Altered p16Ink4a, IL-1β, and Lamin b1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Age and Body Mass Index Do Not Correlate with Senescence Markers
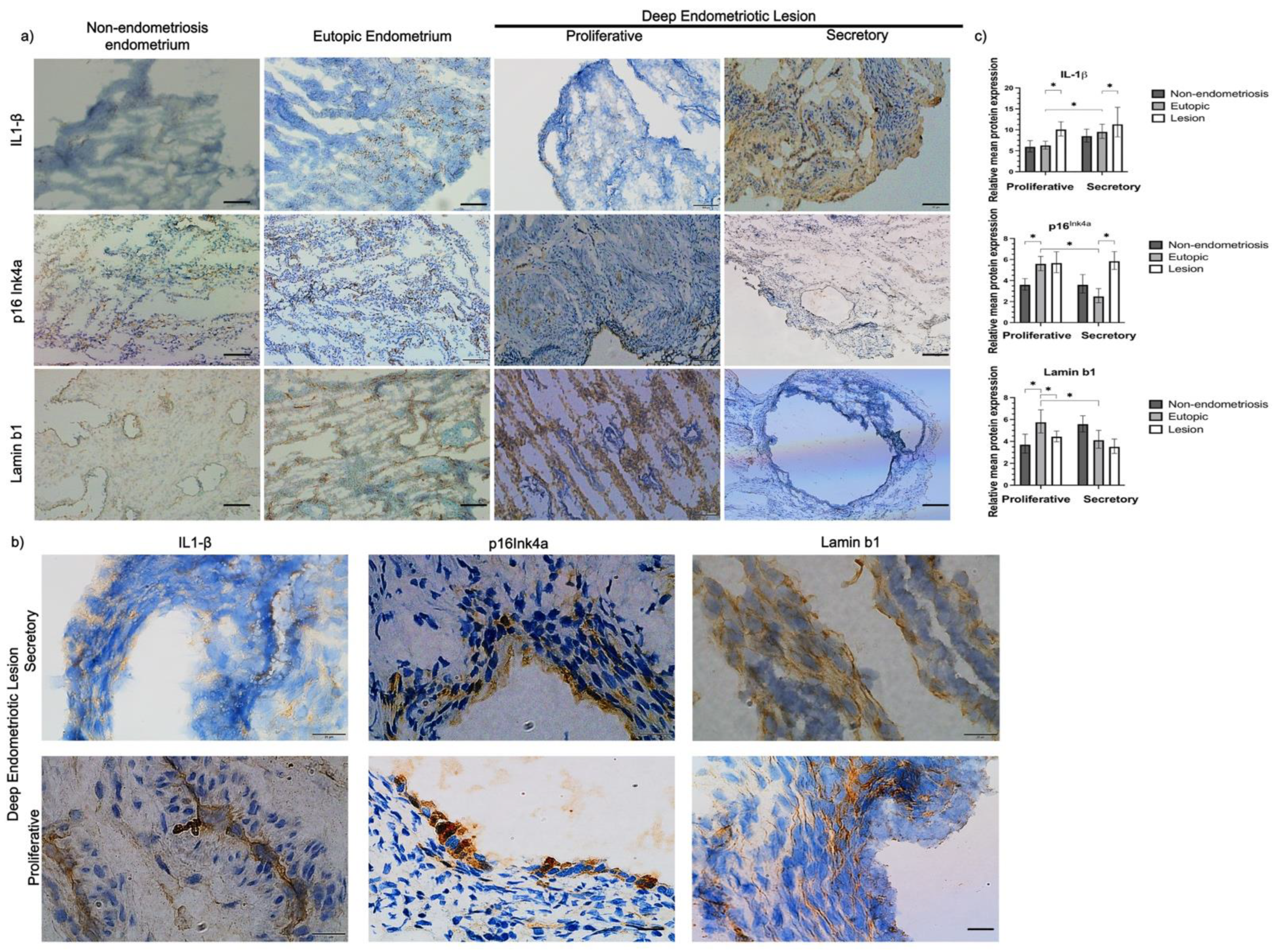
2.3. Increased Expression of Senescence Markers in Endometriosis Lesions Compared to Eutopic Endometrium
2.4. p16Ink4a and Lamin b1 Are Expressed in Both Glandular and Non-Glandular Epithelial Cells
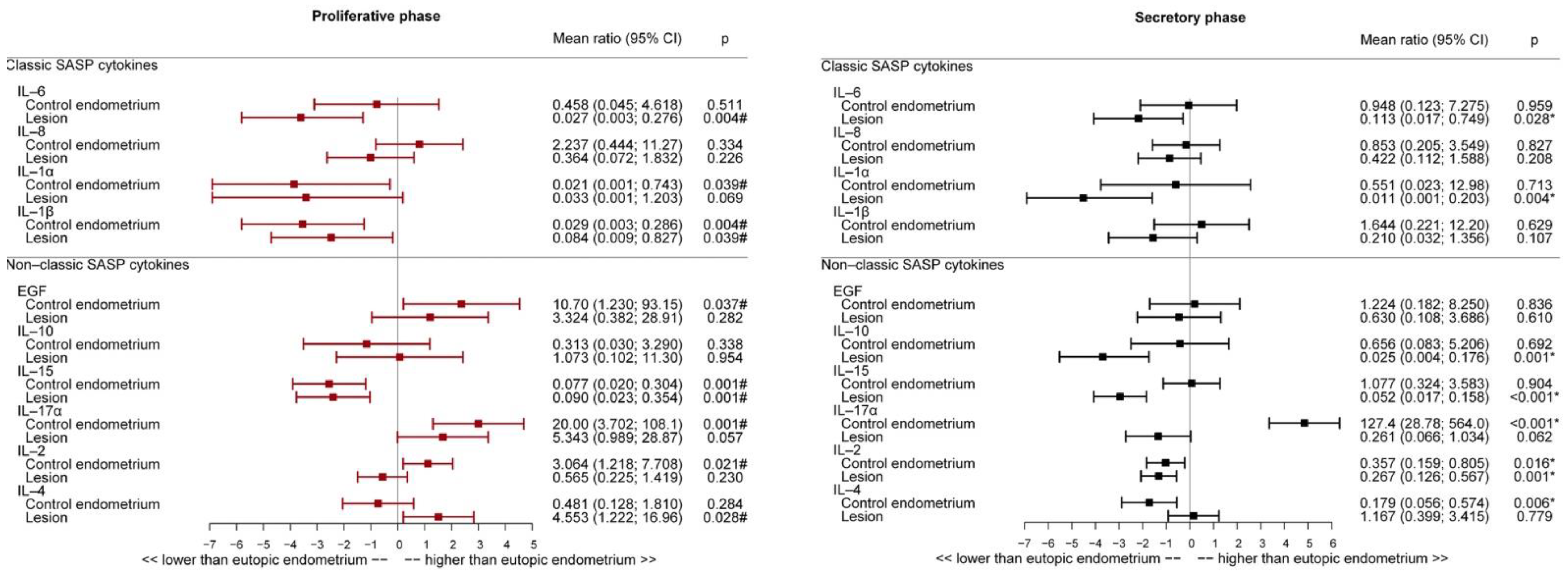
2.5. Inflammatory Cytokine Expression in Endometriosis Lesions Was Lower Than That in Eutopic Endometrium in the Secretory and Proliferative Phases
2.6. Interleukin Expression Profile in Eutopic and Non-Endometriosis Endometrium
2.7. Correlation between Cytokines and Senescence Markers in Eutopic Endometrium and Endometriosis Lesions in the Proliferative and Secretory Phases
3. Discussion
4. Materials and Methods
4.1. Patients and Ethics
4.2. Study Design
4.3. Immunohistochemistry
4.4. Immunofluorescence
4.5. SASP
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dutta, M.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Intrafollicular interleukin-8, interleukin-12, and adrenomedullin are the promising prognostic markers of oocyte and embryo quality in women with endometriosis. J. Assist. Reprod Genet. 2016, 33, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.R.; Marissen, L.M.; Scherjon, S.A.; Hoek, A.; Cantineau, A.E.P. Is there an immune modulating role for follicular fluid in endometriosis? A narrative review. Reproduction 2020, 159, R45–R54. [Google Scholar] [CrossRef]
- Guo, M.; Bafligil, C.; Tapmeier, T.; Hubbard, C.; Manek, S.; Shang, C.; Martinez, F.O.; Schmidt, N.; Obendorf, M.; Hess-Stumpp, H.; et al. Mass cytometry analysis reveals a distinct immune environment in peritoneal fluid in endometriosis: A characterisation study. BMC Med. 2020, 18, 1–16. [Google Scholar] [CrossRef]
- Dorien, F.O.; Roskams, T.; Van Den Eynde, K.; Vanhie, A.; Peterse, D.P.; Meuleman, C.; Tomassetti, C.; Peeraer, K.; D’Hooghe, T.M.; Fassbender, A. The Presence of Endometrial Cells in Peritoneal Fluid of Women with and Without Endometriosis. Reprod. Sci. 2017, 24, 242–251. [Google Scholar] [CrossRef]
- Bellelis, P.; Barbeiro, D.F.; Gueuvoghlanian-Silva, B.Y.; Kalil, J.; Abrão, M.S.; Podgaec, S. Interleukin-15 and Interleukin-7 are the Major Cytokines to Maintain Endometriosis. Gynecol. Obstet. Investig. 2019, 84, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, C.; Gueuvoghlanian-Silva, B.Y.; Monnaka, V.U.; Ribeiro, N.M.; Pereira, W.d.O.; Podgaec, S. Regulatory T cells isolated from endometriotic peritoneal fluid express a different number of Toll-like receptors. Einstein 2020, 18, eAO5294. [Google Scholar] [CrossRef]
- Hey-Cunningham, A.J.; Peters, K.M.; Zevallos, H.B.-V.; Berbic, M.; Markham, R.; Fraser, I.S. Angiogenesis, lymphangiogenesis and neurogenesis in endometriosis. Front. Biosci. (Elite Ed.) 2013, 5, 1033–1056. [Google Scholar] [CrossRef]
- García Manero, M.; Olartecoechea, B.; Aubá, M.; Alcázar, J.L.; López, G. Angiogenesis and endometriosis. Rev. Med. La Univ. Navar. 2009, 53, 8–13. [Google Scholar]
- Klemmt, P.A.B.; Carver, J.G.; Koninckx, P.; McVeigh, E.J.; Mardon, H.J. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: Towards a mechanistic model for endometriosis progression. Hum. Reprod. 2007, 22, 3139–3147. [Google Scholar] [CrossRef]
- Králíčková, M.; Losan, P.; Vetvicka, V. Endometriosis and cancer. Womens Health 2014, 10, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Pavone, M.E.; Lyttle, B.M. Endometriosis and ovarian cancer: Links, risks, and challenges faced. Int. J. Womens Health 2015, 7, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, D.; Cosson, M.; Dufour, P. Is endometriosis an endometrial disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 91, 113–125. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, B.; Yu, J.J.; Wei, C.Y.; Zhou, W.J.; Chang, K.K.; Yang, H.L.; Jin, L.P.; Zhu, X.Y.; Li, M.Q. Macrophages promote the growth and invasion of endometrial stromal cells by downregulating IL-24 in endometriosis. Reproduction 2016, 152, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Thiruchelvam, U.; Wingfield, M.; O’Farrelly, C. Natural Killer Cells: Key Players in Endometriosis. Am. J. Reprod. Immunol. 2015, 74, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.A., Jr.; Podgaec, S.; de Oliveira, R.M.; Carnevale Marin, M.L.; Baracat, E.C.; Abrão, M.S. Patients with Endometriosis of the Rectosigmoid Have a Higher Percentage of Natural Killer Cells in Peripheral Blood. J. Minim. Invasive Gynecol. 2012, 19, 317–324. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lai, M.D.; Lei, H.Y.; Wing, L.Y.C. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology 2006, 147, 1278–1286. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Abrão, M.S.; Mechsner, S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum. Reprod. 2014, 29, 253–266. [Google Scholar] [CrossRef]
- Saretzki, G. Cellular Senescence in the Development and Treatment of Cancer. Curr. Pharm. Des. 2010, 16, 79–100. [Google Scholar] [CrossRef]
- Lasry, A.; Ben-Neriah, Y. Senescence-associated inflammatory responses: Aging and cancer perspectives. Trends Immunol. 2015, 36, 217–228. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef]
- Dutta, E.H.; Behnia, F.; Boldogh, I.; Saade, G.R.; Taylor, B.D.; Kacerovský, M.; Menon, R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol. Hum. Reprod. 2015, 22, 143–157. [Google Scholar] [CrossRef]
- Wu, G.; Bersinger, N.A.; Mueller, M.D.; von Wolff, M. Intrafollicular inflammatory cytokines but not steroid hormone concentrations are increased in naturally matured follicles of women with proven endometriosis. J. Assist. Reprod. Genet. 2017, 34, 357–364. [Google Scholar] [CrossRef]
- Malvezzi, H.; Viana, B.G.; Dobo, C.; Filippi, R.Z.; Podgaec, S.; Piccinato, C.A. Depleted lamin B1: A possible marker of the involvement of senescence in endometriosis? Arch. Gynecol. Obstet. 2018, 297, 977–984. [Google Scholar] [CrossRef]
- Ohtani, N.; Hara, E. Roles and mechanisms of cellular senescence in regulation of tissue homeostasis. Cancer Sci. 2013, 104, 525–530. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Malvezzi, H.; Hernandes, C.; Piccinato, C.A.; Podgaec, S. Interleukin in endometriosis-associated infertility-pelvic pain: Systematic review and meta-analysis. Reproduction 2019, 158, 1–12. [Google Scholar] [CrossRef]
- Dodig, S.; Čepelak, I.; Pavić, I. Hallmarks of senescence and aging. Biochem. Med. 2019, 29, 1–15. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer. 2015, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Dürr, P.; Wlaschek, M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef]
- Wang, A.S.; Ong, P.F.; Chojnowski, A.; Clavel, C.; Dreesen, O. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Lau, L.; Porciuncula, A.; Yu, A.; Iwakura, Y.; David, G. Uncoupling the Senescence-Associated Secretory Phenotype from Cell Cycle Exit via Interleukin-1 Inactivation Unveils Its Protumorigenic Role. Mol. Cell Biol. 2019, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- González-Puertos, V.Y.; Maciel-Barón, L.Á.; Barajas-Gómez, B.A.; López-Diazguerrero, N.E.; Diazguerrero, M. Senescence-Associated Secretory Phenotype (SASP) involvement in the development of cancer, aging and age related diseases. Gac. Médica México 2015, 151, 460–468. [Google Scholar]
- Wiggins, K.A.; Parry, A.J.; Cassidy, L.D.; Humphry, M.; Webster, S.J.; Goodall, J.C.; Narita, M.; Clarke, M.C.H. IL-1α cleavage by inflammatory caspases of the noncanonical inflammasome controls the senescence-associated secretory phenotype. Aging Cell 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef]
- Liu, J.Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; Sharpless, N.E. Cells exhibiting strong p16 INK4a promoter activation in vivo display features of senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, B.D.; Gilbert, P.M.; Porpiglia, E.; Mourkioti, F.; Lee, S.P.; Corbel, S.Y.; Llewellyn, M.E.; Delp, S.L.; Blau, H.M. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 2014, 20, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Johnson, S.M.; Fedoriw, Y.; Rogers, A.B.; Yuan, H.; Krishnamurthy, J.; Sharpless, N.E. Expression of p16INK4a prevents cancer and promotes aging in lymphocytes. Blood 2011, 117, 3257–3267. [Google Scholar] [CrossRef]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Yoon, N.; Yoon, G.; Park, C.K.; Kim, H.-S. Stromal p16 expression is significantly increased in malignant ovarian neoplasms. Oncotarget 2016, 4, 64665–64673. [Google Scholar] [CrossRef] [PubMed]
- Moritani, S.; Ichihara, S.; Hasegawa, M.; Iwakoshi, A.; Murakami, S.; Sato, T.; Okamoto, T.; Mori, Y.; Kuhara, H.; Silverberg, S.G. Stromal p16 expression differentiates endometrial polyp from endometrial hyperplasia. Virchows Arch. 2012, 461, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Koh, C.W.; Yoon, N.; Kim, J.Y.; Kim, H.S. Stromal p16 expression is significantly increased in endometrial carcinoma. Oncotarget 2017, 8, 4826–4836. [Google Scholar] [CrossRef]
- Yu, C.X.; Song, J.H.; Li, Y.F.; Tuo, Y.; Zheng, J.J.; Miao, R.J.; Wang, F.; Liu, J.; Liang, L. Correlation between replicative senescence of endometrial gland epithelial cells in shedding and non-shedding endometria and endometriosis cyst during menstruation. Gynecol Endocrinol. 2018, 34, 981–986. [Google Scholar] [CrossRef]
- Parvanov, D.; Ganeva, R.; Vidolova, N.; Stamenov, G. Decreased number of p16-positive senescent cells in human endometrium as a marker of miscarriage. J. Assist. Reprod. Genet. 2021, 38, 2087–2095. [Google Scholar] [CrossRef]
- Hapangama, D.K.; Kamal, A.; Saretzki, G. Implications of telomeres and telomerase in endometrial pathology. Hum. Reprod. Update 2017, 23, 166–187. [Google Scholar] [CrossRef]
- Saito, M.; Nakagawa, K.; Hamada, K.; Hirose, S.; Harada, H.; Kohno, S.; Nagato, S.; Ohnishi, T. Introduction of p16INK4a inhibits telomerase activity through transcriptional suppression of human telomerase reverse transcriptase expression in human gliomas. Int. J. Oncol. 2004, 24, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Solovei, I.; Wang, A.S.; Thanisch, K.; Schmidt, C.S.; Krebs, S.; Zwerger, M.; Cohen, T.V.; Devys, D.; Foisner, R.; Peichl, L.; et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 2013, 152, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Donahue, G.; Otte, G.L.; Capell, B.C.; Nelson, D.M.; Cao, K.; Aggarwala, V.; Cruickshanks, H.A.; Rai, T.S.; McBryan, T.; et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, O.; Ong, P.F.; Chojnowski, A.; Colman, A. The contrasting roles of lamin B1 in cellular aging and human disease. Nucleus 2013, 4, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, O.; Chojnowski, A.; Ong, P.F.; Zhao, T.Y.; Common, J.E.; Lunny, D.; Lane, E.B.; Lee, S.J.; Vardy, L.A.; Stewart, C.L.; et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 2013, 200, 605–617. [Google Scholar] [CrossRef]
- En, A.; Takauji, Y.; Ayusawa, D.; Fujii, M. The role of lamin B receptor in the regulation of senescence-associated secretory phenotype (SASP). Exp. Cell Res. 2020, 390, 111927. [Google Scholar] [CrossRef]
- Dalle Pezze, P.; Nelson, G.; Otten, E.G.; Korolchuk, V.I.; Kirkwood, T.B.L.; von Zglinicki, T.; Shanley, D.P. Dynamic Modelling of Pathways to Cellular Senescence Reveals Strategies for Targeted Interventions. PLoS Comput. Biol. 2014, 10, e1003728. [Google Scholar] [CrossRef]
- Fitzgerald, H.C.; Schust, D.J.; Spencer, T.E. In vitro models of the human endometrium: Evolution and application for women’s health. Biol. Reprod. 2021, 104, 282–293. [Google Scholar] [CrossRef]
- Maybin, J.A.; Critchley, H.O.D. Menstrual physiology: Implications for endometrial pathology and beyond. Hum. Reprod. Update 2015, 21, 748–761. [Google Scholar] [CrossRef]
- Monsanto, S.P.; Edwards, A.K.; Zhou, J.; Nagarkatti, P.; Nagarkatti, M.; Young, S.L.; Lessey, B.A.; Tayade, C. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil. Steril. 2016, 105, 968–977. [Google Scholar] [CrossRef]
- Volpato, L.K.; Horewicz, V.V.; Bobinski, F.; Martins, D.F.; Piovezan, A.P. Annexin A1, FPR2/ALX, and inflammatory cytokine expression in peritoneal endometriosis. J. Reprod. Immunol. 2018, 129, 30–35. [Google Scholar] [CrossRef]
- Kato, T.; Yasuda, K.; Matsushita, K.; Ishii, K.J.; Hirota, S.; Yoshimoto, T.; Shibahara, H. Interleukin-1/-33 signaling pathways as therapeutic targets for endometriosis. Front. Immunol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, M.S.; Lim, H.X.; Cho, D.; Kim, T.S. IL-33-matured dendritic cells promote Th17 cell responses via IL-1β and IL-6. Cytokine 2017, 99, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.W.; van Diest, P.J.; Williams, R.; Gallagher, A.G. Do we see what we think we see? The complexities of morphological assessment. J. Pathol. 2009, 218, 285–291. [Google Scholar] [CrossRef]
- Enciso, F.B.; Crespo, L.P.; Acuña, J.C. Clasificación y nomenclatura de las alteraciones menstruales. Ginecol. Obstet. Mex. 2007, 75, 641–651. [Google Scholar]
- Dunstan, R.W.; Wharton, K.A., Jr.; Quigley, C.; Lowe, A. The use of immunohistochemistry for biomarker assessment—Can itcompete with other technologies? Toxicol. Pathol. 2011, 39, 988–1002, Erratum in Toxicol. Pathol. 2012, 40, 127. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.K.; Critchley, H.O.D.; Henderson, T.A.; Baird, D.T. Mifepristone-induced vaginal bleeding is associated with increased immunostaining for cyclooxygenase-2 and decrease in prostaglandin dehydrogenase in luteal phase endometrium. J. Clin. Endocrinol. Metab. 2002, 87, 5229–5234. [Google Scholar] [CrossRef][Green Version]
- Hapangama, D.K.; Turner, M.A.; Drury, J.A.; Quenby, S.; Saretzki, G.; Martin-Ruiz, C.; Von Zglinicki, T. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum. Reprod. 2008, 63, 711–713. [Google Scholar] [CrossRef]
- Wang, H.; Critchley, H.O.D.; Kelly, R.W.; Shen, D.; Baird, D.T. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol. Hum. Reprod. 1998, 4, 407–412. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; CRC Press: New York, NY, USA, 2016; 399p. [Google Scholar]

| Protein Expression (%) | MR (95% CI) | p-Value |
|---|---|---|
| IL-1β | ||
| Age (years) | 0.978 (0.925–1.034) | 0.436 |
| BMI (kg/m2) | 1.015 (0.958–1.075) | 0.618 |
| p16Ink4a | ||
| Age (years) | 0.969 (0.921–1.019) | 0.223 |
| BMI (kg/m2) | 1.020 (0.964–1.078) | 0.493 |
| Lamin b1 | ||
| Age (years) | 1.003 (0.965–1.042) | 0.893 |
| BMI (kg/m2) | 0.994 (0.937–1.055) | 0.841 |
| Secretory Phase | MR (95% CI) | p-Value |
|---|---|---|
| IL-1β | ||
| Non-endometriosis endometrium | 0.890 (0.669–1.185) | 0.426 |
| Lesion | 1.600 (1.206–2.122) | 0.001 * |
| p16Ink4a | ||
| Non-endometriosis endometrium | 1.158 (0.885–1.515) | 0.284 |
| Lesion | 1.567 (1.206–2.036) | 0.001 * |
| Lamin b1 | ||
| Non-endometriosis endometrium | 1.138 (0.898–1.442) | 0.286 |
| Lesion | 0.816 (0.648–1.028) | 0.084 |
| Proliferative Phase | MR (95% CI) | p-Value |
| IL-1β | ||
| Non-endometriosis endometrium | 1.042 (0.798–1.360) | 0.763 |
| Lesion | 1.720 (1.358–2.179) | <0.001 # |
| p16Ink4a | ||
| Non-endometriosis endometrium | 0.649 (0.496–0.849) | 0.002 # |
| Lesion | 1.182 (0.940–1.487) | 0.154 |
| Lamin b1 | ||
| Non-endometriosis endometrium | 0.645 (0.508–0.820) | <0.001 # |
| Lesion | 0.643 (0.523–0.791) | <0.001 # |
| Secretory Phase | MR (95% CI) | p-Value |
|---|---|---|
| p16Ink4a | ||
| IL-17A | 1.036 (1.003–1.069) | 0.034 * |
| Lamin b1 | ||
| IL-1A | 0.999 (0.998–1.000) | 0.039 * |
| IL-2 | 0.300 (0.101–0.893) | 0.035 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malvezzi, H.; Dobo, C.; Filippi, R.Z.; Mendes do Nascimento, H.; Palmieri da Silva e Sousa, L.; Meola, J.; Piccinato, C.A.; Podgaec, S. Altered p16Ink4a, IL-1β, and Lamin b1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions. Int. J. Mol. Sci. 2022, 23, 2476. https://doi.org/10.3390/ijms23052476
Malvezzi H, Dobo C, Filippi RZ, Mendes do Nascimento H, Palmieri da Silva e Sousa L, Meola J, Piccinato CA, Podgaec S. Altered p16Ink4a, IL-1β, and Lamin b1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions. International Journal of Molecular Sciences. 2022; 23(5):2476. https://doi.org/10.3390/ijms23052476
Chicago/Turabian StyleMalvezzi, Helena, Cristine Dobo, Renee Zon Filippi, Helen Mendes do Nascimento, Laura Palmieri da Silva e Sousa, Juliana Meola, Carla Azevedo Piccinato, and Sérgio Podgaec. 2022. "Altered p16Ink4a, IL-1β, and Lamin b1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions" International Journal of Molecular Sciences 23, no. 5: 2476. https://doi.org/10.3390/ijms23052476
APA StyleMalvezzi, H., Dobo, C., Filippi, R. Z., Mendes do Nascimento, H., Palmieri da Silva e Sousa, L., Meola, J., Piccinato, C. A., & Podgaec, S. (2022). Altered p16Ink4a, IL-1β, and Lamin b1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions. International Journal of Molecular Sciences, 23(5), 2476. https://doi.org/10.3390/ijms23052476







