–8806. [Google Scholar] [CrossRef]
- Jennings, J.A.; Carpenter, D.P.; Troxel, K.S.; Beenken, K.E.; Smeltzer, M.; Courtney, H.S.; Haggard, W.O. Novel antibiotic-loaded point-of-care implant coating inhibits biofilm. Clin. Orthop. Relat. Res. 2015, 473, 2270–2282. [Google Scholar] [CrossRef]
- He, S.; Zhou, P.; Wang, L.; Xiong, X.; Zhang, Y.; Deng, Y.; Wei, S. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface 2014, 11, 20140169. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q.; Xiao, D.; Guo, T.; Ma, Y.; Duan, K.; Wang, J.; Lu, X.; Feng, B.; Weng, J. Microparticle entrapment for drug release from porous-surfaced bone implants. J. Microencapsul. 2015, 32, 443–449. [Google Scholar] [CrossRef]
- Ordikhani, F.; Tamjid, E.; Simchi, A. Characterization and antibacterial performance of electrodeposited chitosan–vancomycin composite coatings for prevention of implant-associated infections. Mater. Sci. Eng. C 2014, 41, 240–248. [Google Scholar] [CrossRef]
- Rožanc, J.; Žižek, M.; Milojević, M.; Maver, U.; Finšgar, M. Dexamethasone-loaded bioactive coatings on medical grade stainless steel promote osteointegration. Pharmaceutics 2021, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Mastnak, T.; Mihelič, M.; Maver, U.; Finšgar, M. Clindamycin-based 3D-printed and electrospun coatings for treatment of implant-related infections. Materials 2021, 14, 1464. [Google Scholar] [CrossRef]
- Kossover, O.; Cohen, N.; Lewis, J.A.; Berkovitch, Y.; Peled, E.; Seliktar, D. Growth factor delivery for the repair of a critical size tibia defect using an acellular, biodegradable polyethylene glycol–albumin hydrogel implant. ACS Biomater. Sci. Eng. 2020, 6, 100–111. [Google Scholar] [CrossRef]
- Bjelić, D.; Finšgar, M. The role of growth factors in bioactive coatings. Pharmaceutics 2021, 13, 1083. [Google Scholar] [CrossRef]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Nejadnik, M.R. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ni, Y.; Liu, Y.; Zeng, B.; Xu, Y.; Ge, W. The role of simvastatin in the osteogenesis of injectable tissue-engineered bone based on human adipose-derived stromal cells and platelet-rich plasma. Biomaterials 2010, 31, 5325–5335. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Al-Rubeai, M. A multifunctional dexamethasone-delivery implant fabricated using atmospheric plasma and its effects on apoptosis, osteogenesis and inflammation. Drug Deliv. Transl. Res. 2021, 11, 86–102. [Google Scholar] [CrossRef]
- Liang, Y.; Kiick, K.L. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. A polydopamine-based platform for anti-cancer drug delivery. Biomater. Sci. 2019, 7, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, Z.; Li, Z. Polydopamine-based nanocarriers for photosensitizer delivery. Front. Chem. 2019, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Li, C.; Qin, Y.; Zhong, L.; Chen, B.; Li, Z.; Liu, H.; Chang, F.; Wang, J. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: A review. J. Alloy. Compd. 2017, 717, 271–285. [Google Scholar] [CrossRef]
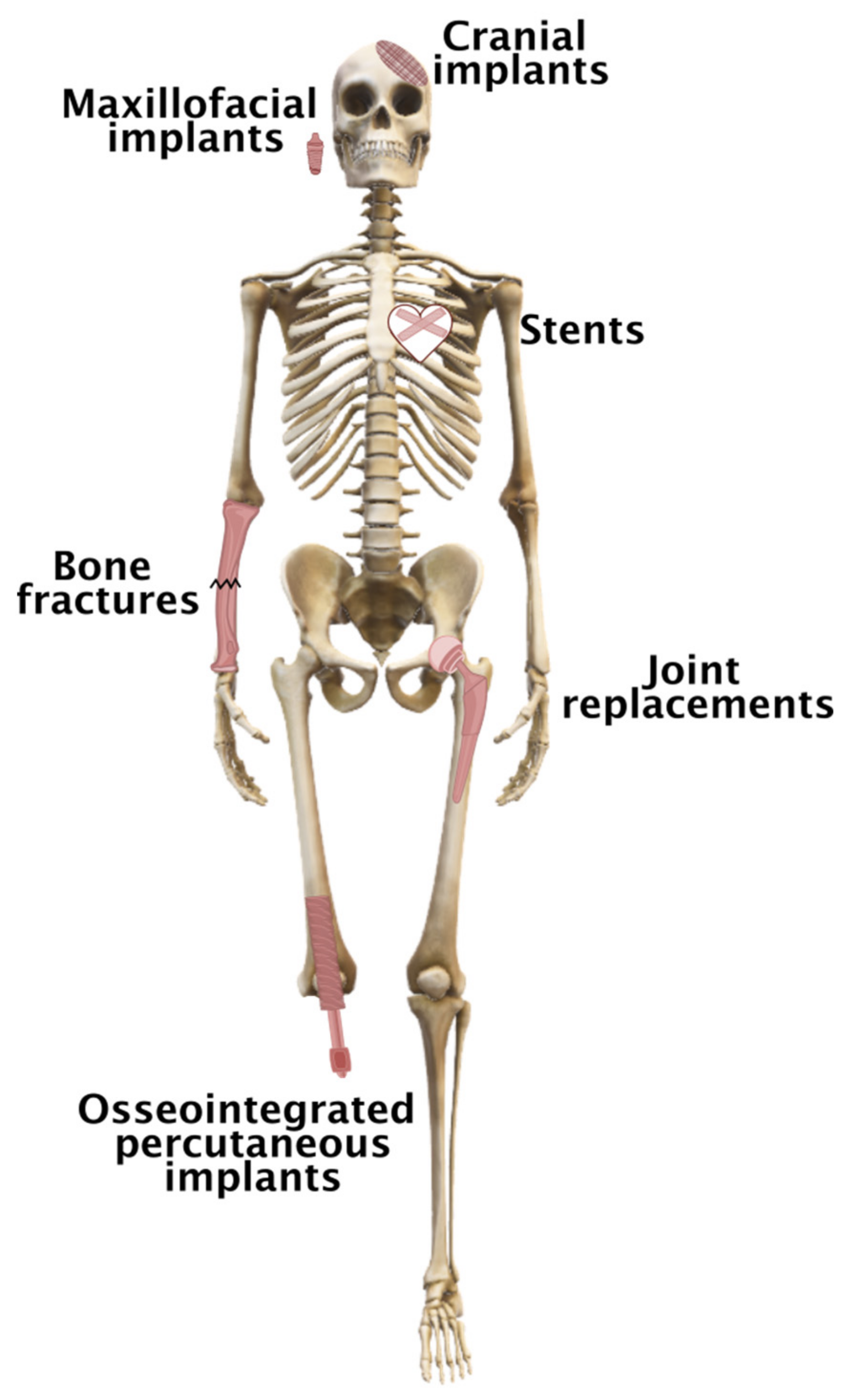
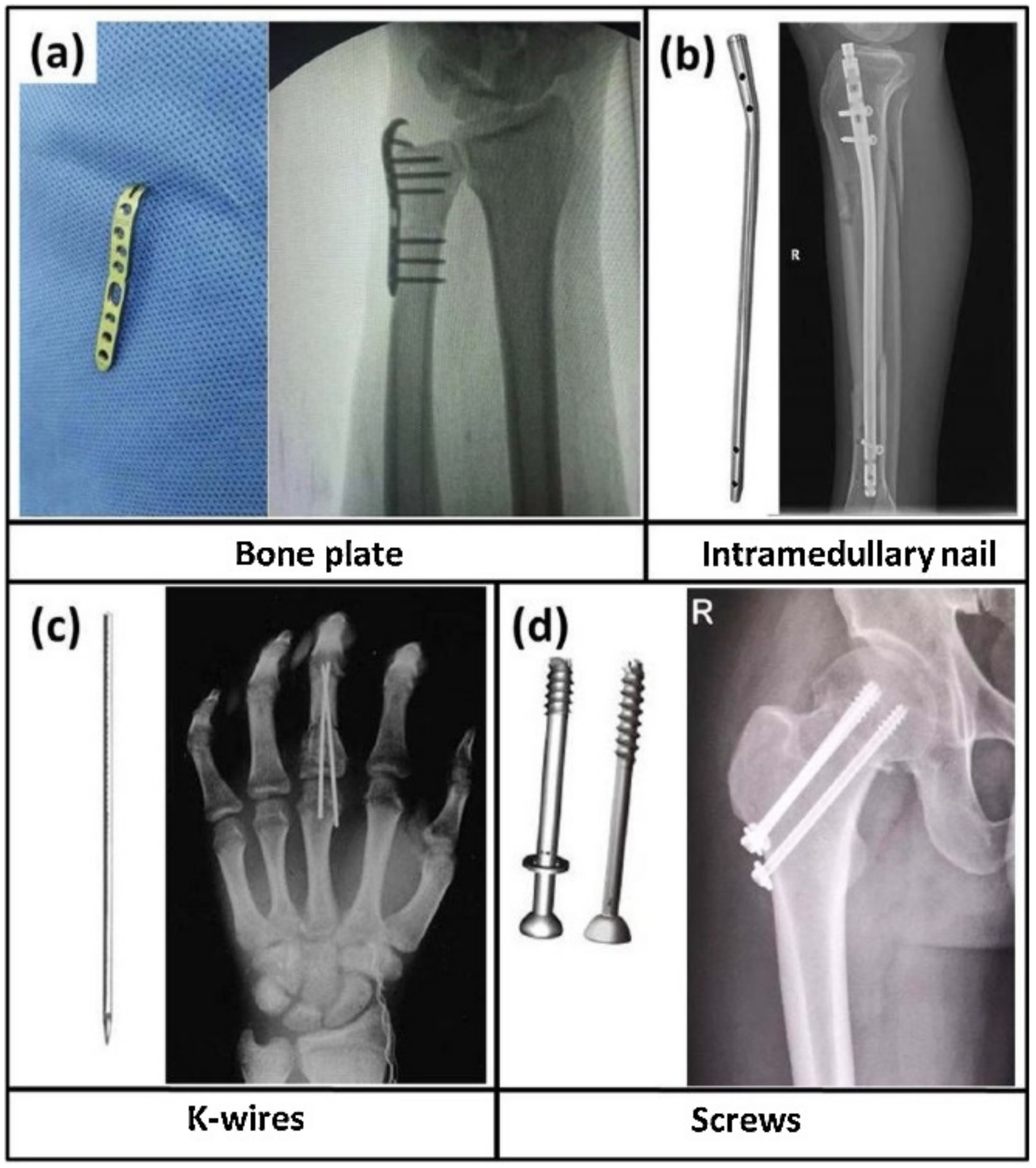
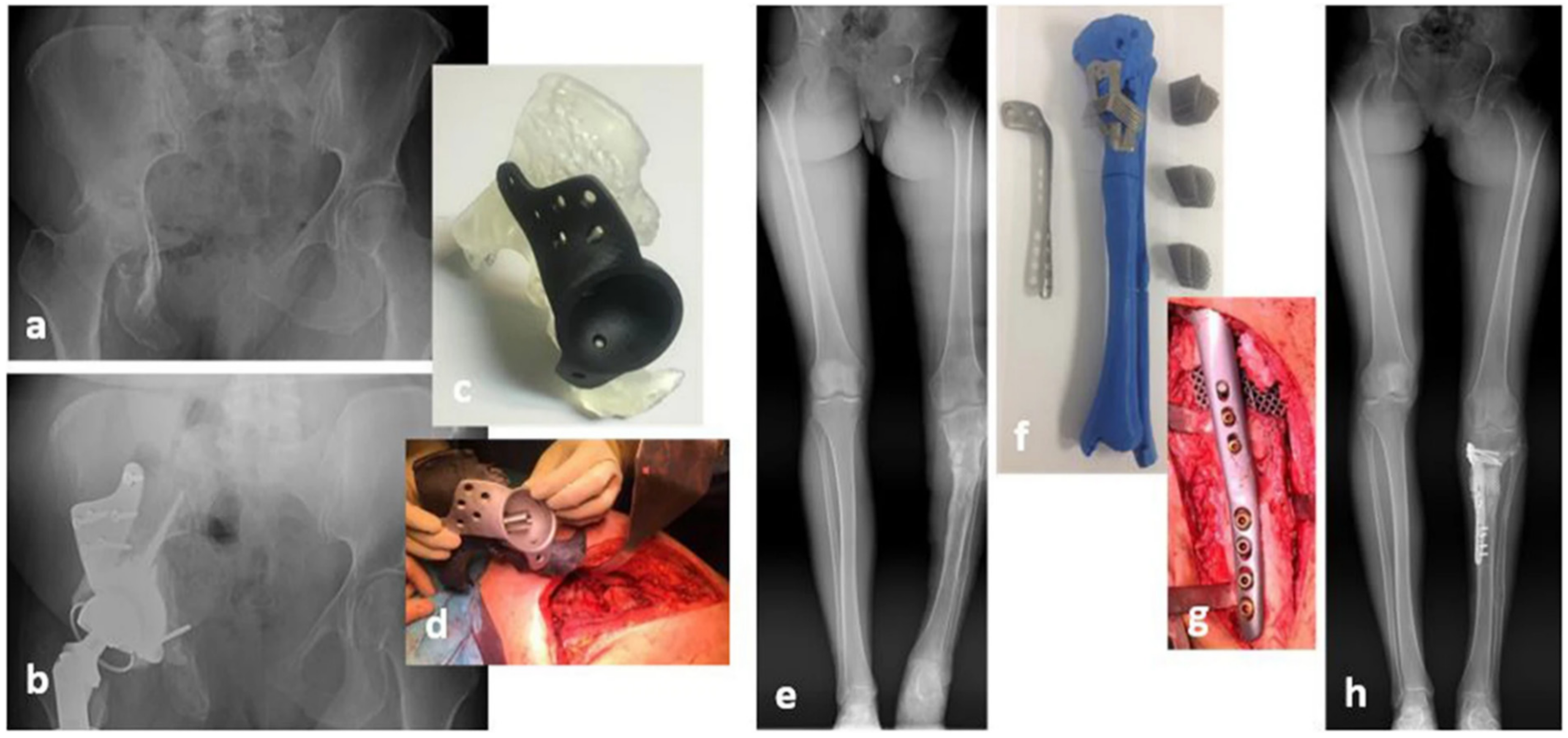
| Country | Hip Replacements per 100,000 Inhabitants | Total Number of Hip Replacements | Knee Replacements per 100,000 Inhabitants | Total Number of Knee Replacements |
|---|---|---|---|---|
| Belgium | 274.6 | 31,303.3 | 207.3 | 23,626.5 |
| Bulgaria | 117.7 | 8241.8 | 31.5 | 2207.8 |
| Czechia | 199.2 | 21,115.2 | 144.7 | 15,337.1 |
| Denmark | 241.4 | 13,952.3 | 181.2 | 10,471.0 |
| Germany | 310.6 | 257,129.2 | 222.8 | 184,431.3 |
| Estonia | 170.2 | 2246.6 | 108.3 | 1429.8 |
| Ireland | 123.3 | 5953.0 | 47.5 | 2295.2 |
| Spain | 121.5 | 56,691.9 | 132.2 | 61,675.2 |
| France | 248.6 | 166,315.3 | 181.8 | 121,612.7 |
| Croatia | 171.0 | 7012.6 | 72.8 | 2984.8 |
| Italy | 184.9 | 111,815.4 | 128.9 | 77,952.7 |
| Cyprus | 55.5 | 660.0 | 54.4 | 646.3 |
| Latvia | 180.4 | 3488.4 | 103.9 | 2010.0 |
| Lithuania | 200.6 | 5616.8 | 124.4 | 3484.0 |
| Luxembourg | 181.6 | 1089.5 | 182.1 | 1092.5 |
| Hungary | 138.8 | 13,466.5 | 88.6 | 8598.1 |
| Malta 1 | 88.9 | 391.1 | 167.3 | 736.2 |
| Netherlands | 222.3 | 37,975.7 | 171.4 | 29,282.0 |
| Austria | 298.5 | 26,332.8 | 229.9 | 20,284.4 |
| Poland | 161.8 | 61,444.0 | 66.8 | 25,385.8 |
| Portugal 2 | 90.6 | 9397.3 | 62.2 | 6448.1 |
| Romania | 71.4 | 13,936.6 | 24.7 | 4816.1 |
| Slovenia | 187.7 | 3753.4 | 132.8 | 2655.8 |
| Slovakia | 129.0 | 7094.5 | 105.9 | 5822.3 |
| Finland | 274.5 | 15,097.5 | 233.4 | 12,838.7 |
| Sweden | 242.0 | 24,487.4 | 130.6 | 13,213.7 |
| United Kingdom | 187.1 | 123,964.7 | 148.4 | 98,371.2 |
| Liechtenstein | 26.2 | 9.9 | 7.8 | 3.0 |
| Norway | 259.6 | 13,863.2 | 130.7 | 6979.9 |
| Switzerland | 307.3 | 26,118.8 | 250.2 | 21,265.3 |
| Total | 5466.6 | 1,069,964.7 | 3874.6 | 767,957.5 |
| Condition | Country | Estimated Cost per Patient | Ref. | Total Number of Hip Replacements | Approximate Costs of Revision in 1% of the Population (Millions) |
|---|---|---|---|---|---|
| Hip prosthetic joint infection | France | EUR (23,757 ± 8235) | 166,315 | EUR (40.0 ± 13.7) | |
| Italy | EUR (60,394 ± 15,886) | [38] | 111,815 | EUR (67.0 ± 1.88) | |
| Germany | EUR 20,166 | [37] | 257,129 | EUR 51.9 | |
| United Kingdom | GBP (21,937 ± 10,965) | [40] | 123,965 | GBP (27.2 ± 13.6) | |
| United States | USD 31,753 | [34] | 438,000 [39] | USD 139.0 | |
| USD 30,300 | [36] | USD 132.7 | |||
| USD 31,312 | [32] | USD 137.1 | |||
| Condition | Country | Estimated cost per patient | Ref. | Total number of knee replacements | Approximate cost of revision in 1% of the population (millions) |
| Knee prosthetic joint infection | Germany | EUR 25,194 | [33] | 184,431 | EUR 46.5 |
| EUR 19,010 | [37] | EUR 35.1 | |||
| United States | USD 25,692 | [34] | 686,000 [40] | USD 176.2 | |
| USD 25,300 | [36] | USD 173.6 |
| Device Type | Material | Device Type | Material |
|---|---|---|---|
| Bone fixation devices | Ti | Soft tissue fixation devices | Ti |
| Ti6Al4V | Ti6Al4V | ||
| Stainless steel | Stainless steel | ||
| NiTi | Ta | ||
| Prostheses | Ti | NiCo | |
| CoCr alloys | NiTi | ||
| Stainless steel | CoCr alloys | ||
| Material | Commercial examples | ||
| Ti | Ti | ||
| STIKTITE | |||
| Ti6Al4V | Ti6Al4V | ||
| Regenerex® | |||
| 4WEB Medical Truss Implant Technology® | |||
| Stainless steel | CarTech® BioDur® 108 Alloy | ||
| Ta | Trabecular Metal™ | ||
| CoCrMo | CoCrMo | ||
| Freedom CoCr® | |||
| Material | Processing | Application | Patient(s) | Reference |
|---|---|---|---|---|
| Ti6Al4V | EBM | Vertebral body replacement | A 12-year-old boy | [76] |
| Ti6Al4V | EBM | Upper cervical spine reconstruction | 2 males and 7 females, 12 to 59 years | [77] |
| Ti | Not specified | Pelvic tumor resection | A 65-year-old male | [78] |
| Ti6Al4V | Not specified | Severe foot and ankle trauma | A 46-year-old female | [79] |
| Ti6Al4V | SLM | Orbital wall injury | A 67-year-old male | [80] |
| Ti6Al4V | SLM | Large cranial defect | A 22-year-old male | [81] |
| Ti | Not specified | Complex midfacial defects | A 50-year-old male | [82] |
| Ti6Al4V | EBM | Wrist arthroplasty | A 34-year-old male, a 39-year-old male | [83] |
| Ti6Al4V | SLM | Upper maxilla waferless repositioning | 10 patients | [84] |
| Commonly Isolated Class of Microorganisms 1 | The Commonest Species | Sensitivity | Approximate Percentage of Infections Caused | Ref. |
|---|---|---|---|---|
| Gram-positive cocci | Staphylococcus aureus *, Staphylococcus epidermidis, Streptococcus species, Enterococcus species | β-lactams (flucloxacillin, cephalosporins, carbapenems), glycopeptide antibiotics (vancomycin, teicoplanin), lincosamide clindamycin, fluroquinolones, aminoglycoside rifampicin | 65 | [30] |
| 54–83 | [169] | |||
| 44–87 | [160] | |||
| 64–82 | [164] | |||
| 70 | [170] | |||
| Gram-negative bacilli | Enterobacteriaceae, Pseudomonas aeruginosa | Usually, a combination of a β-lactam (e.g., carbapenem) and an aminoglycoside or fluoroquinolone [171] | 6 | [30] |
| 10–17 | [169] | |||
| 6–17 | [160] | |||
| 8 | [164,170] | |||
| Anaerobes | Propionibacterium species, Peptostreptococcus species, Finegoldia magna | Metronidazole, carbapenems, chloramphenicol, combinations of penicillin and a beta-lactamase inhibitor, tigecycline and clindamycin [172] | 4 | [30] |
| 2–4 | [169] | |||
| 4–5 | [160] | |||
| Multispecies bacterial infections | Various combinations of bacteria (for example: S. aureus and Streptococcus agalactiae [173] or Propionibacterium acnes [174]) | Species-dependant | 20 | [30] |
| 10–20 | [169,160] | |||
| 10–12 | [164] |
| Anticoagulant | Mode of Action | Disadvantages | Administration |
|---|---|---|---|
| Warfarin | Inhibits several coagulation factors (II, VII, IX, and X) | Constant blood monitoring is required; interactions with multiple foods and drugs | Oral |
| Unfractionated HEP (UHEP) | Binds to antithrombin III (ATIII), inactivating coagulation enzymes XIIa, XIa, IXa, Xa, and thrombin (factor IIa) | Blood monitoring is required; extended use might cause delayed healing, thrombocytopenia, and osteoporosis; [210] variable pharmacokinetic properties [211] | Intravenous infusion (IV) or subcutaneous (SC) injection |
| Low molecular weight heparin (LMWHEP) | Indirect factor Xa inhibitor | Similar to UH but to a lesser extent [210]; dosing depends on creatinine clearance (eliminated by the kidneys) | SC injection |
| Dabigatran | Direct thrombin (factor IIa) inhibitor | May increase the risk of hemorrhagic stroke [212] and gastrointestinal bleeding [213] | Oral |
| Rivaroxaban | Direct factor Xa inhibitors | Dosing depends on creatinine clearance (eliminated by the kidneys) | |
| Apixaban | |||
| Edoxaban | |||
| Betrixaban | |||
| Fondaparinux | Indirect factor Xa inhibitor | Increased risk of major bleeding [214] | SC injection |
| Metal Substrate | Carrier Matrix | Active Ingredient | Results | Testing Model | Ref. |
|---|---|---|---|---|---|
| Ti | HEP/dopamine | HEP | A possible alternative to long-term application in physiological fluid if the anti-erosion capability of the outermost HEP layer could be improved | In Vitro | [221] |
| Ti | Hydroxyapatite-HEP | HEP | Homogeneous incorporation of HEP in the composite films and enhanced bioactivity | In Vitro | [222] |
| Ti6Al4V | A partially sulphated HA functionalized with a dopamine moiety | GEN and VAN | Demonstrated prevention of biofilm formation on the surface of the Ti alloy samples | In Vitro | [223] |
| Ti | Polydopamine coating followed by the deposition of the GO coating loaded with HEP | HEP | Improved blood compatibility of Ti, the promotion of endothelial cell adhesion and proliferation | In Vitro | [224] |
| 316L stainless steel | Polyglycidyl methacrylate grafted with HEP/NONOate nanoparticles | HEP | Improved anticoagulation, anti-restenosis, and enhanced endothelial regeneration | In Vivo | [225] |
| Ti | HEP-grafted surface | Alendronate | Dual bioactivity: enhanced osteoblast differentiation and inhibited osteoclast differentiation | In Vitro | [226] |
| Ti | GO | HEP | The coating improved hemocompatibility and cytocompatibility with endothelial cells | In Vitro | [224] |
| Ti | Hydroxyapatite | HEP and BMP-2 | Sustained release of BMP-2 from the coating, increased bone formation, and osseointegration | In Vitro and in vivo | [227] |
| Ti | GO | Aspirin | Enhanced osteoblast proliferation and osteogenic differentiation, sustained release of aspirin for 3 days | In Vitro | [149] |
| 316L stainless steel | Gelatin nanospheres/CHI | DEX | Inhibited inflammation and stimulated osteogenesis, sustained release of DEX for up to 28 days | In Vitro | [228] |
| Ti6Al7Nb | Polylactic-co-glycolic acid, dipalmitoyl phosphatidyl choline, and distearoyl phosphatidyl choline | Doxycycline | Protection against doxycycline-resistant MRSA, release of doxycycline for up to 28 days | In Vitro and in vivo | [20] |
| CoCrMo | Silk fibroin | GEN | Enhanced initial osteoblastic response on coated substrates, antibacterial effect within 1 week | In Vitro | [229] |
| Ti | Ca-P | Simvastatin (SIM) and metronidazole (MNZ) | Controlled release of both SIM and MNZ, increased osteogenic cell differentiation, and the inhibition of bacterial growth | In Vitro | [230] |
| AISI 316L stainless steel | CHI/bioactive glass | GEN | Sustained drug delivery over a period of 8 weeks, inhibited bacterial growth for the first 2 days, and support of cellular proliferation for up to 10 days | In Vitro | [231] |
| Ti6Al4V and 316L stainless steel | Phosphatidylcholine coatings loaded with either one or both of the antibiotics | Amikacin and VAN | The eluted antibiotics showed prevention of biofilm formation | In Vitro and in vivo | [232] |
| Ti | Polydopamine | Cefotaxime sodium (CS) | The CS-grafted Ti substrate was biocompatible, haemocompatible, and could effectively prevent adhesion and the proliferation of E. coli and S. mutans | In Vitro | [233] |
| Ti6Al4V | Collagen/hydroxyapatite layers | VAN | The coating enhanced osteointegration; local VAN release 7 days following implantation | In Vitro and in vivo | [19] |
| Ti | CHI microspheres and ALG microspheres | GEN (CHI microspheres) and VAN (ALG microspheres) | Antibiotic-loaded CHI and ALG microparticles were entrapped in porous-coated Ti to produce local drug release and inhibit adjacent bacterial growth | In Vitro | [234] |
| Ti | CHI | VAN | The coatings were biocompatible and provided an antibacterial effect, while reducing the rate of corrosion; release of VAN for up to 6 days | In Vitro | [235] |
| 316LVM stainless steel | Alternating layers of CHI and the pharmaceutical | DCF | The coatings were biocompatible, provided a certain degree of corrosion protection, and improved osteointegration; controlled release of DCF | In Vitro | [18], [95] |
| 316LVM stainless steel | Alternating layers of CMC and the pharmaceutical | DCF | The coatings were biocompatible, they improved osteointegration, and did not influence the corrosion susceptibility of stainless steel; controlled release of DCF | In Vitro | [17] |
| 316LVM stainless steel | Alternating layers of CMC and the pharmaceutical; β-cyclodextrin for increasing the DEX dosage | DEX | The coatings were biocompatible and showed an osteointegrative potential; their application did not increase the corrosion susceptibility of stainless steel; release of DEX for up to 3 days | In Vitro | [236] |
| 316LVM stainless steel and Ti6Al4V | Cellulose nanofibril suspension, ALG, and CMC | CLIN | The coatings were biocompatible; complete release of CLIN after 3 days | In Vitro | [237] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastnak, T.; Maver, U.; Finšgar, M. Addressing the Needs of the Rapidly Aging Society through the Development of Multifunctional Bioactive Coatings for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 2786. https://doi.org/10.3390/ijms23052786
Mastnak T, Maver U, Finšgar M. Addressing the Needs of the Rapidly Aging Society through the Development of Multifunctional Bioactive Coatings for Orthopedic Applications. International Journal of Molecular Sciences. 2022; 23(5):2786. https://doi.org/10.3390/ijms23052786
Chicago/Turabian StyleMastnak, Tinkara, Uroš Maver, and Matjaž Finšgar. 2022. "Addressing the Needs of the Rapidly Aging Society through the Development of Multifunctional Bioactive Coatings for Orthopedic Applications" International Journal of Molecular Sciences 23, no. 5: 2786. https://doi.org/10.3390/ijms23052786
APA StyleMastnak, T., Maver, U., & Finšgar, M. (2022). Addressing the Needs of the Rapidly Aging Society through the Development of Multifunctional Bioactive Coatings for Orthopedic Applications. International Journal of Molecular Sciences, 23(5), 2786. https://doi.org/10.3390/ijms23052786








