Bortezomib-Induced Epigenetic Alterations in Nerve Cells: Focus on the Mechanisms Contributing to the Peripheral Neuropathy Development
Abstract
1. Introduction
2. Results
2.1. Cell Viability
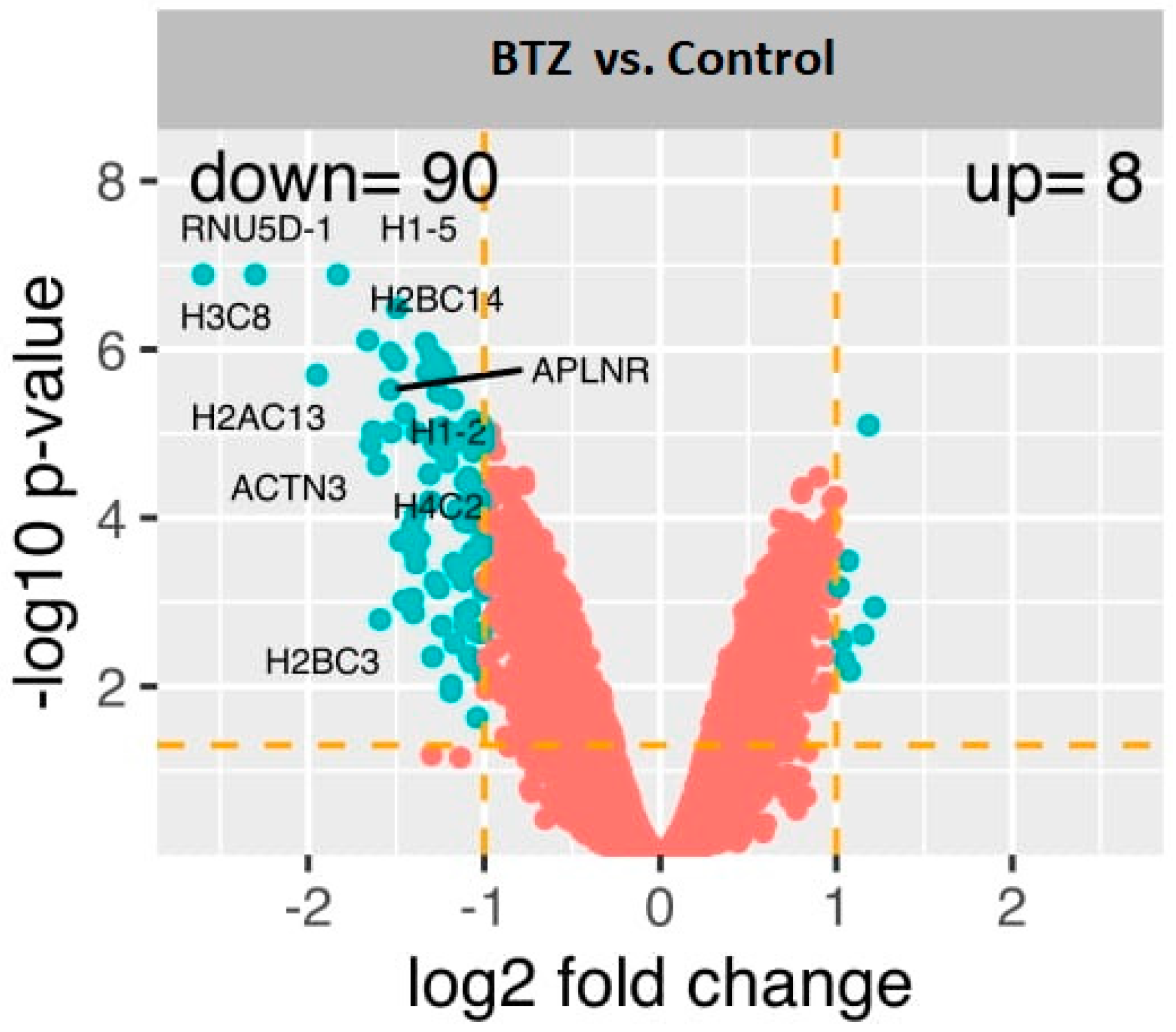
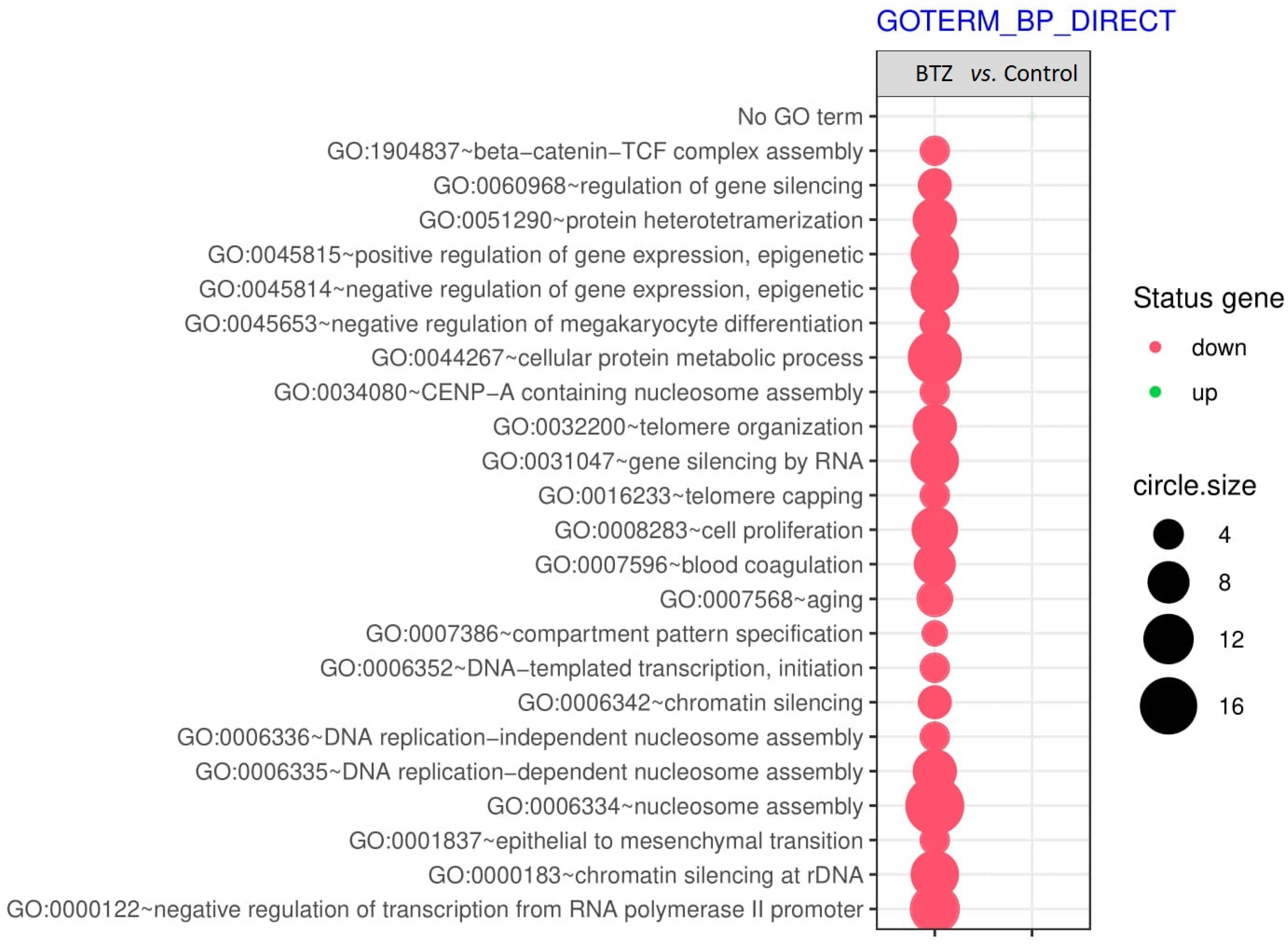
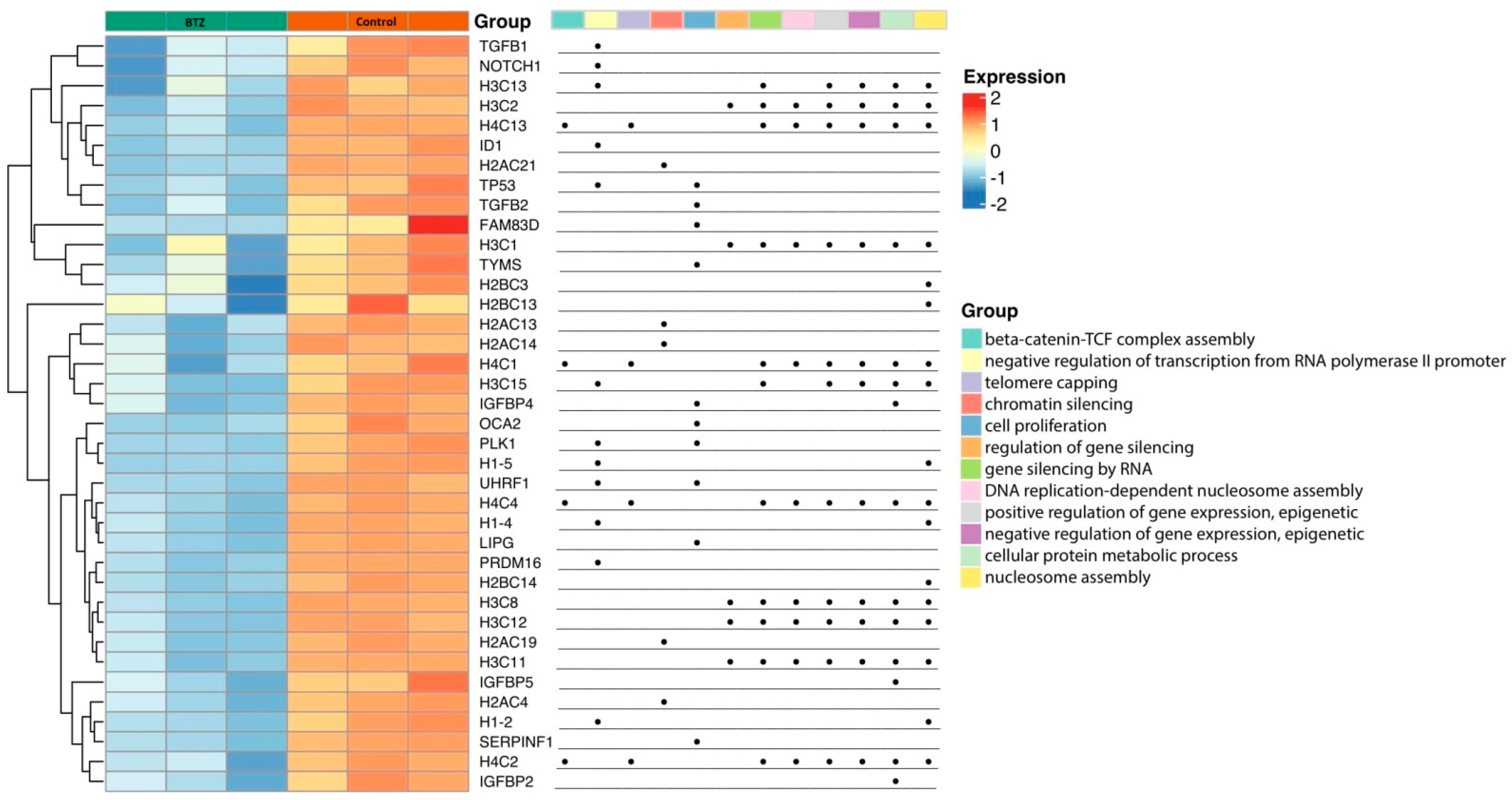
2.2. Gene Expression Profile in Neuronal Cells
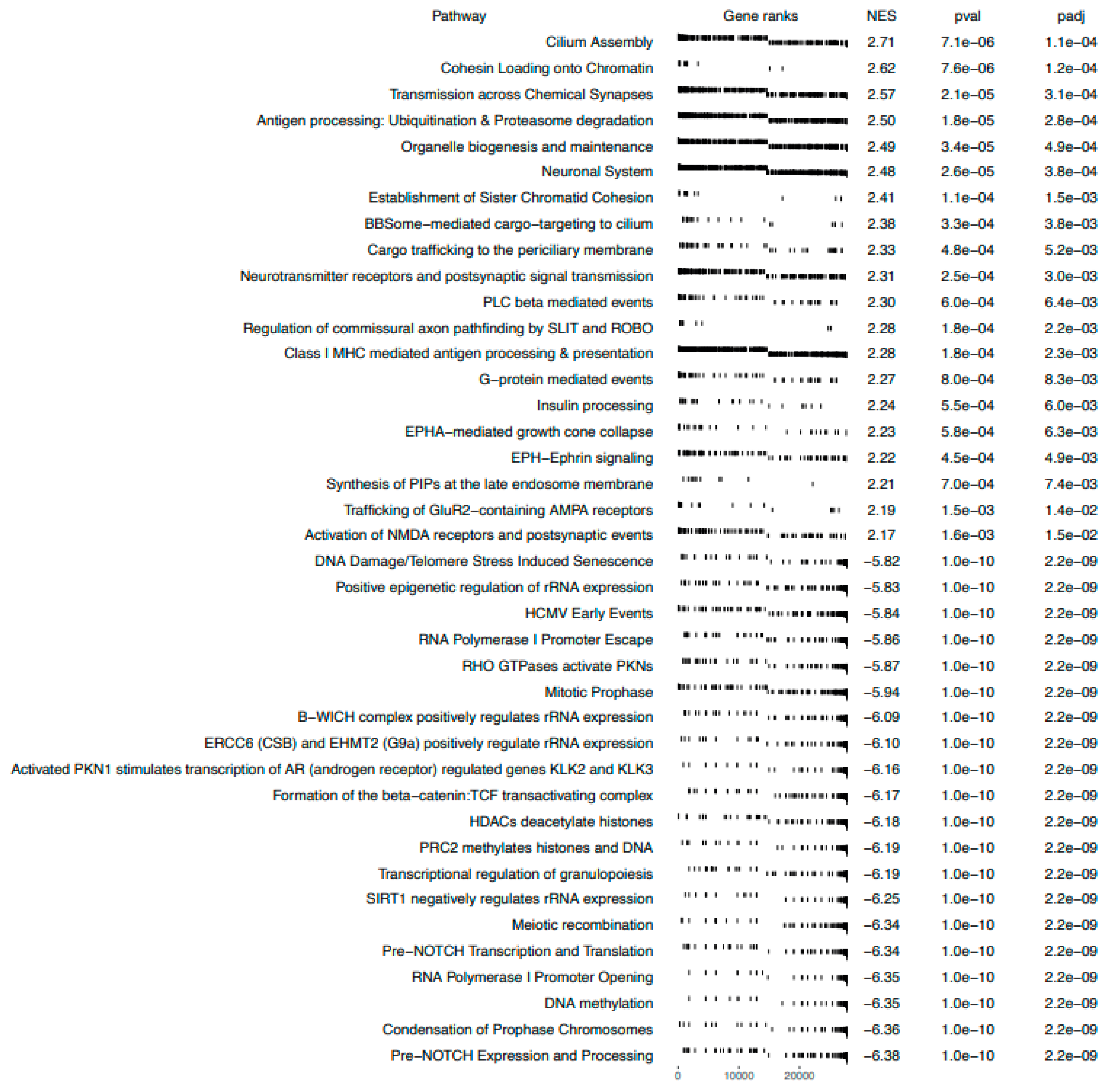
Gene Set Enrichment Analysis
2.3. Validation of mRNA Microarray
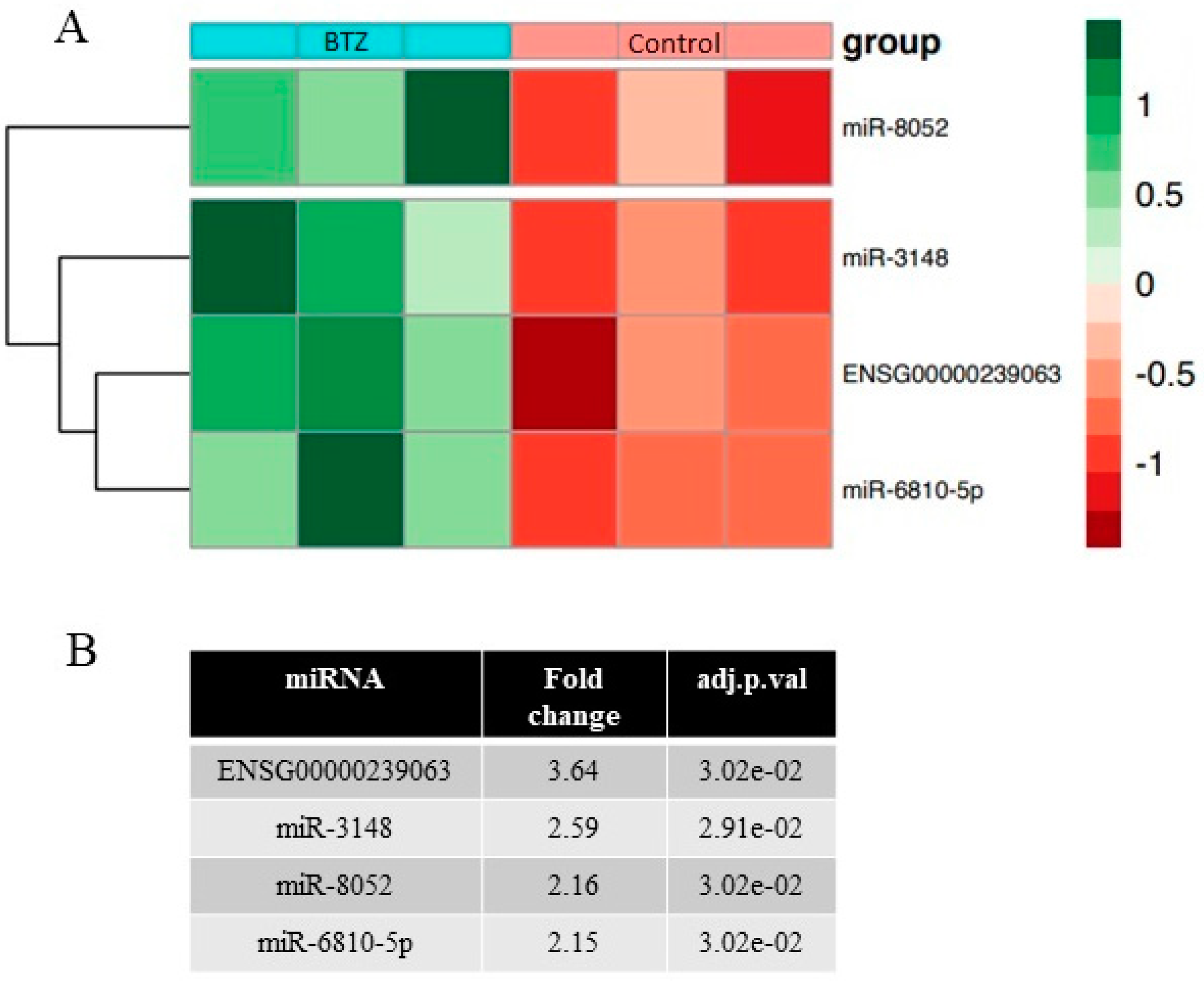
2.4. miRNAs Expression Profile in Neuronal Cells
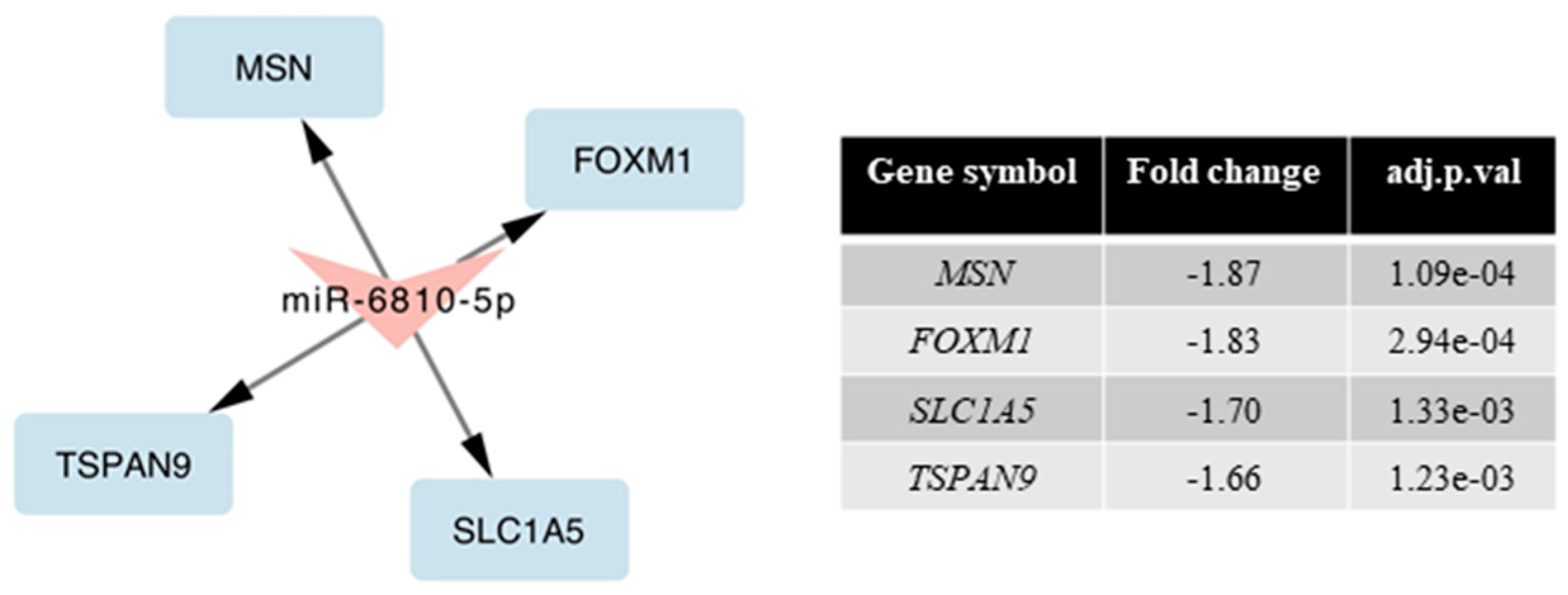
2.5. Validation of miRNA Microarray
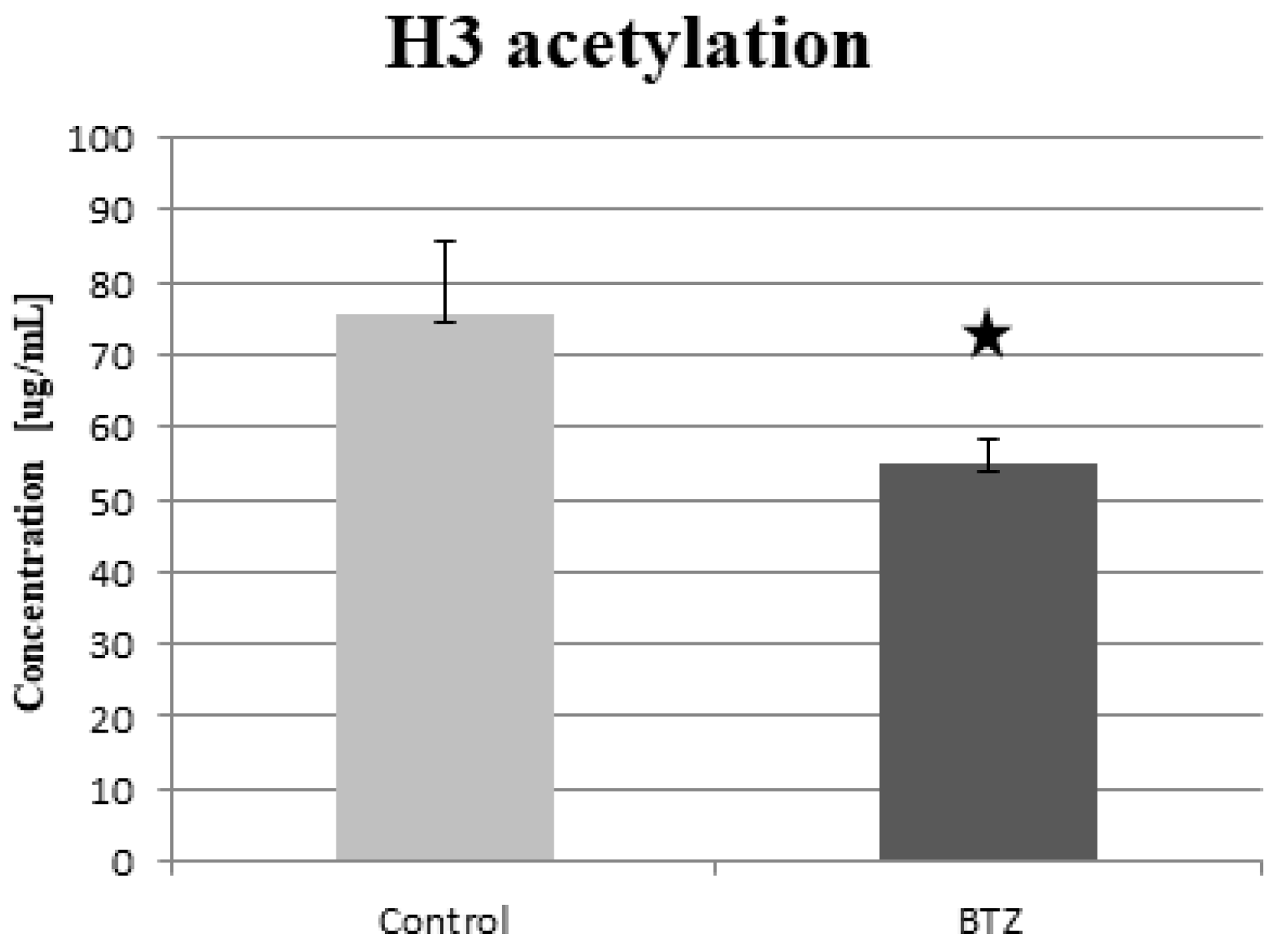
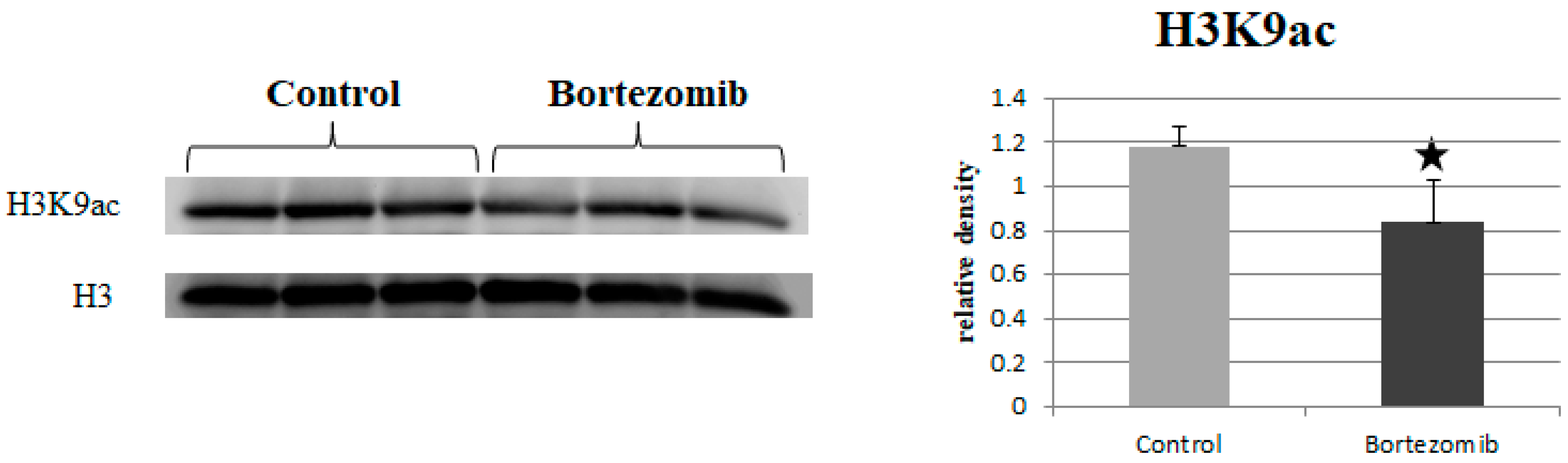
2.6. Histone Acetylation
2.6.1. ELISA
2.6.2. Western Blot
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Cell Culture and Differentiation
4.2. The Course of the Experiment and the Determination of the BTZ Dose
4.3. RNA Isolation
4.4. Histone Isolation
4.5. Cell Viability
4.6. Affymetrix GeneChip Microarray and Data Analysis
4.7. Affymetrix GeneChip miRNA Microarray
4.8. Validation of Data Obtained from Microarrays
4.8.1. Validation of mRNA Microarray
4.8.2. Validation of miRNA Microarray
4.9. Histone Acetylation
4.9.1. ELISA
4.9.2. Western Blot
4.10. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamamoto, S.; Egashira, N. Pathological Mechanisms of BTZ-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2021, 22, 888. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Mitsiades, C.; Hideshima, T.; Anderson, K.C. BTZ: Proteasome inhibition as an effective anticancer therapy. Ann. Rev. Med. 2006, 57, 33–47. [Google Scholar] [CrossRef]
- Moreau, P.; Pylypenko, H.; Grosicki, S.; Karamanesht, I.; Leleu, X.; Grishunina, M.; Rekhtman, G.; Masliak, Z.; Robak, T.; Shubina, A.; et al. Subcutaneous versus intravenous administration of BTZ in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011, 12, 431–440. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Tamaskar, A.; Wang, H.; Zhao, J.; Ma, H.; Zhao, Y. Proteasome Inhibitors Diminish c-Met Expression and Induce Cell Death in Non-Small Cell Lung Cancer Cells. Oncol. Res. 2020, 28, 497–507. [Google Scholar] [CrossRef]
- Hideshima, H.; Yoshida, Y.; Ikeda, H.; Hide, M.; Iwasaki, A.; Anderson, K.C.; Hideshima, T. IKKβ inhibitor in combination with BTZ induces cytotoxicity in breast cancer cells. Int. J. Oncol. 2014, 44, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Wu, W.S.; Lin, L.C.; Liu, C.S.; Ho, S.Y.; Wang, B.J.; Huang, B.M.; Yeh, Y.L.; Chiu, H.W.; Yang, W.L.; et al. BTZ enhances radiosensitivity in oral cancer through inducing autophagy-mediated TRAF6 oncoprotein degradation. J. Exp. Clin. Cancer Res. 2018, 37, 91. [Google Scholar] [CrossRef]
- Pancheri, E.; Guglielmi, V.; Wilczynski, G.M.; Malatesta, M.; Tonin, P.; Tomelleri, G.; Nowis, D.; Vattemi, G. Non-Hematologic Toxicity of BTZ in Multiple Myeloma: The Neuromuscular and Cardiovascular Adverse Effects. Cancers 2020, 12, 2540. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Iconomou, G.; Kalofonos, H.P. BTZ-induced peripheral neuropathy in multiple myeloma: A comprehensive review of the literature. Blood 2008, 112, 1593–1599. [Google Scholar] [CrossRef]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; Reece, D.; et al. Reversibility of symptomatic peripheral neuropathy with BTZ in the phase III APEX trial in relapsed multiple myeloma: Impact of a dose-modification guideline. Br. J. Haematol. 2009, 144, 895–903. [Google Scholar] [CrossRef]
- Perera, B.P.U.; Faulk, C.; Svoboda, L.K.; Goodrich, J.M.; Dolinoy, D.C. The role of environmental exposures and the epigenome in health and disease. Environ. Mol. Mutagenesis 2020, 1, 176–192. [Google Scholar] [CrossRef]
- Guo, K.; Elzinga, S.; Eid, S.; Figueroa-Romero, C.; Hinder, L.M.; Pacut, C.; Feldman, E.L.; Hur, J. Genome-wide DNA methylation profiling of human diabetic peripheral neuropathy in subjects with type 2 diabetes mellitus. Epigenetics 2019, 14, 766–779. [Google Scholar] [CrossRef]
- Kober, K.M.; Lee, M.C.; Olshen, A.; Conley, Y.P.; Sirota, M.; Keiser, M.; Hammer, M.J.; Abrams, G.; Schumacher, M.; Levine, J.D.; et al. Differential methylation and expression of genes in the hypoxia-inducible factor 1 signaling pathway are associated with paclitaxel-induced peripheral neuropathy in breast cancer survivors and with preclinical models of chemotherapy-induced neuropathic pain. Mol. Pain 2020, 16, 1744806920936502. [Google Scholar] [PubMed]
- Łuczkowska, K.; Rutka, M.; Rogińska, D.; Paczkowska, E.; Baumert, B.; Milczarek, S.; Górska, M.; Kulig, P.; Osękowska, B.; Janowski, M.; et al. The Potential Role of Proinflammatory Cytokines and Complement Components in the Development of Drug-Induced Neuropathy in Patients with Multiple Myeloma. J. Clin. Med. 2021, 10, 4584. [Google Scholar] [CrossRef] [PubMed]
- Łuczkowska, K.; Rogińska, D.; Ulańczyk, Z.; Machaliński, B. Effect of BTZ on Global Gene Expression in PC12-Derived Nerve Cells. Int. J. Mol. Sci. 2020, 21, 751. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, B. Epigenetics: The science of change. Environ. Health Perspect. 2006, 114, A160–A167. [Google Scholar] [CrossRef] [PubMed]
- Tchurikov, N.A. Molecular mechanisms of epigenetics. Biochemistry 2005, 70, 406–423. [Google Scholar] [CrossRef]
- Jagannath, S.; Barlogie, B.; Berenson, J.; Siegel, D.; Irwin, D.; Richardson, P.G.; Niesvizky, R.; Alexanian, R.; Limentani, S.A.; Alsina, M.; et al. A phase 2 study of two doses of BTZ in relapsed or refractory myeloma. Br. J. Haematol. 2004, 127, 165–172. [Google Scholar] [CrossRef]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A phase 2 study of BTZ in relapsed, refractory myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef]
- Grammatico, S.; Cesini, L.; Petrucci, M.T. Managing treatment-related peripheral neuropathy in patients with multiple myeloma. Blood Lymphat. Cancer 2016, 6, 37–47. [Google Scholar]
- Schey, S.A.; Yong, K.L.; Marcus, R.; Anderson, K.C. (Eds.) Myeloma: Pathology, Diagnosis, and Treatment, 1st ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Field-Smith, A.; Morgan, G.J.; Davies, F.E. BTZ (Velcade™) in the Treatment of Multiple Myeloma. Ther. Clin. Risk Manag. 2006, 2, 271–279. [Google Scholar] [CrossRef]
- Curran, M.P.; McKeage, K. BTZ: A review of its use in patients with multiple myeloma. Drugs 2009, 69, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Reece, D.E.; Sullivan, D.; Lonial, S.; Mohrbacher, A.F.; Chatta, G.; Shustik, C.; Burris, H., 3rd; Venkatakrishnan, K.; Neuwirth, R.; Riordan, W.J.; et al. Pharmacokinetic and pharmacodynamic study of two doses of BTZ in patients with relapsed multiple myeloma. Cancer Chemother. Pharmacol. 2011, 67, 57–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernandez de Larrea, C.; Martin-Antonio, B.; Cibeira, M.T.; Navarro, A.; Tovar, N.; Diaz, T.; Rosinol, L.; Monzo, M.; Urbano-Ispizua, A.; Blade, J. Impact of global and gene-specific DNA methylation pattern in relapsed multiple myeloma patients treated with BTZ. Leuk. Res. 2013, 37, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.Y.; Ko, C.Y.; Wang, S.M.; Lin, P.I.; Wang, H.Y.; Lin, W.C.; Wu, D.Y.; Wang, L.H.; Wang, J.M. BTZ-induced miRNAs direct epigenetic silencing of locus genes and trigger apoptosis in leukemia. Cell Death Dis. 2017, 8, e3167. [Google Scholar] [CrossRef]
- Łuczkowska, K.; Rogińska, D.; Ulańczyk, Z.; Paczkowska, E.; Schmidt, C.A.; Machaliński, B. Molecular Mechanisms of BTZ Action: Novel Evidence for the miRNA-mRNA Interaction Involvement. Int. J. Mol. Sci. 2020, 21, 350. [Google Scholar] [CrossRef]
- Łuczkowska, K.; Sokolowska, K.E.; Taryma-Lesniak, O.; Pastuszak, K.; Supernat, A.; Bybjerg-Grauholm, J.; Hansen, L.L.; Paczkowska, E.; Wojdacz, T.K.; Machaliński, B. BTZ induces methylation changes in neuroblastoma cells that appear to play a significant role in resistance development to this compound. Sci. Rep. 2021, 11, 9846. [Google Scholar] [CrossRef]
- Hisahara, S.; Chiba, S.; Matsumoto, H.; Tanno, M.; Yagi, H.; Shimohama, S.; Sato, M.; Horio, Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc. Natl. Acad. Sci. USA 2008, 105, 15599–15604. [Google Scholar] [CrossRef]
- Romeo-Guitart, D.; Leiva-Rodríguez, T.; Espinosa-Alcantud, M.; Sima, N.; Vaquero, A.; Domínguez-Martín, H.; Ruano, D.; Casas, C. SIRT1 activation with neuroheal is neuroprotective but SIRT2 inhibition with AK7 is detrimental for disconnected motoneurons. Cell Death Dis. 2018, 9, 531. [Google Scholar] [CrossRef]
- Romeo-Guitart, D.; Leiva-Rodriguez, T.; Forés, J.; Casas, C. Improved Motor Nerve Regeneration by SIRT1/Hif1a-Mediated Autophagy. Cells 2019, 8, 1354. [Google Scholar] [CrossRef]
- Chen, K.; Fan, J.; Luo, Z.F.; Yang, Y.; Xin, W.J.; Liu, C.C. Reduction of SIRT1 epigenetically upregulates NALP1 expression and contributes to neuropathic pain induced by chemotherapeutic drug BTZ. J. Neuroinflamm. 2018, 15, 292. [Google Scholar] [CrossRef]
- Vintermist, A.; Böhm, S.; Sadeghifar, F.; Louvet, E.; Mansén, A.; Percipalle, P.; Ostlund Farrants, A.K. The chromatin remodelling complex B-WICH changes the chromatin structure and recruits histone acetyl-transferases to active rRNA genes. PLoS ONE 2011, 6, e19184. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhou, T.; Xie, W.; Ling, T.; Zhu, Q.; Zong, L.; Lyu, G.; Gao, Q.; Zhang, F.; Tao, W. The chromatin remodeling factor CSB recruits histone acetyltransferase PCAF to rRNA gene promoters in active state for transcription initiation. PLoS ONE 2013, 8, e62668. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, M. Physiological Role of b-Catenin/TCF Signaling in Neurons of the Adult Brain. Neurochem. Res. 2013, 38, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Guo, X.; Yang, C. Effect of BTZ on proliferation and apoptosis of myeloma cells by activating Wnt/β-catenin signaling pathway. Oncol Lett. 2020, 20, 1295–1299. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Wei, W.; Zhang, G.; Yan, S.; Dai, R.; Sun, Y.; Su, D.; Lv, S.; Xia, Y.; et al. BTZ potentiates antitumor activity of mitoxantrone through dampening Wnt/β-catenin signal pathway in prostate cancer cells. BMC Cancer 2021, 21, 1101. [Google Scholar] [CrossRef]
- Powers, G.L.; Rajbhandari, P.; Solodin, N.M.; Bickford, B.; Alarid, E.T. The proteasome inhibitor BTZ induces an inhibitory chromatin environment at a distal enhancer of the estrogen receptor-α gene. PLoS ONE 2013, 8, e81110. [Google Scholar] [CrossRef]
- Mannava, S.; Zhuang, D.; Nair, J.R.; Bansal, R.; Wawrzyniak, J.A.; Zucker, S.N.; Fink, E.E.; Moparthy, K.C.; Hu, Q.; Liu, S.; et al. KLF9 is a novel transcriptional regulator of BTZ- and LBH589-induced apoptosis in multiple myeloma cells. Blood 2012, 119, 1450–1458. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, J.; Li, J.; Pang, X.; Wang, H. Inhibition of microRNA-155 Reduces Neuropathic Pain During Chemotherapeutic Bortezomib via Engagement of Neuroinflammation. Front. Oncol. 2020, 10, 416. [Google Scholar] [CrossRef]
- Łuczkowska, K.; Rogińska, D.; Ulańczyk, Z.; Safranow, K.; Paczkowska, E.; Baumert, B.; Milczarek, S.; Osękowska, B.; Górska, M.; Borowiecka, E.; et al. microRNAs as the biomarkers of chemotherapy-induced peripheral neuropathy in patients with multiple myeloma. Leuk. Lymphoma 2021, 62, 2768–2776. [Google Scholar] [CrossRef]
- Pelzer, D.; Phipps, L.S.; Thuret, R.; Gallardo-Dodd, C.J.; Baker, S.M.; Dorey, K. Foxm1 regulates neural progenitor fate during spinal cord regeneration. EMBO Rep. 2021, 22, e50932. [Google Scholar] [CrossRef]
- Besharat, Z.M.; Abballe, L.; Cicconardi, F.; Bhutkar, A.; Grassi, L.; Le Pera, L.; Moretti, M.; Chinappi, M.; D’Andrea, D.; Mastronuzzi, A.; et al. Foxm1 controls a pro-stemness microRNA network in neural stem cells. Sci. Rep. 2018, 8, 3523. [Google Scholar] [CrossRef] [PubMed]
- Bhat, U.G.; Halasi, M.; Gartel, A.L. FoxM1 is a general target for proteasome inhibitors. PLoS ONE 2009, 4, e6593. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Jing, X.; Holman, C.; Sompallae, R.; Zhan, F.; Tricot, G.; Yang, Y.; Janz, S. Upregulation of FOXM1 leads to diminished drug sensitivity in myeloma. BMC Cancer 2018, 18, 1152. [Google Scholar] [CrossRef] [PubMed]
- Colas, C.; Grewer, C.; Otte, N.J.; Gameiro, A.; Albers, T.; Singh, K.; Shere, H.; Bonomi, M.; Holst, J.; Schlessinger, A. Ligand Discovery for the Alanine-Serine-Cysteine Transporter (ASCT2, SLC1A5) from Homology Modeling and Virtual Screening. PLoS Comput. Biol. 2015, 11, e1004477. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Bang, O.S.; Park, E.K.; Yang, S.I.; Lee, S.R.; Franke, T.F.; Kang, S.S. Overexpression of Akt inhibits NGF-induced growth arrest and neuronal differentiation of PC12 cells. J. Cell Sci. 2001, 114 Pt 1, 81–88. [Google Scholar] [CrossRef]
- Sugars, K.L.; Brown, R.; Cook, L.J.; Swartz, J.; Rubinsztein, D.C. Decreased cAMP response element-mediated transcription: An early event in exon 1 and full-length cell models of Huntington’s disease that contributes to polyglutamine pathogenesis. J. Biol. Chem. 2004, 279, 4988–4999. [Google Scholar] [CrossRef]
- Katoh, S.; Mitsui, Y.; Kitani, K.; Suzuki, T. Hyperoxia induces the neuronal differentiated phenotype of PC12 cells via a sustained activity of mitogen-activated protein kinase induced by Bcl-2. Biochem. J. 1999, 338 Pt 2, 465–470. [Google Scholar] [CrossRef]
- Tong, Z.B.; Hogberg, H.; Kuo, D.; Sakamuru, S.; Xia, M.; Smirnova, M.; Hartung, T.; Gerhold, D. Characterization of three human cell line models for high-throughput neuronal cytotoxicity screening. J. Appl. Toxicol. 2017, 37, 167–180. [Google Scholar] [CrossRef]
- Harris, G.; Hogberg, H.; Hartung, T.; Smirnova, L. 3D Differentiation of LUHMES Cell Line to Study Recovery and Delayed Neurotoxic Effects. Curr. Protoc. Toxicol. 2017, 73, 11.23.1–11.23.28. [Google Scholar] [CrossRef]
| Day of the Experiment | Action |
|---|---|
| 1st | medium with BTZ (0.15 nM) for 24 h |
| 2nd | change the medium to BTZ-free |
| 3rd | |
| 4th | medium with BTZ (0.15 nM) for 24 h |
| 5th | change the medium to BTZ-free |
| 6th | |
| 7th | change the medium to BTZ-free |
| 8th | medium with BTZ (0.15 nM) for 24 h |
| 9th | change the medium to BTZ-free |
| 10th | |
| 11th | medium with BTZ (0.15 nM) for 24 h |
| 12th | RNA/miRNA and histone isolation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuczkowska, K.; Rogińska, D.; Kulig, P.; Bielikowicz, A.; Baumert, B.; Machaliński, B. Bortezomib-Induced Epigenetic Alterations in Nerve Cells: Focus on the Mechanisms Contributing to the Peripheral Neuropathy Development. Int. J. Mol. Sci. 2022, 23, 2431. https://doi.org/10.3390/ijms23052431
Łuczkowska K, Rogińska D, Kulig P, Bielikowicz A, Baumert B, Machaliński B. Bortezomib-Induced Epigenetic Alterations in Nerve Cells: Focus on the Mechanisms Contributing to the Peripheral Neuropathy Development. International Journal of Molecular Sciences. 2022; 23(5):2431. https://doi.org/10.3390/ijms23052431
Chicago/Turabian StyleŁuczkowska, Karolina, Dorota Rogińska, Piotr Kulig, Anna Bielikowicz, Bartłomiej Baumert, and Bogusław Machaliński. 2022. "Bortezomib-Induced Epigenetic Alterations in Nerve Cells: Focus on the Mechanisms Contributing to the Peripheral Neuropathy Development" International Journal of Molecular Sciences 23, no. 5: 2431. https://doi.org/10.3390/ijms23052431
APA StyleŁuczkowska, K., Rogińska, D., Kulig, P., Bielikowicz, A., Baumert, B., & Machaliński, B. (2022). Bortezomib-Induced Epigenetic Alterations in Nerve Cells: Focus on the Mechanisms Contributing to the Peripheral Neuropathy Development. International Journal of Molecular Sciences, 23(5), 2431. https://doi.org/10.3390/ijms23052431






