Fatty Acid Amide Hydrolase (FAAH) Inhibition Plays a Key Role in Counteracting Acute Lung Injury
Abstract
1. Introduction
2. Results
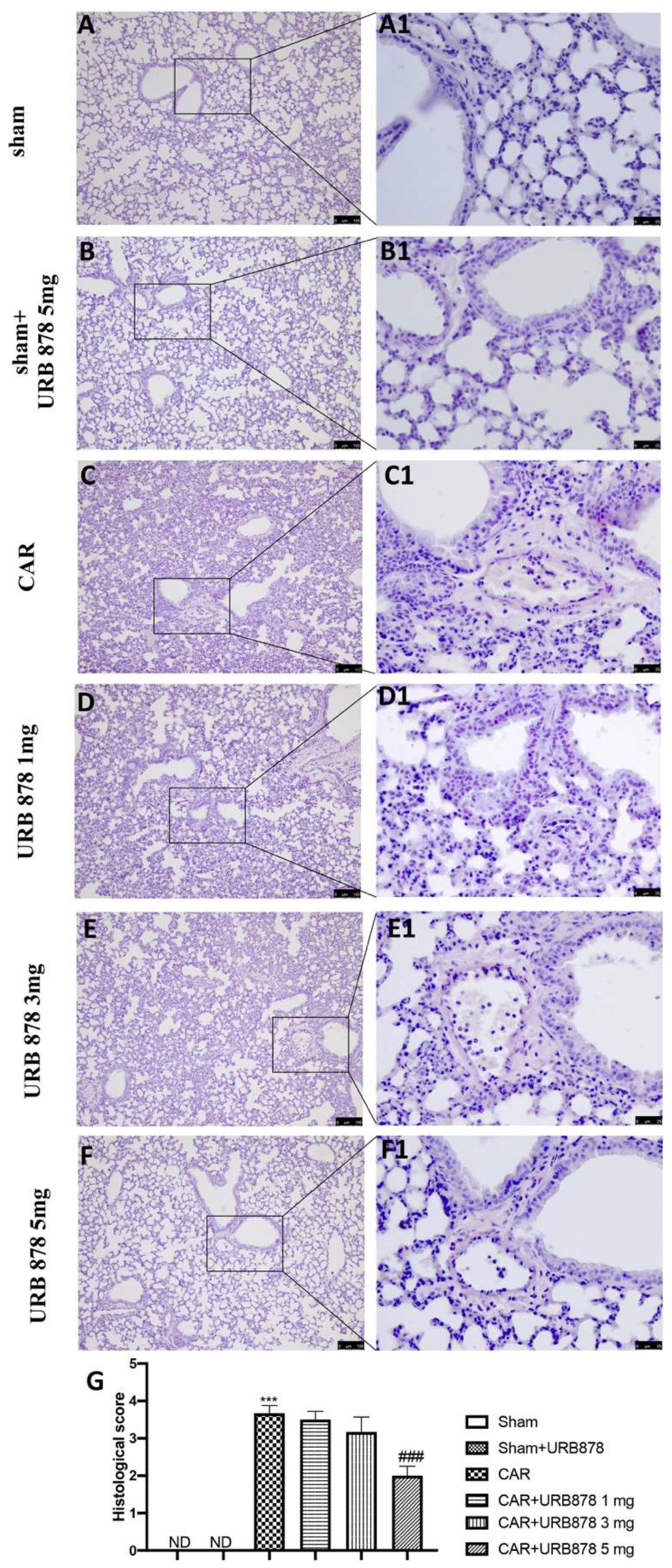
2.1. URB878 Reduces CAR-Induced Lung Injury
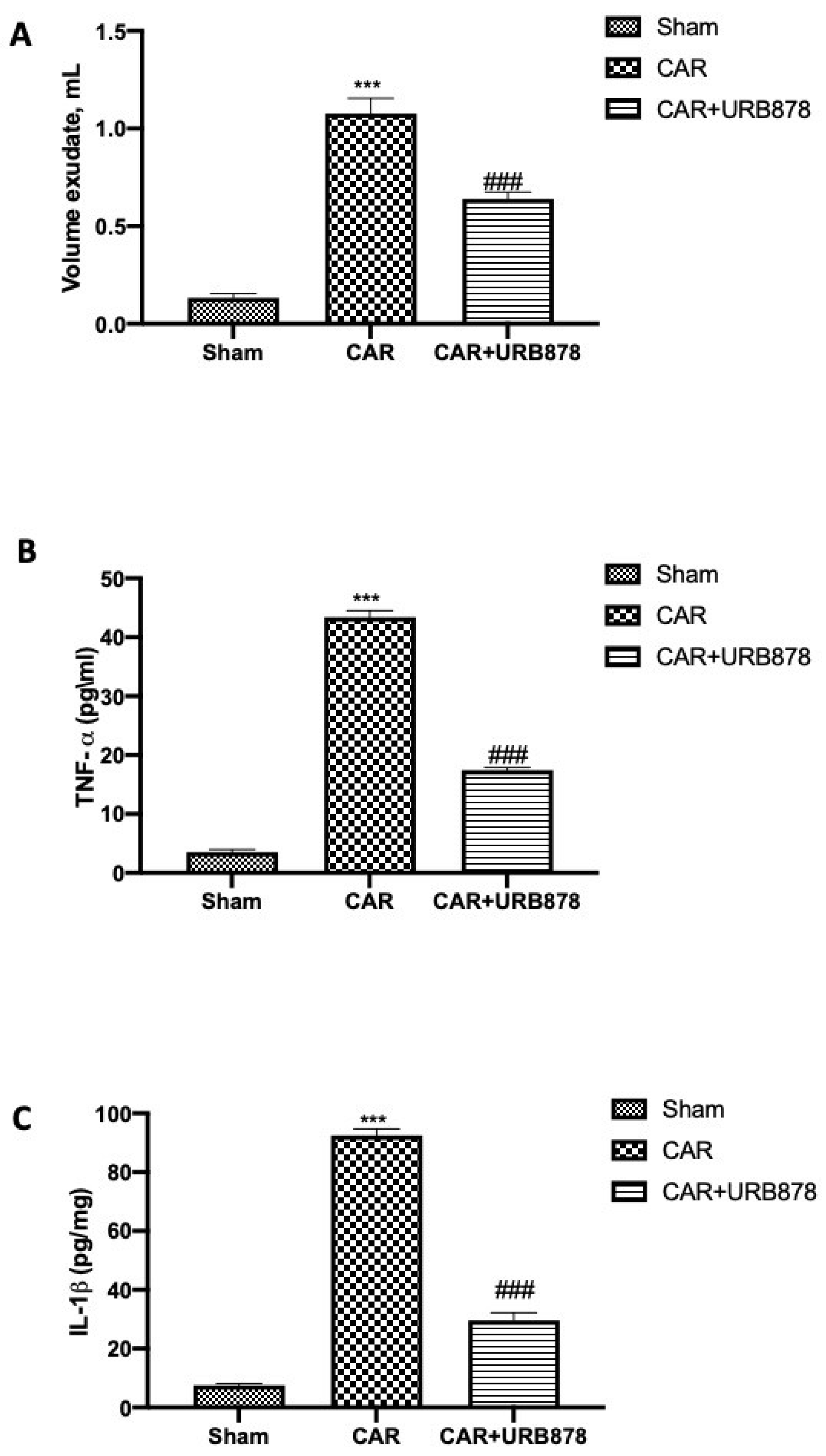
2.2. URB878 Inhibits CAR-Induced Mast Cell Degranulation, PMN Infiltration and MPO Production
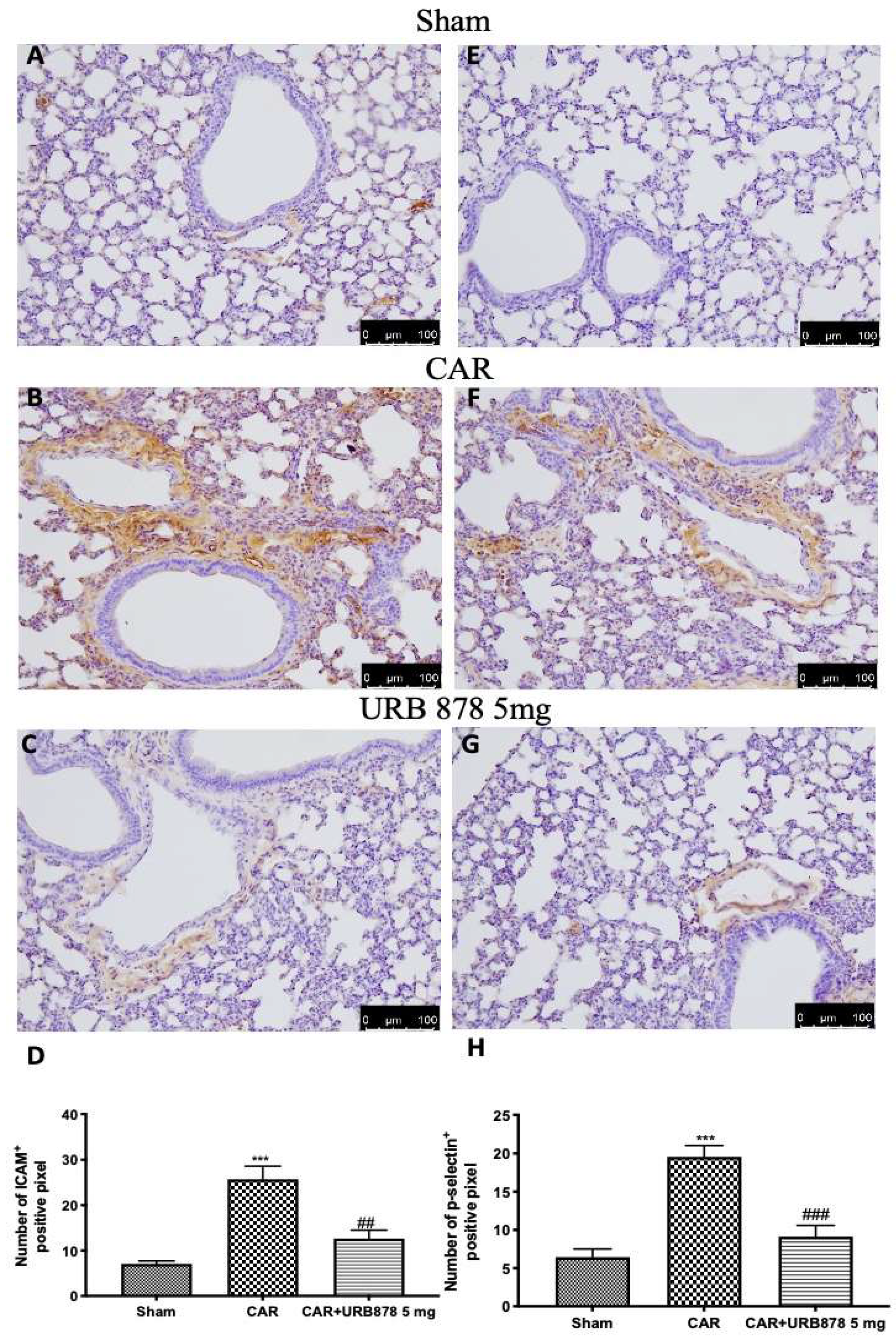
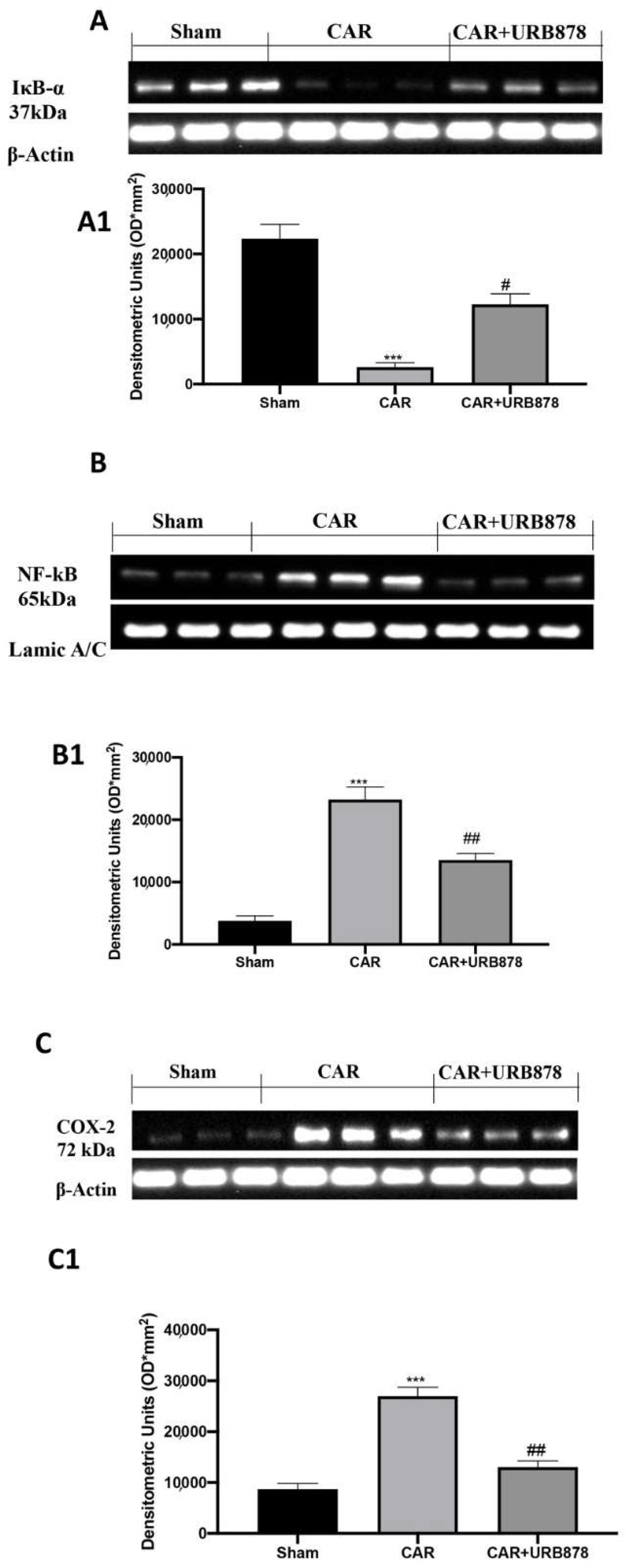
2.3. URB878 Reduces the Expressions of the Adhesion Molecules ICAM and P-Selectin
2.4. Effect of URB878 Administration on CAR-Induced Nitrosative Stress
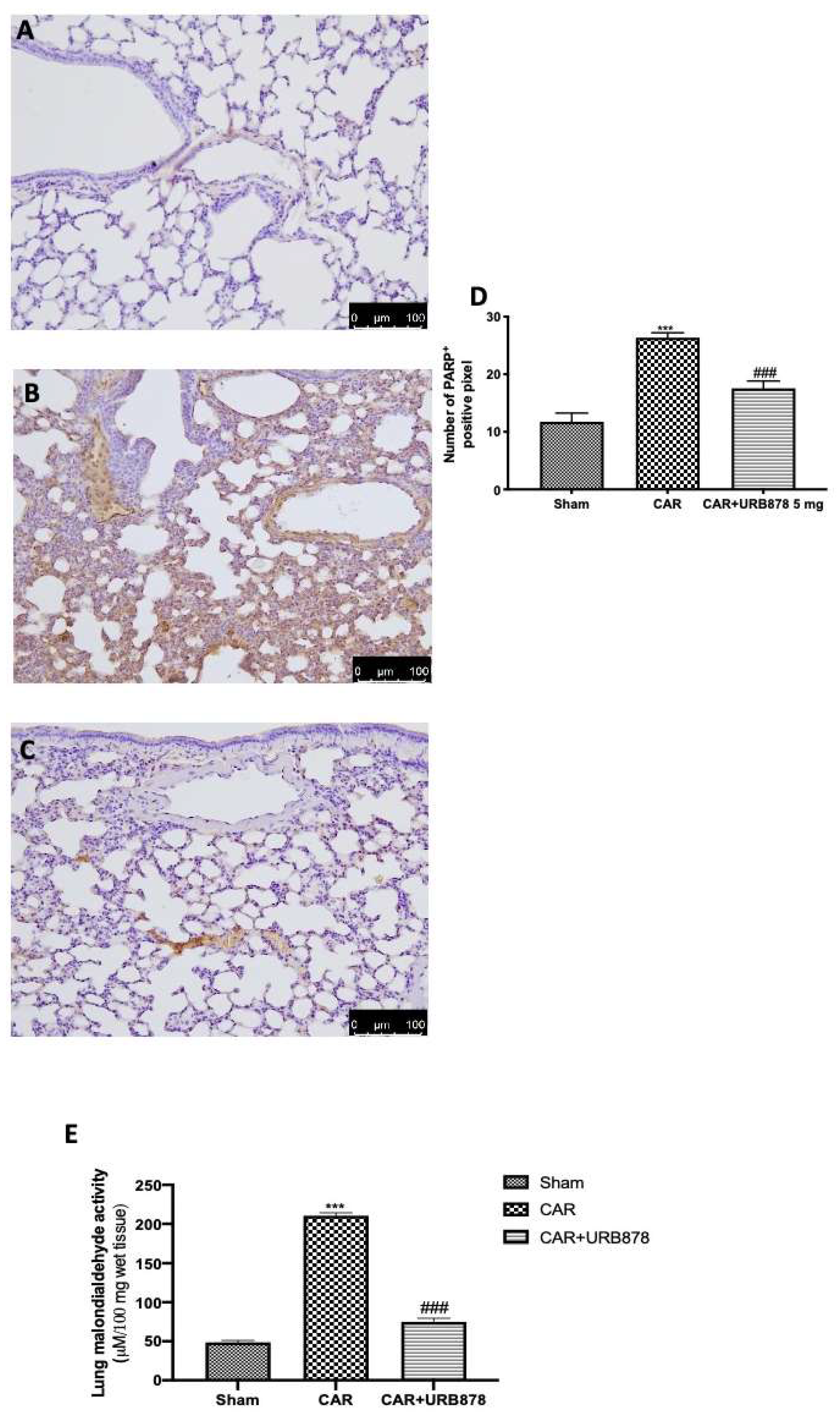
2.5. FAAH Inihibition Reduces DNA Damage and Lipid Peroxidation
2.6. URB878 Reduces CAR-Induced Lung Inflammation
3. Discussion and Conclusions
4. Materials and Methods
4.1. Animals
4.2. Experimental Design and Groups
- (1)
- CAR: mice were subjected to the CAR injection described above, and treated with saline solution;
- (2)
- CAR+URB878: mice were subjected to the CAR injection described above, and treated orally 1 h after with URB878 dissolved in a vehicle consisting of 10% PEG-400, 10% Tween-80 and 80% saline at different doses;
- (3)
- Sham groups: animals were subjected to an injection of saline solution;
- (4)
- Sham groups+URB878: animals were subjected to URB878 treatment at different doses (data not shown) (in our results, there were not significant difference observed between Sham and Sham+URB878).
4.3. Exudate and Leukocytes Count
4.4. Western Blot Analysis of Cytosolic and Nuclear Extracts
4.5. Evaluation of Tissue Lipid Peroxidation
4.6. Cytokine and Nitrite/Nitrate Measurement
4.7. Histopathological Evaluation with Hematoxylin/Eosin and Toluidine Blue
4.8. Immunohistochemical Localization of Nitrotyrosine, Poly(ADP-ribose), ICAM and P-Selectin
4.9. Materials
4.10. Statistical Evaluation
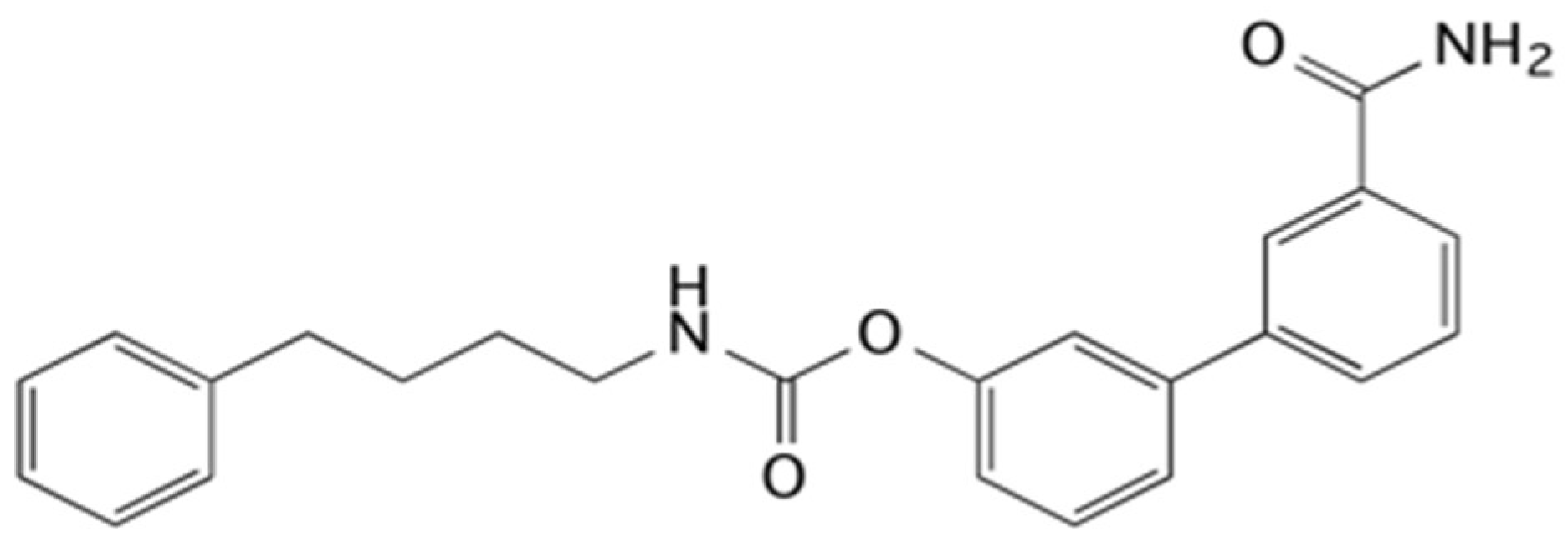
4.11. Synthesis of URB878
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Ragaller, M.; Richter, T. Acute lung injury and acute respiratory distress syndrome. J. Emerg. Trauma Shock 2010, 3, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Mascia, L.; Del Turco, M.; Malacarne, P.; Giunta, F.; Brochard, L.; Slutsky, A.S.; Marco Ranieri, V. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 2002, 96, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.; Leiner, T.; Mikor, A.; Szakmany, T.; Bogar, L.; Molnar, Z. Hemodynamic and respiratory changes during lung recruitment and descending optimal positive end-expiratory pressure titration in patients with acute respiratory distress syndrome. Crit. Care. Med. 2007, 35, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Bein, T.; Kuhr, L.P.; Bele, S.; Ploner, F.; Keyl, C.; Taeger, K. Lung recruitment maneuver in patients with cerebral injury: Effects on intracranial pressure and cerebral metabolism. Intensive. Care. Med. 2002, 28, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.C.; Guldner, A.; Beda, A.; Rentzsch, I.; Uhlig, C.; Dittrich, S.; Spieth, P.M.; Wiedemann, B.; Kasper, M.; Koch, T.; et al. Higher levels of spontaneous breathing reduce lung injury in experimental moderate acute respiratory distress syndrome. Crit. Care. Med. 2014, 42, e702–e715. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xiu, Y.; Zhou, P.; Qiu, Y.; Li, Y. A New Use for an Old Drug: Carmofur Attenuates Lipopolysaccharide (LPS)-Induced Acute Lung Injury via Inhibition of FAAH and NAAA Activities. Front. Pharm. 2019, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Wortley, M.A.; Adcock, J.J.; Dubuis, E.D.; Maher, S.A.; Bonvini, S.J.; Delescluse, I.; Kinloch, R.; McMurray, G.; Perros-Huguet, C.; Papakosta, M.; et al. Targeting fatty acid amide hydrolase as a therapeutic strategy for antitussive therapy. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, R.; Sasso, O.; Piomelli, D. A Double Whammy: Targeting Both Fatty Acid Amide Hydrolase (FAAH) and Cyclooxygenase (COX) To Treat Pain and Inflammation. Chemmedchem 2016, 11, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Dider, S.; Ji, J.D.; Zhao, Z.; Xie, L. Molecular mechanisms involved in the side effects of fatty acid amide hydrolase inhibitors: A structural phenomics approach to proteome-wide cellular off-target deconvolution and disease association. Npj Syst. Biol. Appl. 2016, 2, 16023. [Google Scholar] [CrossRef]
- Graves, R.S.; Pryce, G.; Cabranes, A.; Fernandez-Ruiz, J.; Bisogno, T.; Di Marzo, V.; Cravatt, B.F.; Michael, G.J.; Giovannoni, G.; Elphick, M.R.; et al. Fatty acid amide hydrolase as a target for neuroprotection. Mult. Scler. J. 2011, 17, S431. [Google Scholar]
- Ahn, K.; Johnson, D.S.; Cravatt, B.F. Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders. Expert Opin. Drug Discov. 2009, 4, 763–784. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Kinsey, S.G.; Lichtman, A.H. Targeting Fatty Acid Amide Hydrolase (FAAH) to Treat Pain and Inflammation. Aaps J. 2009, 11, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. Fatty acid amide hydrolase: A potential target for next generation therapeutics. Curr. Pharm. Des. 2006, 12, 759–772. [Google Scholar] [CrossRef]
- Parker, L.A.; Limebeer, C.L.; Rock, E.M.; Litt, D.L.; Kwiatkowska, M.; Piomelli, D. The FAAH inhibitor URB-597 interferes with cisplatin- and nicotine-induced vomiting in the Suncus murinus (house musk shrew). Physiol. Behav. 2009, 97, 121–124. [Google Scholar] [CrossRef]
- Manwell, L.A.; Satvat, E.; Lang, S.T.; Allen, C.P.; Leri, F.; Parker, L.A. FAAH inhibitor, URB-597, promotes extinction and CB(1) antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharm. Biochem. Behav. 2009, 94, 154–162. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Mechoulam, R.; Piomelli, D.; Parker, L.A. The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology 2008, 196, 389–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, S.H.; Wang, Y.Q.; Wu, Y.F.; Wang, D.P.; Lin, Q.; Hai, J. Cannabinoid receptor agonist WIN55,212-2 and fatty acid amide hydrolase inhibitor URB597 may protect against cognitive impairment in rats of chronic cerebral hypoperfusion via PI3K/AKT signaling. Behav. Brain. Res. 2016, 313, 334–344. [Google Scholar] [CrossRef]
- Piomelli, D.; Tarzia, G.; Duranti, A.; Tontini, A.; Mor, M.; Compton, T.R.; Dasse, O.; Monaghan, E.P.; Parrott, J.A.; Putman, D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug. Rev. 2006, 12, 21–38. [Google Scholar] [CrossRef]
- Mor, M.; Rivara, S.; Lodola, A.; Plazzi, P.V.; Tarzia, G.; Duranti, A.; Tontini, A.; Piersanti, G.; Kathuria, S.; Piomelli, D. Cyclohexylcarbamic acid 3’–or 4’-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: Synthesis, quantitative structure-activity relationships, and molecular modeling studies. J. Med. Chem. 2004, 47, 4998–5008. [Google Scholar] [CrossRef]
- Kathuria, S.; Gaetani, S.; Fegley, D.; Valino, F.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; La Rana, G.; Calignano, A.; et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003, 9, 76–81. [Google Scholar] [CrossRef]
- Gobbi, G.; Bambico, F.R.; Mangieri, R.; Bortolato, M.; Campolongo, P.; Solinas, M.; Cassano, T.; Morgese, M.G.; Debonnel, G.; Duranti, A.; et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. USA 2005, 102, 18620–18625. [Google Scholar] [CrossRef] [PubMed]
- Bambico, F.R.; Duranti, A.; Nobrega, J.N.; Gobbi, G. The fatty acid amide hydrolase inhibitor URB597 modulates serotonin-dependent emotional behaviour, and serotonin1A and serotonin2A/C activity in the hippocampus. Eur. Neuropsychopharmacol. 2016, 26, 578–590. [Google Scholar] [CrossRef]
- Russo, R.; Loverme, J.; La Rana, G.; Compton, T.R.; Parrott, J.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; Calignano, A.; et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J. Pharm. Exp. 2007, 322, 236–242. [Google Scholar] [CrossRef]
- Ebrahimi-Ghiri, M.; Shahini, F.; Zarrindast, M.R. The effect of URB597, exercise or their combination on the performance of 6-OHDA mouse model of Parkinson disease in the elevated plus maze, tail suspension test and step-down task. Metab. Brain. Dis. 2021, 36, 2579–2588. [Google Scholar] [CrossRef]
- Alteba, S.; Mizrachi Zer-Aviv, T.; Tenenhaus, A.; Ben David, G.; Adelman, J.; Hillard, C.J.; Doron, R.; Akirav, I. Antidepressant-like effects of URB597 and JZL184 in male and female rats exposed to early life stress. Eur. Neuropsychopharmacol. 2020, 39, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Bedse, G.; Colangeli, R.; Lavecchia, A.M.; Romano, A.; Altieri, F.; Cifani, C.; Cassano, T.; Gaetani, S. Role of the basolateral amygdala in mediating the effects of the fatty acid amide hydrolase inhibitor URB597 on HPA axis response to stress. Eur. Neuropsychopharmacol. 2014, 24, 1511–1523. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozlowska, H.; Kloza, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Beneficial Changes in Rat Vascular Endocannabinoid System in Primary Hypertension and under Treatment with Chronic Inhibition of Fatty Acid Amide Hydrolase by URB597. Int. J. Mol. Sci. 2021, 22, 4833. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozlowska, H.; Kloza, M.; Karpinska, O.; Toczek, M.; Harasim, E.; Kasacka, I.; Malinowska, B. Protective role of cannabinoid CB1 receptors and vascular effects of chronic administration of FAAH inhibitor URB597 in DOCA-salt hypertensive rats. Life Sci. 2016, 151, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.; Ambrozewicz, E.; Gegotek, A.; Toczek, M.; Bielawska, K.; Skrzydlewska, E. Redox system and phospholipid metabolism in the kidney of hypertensive rats after FAAH inhibitor URB597 administration. Redox. Biol. 2018, 15, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.; Ambrozewicz, E.; Gegotek, A.; Toczek, M.; Skrzydlewska, E. Long-term administration of fatty acid amide hydrolase inhibitor (URB597) to rats with spontaneous hypertension disturbs liver redox balance and phospholipid metabolism. Adv. Med. Sci. 2019, 64, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.; Baranowska-Kuczko, M.; Niklinska, G.N.; Skrzydlewska, E. The FAAH Inhibitor URB597 Modulates Lipid Mediators in the Brain of Rats with Spontaneous Hypertension. Biomolecules 2020, 10, 1022. [Google Scholar] [CrossRef]
- Biernacki, M.; Luczaj, W.; Gegotek, A.; Toczek, M.; Bielawska, K.; Skrzydlewska, E. Crosstalk between liver antioxidant and the endocannabinoid systems after chronic administration of the FAAH inhibitor, URB597, to hypertensive rats. Toxicol. Appl. Pharmacol. 2016, 301, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.; Luczaj, W.; Jarocka-Karpowicz, I.; Ambrozewicz, E.; Toczek, M.; Skrzydlewska, E. The Effect of Long-Term Administration of Fatty Acid Amide Hydrolase Inhibitor URB597 on Oxidative Metabolism in the Heart of Rats with Primary and Secondary Hypertension. Molecules 2018, 23, 2350. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, D.; Matthey, M.; Bindila, L.; Lerner, R.; Lutz, B.; Zimmer, A.; Fleischmann, B.K. Endocannabinoid anandamide mediates hypoxic pulmonary vasoconstriction. Proc. Natl. Acad. Sci. USA 2013, 110, 18710–18715. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Ramer, R.; Dithmer, S.; Ivanov, I.; Merkord, J.; Hinz, B. Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells. Oncotarget 2016, 7, 15047–15064. [Google Scholar] [CrossRef]
- Ravi, J.; Sneh, A.; Shilo, K.; Nasser, M.W.; Ganju, R.K. FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway. Oncotarget 2014, 5, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, L.; Chen, L.; Li, Y.; Chen, H.; Li, Y.; Ji, G.; Lin, D.; Liu, Z.; Qiu, Y. Potential analgesic effects of a novel N-acylethanolamine acid amidase inhibitor F96 through PPAR-α. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, Y.; Ke, H.; Li, Y.; Yang, L.; Yu, H.; Huang, R.; Lu, C.; Qiu, Y. Design, synthesis, and biological evaluation of oxazolidone derivatives as highly potent N-acylethanolamine acid amidase (NAAA) inhibitors. RSC Adv. 2017, 7, 12455–12463. [Google Scholar] [CrossRef]
- Clapper, J.R.; Vacondio, F.; King, A.R.; Duranti, A.; Tontini, A.; Silva, C.; Sanchini, S.; Tarzia, G.; Mor, M.; Piomelli, D. A second generation of carbamate-based fatty acid amide hydrolase inhibitors with improved activity in vivo. ChemMedChem 2009, 4, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Vacondio, F.; Silva, C.; Lodola, A.; Carmi, C.; Rivara, S.; Duranti, A.; Tontini, A.; Sanchini, S.; Clapper, J.R.; Piomelli, D.; et al. Biphenyl-3-yl alkylcarbamates as fatty acid amide hydrolase (FAAH) inhibitors: Steric effects of N-alkyl chain on rat plasma and liver stability. Eur. J. Med. Chem. 2011, 46, 4466–4473. [Google Scholar] [CrossRef]
- Mor, M.; Lodola, A.; Rivara, S.; Vacondio, F.; Duranti, A.; Tontini, A.; Sanchini, S.; Piersanti, G.; Clapper, J.R.; King, A.R.; et al. Synthesis and quantitative structure-activity relationship of fatty acid amide hydrolase inhibitors: Modulation at the N-portion of biphenyl-3-yl alkylcarbamates. J. Med. Chem. 2008, 51, 3487–3498. [Google Scholar] [CrossRef][Green Version]
- Tarzia, G.; Duranti, A.; Tontini, A.; Piersanti, G.; Mor, M.; Rivara, S.; Plazzi, P.V.; Park, C.; Kathuria, S.; Piomelli, D. Design, synthesis, and structure-activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors. J. Med. Chem. 2003, 46, 2352–2360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vozella, V.; Ahmed, F.; Choobchian, P.; Merrill, C.B.; Zibardi, C.; Tarzia, G.; Mor, M.; Duranti, A.; Tontini, A.; Rivara, S.; et al. Pharmacokinetics, pharmacodynamics and safety studies on URB937, a peripherally restricted fatty acid amide hydrolase inhibitor, in rats. J. Pharm. Pharmacol. 2019, 71, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Virk, H.; Arthur, G.; Bradding, P. Mast cells and their activation in lung disease. Transl. Res. 2016, 174, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E. Neutrophils and acute lung injury. Crit. Care Med. 2003, 31, S195–S199. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef]
- Malik, Z.; Baik, D.; Schey, R. The role of cannabinoids in regulation of nausea and vomiting, and visceral pain. Curr. Gastroenterol. Rep. 2015, 17, 429. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Darmani, N.A.; Parker, L.A. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur. J. Pharmacol. 2014, 722, 134–146. [Google Scholar] [CrossRef]
- Parker, L.A.; Rock, E.M.; Limebeer, C.L. Regulation of nausea and vomiting by cannabinoids. Br. J. Pharmacol. 2011, 163, 1411–1422. [Google Scholar] [CrossRef]
- Sagar, D.R.; Kendall, D.A.; Chapman, V. Inhibition of fatty acid amide hydrolase produces PPAR-alpha-mediated analgesia in a rat model of inflammatory pain. Br. J. Pharmacol. 2008, 155, 1297–1306. [Google Scholar] [CrossRef]
- Bortolato, M.; Mangieri, R.A.; Fu, J.; Kim, J.H.; Arguello, O.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; Piomelli, D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol. Psychiatry. 2007, 62, 1103–1110. [Google Scholar] [CrossRef]
- Celorrio, M.; Fernandez-Suarez, D.; Rojo-Bustamante, E.; Echeverry-Alzate, V.; Ramirez, M.J.; Hillard, C.J.; Lopez-Moreno, J.A.; Maldonado, R.; Oyarzabal, J.; Franco, R.; et al. Fatty acid amide hydrolase inhibition for the symptomatic relief of Parkinson’s disease. Brain Behav. Immun. 2016, 57, 94–105. [Google Scholar] [CrossRef]
- Selvaraj, P.; Wen, J.; Tanaka, M.; Zhang, Y. Therapeutic Effect of a Novel Fatty Acid Amide Hydrolase Inhibitor PF04457845 in the Repetitive Closed Head Injury Mouse Model. J. Neurotrauma. 2019, 36, 1655–1669. [Google Scholar] [CrossRef]
- Tchantchou, F.; Tucker, L.B.; Fu, A.H.; Bluett, R.J.; McCabe, J.T.; Patel, S.; Zhang, Y. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology 2014, 85, 427–439. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, G.P. Advances in the diagnosis and treatment of pediatric acute respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi 2018, 20, 717–723. [Google Scholar] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Ward, P.A. Regulation of experimental lung inflammation. Respir. Physiol. 2001, 128, 17–22. [Google Scholar] [CrossRef]
- Salvemini, D.; Wang, Z.Q.; Bourdon, D.M.; Stern, M.K.; Currie, M.G.; Manning, P.T. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J. Pharmacol. 1996, 303, 217–220. [Google Scholar] [CrossRef]
- Corsini, E.; Di Paola, R.; Viviani, B.; Genovese, T.; Mazzon, E.; Lucchi, L.; Marinovich, M.; Galli, C.L.; Cuzzocrea, S. Increased carrageenan-induced acute lung inflammation in old rats. Immunology 2005, 115, 253–261. [Google Scholar] [CrossRef]
- Guidot, D.M.; Folkesson, H.G.; Jain, L.; Sznajder, J.I.; Pittet, J.F.; Matthay, M.A. Integrating acute lung injury and regulation of alveolar fluid clearance. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2006, 291, L301–L306. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, S.E.; Mavrommati, I.; Korovesi, I.; Roussos, C. Pulmonary endothelium in acute lung injury: From basic science to the critically ill. Intensive Care Med. 2004, 30, 1702–1714. [Google Scholar] [CrossRef]
- Ishida, Y.; Takayasu, T.; Kimura, A.; Hayashi, T.; Kakimoto, N.; Miyashita, T.; Kondo, T. Gene expression of cytokines and growth factors in the lungs after paraquat administration in mice. Leg. Med. 2006, 8, 102–109. [Google Scholar] [CrossRef]
- Amirshahrokhi, K.; Bohlooli, S. Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice. Inflammation 2013, 36, 1111–1121. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Wang, H.D.; Lu, D.X.; Qi, R.B.; Wang, Y.P.; Yan, Y.X.; Fu, Y.M. Berberine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the alpha2-adrenergic receptor in mice. Shock 2008, 29, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Roux, J.; Kawakatsu, H.; Gartland, B.; Pespeni, M.; Sheppard, D.; Matthay, M.A.; Canessa, C.M.; Pittet, J.F. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J. Biol. Chem. 2005, 280, 18579–18589. [Google Scholar] [CrossRef]
- Frank, J.; Roux, J.; Kawakatsu, H.; Su, G.; Dagenais, A.; Berthiaume, Y.; Howard, M.; Canessa, C.M.; Fang, X.; Sheppard, D.; et al. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J. Biol. Chem. 2003, 278, 43939–43950. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Lee, Y.M.; Cho, H.G.; Cho, O.J.; Repine, J.E. Alveolar type II cell abnormalities and peroxide formation in lungs of rats given IL-1 intratracheally. Inflammation 2000, 24, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Hybertson, B.M.; Cho, H.G.; Terada, L.S.; Cho, O.; Repine, A.J.; Repine, J.E. Platelet-activating factor contributes to acute lung leak in rats given interleukin-1 intratracheally. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2000, 279, L75–L80. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The acute respiratory distress syndrome: From mechanism to translation. J. Immunol. 2015, 194, 855–860, Correction in J. Immunol. 2015, 194, 5569. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 5533. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, W.; Hu, D.; Han, X.; Wang, H.; Yang, J.; Xu, Y.; Li, Y.; Yao, W.; Chen, C. Resveratrol efficiently improves pulmonary function via stabilizing mast cells in a rat intestinal injury model. Life Sci. 2017, 185, 30–37. [Google Scholar] [CrossRef]
- Lange, M.; Connelly, R.; Traber, D.L.; Hamahata, A.; Nakano, Y.; Esechie, A.; Jonkam, C.; von Borzyskowski, S.; Traber, L.D.; Schmalstieg, F.C.; et al. Time course of nitric oxide synthases, nitrosative stress, and poly(ADP ribosylation) in an ovine sepsis model. Crit. Care 2010, 14, R129. [Google Scholar] [CrossRef]
- Talero, E.; Di Paola, R.; Mazzon, E.; Esposito, E.; Motilva, V.; Cuzzocrea, S. Anti-inflammatory effects of adrenomedullin on acute lung injury induced by Carrageenan in mice. Mediat. Inflamm. 2012, 2012, 717851. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Ward, P.A. Activation and regulation of NFkappaB during acute inflammation. Clin. Chem. Lab. Med. 1999, 37, 205–208. [Google Scholar] [CrossRef]
- Hawiger, J. Innate immunity and inflammation: A transcriptional paradigm. Immunol. Res. 2001, 23, 99–109. [Google Scholar] [CrossRef]
- Huxford, T.; Ghosh, G. A structural guide to proteins of the NF-kappaB signaling module. Cold Spring Harb. Perspect. Biol. 2009, 1, a000075. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Mazzon, E.; Muia, C.; Genovese, T.; Menegazzi, M.; Zaffini, R.; Suzuki, H.; Cuzzocrea, S. Green tea polyphenol extract attenuates lung injury in experimental model of carrageenan-induced pleurisy in mice. Respir. Res. 2005, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Gugliandolo, E.; Biundo, F.; Campolo, M.; Di Paola, R.; Cuzzocrea, S. Inhibition of inflammasome activation improves lung acute injury induced by carrageenan in a mouse model of pleurisy. FASEB. J. 2017, 31, 3497–3511. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Paterniti, I.; Siracusa, R.; Impellizzeri, D.; Esposito, E.; Cuzzocrea, S. KU0063794, a Dual mTORC1 and mTORC2 Inhibitor, Reduces Neural Tissue Damage and Locomotor Impairment After Spinal Cord Injury in Mice. Mol. Neurobiol. 2017, 54, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Esposito, E.; Petrosino, S.; Cuzzocrea, S. Anti-Inflammatory and Neuroprotective Effects of Co-UltraPEALut in a Mouse Model of Vascular Dementia. Front. Neurol. 2017, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Esposito, E.; Ahmad, A.; Di Paola, R.; Paterniti, I.; Cordaro, M.; Bruschetta, G.; Wallace, J.L.; Cuzzocrea, S. Hydrogen sulfide-releasing cyclooxygenase inhibitor ATB-346 enhances motor function and reduces cortical lesion volume following traumatic brain injury in mice. J. Neuroinflamm. 2014, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Di Paola, R.; Campolo, M.; Siracusa, R.; Cordaro, M.; Bruschetta, G.; Tremolada, G.; Maestroni, A.; Bandello, F.; Esposito, E.; et al. Palmitoylethanolamide treatment reduces retinal inflammation in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2015, 769, 313–323. [Google Scholar] [CrossRef]
- Cordaro, M.; Impellizzeri, D.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a Palmitoylethanolamide Analogue, as a New Pharmacological Treatment for the Management of Inflammatory Bowel Disease. Mol. Pharmacol. 2016, 90, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Cordaro, M.; Crupi, R.; Siracusa, R.; Campolo, M.; Bruschetta, G.; Fusco, R.; Pugliatti, P.; Esposito, E.; Cuzzocrea, S. Protective Effects of Ultramicronized Palmitoylethanolamide (PEA-um) in Myocardial Ischaemia and Reperfusion Injury in VIVO. Shock 2016, 46, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Impellizzeri, D.; Fusco, R.; Cordaro, M.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Ultramicronized palmitoylethanolamide (PEA-um((R))) in the treatment of idiopathic pulmonary fibrosis. Pharmacol. Res. 2016, 111, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S. A new co-micronized composite containing palmitoylethanolamide and polydatin shows superior oral efficacy compared to their association in a rat paw model of carrageenan-induced inflammation. Eur. J. Pharmacol. 2016, 782, 107–118. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Crupi, R.; Pascali, J.; Alfonsi, D.; Marcolongo, G.; Cuzzocrea, S. 2-pentadecyl-2-oxazoline: Identification in coffee, synthesis and activity in a rat model of carrageenan-induced hindpaw inflammation. Pharmacol. Res. 2016, 108, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; D’Amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355–31366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Cordaro, M.; Crupi, R.; Mandalari, G.; Impellizzeri, D.; et al. The Role of Cashew (Anacardium occidentale L.) Nuts on an Experimental Model of Painful Degenerative Joint Disease. Antioxidants 2020, 9, 511. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Peritore, A.F.; Cordaro, M.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. The Antioxidant and Anti-Inflammatory Properties of Anacardium occidentale L. Cashew Nuts in a Mouse Model of Colitis. Nutrients 2020, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis. Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Crisafulli, C.; Mazzon, E.; Genovese, T.; Paterniti, I.; Bramanti, P.; Cuzzocrea, S. Effect of PD98059, a selective MAPK3/MAPK1 inhibitor, on acute lung injury in mice. Int. J. Immunopathol. Pharmacol. 2009, 22, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, S.; Xie, W.; He, H. Ketamine ameliorates oxidative stress-induced apoptosis in experimental traumatic brain injury via the Nrf2 pathway. Drug Des. Devel. Ther. 2018, 12, 845–853. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Li, Y.; Chen, L.; Xiang, J. Tenacissoside H Promotes Neurological Recovery of Cerebral Ischemia-reperfusion Injury in Mice by Modulating Inflammation and Oxidative stress via TrkB Pathway. Clin. Exp. Pharmacol. Physiol. 2020, 48, 757–769. [Google Scholar] [CrossRef]
- Shi, D.D.; Huang, Y.H.; Lai, C.S.W.; Dong, C.M.; Ho, L.C.; Wu, E.X.; Li, Q.; Wang, X.M.; Chung, S.K.; Sham, P.C.; et al. Chemotherapy-Induced Cognitive Impairment Is Associated with Cytokine Dysregulation and Disruptions in Neuroplasticity. Mol. Neurobiol. 2019, 56, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Liang, X.; Zhang, M.; Yin, X.; Yang, H.; Zhi, Y.; Ying, G.; Zou, J.; Chen, L.; Yao, X.; et al. Astaxanthin protects cognitive function of vascular dementia. Behav. Brain. Funct. 2020, 16, 10. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef] [PubMed]
- D’Amicoa, R.; Monacob, F.; Fuscoa, R.; Peritorea, A.F.; Genovesea, T.; Impellizzeria, D.; Crupic, R.; Interdonatoa, L.; Sforzaa, A.M.; Gugliandoloc, E. Exposure to Atrazine Induces Lung Inflammation through Nrf2-HO1 and Beclin 1/LC3 Pathways. Cell Physiol. Biochem. 2021, 55, 413–427. [Google Scholar]
- Conte, E.; Fagone, E.; Gili, E.; Fruciano, M.; Iemmolo, M.; Pistorio, M.P.; Impellizzeri, D.; Cordaro, M.; Cuzzocrea, S.; Vancheri, C. Preventive and therapeutic effects of thymosin beta4 N-terminal fragment Ac-SDKP in the bleomycin model of pulmonary fibrosis. Oncotarget 2016, 7, 33841–33854. [Google Scholar] [CrossRef][Green Version]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Scuto, M.; Cuzzocrea, S.; Di Paola, R.; et al. Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome. Int. J. Mol. Sci. 2020, 21, 2144. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Genovese, T.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Crupi, R.; Cuzzocrea, S.; et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants 2020, 9, 601. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Cordaro, M.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Mucosa-Associated Lymphoid Tissue Lymphoma Translocation 1 Inhibitor as a Novel Therapeutic Tool for Lung Injury. Int. J. Mol. Sci. 2020, 21, 7761. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Talero, E.; Siracusa, R.; Alcaide, A.; Cordaro, M.; Maria Zubelia, J.; Bruschetta, G.; Crupi, R.; Esposito, E.; Cuzzocrea, S.; et al. Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. Br. J. Nutr. 2015, 114, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.; Gokulan, R.; Dongre, H.; Vaidya, M.; Chaukar, D.; Prabhash, K.; Ingle, A.; Joshi, S.; Dange, P.; Joshi, S.; et al. Prognostic role of Oct4, CD44 and c-Myc in radio-chemo-resistant oral cancer patients and their tumourigenic potential in immunodeficient mice. Clin. Oral Investig. 2016, 20, 43–56. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, T.; Duranti, A.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Cuzzocrea, S.; Di Paola, R.; et al. Fatty Acid Amide Hydrolase (FAAH) Inhibition Plays a Key Role in Counteracting Acute Lung Injury. Int. J. Mol. Sci. 2022, 23, 2781. https://doi.org/10.3390/ijms23052781
Genovese T, Duranti A, D’Amico R, Fusco R, Impellizzeri D, Peritore AF, Crupi R, Gugliandolo E, Cuzzocrea S, Di Paola R, et al. Fatty Acid Amide Hydrolase (FAAH) Inhibition Plays a Key Role in Counteracting Acute Lung Injury. International Journal of Molecular Sciences. 2022; 23(5):2781. https://doi.org/10.3390/ijms23052781
Chicago/Turabian StyleGenovese, Tiziana, Andrea Duranti, Ramona D’Amico, Roberta Fusco, Daniela Impellizzeri, Alessio Filippo Peritore, Rosalia Crupi, Enrico Gugliandolo, Salvatore Cuzzocrea, Rosanna Di Paola, and et al. 2022. "Fatty Acid Amide Hydrolase (FAAH) Inhibition Plays a Key Role in Counteracting Acute Lung Injury" International Journal of Molecular Sciences 23, no. 5: 2781. https://doi.org/10.3390/ijms23052781
APA StyleGenovese, T., Duranti, A., D’Amico, R., Fusco, R., Impellizzeri, D., Peritore, A. F., Crupi, R., Gugliandolo, E., Cuzzocrea, S., Di Paola, R., Siracusa, R., & Cordaro, M. (2022). Fatty Acid Amide Hydrolase (FAAH) Inhibition Plays a Key Role in Counteracting Acute Lung Injury. International Journal of Molecular Sciences, 23(5), 2781. https://doi.org/10.3390/ijms23052781












