Nanoscale Topographical Effects on the Adsorption Behavior of Bone Morphogenetic Protein-2 on Graphite
Abstract
:1. Introduction
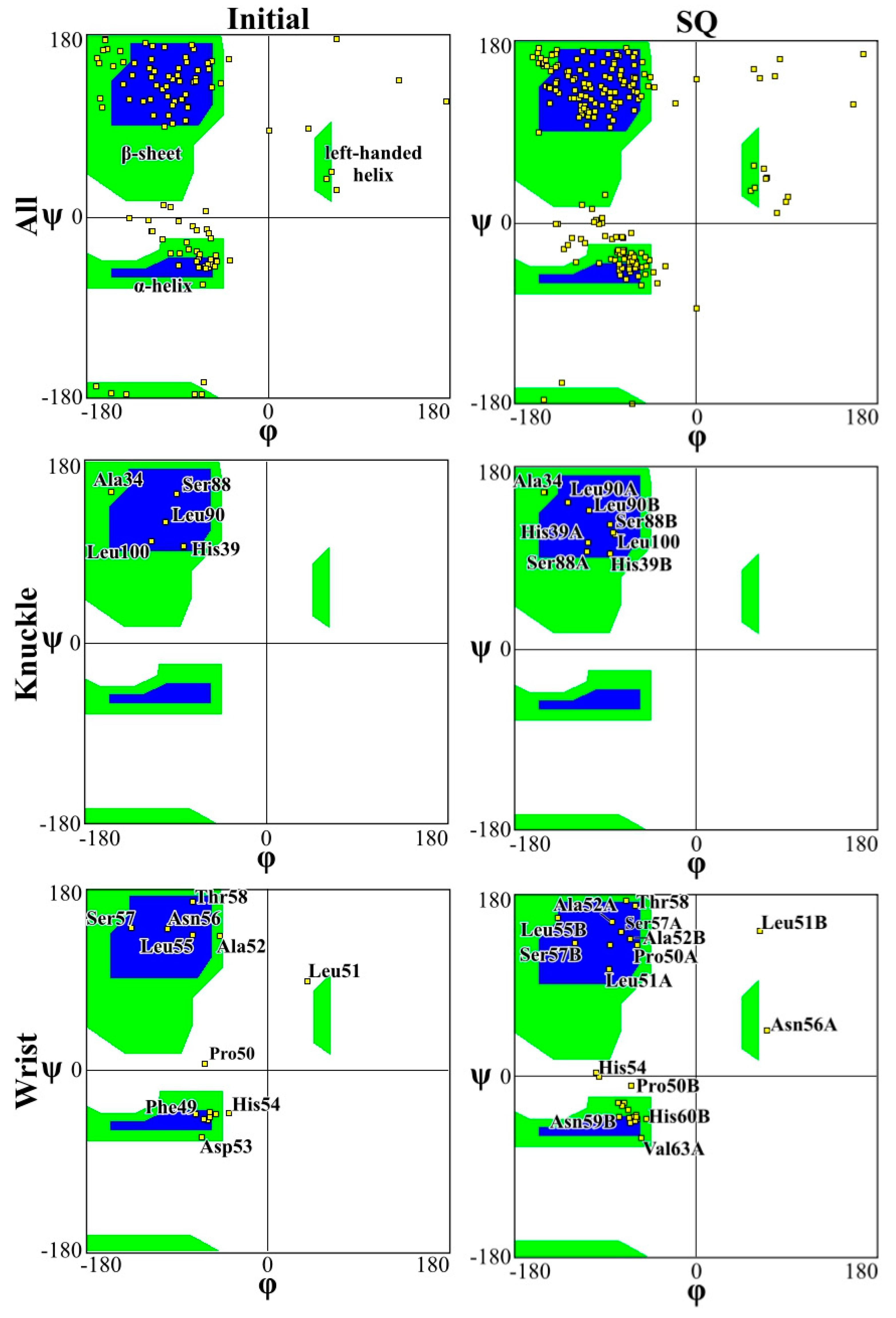
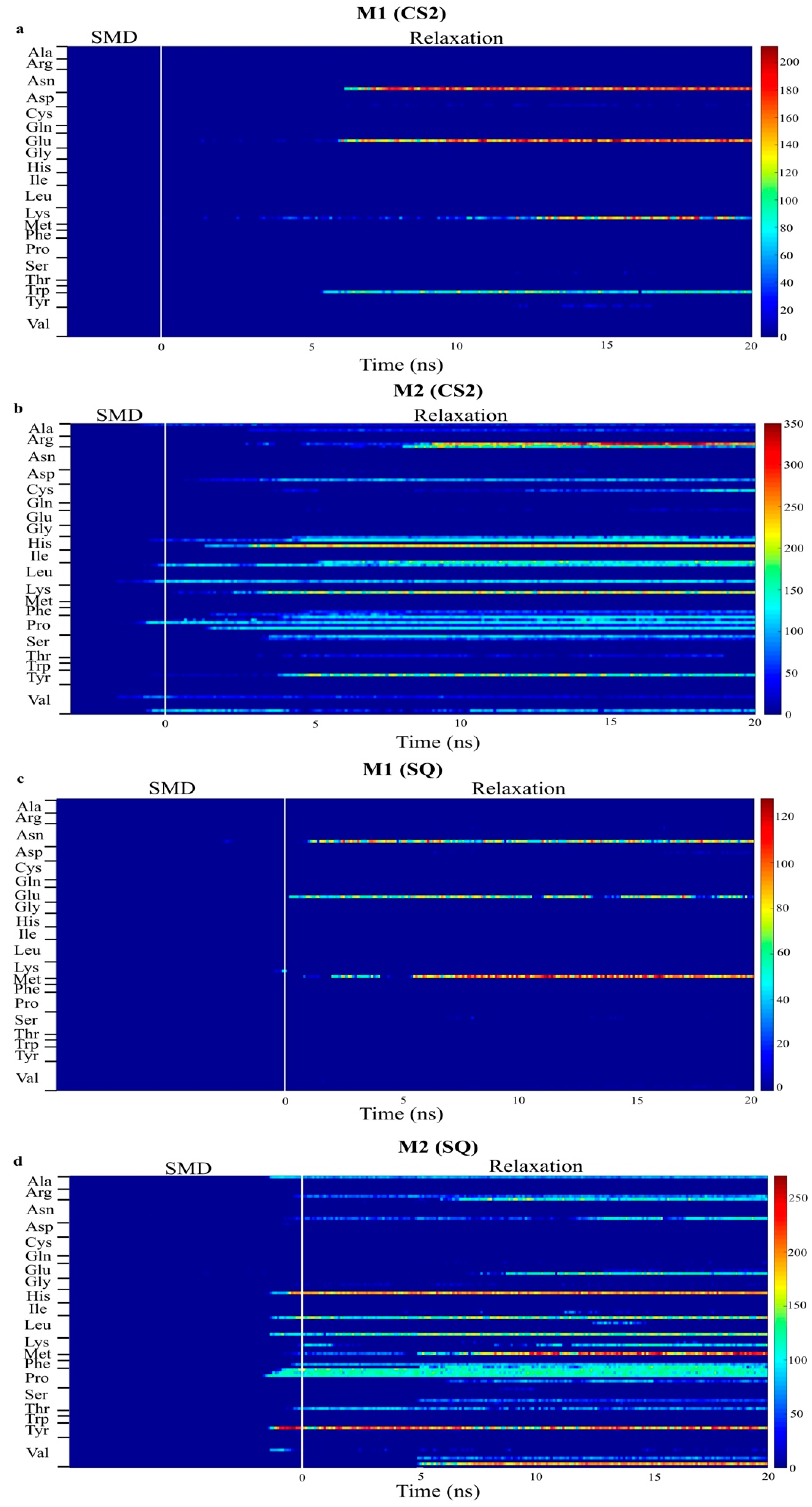
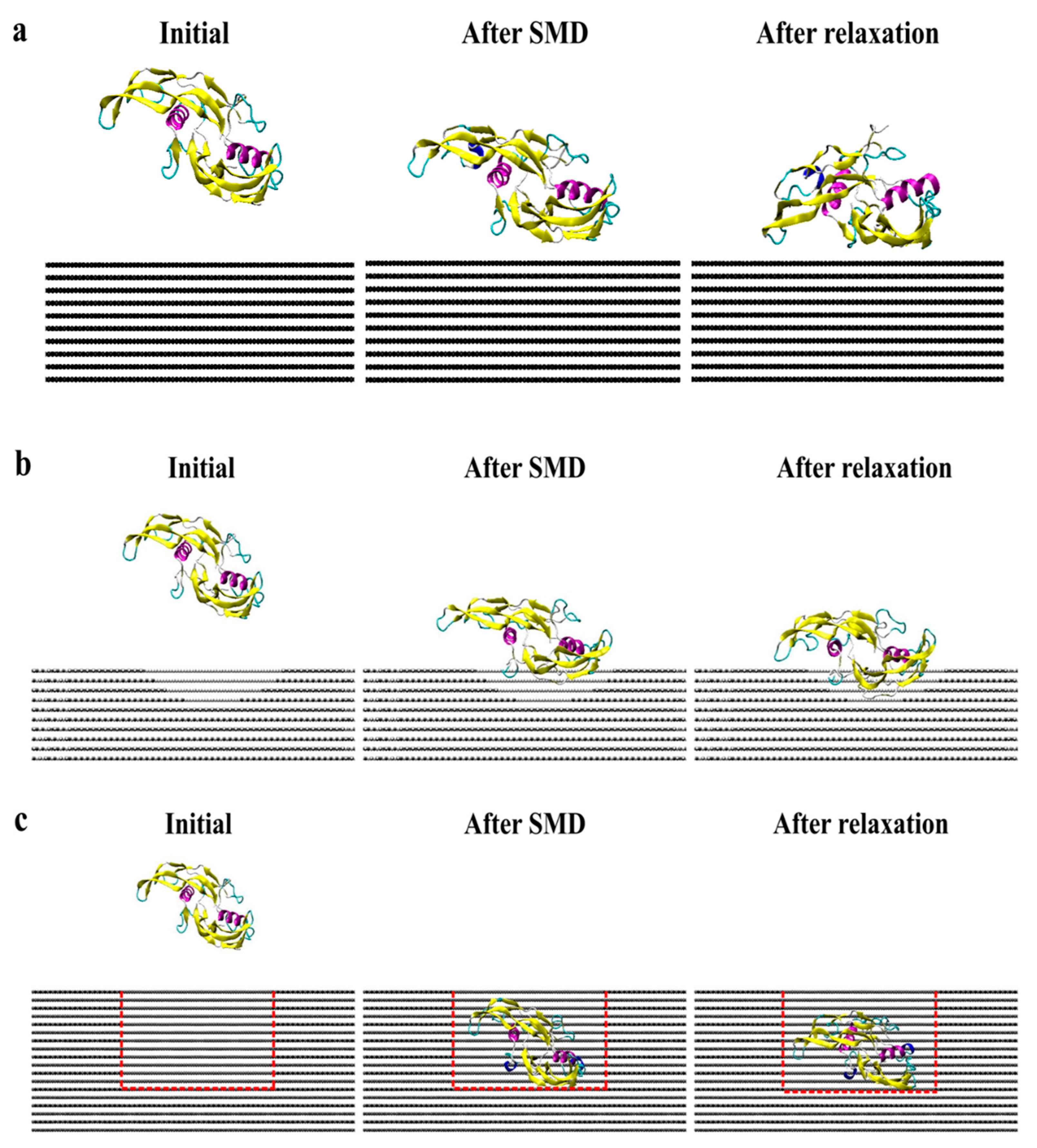
2. Results and Discussion
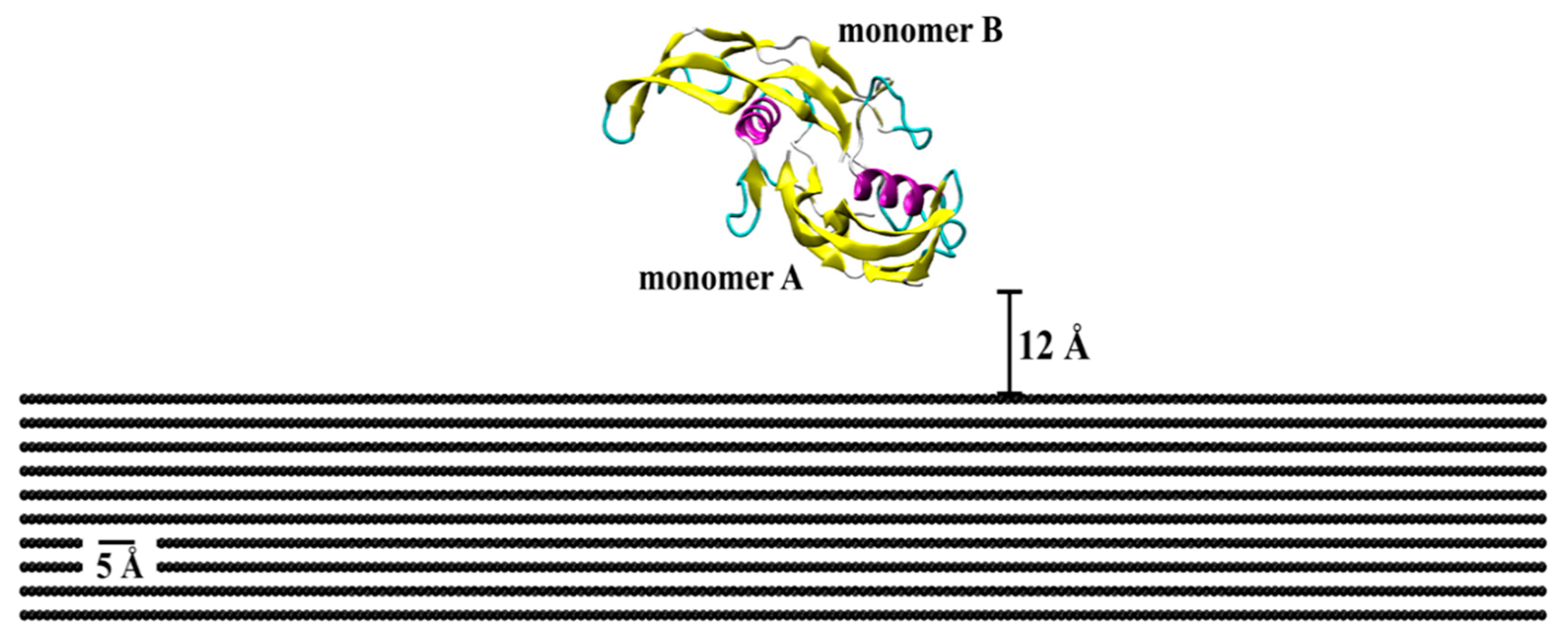
3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, R.R.; Alves, N.M.; Rodríguez-Cabello, J.C.; Mano, J.F. Stimuli-Responsive Surfaces for Biomedical Applications. In Biomaterials Surface Science; Wiley: Hoboken, NJ, USA, 2013; pp. 63–87. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef]
- Ekblad, T.; Liedberg, B. Protein adsorption and surface patterning. Curr. Opin. Colloid Interface Sci. 2010, 15, 499–509. [Google Scholar] [CrossRef]
- Desai, S.; Bidanda, B.; Bártolo, P.J. Emerging Trends in the Applications of Metallic and Ceramic Biomaterials. In Bio-Materials and Prototyping Applications in Medicine; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–17. [Google Scholar] [CrossRef]
- Desai, S.; Shankar, M.R. Emerging Trends in Polymers, Composites, and Nano Biomaterial Applications. In Bio-Materials and Prototyping Applications in Medicine; Springer: Berlin/Heidelberg, Germany, 2020; pp. 19–34. [Google Scholar] [CrossRef]
- Desai, S.; Harrison, B. Direct-Writing of Biomedia for Drug Delivery and Tissue Regeneration. In Printed Biomaterials; Springer: Berlin/Heidelberg, Germany, 2019; pp. 71–89. [Google Scholar] [CrossRef]
- Desai, S.; Perkins, J.; Harrison, B.S.; Sankar, J. Understanding release kinetics of biopolymer drug delivery microcapsules for biomedical applications. Mater. Sci. Eng. B 2010, 168, 127–131. [Google Scholar] [CrossRef]
- Desai, S.; Moore, A.; Harrison, B.; Sankar, J. Understanding Microdroplet Formations for Biomedical Applications. ASME Int. Mech. Eng. Congr. Expo. Proc. 2009, 15, 119–123. [Google Scholar] [CrossRef]
- van Osch, G.J.V.M.; Brittberg, M.; Dennis, J.E.; Bastiaansen-Jenniskens, Y.M.; Erben, R.G.; Konttinen, Y.T.; Luyten, F.P. Cartilage repair: Past and future—Lessons for regenerative medicine. J. Cell. Mol. Med. 2009, 13, 792–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivats, A.R.; McDermott, M.C.; Hollinger, J.O. Bone tissue engineering: State of the union. Drug Discov. Today 2014, 19, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, F.; Desai, S.; Andar, A.J. Laser Fabrication of Polymeric Microneedles for Transdermal Drug Delivery. In Proceedings of the 2021 IISE Annual Conference, Online, 22–25 May 2021. [Google Scholar]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Adarkwa, E.; Kotoka, R.; Desai, S. 3D printing of polymeric Coatings on AZ31 Mg alloy Substrate for Corrosion Protection of biomedical implants. Med. Devices Sens. 2021, 4, e10167. [Google Scholar] [CrossRef]
- Marquetti, I.; Desai, S. Adsorption Behavior of Bone Morphogenic Protein (BMP-2) on Nanoscale Topographies. In Proceedings of the ASME NanoEngineering for Medicine and Biology Conference, Los Angeles, CA, USA, 20–24 August 2018. [Google Scholar]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Briggs, D.; Brewis, D.M.; Dahm, R.H.; Fletcher, I.W. Analysis of the surface chemistry of oxidized polyethylene: Comparison of XPS and ToF-SIMS. Surf. Interface Anal. 2003, 35, 156–167. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Gao, L.; Jing, L.; Zhou, Q.; Chang, J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 73, 509–521. [Google Scholar] [CrossRef]
- Dutta, D.; Sundaram, S.K.; Teeguarden, J.G.; Riley, B.J.; Fifield, L.S.; Jacobs, J.M.; Addleman, S.R.; Kaysen, G.A.; Moudgil, B.M.; Weber, T.J. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol. Sci. Off. J. Soc. Toxicol. 2007, 100, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainz, M.A.; Serena, S.; Belmonte, M.; Miranzo, P.; Osendi, M.I. Protein adsorption and in vitro behavior of additively manufactured 3D-silicon nitride scaffolds intended for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 110734. [Google Scholar] [CrossRef] [PubMed]
- Latour, R.A. Molecular simulation of protein-surface interactions: Benefits, problems, solutions, and future directions (Review). Biointerphases 2008, 3, FC2–FC12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klepeis, J.L.; Lindorff-Larsen, K.; Dror, R.O.; Shaw, D.E. Long-timescale molecular dynamics simulations of protein structure and function. Curr. Opin. Struct. Biol. 2009, 19, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, S. Biodegradable Gelatin Microcarriers in Tissue Engineering. Ph.D. Thesis, Linköping University, Linköping, Sweden, 2009. [Google Scholar]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone Morphogenetic Proteins: A critical review. Cell. Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Gemming, S.; Seifert, G. Conformational Analysis of Aqueous BMP-2 Using Atomistic Molecular-Dynamics Simulations. J. Phys. Chem. B 2011, 115, 1122–1130. [Google Scholar] [CrossRef]
- Schwab, E.H.; Pohl, T.L.M.; Haraszti, T.; Schwaerzer, G.K.; Hiepen, C.; Spatz, J.P.; Knaus, P.; Cavalcanti-Adam, E.A. Nanoscale control of surface immobilized BMP-2: Toward a quantitative assessment of BMP-mediated signaling events. Nano Lett. 2015, 15, 1526–1534. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Y.; Ding, S.; Li, J.; Ren, J.; Feng, B.; Li, T.; Gu, Y.; Liu, C. Nanostructured hydroxyapatite surfaces-mediated adsorption alters recognition of BMP receptor IA and bioactivity of bone morphogenetic protein-2. Acta Biomater. 2015, 27, 275–285. [Google Scholar] [CrossRef]
- Huang, B.; Lou, Y.; Li, T.; Lin, Z.; Sun, S.; Yuan, Y.; Liu, C.; Gu, Y. Molecular dynamics simulations of adsorption and desorption of bone morphogenetic protein-2 on textured hydroxyapatite surfaces. Acta Biomater. 2018, 80, 121–130. [Google Scholar] [CrossRef]
- Mücksch, C.; Urbassek, H.M. Adsorption of BMP-2 on a hydrophobic graphite surface: A molecular dynamics study. Chem. Phys. Lett. 2011, 510, 252–256. [Google Scholar] [CrossRef]
- Mücksch, C.; Urbassek, H.M. Mucksch. Enhancing protein adsorption simulations by using accelerated molecular dynamics. PLoS ONE 2013, 8, e64883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquetti, I.; Desai, S. Orientation effects on the nanoscale adsorption behavior of bone morphogenetic protein-2 on hydrophilic silicon dioxide. RSC Adv. 2019, 9, 906–916. [Google Scholar] [CrossRef] [Green Version]
- Marquetti, I.; Desai, S. Molecular modeling the adsorption behavior of bone morphogenetic protein-2 on hydrophobic and hydrophilic substrates. Chem. Phys. Lett. 2018, 706, 285–294. [Google Scholar] [CrossRef]
- Marquetti, I.; Desai, S. An Atomistic Investigation of Adsorption of Bone Morphogenetic Protein-2 on Gold with Nanoscale Topographies. Surfaces 2022, 5, 176–185. [Google Scholar] [CrossRef]
- Marquetti, I.; Desai, S. Adsorption Behavior of Bone Morphogenetic Protein-2 on a Graphite Substrate for Biomedical Applications. Am. J. Eng. Appl. Sci. 2018, 11, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Mücksch, C.; Urbassek, H.M. Accelerated Molecular Dynamics Study of the Effects of Surface Hydrophilicity on Protein Adsorption. Langmuir 2016, 32, 9156–9162. [Google Scholar] [CrossRef]
- Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475–480. [Google Scholar] [CrossRef]
- Rampazzo, E.; Dettin, M.; Maule, F.; Scabello, A.; Calvanese, L.; D’Auria, G.; Falcigno, L.; Porcù, E.; Zamuner, A.; Della Puppa, A.; et al. A synthetic BMP-2 mimicking peptide induces glioblastoma stem cell differentiation. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 2282–2292. [Google Scholar] [CrossRef]
- Utesch, T.; Daminelli, G.; Mroginski, M.A. Molecular dynamics simulations of the adsorption of bone morphogenetic protein-2 on surfaces with medical relevance. Langmuir ACS J. Surf. Colloids 2011, 27, 13144–13153. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.; Kuppa, V.K. Polymer adsorption on rough surfaces. Curr. Opin. Chem. Eng. 2018, 19, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. Available online: https://www.academia.edu/13619601/Scalable_molecular_dynamics_with_NAMD (accessed on 26 January 2022). [CrossRef] [PubMed] [Green Version]
- Towns, J.; Cockerill, T.; Dahan, M.; Foster, I.; Gaither, K.; Grimshaw, A.; Hazlewood, V.; Lathrop, S.; Lifka, D.; Peterson, G.; et al. XSEDE: Accelerating scientific discovery. Comput. Sci. Eng. 2014, 16, 62–74. [Google Scholar] [CrossRef]
- Marquetti, I.; Rodrigues, J.; Desai, S.S. Ecological Impact of Green Computing Using Graphical Processing Units in Molecular Dynamics Simulations. Int. J. Green Comput. 2018, 9, 38–45. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKerell, A.D., Jr.; Bashford, D.; Bellott, M.L.; Dunbrack, R.L., Jr.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Kotzsch, A.; Nickel, J.; Harth, S.; Seher, A.; Mueller, U.; Sebald, W.; Mueller, T.D. A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct. Biol. 2007, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Marquetti, I.J. Molecular modeling of bone morphogenetic protein for tissue engineering applications. In Proceedings of the 2018 IISE Annual Conference, Orlando, FL, USA, 19–22 May 2018. [Google Scholar]
- Smith, L.J.; Fiebig, K.M.; Schwalbe, H.; Dobson, C.M. The concept of a random coil. Residual structure in peptides and denatured proteins. Fold. Des. 1996, 1, R95–R106. [Google Scholar] [CrossRef] [Green Version]
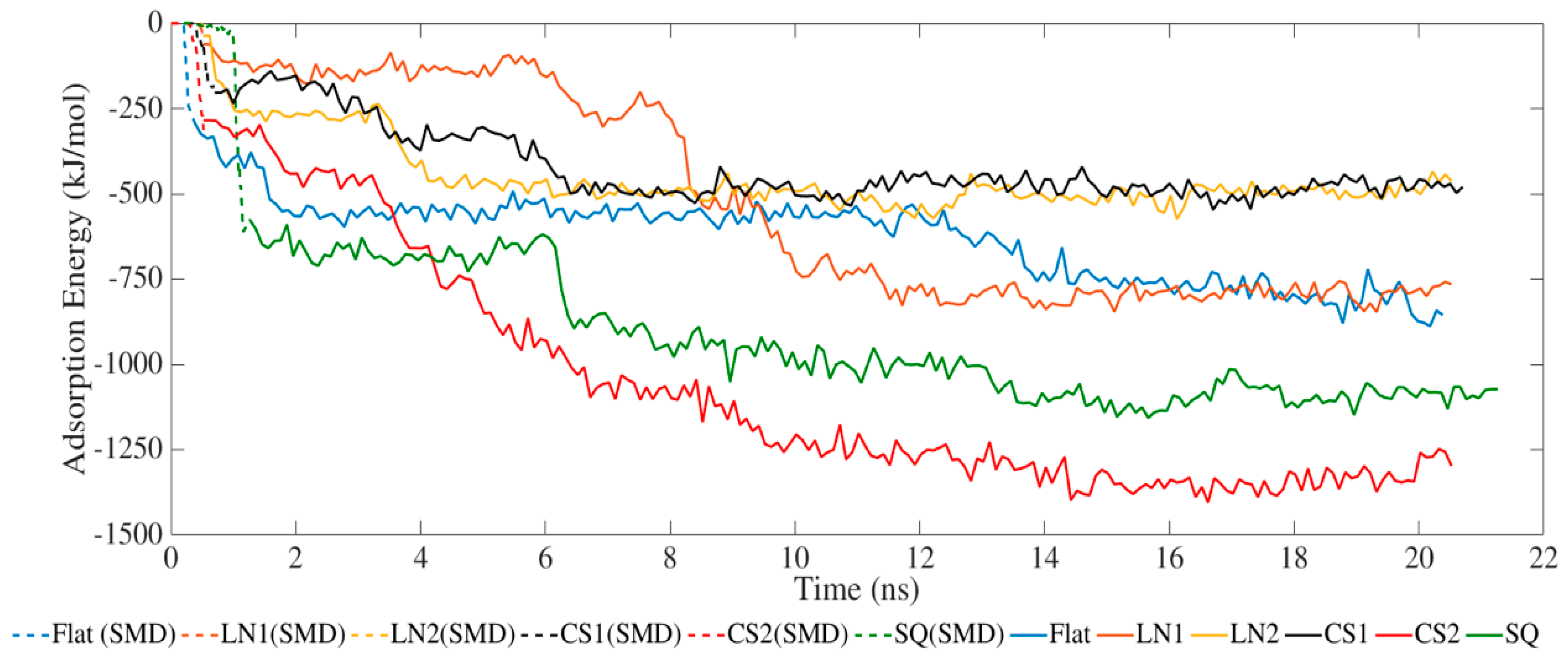
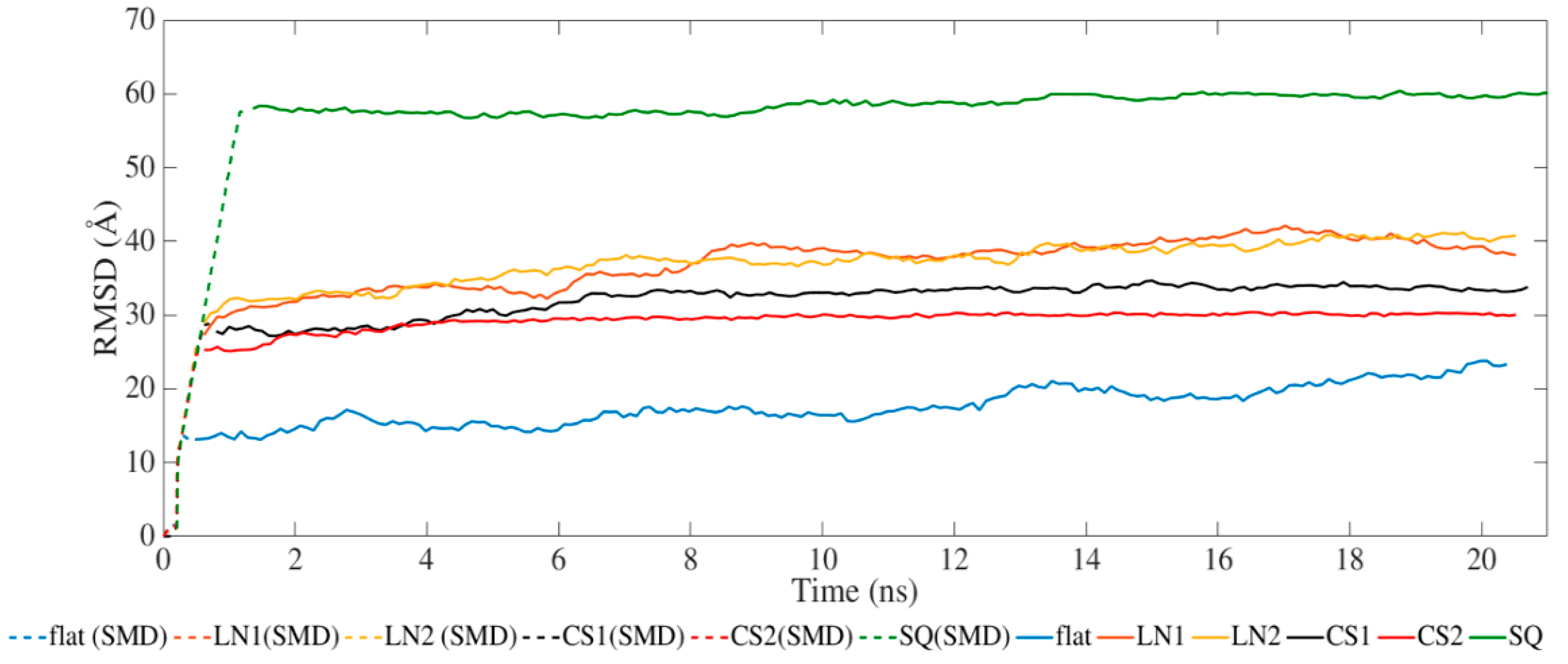
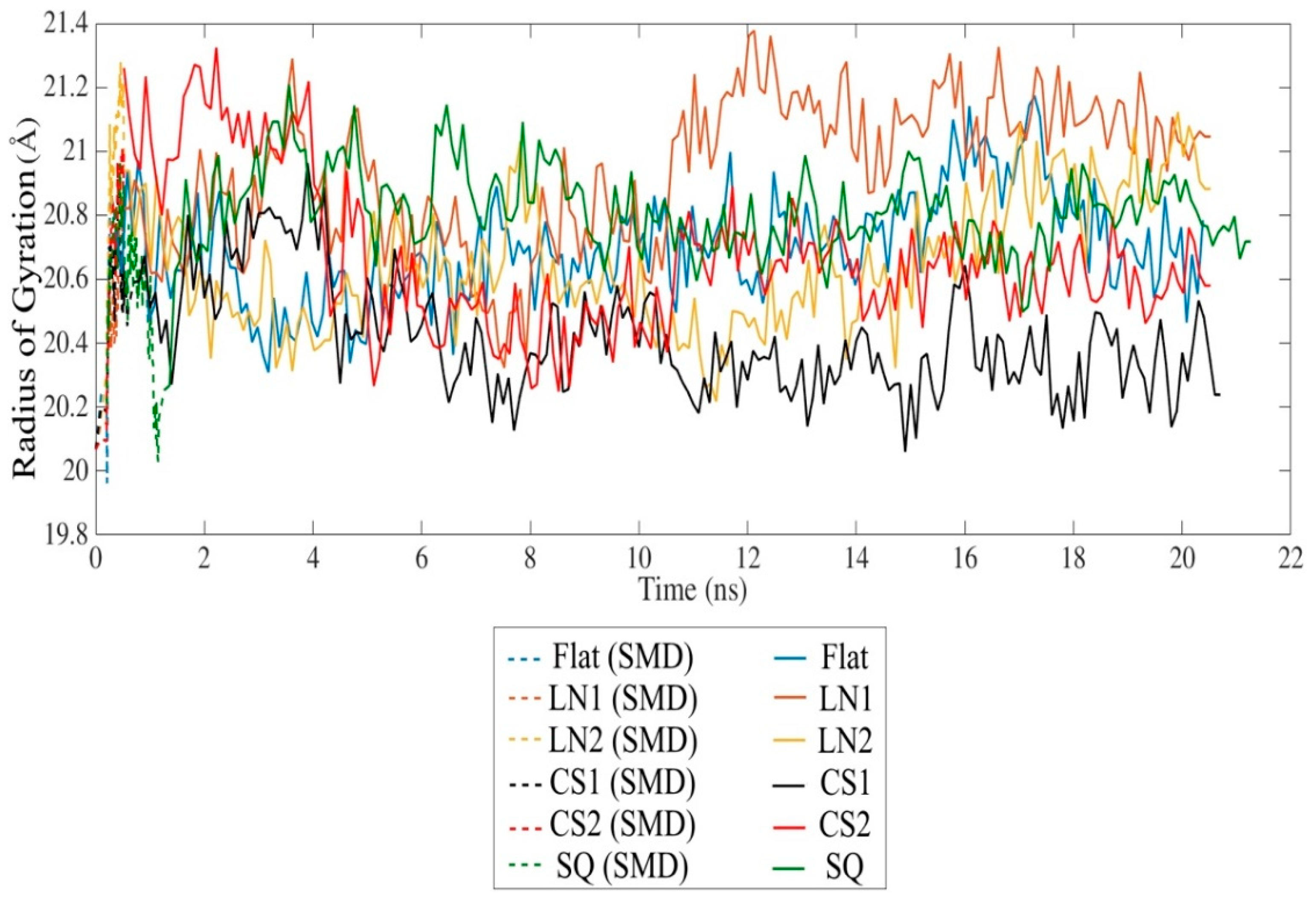
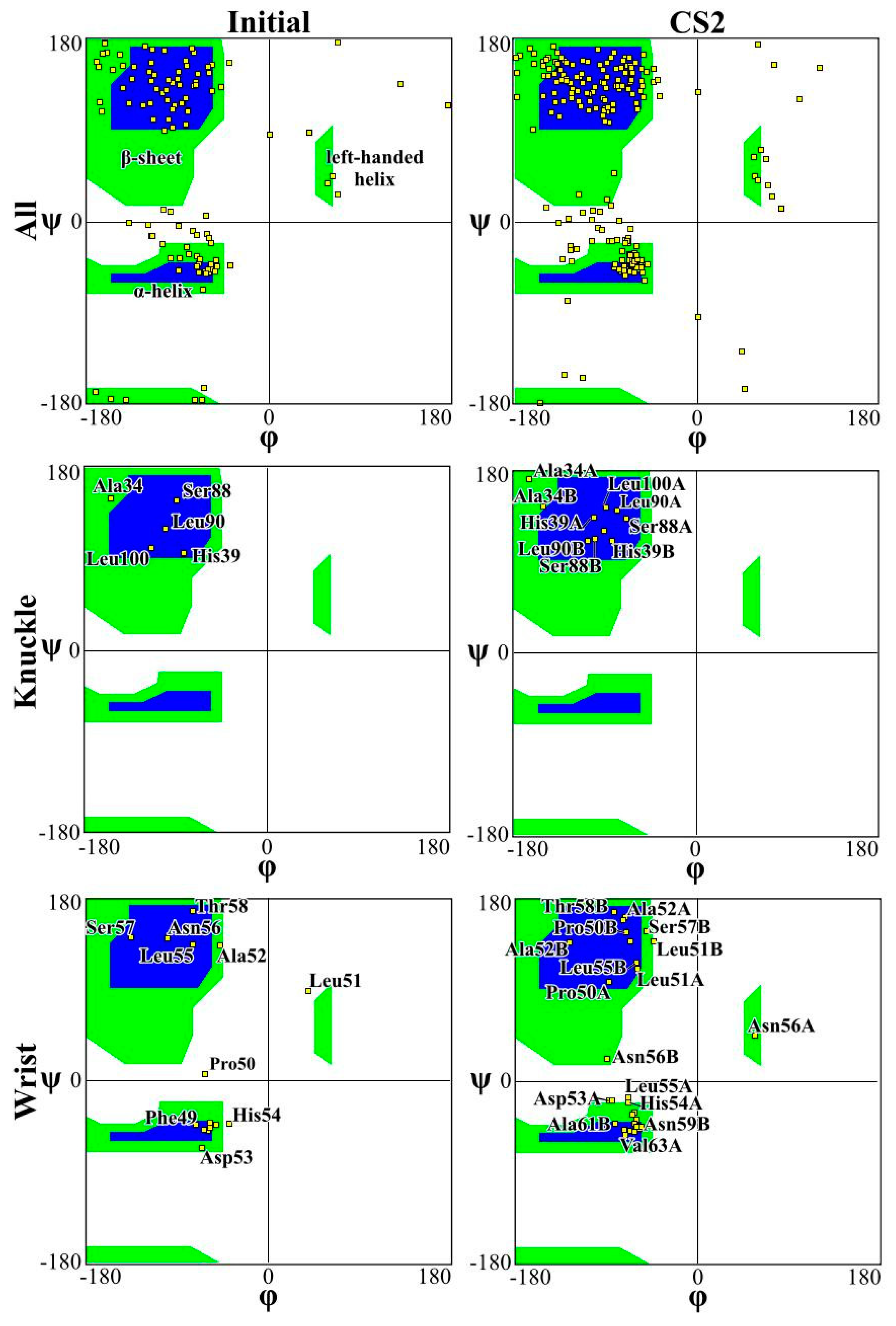

| Secondary Structure | Initial Structure | Flat | LN1 | LN2 | CS1 | CS2 | SQ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | A | B | A | B | ||
| α-helix | 10.38 | 9.43 | 11.32 | 11.32 | 11.32 | 15.09 | 11.32 | 9.43 | 11.32 | 11.32 | 11.32 | 10.38 | 11.32 |
| 310-helix | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| β-sheets | 44.34 | 40.57 | 42.45 | 38.68 | 42.45 | 44.34 | 44.34 | 40.57 | 42.45 | 35.85 | 44.35 | 37.74 | 44.34 |
| Type of Patterns | Timestep | Time (ps) |
|---|---|---|
| Flat | 40,000 | 8 |
| Linear grating 1 (LN1) | 160,000 | 32 |
| Linear grating 2 (LN2) | 160,000 | 32 |
| Circular slot 1 (CS1) | 200,000 | 40 |
| Circular slot 2 (CS2) | 160,000 | 32 |
| Square slot (SQ) | 480,000 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquetti, I.; Desai, S. Nanoscale Topographical Effects on the Adsorption Behavior of Bone Morphogenetic Protein-2 on Graphite. Int. J. Mol. Sci. 2022, 23, 2432. https://doi.org/10.3390/ijms23052432
Marquetti I, Desai S. Nanoscale Topographical Effects on the Adsorption Behavior of Bone Morphogenetic Protein-2 on Graphite. International Journal of Molecular Sciences. 2022; 23(5):2432. https://doi.org/10.3390/ijms23052432
Chicago/Turabian StyleMarquetti, Izabele, and Salil Desai. 2022. "Nanoscale Topographical Effects on the Adsorption Behavior of Bone Morphogenetic Protein-2 on Graphite" International Journal of Molecular Sciences 23, no. 5: 2432. https://doi.org/10.3390/ijms23052432
APA StyleMarquetti, I., & Desai, S. (2022). Nanoscale Topographical Effects on the Adsorption Behavior of Bone Morphogenetic Protein-2 on Graphite. International Journal of Molecular Sciences, 23(5), 2432. https://doi.org/10.3390/ijms23052432







