Hierarchically Structured Surfaces Prepared by Phase Separation: Tissue Mimicking Culture Substrate
Abstract
1. Introduction
2. Results and Discussion
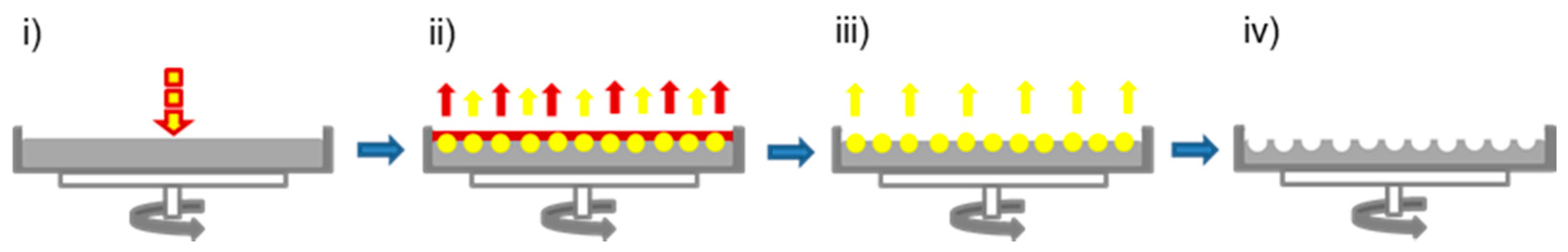
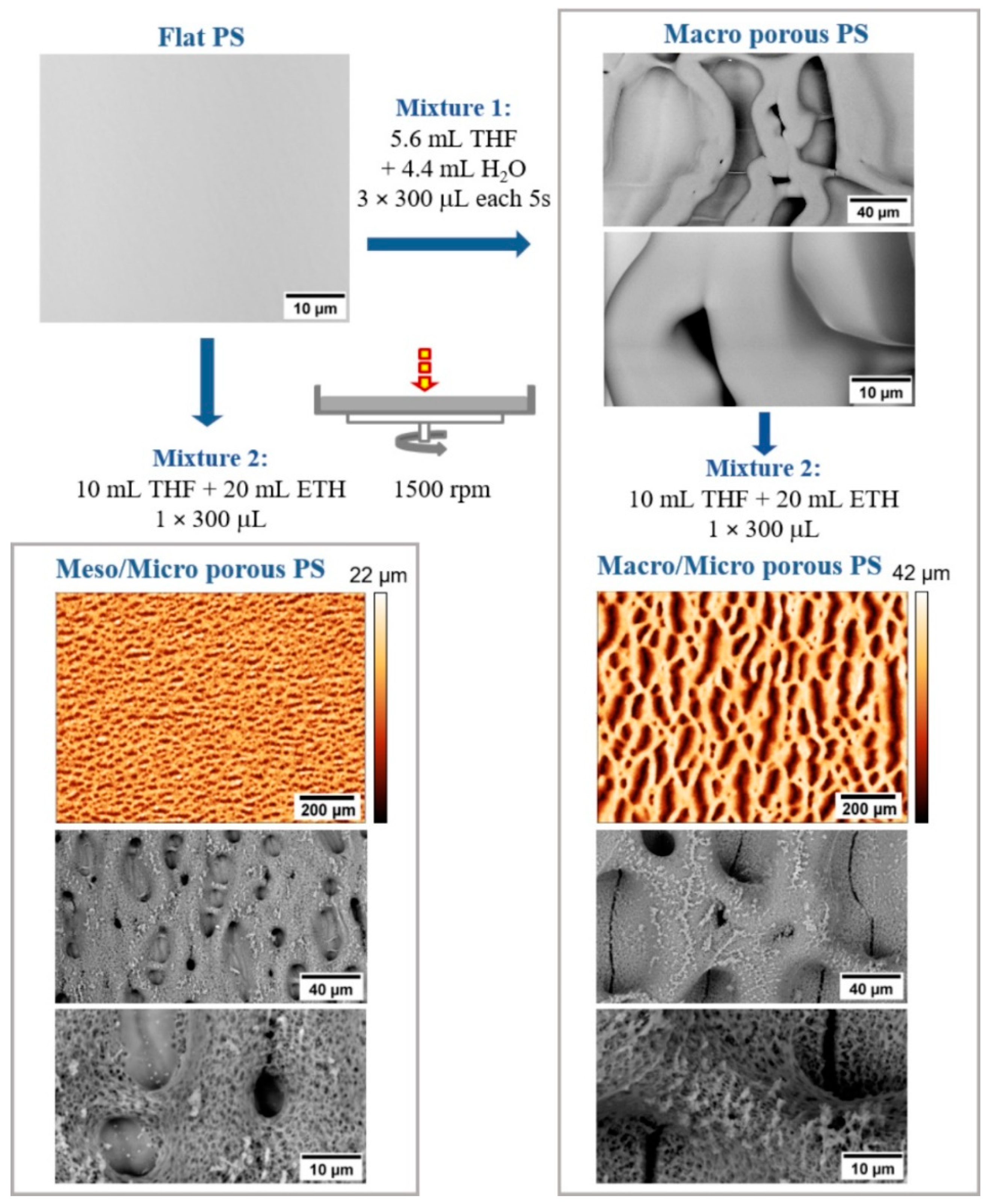
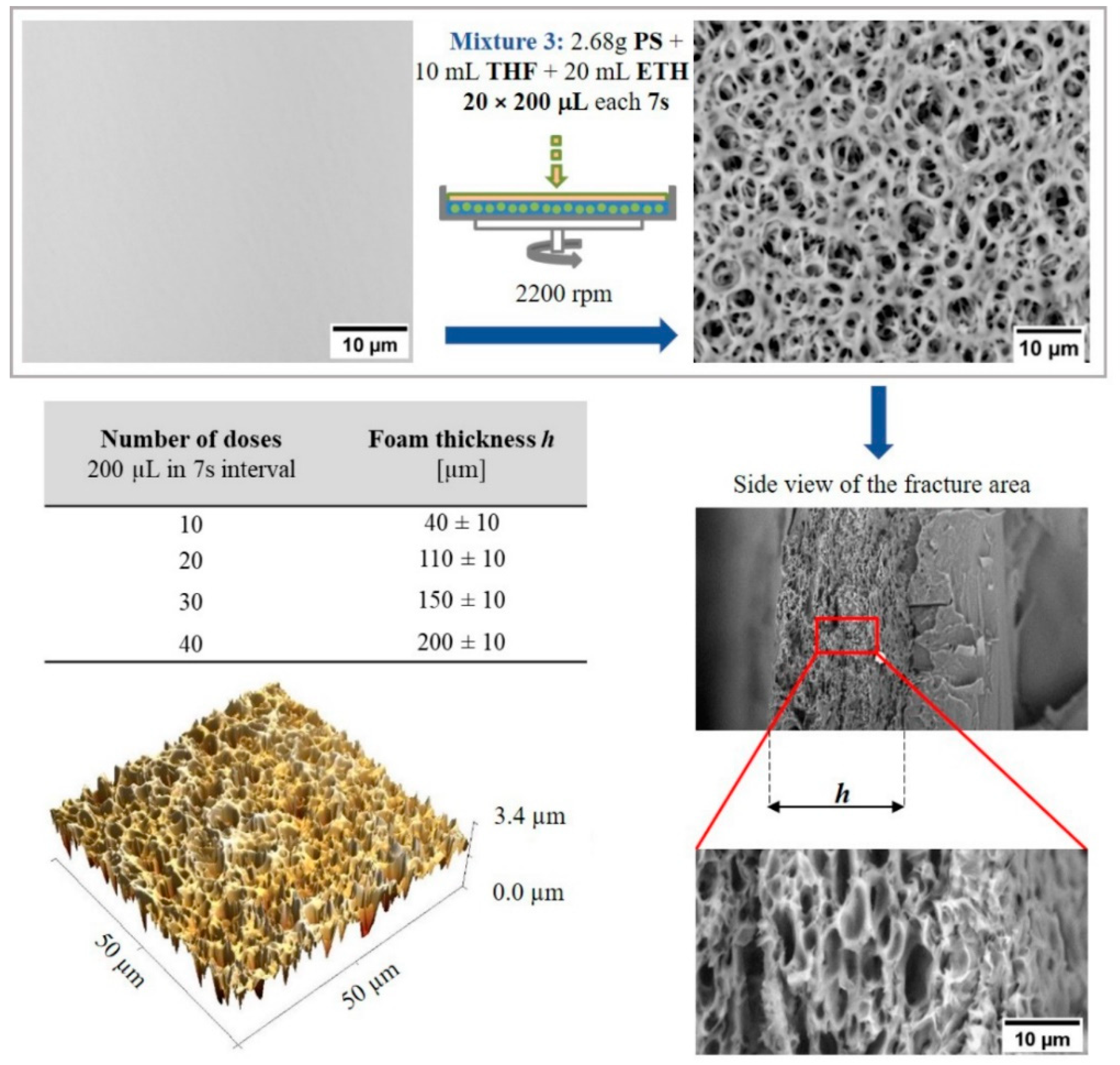
2.1. Hierarchically Structured Surfaces
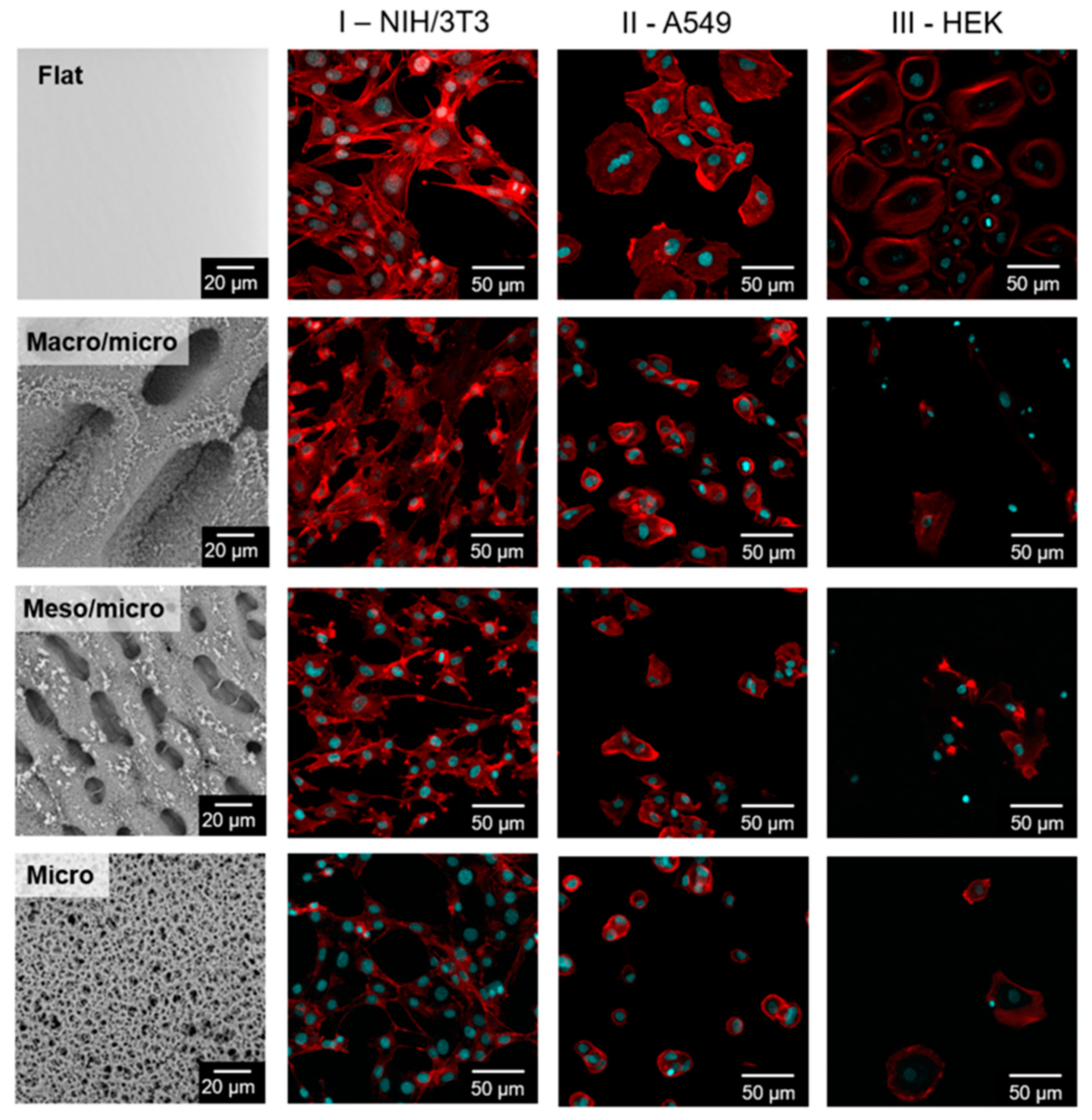
2.2. Influence of Structured Surfaces on Cell Behavior
3. Materials and Methods
3.1. Materials and Reagents
3.2. Solutions Preparation
3.3. Preparation and Characterization of Hierarchically Structured Substrates
3.4. Proliferation of Cells
3.5. Quantification of Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de León, A.S.; del Campo, A.; Fernández-García, M.; Rodríguez-Hernández, J.; Muñoz-Bonilla, A. Hierarchically Structured Multifunctional Porous Interfaces through Water Templated Self-Assembly of Ternary Systems. Langmuir 2012, 28, 9778–9787. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M.; Rodríguez-Hernández, J. Towards hierarchically ordered functional porous polymeric surfaces prepared by the breath figures approach. Prog. Polym. Sci. 2014, 39, 510–554. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Ibarboure, E.; Papon, E.; Rodriguez-Hernandez, J. Self-Organized Hierarchical Structures in Polymer Surfaces: Self-Assembled Nanostructures within Breath Figures. Langmuir 2009, 25, 6493–6499. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Bormashenko, E. Wetting of Real Surfaces; De Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Wasser, L.; Dalle Vacche, S.; Karasu, F.; Müller, L.; Castellino, M.; Vitale, A.; Bongiovanni, R.; Leterrier, Y. Bio-Inspired Fluorine-Free Self-Cleaning Polymer Coatings. Coatings 2018, 8, 436. [Google Scholar] [CrossRef]
- Brown, P.S.; Talbot, E.L.; Wood, T.J.; Bain, C.D.; Badyal, J.P.S. Superhydrophobic Hierarchical Honeycomb Surfaces. Langmuir 2012, 28, 13712–13719. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnology 2017, 15, 64. [Google Scholar] [CrossRef]
- Richert, L.; Vetrone, F.; Yi, J.; Zalzal, S.; Wuest, J.; Rosei, F.; Nanci, A. Surface nanopatterning to control cell growth. Adv. Mater. 2008, 20, 1488–1492. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, X.; Dan, Y.; Moon, J.H.; Chen, V.W.; Johnson, A.T.; Perry, J.W.; Yang, S. Electrodeposition of Three-Dimensional Titania Photonic Crystals from Holographically Patterned Microporous Polymer Templates. Chem. Mater. 2008, 20, 1816–1823. [Google Scholar] [CrossRef]
- Kyu, T.; Nwabunma, D. Simulations of Microlens Arrays Formed by Pattern-Photopolymerization-Induced Phase Separation of Liquid Crystal/Monomer Mixtures. Macromolecules 2001, 34, 9168–9172. [Google Scholar] [CrossRef]
- De Leon, A.; Garnier, T.; Jierry, L.; Boulmedais, F.; Munoz-Bonilla, A.; Rodriguez-Hernandez, J. Enzymatic Catalysis Combining the Breath Figures and Layer-by-Layer Techniques: Toward the Design of Microreactors. Acs Appl. Mater. Interfaces 2015, 7, 12210–12219. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Li, J.; Zhang, A.; Liu, X.; Xu, B.; Gao, S.; Jin, G.; Ma, Z. Robust and hydrophilic polymeric films with honeycomb pattern and their cell scaffold applications. J. Mater. Chem. 2009, 19, 2789–2796. [Google Scholar] [CrossRef]
- Ma, Z.; Mao, Z.; Gao, C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf. B-Biointerfaces 2007, 60, 137–157. [Google Scholar] [CrossRef]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef]
- Ko, T.; Kim, E.; Nagashima, S.; Oh, K.; Lee, K.; Kim, S.; Moon, M. Adhesion behavior of mouse liver cancer cells on nanostructured superhydrophobic and superhydrophilic surfaces. Soft Matter 2013, 9, 8705–8711. [Google Scholar] [CrossRef]
- DeRosa, M.E.; Yulong, H.; Faris, R.A.; Hongwei, R. Microtextured polystyrene surfaces for three-dimensional cell culture made by a simple solvent treatment method. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Matsuzaka, K.; Walboomers, X.; Yoshinari, M.; Inoue, T.; Jansen, J. The attachment and growth behavior of osteoblast-like cells on microtextured surfaces. Biomaterials 2003, 24, 2711–2719. [Google Scholar] [CrossRef]
- Martinez, E.; Engel, E.; Planell, J.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat.—Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef]
- Lucchetta, G.; Sorgato, M.; Zanchetta, E.; Brusatin, G.; Guidi, E.; Di Liddo, R.; Conconi, M. Effect of injection molded micro-structured polystyrene surfaces on proliferation of MC3T3-E1 cells. Express Polym. Lett. 2015, 9, 354–361. [Google Scholar] [CrossRef]
- Jeon, H.; Simon, C.G.; Kim, G. A mini-review: Cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J. Biomed. Mater. Res. Part B 2014, 102, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Papenburg, B.J.; Vogelaar, L.; Bolhuis-Versteeg, L.A.M.; Lammertink, R.G.H.; Stamatialis, D.; Wessling, M. One-step fabrication of porous micropatterned scaffolds to control cell behavior. Biomaterials 2007, 28, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Gerecht, S.; Bettinger, C.J.; Zhang, Z.; Borenstein, J.T.; Vuniak-Novakovic, G.; Langer, R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials 2007, 28, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, A.; Tremblay, D.; Hadjiantoniou, S.; Bukoreshtliev, N.V.; Rogowski, J.L.; Godin, M.; Pelling, A.E. Three dimensional spatial separation of cells in response to microtopography. Biomaterials 2013, 34, 8097–8104. [Google Scholar] [CrossRef]
- Connal, L.A.; Qiao, G.G. Preparation of Porous Poly(dimethylsiloxane)-Based Honeycomb Materials with Hierarchal Surface Features and Their Use as Soft-Lithography Templates. Adv. Mater. 2006, 18, 3024–3028. [Google Scholar] [CrossRef]
- Galeotti, F.; Andicsova, A.; Yunus, S.; Botta, C. Precise surface patterning of silk fibroin films by breath figures. Soft Matter 2012, 8, 4815–4821. [Google Scholar] [CrossRef]
- Tanaka, H. Formation of Network and Cellular Structures by Viscoelastic Phase Separation. Adv. Mater. 2009, 21, 1872–1880. [Google Scholar] [CrossRef]
- Xue, L.J.; Zhang, J.L.; Han, Y.C. Phase separation induced ordered patterns in thin polymer blend films. Prog. Polym. Sci. 2012, 37, 564–594. [Google Scholar] [CrossRef]
- Sun, N.; Chen, J.; Jiang, C.; Zhang, Y.; Shi, F. Enhanced Wet-Chemical Etching To Prepare Patterned Silicon Mask with Controlled Depths by Combining Photolithography with Galvanic Reaction. Ind. Eng. Chem. Res. 2012, 51, 788–794. [Google Scholar] [CrossRef]
- Zhai, W.T.; Feng, W.W.; Ling, J.Q.; Zheng, W.G. Fabrication of Lightweight Microcellular Polyimide Foams with Three-Dimensional Shape by CO2 Foaming and Compression Molding. Ind. Eng. Chem. Res. 2012, 51, 12827–12834. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Wrzecionko, E.; Minarik, A.; Smolka, P.; Minarik, M.; Humpolicek, P.; Rejmontova, P.; Mracek, A.; Minarikova, M.; Grundelova, L. Variations of Polymer Porous Surface Structures via the Time Sequenced Dosing of Mixed Solvents. Acs Appl. Mater. Interfaces 2017, 9, 6472–6481. [Google Scholar] [CrossRef] [PubMed]
- Bunz, U.H.F. Breath Figures as a Dynamic Templating Method for Polymers and Nanomaterials. Adv. Mater. 2006, 18, 973–989. [Google Scholar] [CrossRef]
- Tsay, C.S.; McHugh, A.J. Mass transfer modeling of asymmetric membrane formation by phase inversion. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1327–1365. [Google Scholar] [CrossRef]
- Kimmerle, K.; Strathmann, H. Analysis of the structure-determining process of phase inversion membranes. Desalination 1990, 79, 283–302. [Google Scholar] [CrossRef]
- Altinkaya, S.A.; Ozbas, B. Modeling of asymmetric membrane formation by dry-casting method. J. Membr. Sci. 2004, 230, 71–89. [Google Scholar] [CrossRef][Green Version]
- Matsuzaka, K.; Jinnai, H.; Koga, T.; Hashimoto, T. Effect of Oscillatory Shear Deformation on Demixing Processes of Polymer Blends. Macromolecules 1997, 30, 1146–1152. [Google Scholar] [CrossRef]
- Xuyun, W.; Lin, Z.; Dahai, S.; Quanfu, A.; Huanlin, C. Effect of coagulation bath temperature on formation mechanism of poly(vinylidene fluoride) membrane. J. Appl. Polym. Sci. 2008, 110, 1656–1663. [Google Scholar]
- Li, W.; Ryan, A.J.; Meier, I.K. Morphology Development via Reaction-Induced Phase Separation in Flexible Polyurethane Foam. Macromolecules 2002, 35, 5034–5042. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Samuel, A.; Umapathy, S.; Ramakrishnan, S. Functionalized and Postfunctionalizable Porous Polymeric Films through Evaporation-Induced Phase Separation Using Mixed Solvents. Acs Appl. Mater. Interfaces 2011, 3, 3293–3299. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H.; Kock, K. The formation mechanism of phase inversion membranes. Desalination 1977, 21, 241–255. [Google Scholar] [CrossRef]
- Farnaz, F.; Behzad, P.; Mehdi, S. Direct breath figure formation on PMMA and superhydrophobic surface using in situ perfluoro-modified silica nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 441–451. [Google Scholar]
- Temenoff, J.S.; Mikos, A.G. Biomaterials: The Intersection of Biology and Materials Science; Pearson Prentice Hall: Upper Saddle River, NJ, USA; London, UK, 2008. [Google Scholar]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Bui, V.; Ko, S.; Choi, H. Large-Scale Fabrication of Commercially Available, Nonpolar Linear Polymer Film with a Highly Ordered Honeycomb Pattern. Acs Appl. Mater. Interfaces 2015, 7, 10541–10547. [Google Scholar] [CrossRef]
- Li, J.; Peng, J.; Huang, W.; Wu, Y.; Fu, J.; Cong, Y.; Xue, L.; Han, Y. Ordered honeycomb-structured gold nanoparticle films with changeable pore morphology: From circle to ellipse. Langmuir 2005, 21, 2017–2021. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.-S. Molecular elucidation of morphology and mechanical properties of PVDF hollow fiber membranes from aspects of phase inversion, crystallization and rheology. J. Membr. Sci. 2009, 340, 192–205. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, K.-H. Effect of PEG additive on membrane formation by phase inversion. J. Membr. Sci. 1998, 138, 153–163. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Samusenko, I.; Zorin, I.; Kulthareva, L.; Bilibin, A.; Blinova, M. Poly(D,L-lactide)/PEG blend films for keratinocyte cultivation and skin reconstruction. Biomed. Mater. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Jhala, D.; Vasita, R. A Review on Extracellular Matrix Mimicking Strategies for an Artificial Stem Cell Niche. Polym. Rev. 2015, 55, 561–595. [Google Scholar] [CrossRef]
- Kawano, T.; Sato, M.; Yabu, H.; Shimomura, M. Honeycomb-shaped surface topography induces differentiation of human mesenchymal stem cells (hMSCs): Uniform porous polymer scaffolds prepared by the breath figure technique. Biomater. Sci. 2014, 2, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Jones, S.R.; Yousaf, M.N. Geometric Control of Stem Cell Differentiation Rate on Surfaces. Langmuir 2008, 24, 12129–12133. [Google Scholar] [CrossRef] [PubMed]
- Ankam, S.; Suryana, M.; Chan, L.Y.; Moe, A.A.K.; Teo, B.K.K.; Law, J.B.K.; Sheetz, M.P.; Low, H.Y.; Yim, E.K.F. Substrate topography and size determine the fate of human embryonic stem cells to neuronal or glial lineage. Acta Biomater. 2013, 9, 4535–4545. [Google Scholar] [CrossRef] [PubMed]
- Markert, L.D.; Lovmand, J.; Foss, M.; Lauridsen, R.H.; Lovmand, M.; Fuchtbauer, E.M.; Fuchtbauer, A.; Wertz, K.; Besenbacher, F.; Pedersen, F.S.; et al. Identification of Distinct Topographical Surface Microstructures Favoring Either Undifferentiated Expansion or Differentiation of Murine Embryonic Stem Cells. Stem Cells Dev. 2009, 18, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Minarik, M.; Wrzecionko, E.; Minarik, A.; Grulich, O.; Smolka, P.; Musilova, L.; Junkar, I.; Primc, G.; Ptoskova, B.; Mozetic, M.; et al. Preparation of Hierarchically Structured Polystyrene Surfaces with Superhydrophobic Properties by Plasma-Assisted Fluorination. Coatings 2019, 9, 201. [Google Scholar] [CrossRef]
- Minarik, A.; Rafajova, M.; Rajnohova, E.; Smolka, P.; Mracek, A. Self-organised patterns in polymeric films solidified from diluted solutions—The effect of the substrate surface properties. Int. J. Heat Mass Transf. 2014, 78, 615–623. [Google Scholar] [CrossRef]
- Chvatalova, L.; Cermak, R.; Mracek, A.; Grulich, O.; Vesel, A.; Ponizil, P.; Minarik, A.; Cvelbar, U.; Benicek, L.; Sajdl, P. The effect of plasma treatment on structure and properties of poly(1-butene) surface. Eur. Polym. J. 2012, 48, 866–874. [Google Scholar] [CrossRef]
- Larrieu, J.; Held, B.; Martinez, H.; Tison, Y. Ageing of atactic and isotactic polystyrene thin films treated by oxygen DC pulsed plasma. Surf. Coat. Technol. 2005, 200, 2310–2316. [Google Scholar] [CrossRef]
- Nagy, A.; Rossant, J.; Nagy, R.; Abramownewerly, W.; Roder, J.C. Derivation of completely cell culture-derived mice from early-passage embryonic stem-cells. Proc. Natl. Acad. Sci. USA 1993, 90, 8424–8428. [Google Scholar] [CrossRef]


| PS Sample | Before Plasma Treatment [°] | After Plasma Treatment [°] | After Plasma Treatment and Application of the Cell Culture Media [°] |
|---|---|---|---|
| Flat | 71 ± 1 | * | * |
| Micro | 104 ± 3 | 28 ± 7 | 23 ± 3 |
| Meso/Micro | 115 ± 8 | 27 ± 2 | 24 ± 4 |
| Macro/Micro | 121 ± 2 | 25 ± 2 | 28 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadlečková, M.; Skopalová, K.; Ptošková, B.; Wrzecionko, E.; Daďová, E.; Kocourková, K.; Mráček, A.; Musilová, L.; Smolka, P.; Humpolíček, P.; et al. Hierarchically Structured Surfaces Prepared by Phase Separation: Tissue Mimicking Culture Substrate. Int. J. Mol. Sci. 2022, 23, 2541. https://doi.org/10.3390/ijms23052541
Kadlečková M, Skopalová K, Ptošková B, Wrzecionko E, Daďová E, Kocourková K, Mráček A, Musilová L, Smolka P, Humpolíček P, et al. Hierarchically Structured Surfaces Prepared by Phase Separation: Tissue Mimicking Culture Substrate. International Journal of Molecular Sciences. 2022; 23(5):2541. https://doi.org/10.3390/ijms23052541
Chicago/Turabian StyleKadlečková, Markéta, Kateřina Skopalová, Barbora Ptošková, Erik Wrzecionko, Eliška Daďová, Karolína Kocourková, Aleš Mráček, Lenka Musilová, Petr Smolka, Petr Humpolíček, and et al. 2022. "Hierarchically Structured Surfaces Prepared by Phase Separation: Tissue Mimicking Culture Substrate" International Journal of Molecular Sciences 23, no. 5: 2541. https://doi.org/10.3390/ijms23052541
APA StyleKadlečková, M., Skopalová, K., Ptošková, B., Wrzecionko, E., Daďová, E., Kocourková, K., Mráček, A., Musilová, L., Smolka, P., Humpolíček, P., & Minařík, A. (2022). Hierarchically Structured Surfaces Prepared by Phase Separation: Tissue Mimicking Culture Substrate. International Journal of Molecular Sciences, 23(5), 2541. https://doi.org/10.3390/ijms23052541









