In Vitro Hepatotoxic and Neurotoxic Effects of Titanium and Cerium Dioxide Nanoparticles, Arsenic and Mercury Co-Exposure
Abstract
:1. Introduction
2. Results
2.1. NPs Characterization
2.2. NP-Metal Adsorption and NP Dissolution
2.3. Cell Viability
2.3.1. Single Exposure
2.3.2. Co-Exposure
2.4. Effects of 7 Days Single and Binary Exposures on Cell Proliferation
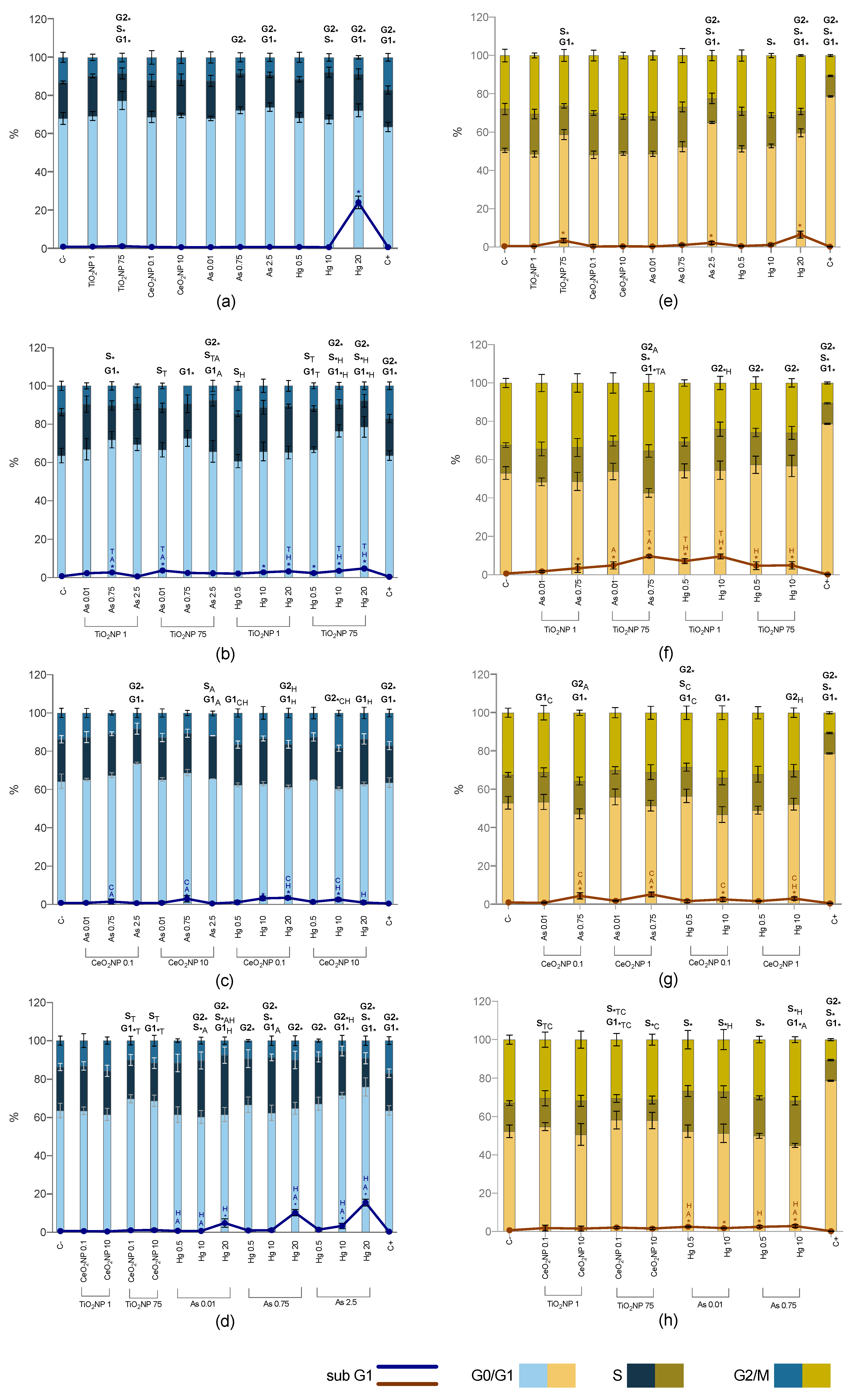
2.5. Cell Cycle Alterations
2.5.1. Single Exposure
2.5.2. Co-Exposure
3. Discussion
3.1. Single Exposures
3.2. Arsenic-Mercury Co-Exposure
3.3. TiO2NPs—CeO2NPs Co-Exposure
3.4. NP-Metal Co-Exposure
4. Materials and Methods
4.1. Materials
4.2. Nanoparticle Dispersion and Solutions
4.3. Nanoparticle Characterization
4.4. NP-Metal Adsorption and NP Dissolution in Cell Culture Media
4.5. Cell Culture
4.6. Exposure to Single or Binary Mixtures of TiO2NPs, CeO2NPs, as and Hg
4.7. Cell Viability by WST-1 Assay
4.8. Cell Proliferation by Clonogenic Assay
4.9. Cell Cycle Analysis—Flow Cytometry
4.10. Statistical Analysis
5. Conclusions
- potentiation effects: if aggregates are of small size and can be easily uptaken by the cell, if adsorption to NP facilitates the entrance of metals, and/or by the toxicity of the metal alone;
- antagonism effects: if the metals cause the formation of large NP aggregates that hinder their uptake by the cells, block the other contaminants’ access to the cell, and/or if the NP (e.g., CeO2NPs) have the ability to act as antioxidant and reduce oxidative stress in the cells.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hull, M.S. Multidimensional Impacts of Nanotechnology on Public Health; Academic Press: Amsterdam, The Netherlands, 2018; ISBN 9780128132906. [Google Scholar]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Remédios, C.; Rosário, F.; Bastos, V. Environmental Nanoparticles Interactions with Plants: Morphological, Physiological, and Genotoxic Aspects. J. Bot. 2012, 2012, 751686. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef] [Green Version]
- Dhasmana, A.; Sajid Jamal, Q.M.; Mir, S.S.; Bhatt, M.L.B.; Rahman, Q.; Gupta, R.; Siddiqui, M.H.; Lohani, M. Titanium Dioxide Nanoparticles as Guardian against Environmental Carcinogen Benzo[alpha]Pyrene. PLoS ONE 2014, 9, e107068. [Google Scholar] [CrossRef] [PubMed]
- Hristovski, K.; Baumgardner, A.; Westerhoff, P. Selecting metal oxide nanomaterials for arsenic removal in fixed bed columns: From nanopowders to aggregated nanoparticle media. J. Hazard. Mater. 2007, 147, 265–274. [Google Scholar] [CrossRef]
- Sharma, R.; Raghav, S.; Nair, M.; Kumar, D. Kinetics and Adsorption Studies of Mercury and Lead by Ceria Nanoparticles Entrapped in Tamarind Powder. ACS Omega 2018, 3, 14606–14619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Meng, X. Size effects of nanocrystalline TiO2 on As (V) and As (III) adsorption and As (III) photooxidation. J. Hazard. Mater. 2009, 168, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Al Bsoul, A.; Hailat, M.; Abdelhay, A.; Tawalbeh, M.; Jum’h, I.; Bani-Melhem, K. Treatment of olive mill effluent by adsorption on titanium oxide nanoparticles. Sci. Total Environ. 2019, 688, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Varner, K. State of the Science Literature Review: Nano Titanium Dioxide; U.S. Environmental Protection Agency: Washington, DC, USA, 2010.
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K. Biological, biomedical and pharmaceutical applications of cerium oxide. In Cerium Oxide (CeO2): Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–358. [Google Scholar]
- Dahle, J.T.; Arai, Y. Environmental geochemistry of cerium: Applications and toxicology of cerium oxide nanoparticles. Int. J. Environ. Res. Public Health 2015, 12, 1253–1278. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Chu, D.; Li, S. Cerium Oxide Nanostructures and their Applications. Funct. Nanomater. 2016, 3, 53–68. [Google Scholar]
- Singh, A.P.; Goel, R.K.; Kaur, T. Mechanisms Pertaining to Arsenic Toxicity. Toxicol. Int. 2011, 18, 87. [Google Scholar] [PubMed] [Green Version]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth edition Incorporating First Addendum, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Mercury; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1997. [Google Scholar]
- European Comission. Ambient Air Pollution by Mercury (Hg) Position Paper; Prepared by the Working Group on Mercury; Office for Official Publications of the European Communities: Luxembourg, 2001. [Google Scholar]
- Zhou, J.; Hou, W.; Qi, P.; Gao, X.; Luo, Z.; Cen, K. CeO2-TiO2 sorbents for the removal of elemental mercury from syngas. Environ. Sci. Technol. 2013, 47, 10056–10062. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, T.; Addo Ntim, S.; Ji, Z.; Lin, S.; Meng, H.; Chung, C.-H.; George, S.; Zhang, H.; Wang, M.; et al. Dispersal State of Multiwalled Carbon Nanotubes Elicits Profibrogenic Cellular Responses That Correlate with Fibrogenesis Biomarkers and Fibrosis in the Murine Lung. ACS Nano 2011, 5, 9772–9787. [Google Scholar] [CrossRef] [Green Version]
- Della Torre, C.; Buonocore, F.; Frenzilli, G.; Corsolini, S.; Brunelli, A.; Guidi, P.; Kocan, A.; Mariottini, M.; Mottola, F.; Nigro, M.; et al. Influence of titanium dioxide nanoparticles on 2,3,7,8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish European sea bass (Dicentrarchus labrax). Environ. Pollut. 2015, 196, 185–193. [Google Scholar] [CrossRef]
- Ferreira, J.L.R.; Lonné, M.N.; França, T.A.; Maximilla, N.R.; Lugokenski, T.H.; Costa, P.G.; Fillmann, G.; Antunes Soares, F.A.; De la Torre, F.R.; Monserrat, J.M. Co-exposure of the organic nanomaterial fullerene C₆₀ with benzo[a]pyrene in Danio rerio (zebrafish) hepatocytes: Evidence of toxicological interactions. Aquat. Toxicol. 2014, 147, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnapongse, S.L.; MacCuspie, R.I.; Hackley, V.A. Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci. Total Environ. 2011, 409, 2443–2450. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Wang, D.; Zhang, C.; Liang, L.; Zhou, X. The effect of different TiO2 nanoparticles on the release and transformation of mercury in sediment. J. Soils Sediments 2017, 17, 536–542. [Google Scholar] [CrossRef]
- Li, M.; Luo, Z.; Yan, Y.; Wang, Z.; Chi, Q.; Yan, C.; Xing, B. Arsenate accumulation, distribution, and toxicity associated with titanium dioxide nanoparticles in Daphnia magna. Environ. Sci. Technol. 2016, 50, 9636–9643. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, X.; Zhang, Z.; Chen, Y.; Crittenden, J.C. Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite. Environ. Pollut. 2009, 157, 1165–1170. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 2010, 79, 928–933. [Google Scholar] [CrossRef]
- Rosenfeldt, R.R.; Seitz, F.; Schulz, R.; Bundschuh, M. Heavy Metal Uptake and Toxicity in the Presence of Titanium Dioxide Nanoparticles: A Factorial Approach Using Daphnia magna. Environ. Sci. Technol. 2014, 48, 6965–6972. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Qiao, J.; Wang, H.; Li, L. Synergistic effects of nano-sized titanium dioxide and zinc on the photosynthetic capacity and survival of Anabaena sp. Int. J. Mol. Sci. 2013, 14, 14395–14407. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Quadros, M.E.; Marr, L.C. Environmental and human health risks of aerosolized silver nanoparticles. J. Air Waste Manag. Assoc. 2010, 60, 770–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiser, M.; Kreyling, W.G. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosário, F.; Duarte, I.F.; Pinto, R.J.B.; Santos, C.; Hoet, P.H.M.; Oliveira, H. Biodistribution and pulmonary metabolic effects of silver nanoparticles in mice following acute intratracheal instillations. Environ. Sci. Pollut. Res. 2020, 28, 2301–2314. [Google Scholar] [CrossRef] [PubMed]
- Cupaioli, F.A.; Zucca, F.A.; Boraschi, D.; Zecca, L. Engineered nanoparticles. How brain friendly is this new guest? Prog. Neurobiol. 2014, 119–120, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Kiliç, G.; Duarte, J.A.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Toxicological assessment of silica-coated iron oxide nanoparticles in human astrocytes. Food Chem. Toxicol. 2018, 118, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Shao, A.; Zhao, Y.; Wang, Z.; Zhang, C.; Sun, Y.; Deng, J.; Chou, L.L. Neurotoxicity of Silver Nanoparticles in Rat Brain After Intragastric Exposure. J. Nanosci. Nanotechnol. 2015, 15, 4215–4223. [Google Scholar] [CrossRef] [PubMed]
- Weldon, B.A.; Faustman, E.M.; Oberdörster, G.; Workman, T.; Griffith, W.C.; Kneuer, C.; Yu, I.J. Occupational exposure limit for silver nanoparticles: Considerations on the derivation of a general health-based value. Nanotoxicology 2016, 10, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Wang, J.; Nie, Y.; Chen, S.; Hei, T.K.; Deng, Z.; Wu, L.; Zhao, G.; Xu, A. Amplification of arsenic genotoxicity by TiO(2) nanoparticles in mammalian cells: New insights from physicochemical interactions and mitochondria. Nanotoxicology 2017, 11, 978–995. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.R.; Bezerra, A.G.; Oliveira Ribeiro, C.A.; Randi, M.A.F.; Voigt, C.L.; Skytte, L.; Rasmussen, K.L.; Kjeldsen, F.; Filipak Neto, F. Toxicological interactions of silver nanoparticles and non-essential metals in human hepatocarcinoma cell line. Toxicol. In Vitro 2017, 40, 134–143. [Google Scholar] [CrossRef]
- Rosário, F.; Bessa, M.J.; Brandão, F.; Costa, C.; Lopes, C.B.; Estrada, A.C.; Tavares, D.S.; Teixeira, J.P.; Reis, A.T. Unravelling the potential cytotoxic effects of metal oxide nanoparticles and metal (Loid) mixtures on a549 human cell line. Nanomaterials 2020, 10, 447. [Google Scholar] [CrossRef] [Green Version]
- Tavares, D.S.; Lopes, C.B.; Almeida, J.C.; Vale, C.; Pereira, E.; Trindade, T. Spinel-type ferrite nanoparticles for removal of arsenic (V) from water. Environ. Sci. Pollut. Res. Int. 2020, 27, 22523–22534. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, M.J.; Aguado, J.; Arencibia, A.; Pascual, R. Mercury removal from aqueous solutions of HgCl2 by heterogeneous photocatalysis with TiO2. Appl. Catal. B Environ. 2011, 104, 220–228. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, S.; Chen, Q.; Huang, X.; Chen, H.; Lu, L.; Zheng, C.; Gao, X.; Wu, T. CeO2 based catalysts for elemental mercury capture. Energy Procedia 2019, 158, 4635–4640. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Tavares, D.S.; Daniel-da-Silva, A.L.; Lopes, C.B.; Pereira, E.; Araújo, J.P.; Sousa, C.T.; Trindade, T. Ferromagnetic sorbents based on nickel nanowires for efficient uptake of mercury from water. ACS Appl. Mater. Interfaces 2014, 6, 8274–8280. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Brandão, F.; Fernández-Bertólez, N.; Rosário, F.; Bessa, M.J.; Fraga, S.; Pásaro, E.; Teixeira, J.P.; Laffon, B.; Valdiglesias, V.; Costa, C. Genotoxicity of TiO(2) Nanoparticles in Four Different Human Cell Lines (A549, HEPG2, A172 and SH-SY5Y). Nanomaterials 2020, 10, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.-L.; Zhao, M.-N.; Zhu, S.-H.; Han, Y.-Y.; Zhang, J.-Y.; Shen, S.-H.; Yu, Z.-S.; Zhang, H. Effects of CeO(2) nanoparticles on the viabilities of neural PC12 and SH-SY5Y cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2020, 36, 62–66. [Google Scholar] [PubMed]
- Sudo, K.; Van Dao, C.; Miyamoto, A.; Shiraishi, M. Comparative analysis of in vitro neurotoxicity of methylmercury, mercury, cadmium, and hydrogen peroxide on SH-SY5Y cells. J. Vet. Med. Sci. 2019, 81, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Barnum, K.J.; O’Connell, M.J. Cell cycle regulation by checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [PubMed] [Green Version]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, H.; Dillon, M.T.; Barker, H.E.; Kyula, J.N.; Schick, U.; Paget, J.T.; Smith, H.G.; Pedersen, M.; McLaughlin, M.; Harrington, K.J. Combined ATR and DNA-PK Inhibition Radiosensitizes Tumor Cells Independently of Their p53 Status. Front. Oncol. 2018, 8, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woynarowski, J.M.; Trevino, A.V.; Salinas, R.; Herman, T.S.; Waters, S.J.; Macdonald, J.R. Cell Cycle Effects and Induction of Premitotic Apoptosis by Irofulven in Synchronized Cancer Cells. Cancer Biol. Ther. 2004, 3, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Kim, Y.S.; Kim, M.S.; Ryu, J.C. The inhibitory mechanism of methylmercury on differentiation of human neuroblastoma cells. Toxicology 2007, 234, 1–9. [Google Scholar] [CrossRef]
- Yousaf, M.N. The Combined Effects or Arsenic Cadmium and Mercury on Hepatocarcinoma and Neuroblastoma Cells In Vitro. Master’s Thesis, University of Pretoria, Pretoria, South Africa, July 2018. [Google Scholar]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Júnior, F.B.; Farina, M.; Viegas, S.; Kempinas, W.D.G. Toxicology of Metals and Metalloids. Biomed. Res. Int. 2014, 2014, 253738. [Google Scholar] [CrossRef]
- Zhu, C.; Mills, K.D.; Ferguson, D.O.; Lee, C.; Manis, J.; Fleming, J.; Gao, Y.; Morton, C.C.; Alt, F.W. Unrepaired DNA Breaks in p53-Deficient Cells Lead to Oncogenic Gene Amplification Subsequent to Translocations. Cell 2002, 109, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Wu, J.; Liu, M.; Zhu, G.; Chen, L.; Chang, Y.; Lu, H. Toxicity of binary mixtures of metal oxide nanoparticles to Nitrosomonas europaea. Chemosphere 2016, 153, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Verrall, K.E.; Warwick, P.; Fairhurst, A.J. Application of the Schulze–Hardy rule to haematite and haematite/humate colloid stability. Colloids Surf. A Physicochem. Eng. Asp. 1999, 150, 261–273. [Google Scholar] [CrossRef]
- Choi, S.Y.; Jeong, S.; Jang, S.H.; Park, J.; Park, J.H.; Ock, K.S.; Lee, S.Y.; Joo, S.-W. In vitro toxicity of serum protein-adsorbed citrate-reduced gold nanoparticles in human lung adenocarcinoma cells. Toxicol. In Vitro 2012, 26, 229–237. [Google Scholar] [CrossRef]
- Zeng, C.; Nguyen, C.; Boitano, S.; Field, J.A.; Shadman, F.; Sierra-Alvarez, R. Cerium dioxide (CeO2) nanoparticles decrease arsenite (As(III)) cytotoxicity to 16HBE14o—Human bronchial epithelial cells. Environ. Res. 2018, 164, 452–458. [Google Scholar] [CrossRef]
- Karakoti, A.; Singh, S.; Dowding, J.M.; Seal, S.; Self, W.T. Redox-active radical scavenging nanomaterials. Chem. Soc. Rev. 2010, 39, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Dowding, J.M.; Song, W.; Bossy, K.; Karakoti, A.; Kumar, A.; Kim, A.; Bossy, B.; Seal, S.; Ellisman, M.H.; Perkins, G.; et al. Cerium oxide nanoparticles protect against Aβ-induced mitochondrial fragmentation and neuronal cell death. Cell Death Differ. 2014, 21, 1622–1632. [Google Scholar] [CrossRef]
- Colon, J.; Hsieh, N.; Ferguson, A.; Kupelian, P.; Seal, S.; Jenkins, D.W.; Baker, C.H. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine 2010, 6, 698–705. [Google Scholar] [CrossRef]
- Wang, J.; Dai, H.; Nie, Y.; Wang, M.; Yang, Z.; Cheng, L.; Liu, Y.; Chen, S.; Zhao, G.; Wu, L.; et al. TiO2 nanoparticles enhance bioaccumulation and toxicity of heavy metals in Caenorhabditis elegans via modification of local concentrations during the sedimentation process. Ecotoxicol. Environ. Saf. 2018, 162, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, J.; Irons, D.R.; Wang, J. Synergistic toxic effect of nano-TiO2 and As (V) on Ceriodaphnia dubia. Sci. Total Environ. 2011, 409, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. 2015, 22, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.M.; Duran-Robles, E.; Goshia, T.; Mesina, M.; Garcia, C.; Young, J.; Sibal, A.; Chiu, M.H.; Chin, W.C. CeO2 nanoparticles attenuate airway mucus secretion induced by TiO2 nanoparticles. Sci. Total Environ. 2018, 631, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Costley, C.T.; Mossop, K.F.; Dean, J.R.; Garden, L.M.; Marshall, J.; Carroll, J. Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal. Chim. Acta 2000, 405, 179–183. [Google Scholar] [CrossRef]
- Reis, A.T.; Coelho, J.P.; Rodrigues, S.M.; Rocha, R.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Development and validation of a simple thermo-desorption technique for mercury speciation in soils and sediments. Talanta 2012, 99, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.; Hankin, S.; Ross, B.; Tran, C.; Stone, V.; Fernandes, T.; Donaldson, K.; Duffin, R.; Chaudhry, Q.; Wilkins, T.; et al. EMERGNANO: A Review of Completed and Near Completed Environment, Health and Safety Research on Nanomaterials and Nanotechnology; Institute of Occupational Medicine: Edinburgh, UK, 2009. [Google Scholar]
- Boxall, A.B.; Tiede, K.; Chaudhry, Q. Engineered nanomaterials in soils and water: How do they behave and could they pose a risk to human health? Nanomedicine 2007, 2, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of titanium dioxide nanoparticles with soil components and plants: Current knowledge and future research needs-a critical review. Environ. Sci. Nano 2018, 5, 257–278. [Google Scholar] [CrossRef]
- Wu, F.; Hicks, A.L. Estimating human exposure to titanium dioxide from personal care products through a social survey approach. Integr. Environ. Assess. Manag. 2020, 16, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, N.; Cummins, E. Ranking initial environmental and human health risk resulting from environmentally relevant nanomaterials. J. Environ. Sci. Health Part A 2010, 45, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Donaldson, K.; Duffin, R.; Tran, L.; Kelly, F.; Mudway, I.; Morin, J.-P.; Guest, R.; Jenkinson, P.; Samaras, Z.; et al. Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive—A case study. Inhal. Toxicol. 2008, 20, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Benameur, L.; Auffan, M.; Cassien, M.; Liu, W.; Culcasi, M.; Rahmouni, H.; Stocker, P.; Tassistro, V.; Bottero, J.-Y.; Rose, J.; et al. DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: Evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicology 2015, 9, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007.
- Commission Regulation (EC) No 1881/2006 of 19 December 2006. Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: http://data.europa.eu/eli/reg/2006/1881/oj (accessed on 8 February 2021).
- Afshar, E.; Mohammadi-Manesh, H.; Dashti Khavidaki, H. Removal of Hg (I) and Hg (II) Ions from Aqueous Solutions, Using TiO2 Nanoparticles. Pollution 2017, 3, 505–516. [Google Scholar]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Monteiro, C.; Pinho, F.; Pinho, S.; Ferreira de Oliveira, J.M.P.; Santos, C. Cadmium-induced genotoxicity in human osteoblast-like cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 775–776, 38–47. [Google Scholar] [CrossRef] [PubMed]

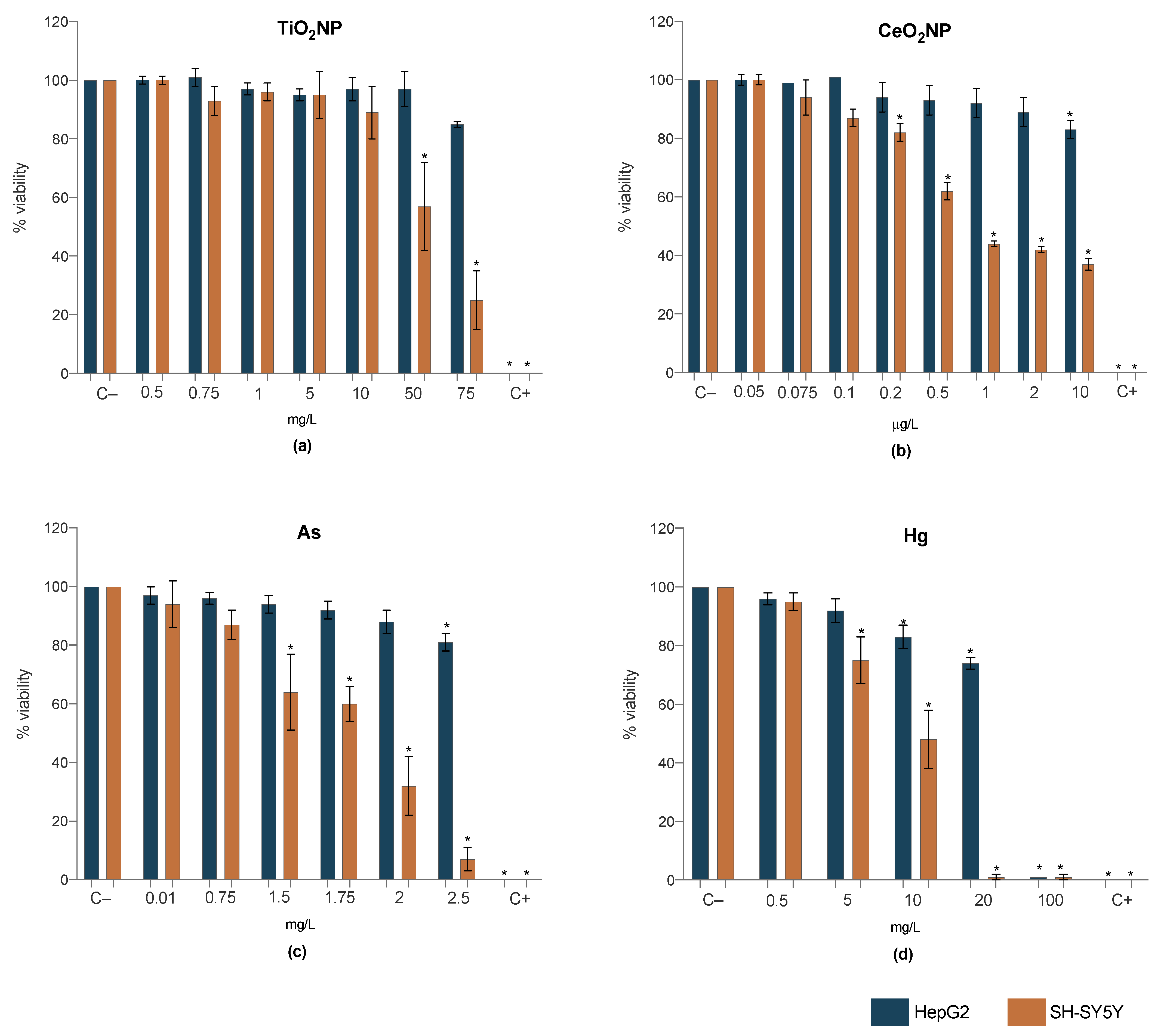


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosário, F.; Costa, C.; Lopes, C.B.; Estrada, A.C.; Tavares, D.S.; Pereira, E.; Teixeira, J.P.; Reis, A.T. In Vitro Hepatotoxic and Neurotoxic Effects of Titanium and Cerium Dioxide Nanoparticles, Arsenic and Mercury Co-Exposure. Int. J. Mol. Sci. 2022, 23, 2737. https://doi.org/10.3390/ijms23052737
Rosário F, Costa C, Lopes CB, Estrada AC, Tavares DS, Pereira E, Teixeira JP, Reis AT. In Vitro Hepatotoxic and Neurotoxic Effects of Titanium and Cerium Dioxide Nanoparticles, Arsenic and Mercury Co-Exposure. International Journal of Molecular Sciences. 2022; 23(5):2737. https://doi.org/10.3390/ijms23052737
Chicago/Turabian StyleRosário, Fernanda, Carla Costa, Cláudia B. Lopes, Ana C. Estrada, Daniela S. Tavares, Eduarda Pereira, João Paulo Teixeira, and Ana Teresa Reis. 2022. "In Vitro Hepatotoxic and Neurotoxic Effects of Titanium and Cerium Dioxide Nanoparticles, Arsenic and Mercury Co-Exposure" International Journal of Molecular Sciences 23, no. 5: 2737. https://doi.org/10.3390/ijms23052737
APA StyleRosário, F., Costa, C., Lopes, C. B., Estrada, A. C., Tavares, D. S., Pereira, E., Teixeira, J. P., & Reis, A. T. (2022). In Vitro Hepatotoxic and Neurotoxic Effects of Titanium and Cerium Dioxide Nanoparticles, Arsenic and Mercury Co-Exposure. International Journal of Molecular Sciences, 23(5), 2737. https://doi.org/10.3390/ijms23052737









