Thermally Stable and Reusable Ceramic Encapsulated and Cross-Linked CalB Enzyme Particles for Rapid Hydrolysis and Esterification
Abstract
:1. Introduction
2. Results and Discussion
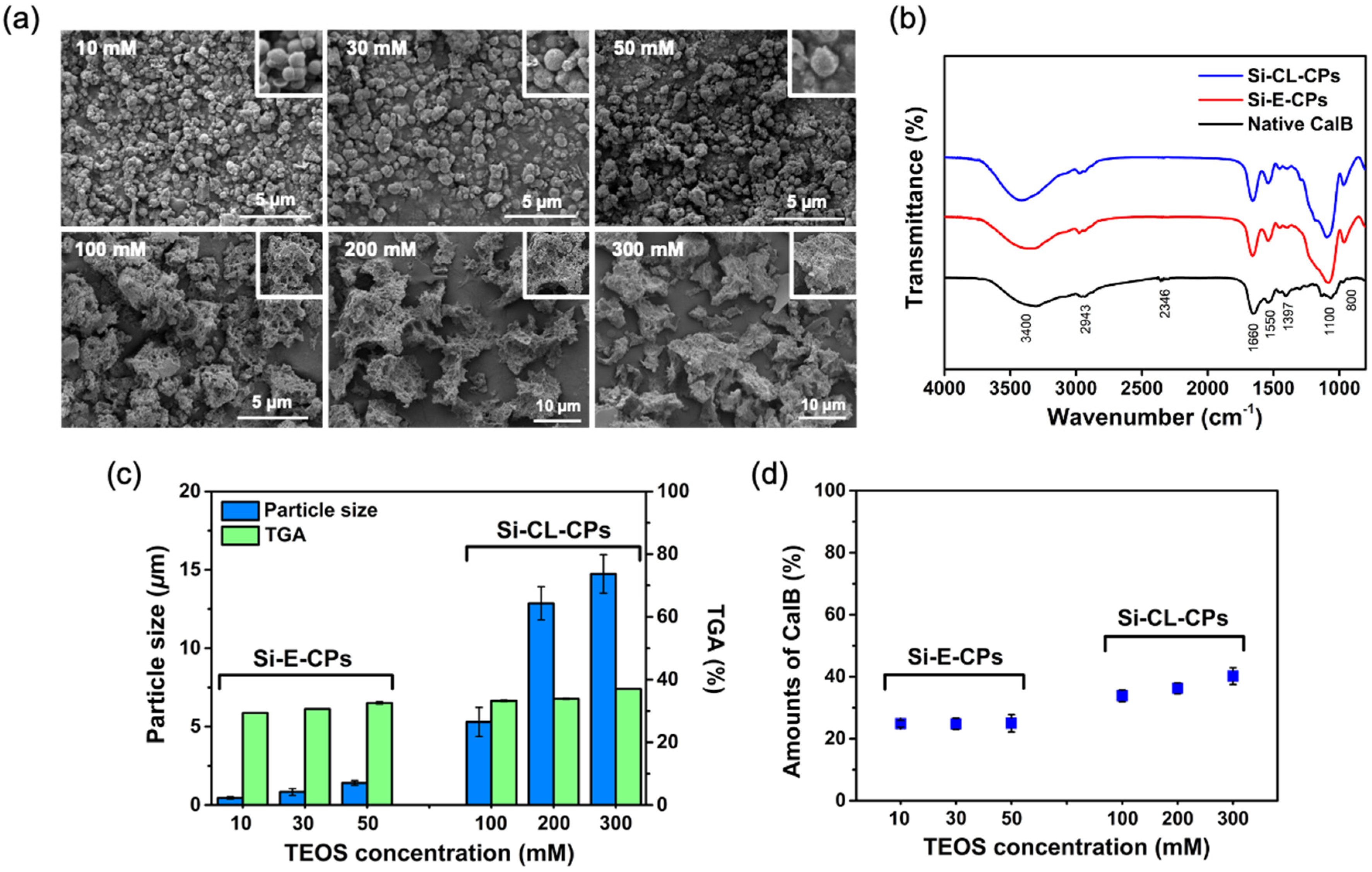
2.1. Preparation and Characterization of Si-E-CPs and Si-CL-CPs
2.2. Thermal Stability, pH Activity, and Reusability of Si-E-CPs and Si-CL-CPs
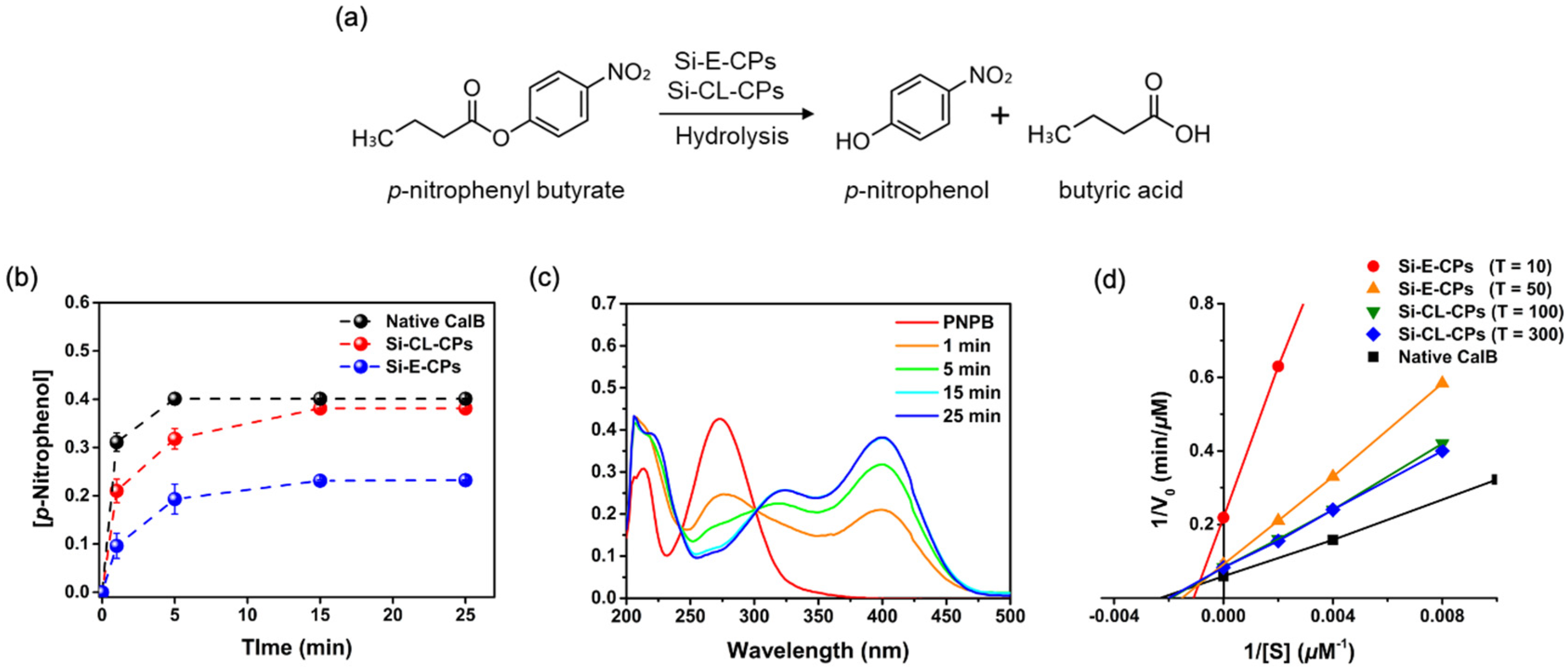
2.3. Enzymatic Hydrolysis for p-Nitrophenyl Butyrate
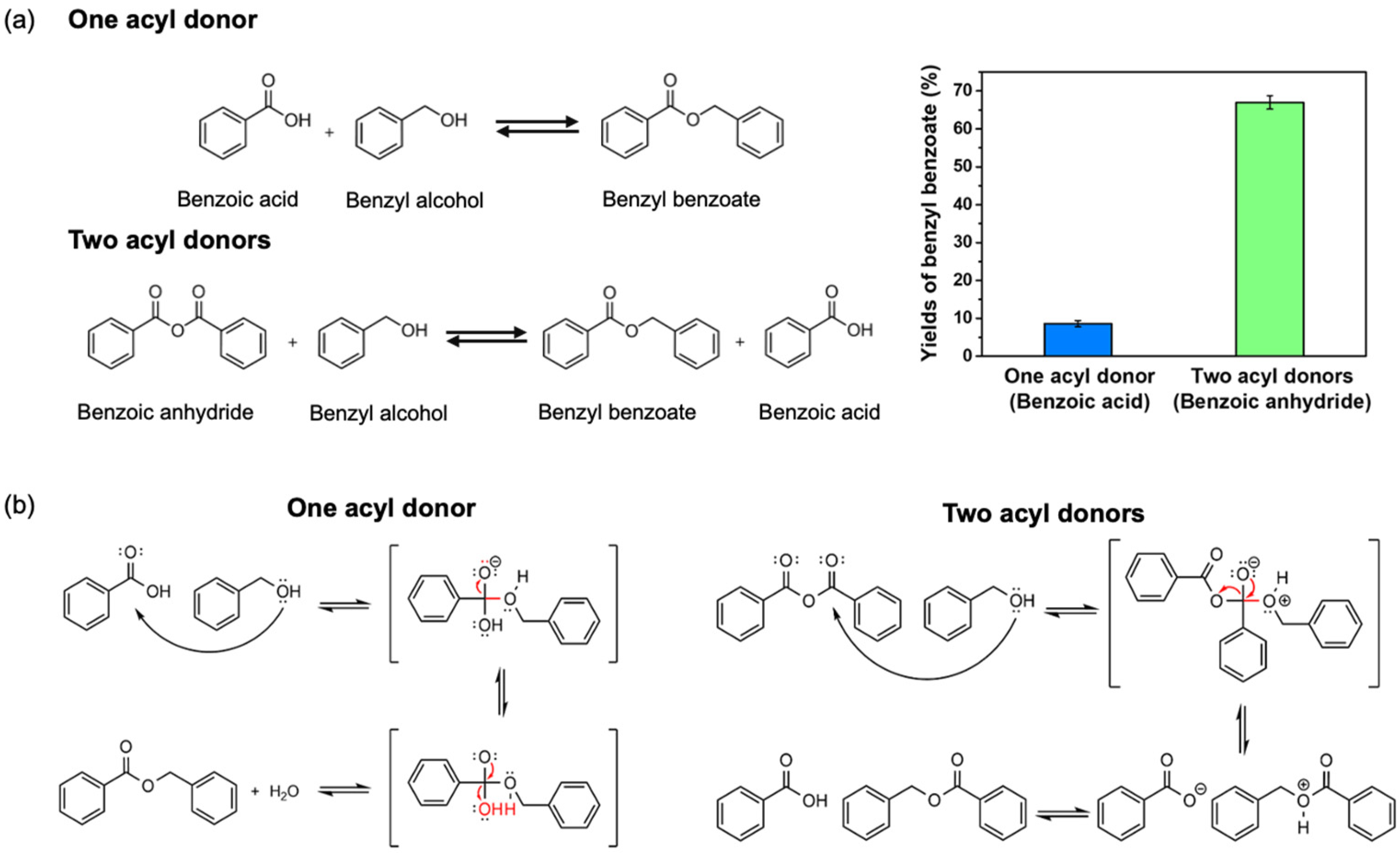
2.4. Enzymatic Esterification of Benzyl Benzoate with Si-CL-CPs
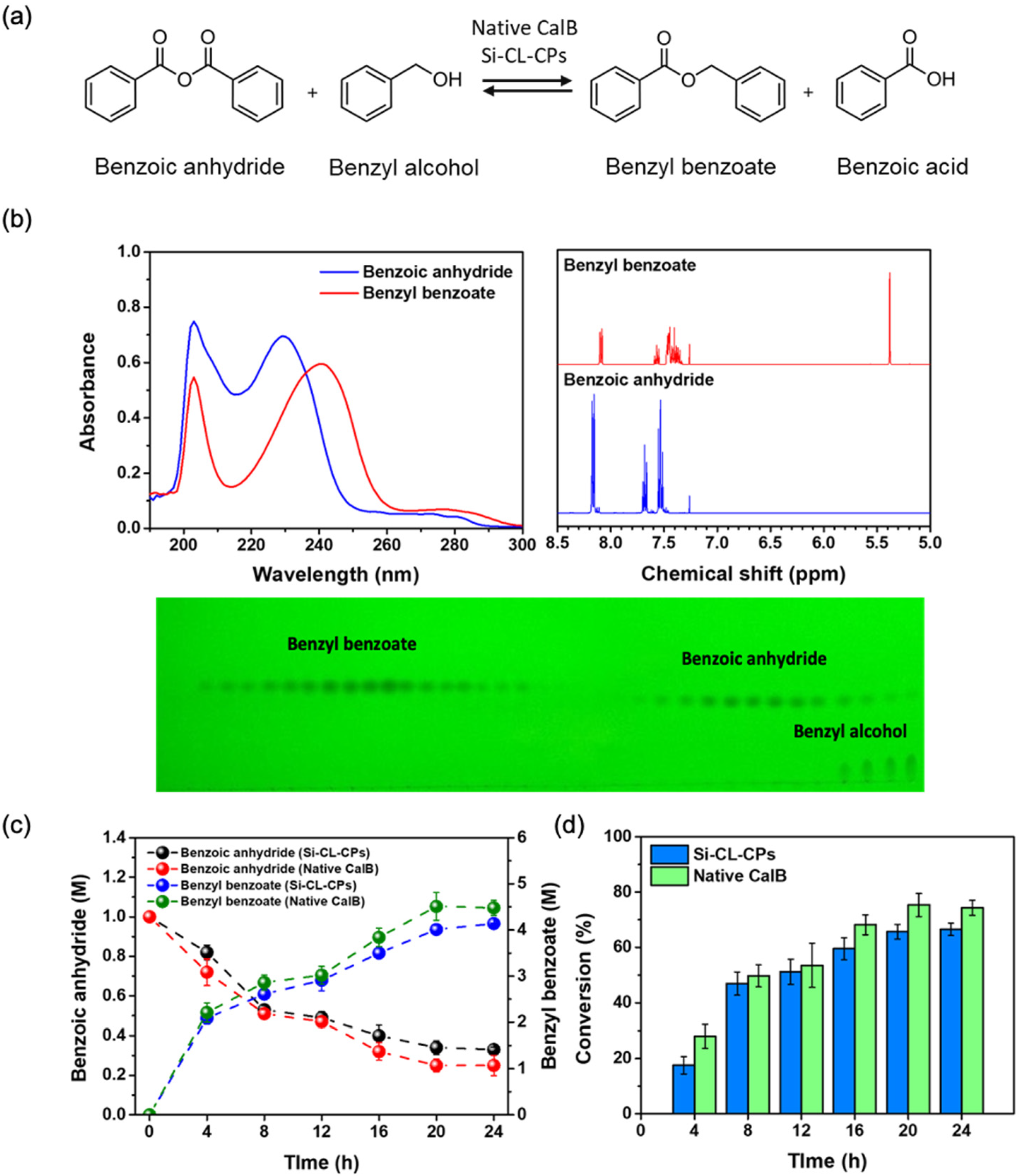
2.5. Conversion of Benzyl Benzoate from Benzoic Anhydride with Native CalB and Si-CL-CPs
3. Materials and Methods
3.1. Materials
3.2. Preparation of Si-E-CPs and Si-CL-CPs
3.3. Determination of CalB Enzyme Amounts in Si-E-CPs and Si-CL-CPs
3.4. Thermal Stability and pH Activity of Native CalB, Si-E-CPs, and Si-CL-CPs
3.5. Reusability of Si-E-CPs and Si-CL-CPs
3.6. Enzymatic Hydrolysis for p-Nitrophenyl Butyrate
3.7. Enzymatic Synthesis for Benzyl Benzoate with Native CalB and Si-CL-CPs
3.8. Instrumental Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, Y.; Caruso, F. Enzyme encapsulation in nanoporous silica spheres. Chem. Comm. 2004, 13, 1528–1529. [Google Scholar] [CrossRef] [PubMed]
- Soldatkin, O.; Kucherenko, I.; Pyeshkova, V.; Kukla, A.; Jaffrezic-Renault, N.; El’Skaya, A.; Dzyadevych, S.; Soldatkin, A. Novel conductometric biosensor based on three-enzyme system for selective determination of heavy metal ions. Bioelectrochemistry 2012, 83, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Ruffi, C.R.; da Cunha, R.H.; Almeida, E.L.; Chang, Y.K.; Steel, C.J. Effect of the emulsifier sodium stearoyl lactylate and of the enzyme maltogenic amylase on the quality of pan bread during storage. LWT 2012, 49, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Schoemaker, H.E.; Mink, D.; Wubbolts, M.G. Dispelling the myths—Biocatalysis in industrial synthesis. Science 2003, 299, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.M.; Robins, K.T.; Kiener, A. Lonza: 20 years of biotransformations. Adv. Synth. Catal. 2003, 345, 425–435. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Rantwijk, F. Biocatalysis for sustainable organic synthesis. Aust. J. Chem. 2004, 57, 281–289. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.; Calvo, L.; Alba, C.; Daneshfar, A.; Ghaziaskar, H. Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in n-hexane. Enzyme Microb. Technol. 2005, 37, 42–48. [Google Scholar] [CrossRef]
- Stergiou, P.-Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Stauch, B.; Fisher, S.J.; Cianci, M. Open and closed states of Candida antarctica lipase B: Protonation and the mechanism of interfacial activation1. J. Lipid Res. 2015, 56, 2348–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst–Many applications: The use of Candida antarctica B-lipase in organic synthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Rotticci, D.; Rotticci-Mulder, J.C.; Denman, S.; Norin, T.; Hult, K. Improved enantioselectivity of a lipase by rational protein engineering. ChemBioChem 2001, 2, 766–770. [Google Scholar] [CrossRef]
- Idris, A.; Bukhari, A. Immobilized Candida antarctica lipase B: Hydration, stripping off and application in ring opening polyester synthesis. Biotechnol. Adv. 2012, 30, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Martinelle, M.; Holmquist, M.; Hult, K. On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metabol. 1995, 1258, 272–276. [Google Scholar] [CrossRef]
- Kundys, A.; Białecka-Florjańczyk, E.; Fabiszewska, A.; Małajowicz, J. Candida antarctica lipase B as catalyst for cyclic esters synthesis, their polymerization and degradation of aliphatic polyesters. J. Polym. Environ. 2018, 26, 396–407. [Google Scholar] [CrossRef]
- Weiser, D.; Boros, Z.; Hornyánszky, G.; Tóth, A.; Poppe, L. Disubstituted dialkoxysilane precursors in binary and ternary sol–gel systems for lipase immobilization. Process Biochem. 2012, 47, 428–434. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Murty, V.R.; Bhat, J.; Muniswaran, P. Hydrolysis of oils by using immobilized lipase enzyme: A review. Biotechnol. Bioprocess Eng. 2002, 7, 57–66. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent bio-applications of sol–gel materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Malcata, F.X.; Hill, C.G., Jr.; Amundson, C.H. Use of a lipase immobilized in a membrane reactor to hydrolyze the glycerides of butteroil. Biotechno. Bioeng. 1991, 38, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R. Cross-linked enzyme aggregates (CLEA® s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Uyanik, A.; Sen, N.; Yilmaz, M. Improvement of catalytic activity of lipase from Candida rugosa via sol–gel encapsulation in the presence of calix (aza) crown. Bioresour. Technol. 2011, 102, 4313–4318. [Google Scholar] [CrossRef]
- Yang, G.; Wu, J.; Xu, G.; Yang, L. Improvement of catalytic properties of lipase from Arthrobacter sp. by encapsulation in hydrophobic sol–gel materials. Bioresour. Technol. 2009, 100, 4311–4316. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional polyimide/polyhedral oligomeric silsesquioxane nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.G.; Kuo, S.W. Functional silica and carbon nanocomposites based on polybenzooxazines. Macromol. Chem. Phys. 2019, 220, 1800306. [Google Scholar] [CrossRef]
- Cui, J.; Jia, S.; Liang, L.; Zhao, Y.; Feng, Y. Mesoporous CLEAs-silica composite microparticles with high activity and enhanced stability. Sci. Rep. 2015, 5, 14203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luckarift, H.R.; Spain, J.C.; Naik, R.R.; Stone, M.O. Enzyme immobilization in a biomimetic silica support. Nat. Biotechnol. 2004, 22, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Pierre, A. The sol-gel encapsulation of enzymes. Biocatal. Biotransform. 2004, 22, 145–170. [Google Scholar] [CrossRef]
- Soares, C.M.; dos Santos, O.A.; Olivo, J.E.; de Castro, H.F.; de Moraes, F.F.; Zanin, G.M. Influence of the alkyl-substituted silane precursor on sol–gel encapsulated lipase activity. J. Mol. Catal. B Enzym. 2004, 29, 69–79. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Babich, L. Enzymatic Cascade Reactions Involving Phosphorylated Intermediates: Immobilization and Process Optimization. Ph.D. Thesis, Universiteit van Amsterdam, Amsterdam, The Netherlands, 2013. [Google Scholar]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Proc. Res. Develop. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Jegan Roy, J.; Emilia Abraham, T. Strategies in making cross-linked enzyme crystals. Chem. Rev. 2004, 104, 3705–3722. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Zikry, A.; Sharaf, M.A. Preparation of spherical silica nanoparticles: Stober silica. J. Am. Sci. 2010, 6, 985–989. [Google Scholar]
- Lindberg, R.; Sjöblom, J.; Sundholm, G. Preparation of silica particles utilizing the sol-gel and the emulsion-gel processes. Colloids Surf. A Physicochem. Eng. Asp. 1995, 99, 79–88. [Google Scholar] [CrossRef]
- Lindberg, R.; Pettersen, B.; Sjo, J.; Friberg, S.E. Multivariate analysis of the size dependence of monodisperse silica particles prepared according to the sol-gel technique. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123, 549–560. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Smit, M.; Dunn, B.; Zink, J.I. Stabilization of creatine kinase encapsulated in silicate sol-gel materials and unusual temperature effects on its activity. Chem. Mater. 2002, 14, 4300–4306. [Google Scholar] [CrossRef]
- Duman, Y.A.; Tekin, N. Kinetic and thermodynamic properties of purified alkaline protease from Bacillus pumilus Y7 and non-covalent immobilization to poly (vinylimidazole)/clay hydrogel. Eng. Life Sci. 2020, 20, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Torres, S.; Castro, G.R. Non-aqueous biocatalysis in homogeneous solvent systems. Food Technol. Biotechnol. 2004, 42, 271–277. [Google Scholar]
- Gandhi, N.N.; Patil, N.S.; Sawant, S.B.; Joshi, J.B.; Wangikar, P.P.; Mukesh, D. Lipase-catalyzed esterification. Catal. Rev. 2000, 42, 439–480. [Google Scholar] [CrossRef]
- Politzer, I.; Griffin, G.; Dowty, B.; Laseter, J. Enhancement of ultraviolet detectability of fatty acids for purposes of liquid chromatographic-mass spectrometric analyses. Anal. Lett. 1973, 6, 539–546. [Google Scholar] [CrossRef]
- de Meneses, A.C.; Balen, M.; de Andrade Jasper, E.; Korte, I.; de Araújo, P.H.H.; Sayer, C.; de Oliveira, D. Enzymatic synthesis of benzyl benzoate using different acyl donors: Comparison of solvent-free reaction techniques. Process Biochem. 2020, 92, 261–268. [Google Scholar] [CrossRef]
- Reetz, M.T. Entrapment of biocatalysts in hydrophobic sol-gel materials for use in organic chemistry. Adv. Mater. 1997, 9, 943–954. [Google Scholar] [CrossRef]
- Chen, Q.; Kenausis, G.L.; Heller, A. Stability of oxidases immobilized in silica gels. J. Am. Chem. Soc. 1998, 120, 4582–4585. [Google Scholar] [CrossRef]
- Obert, R.; Dave, B.C. Enzymatic conversion of carbon dioxide to methanol: Enhanced methanol production in silica sol-gel matrices. J. Am. Chem. Soc 1999, 121, 12192–12193. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, O.; Zor, T. Linearization of the Bradford protein assay. J. Vis. Exp. 2010, 38, e1918. [Google Scholar] [CrossRef] [PubMed]


| [TEOS] (mM) | Km (μM) | Vmax (μM ⸱ min−1) | Kcat (min−1) | Kcat/Km (mM−1 ⸱ min−1) | |
|---|---|---|---|---|---|
| Native CalB | 0 | 432.60 | 16.86 | 15.03 | 34.76 |
| Si−E−CPs | 10 | 915.32 | 4.57 | 5.03 | 5.50 |
| 50 | 588.31 | 9.94 | 9.90 | 16.83 | |
| Si−CL−CPs | 100 | 517.31 | 12.02 | 10.70 | 20.68 |
| 300 | 497.86 | 12.06 | 10.73 | 21.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Chang, J.-H. Thermally Stable and Reusable Ceramic Encapsulated and Cross-Linked CalB Enzyme Particles for Rapid Hydrolysis and Esterification. Int. J. Mol. Sci. 2022, 23, 2459. https://doi.org/10.3390/ijms23052459
Song M, Chang J-H. Thermally Stable and Reusable Ceramic Encapsulated and Cross-Linked CalB Enzyme Particles for Rapid Hydrolysis and Esterification. International Journal of Molecular Sciences. 2022; 23(5):2459. https://doi.org/10.3390/ijms23052459
Chicago/Turabian StyleSong, Min, and Jeong-Ho Chang. 2022. "Thermally Stable and Reusable Ceramic Encapsulated and Cross-Linked CalB Enzyme Particles for Rapid Hydrolysis and Esterification" International Journal of Molecular Sciences 23, no. 5: 2459. https://doi.org/10.3390/ijms23052459
APA StyleSong, M., & Chang, J.-H. (2022). Thermally Stable and Reusable Ceramic Encapsulated and Cross-Linked CalB Enzyme Particles for Rapid Hydrolysis and Esterification. International Journal of Molecular Sciences, 23(5), 2459. https://doi.org/10.3390/ijms23052459





