A Connecting Link between Hyaluronan Synthase 3-Mediated Hyaluronan Production and Epidermal Function
Abstract
:1. Introduction
2. Results
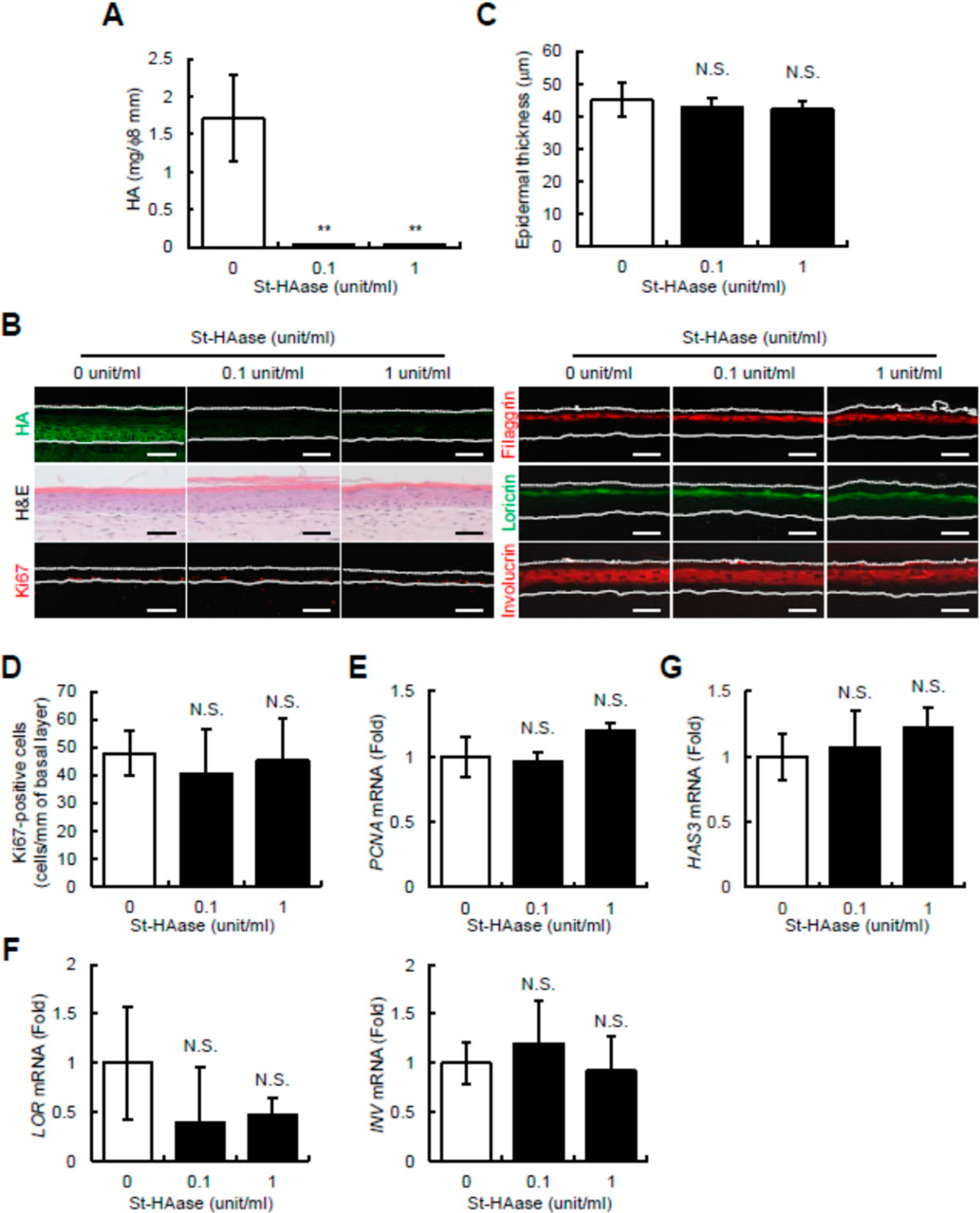
2.1. Streptomyces Hyaluronidase Does Not Change Epidermal Thickness, Keratinocyte Proliferation and Differentiation, or HAS3 mRNA Expression in Reconstructed Human Skin Equivalents
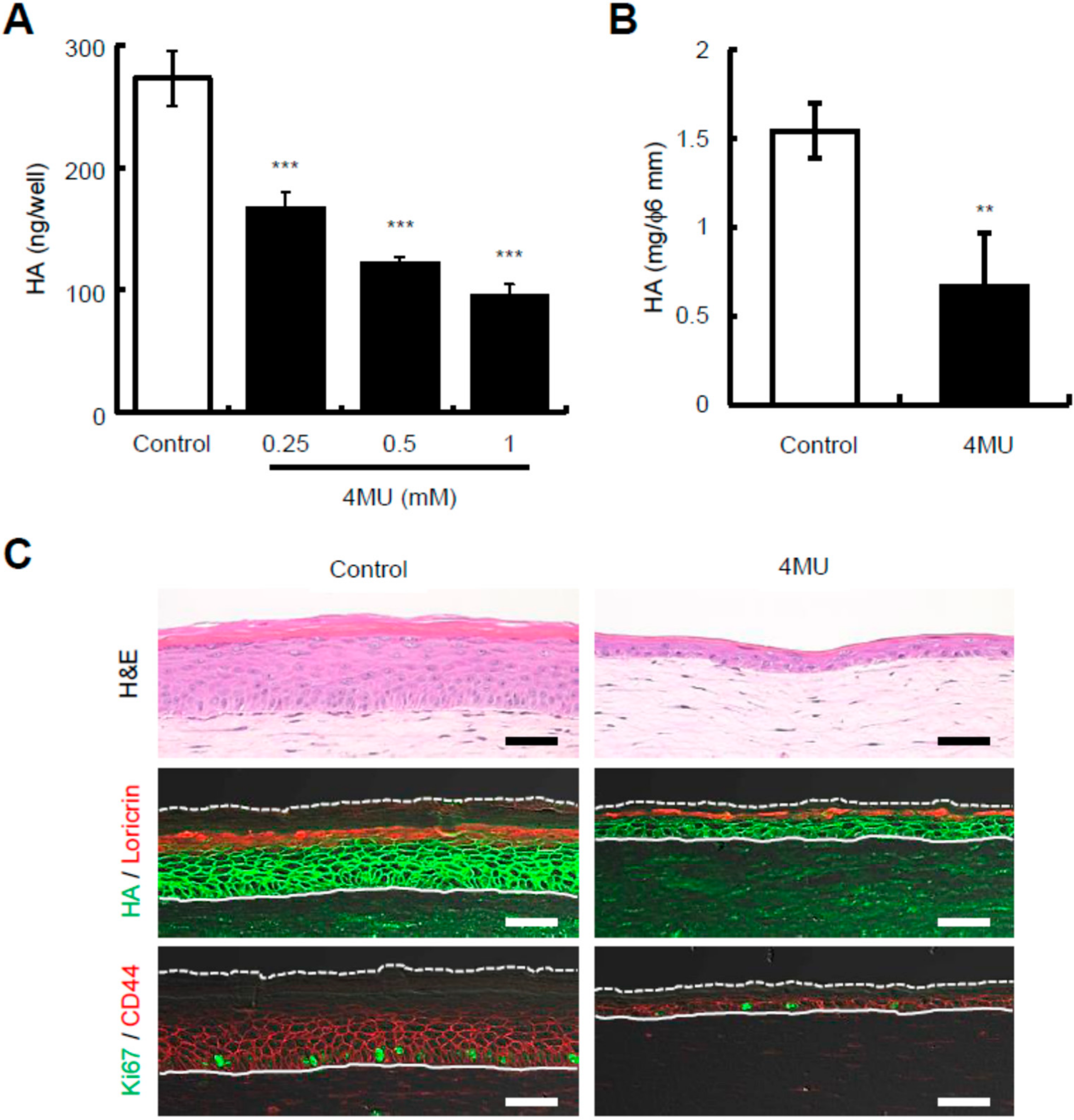
2.2. Presence of 4-Methylumbelliferone Decreases Epidermal Thickness and Keratinocyte Proliferation in Reconstructed Human Skin Equivalents
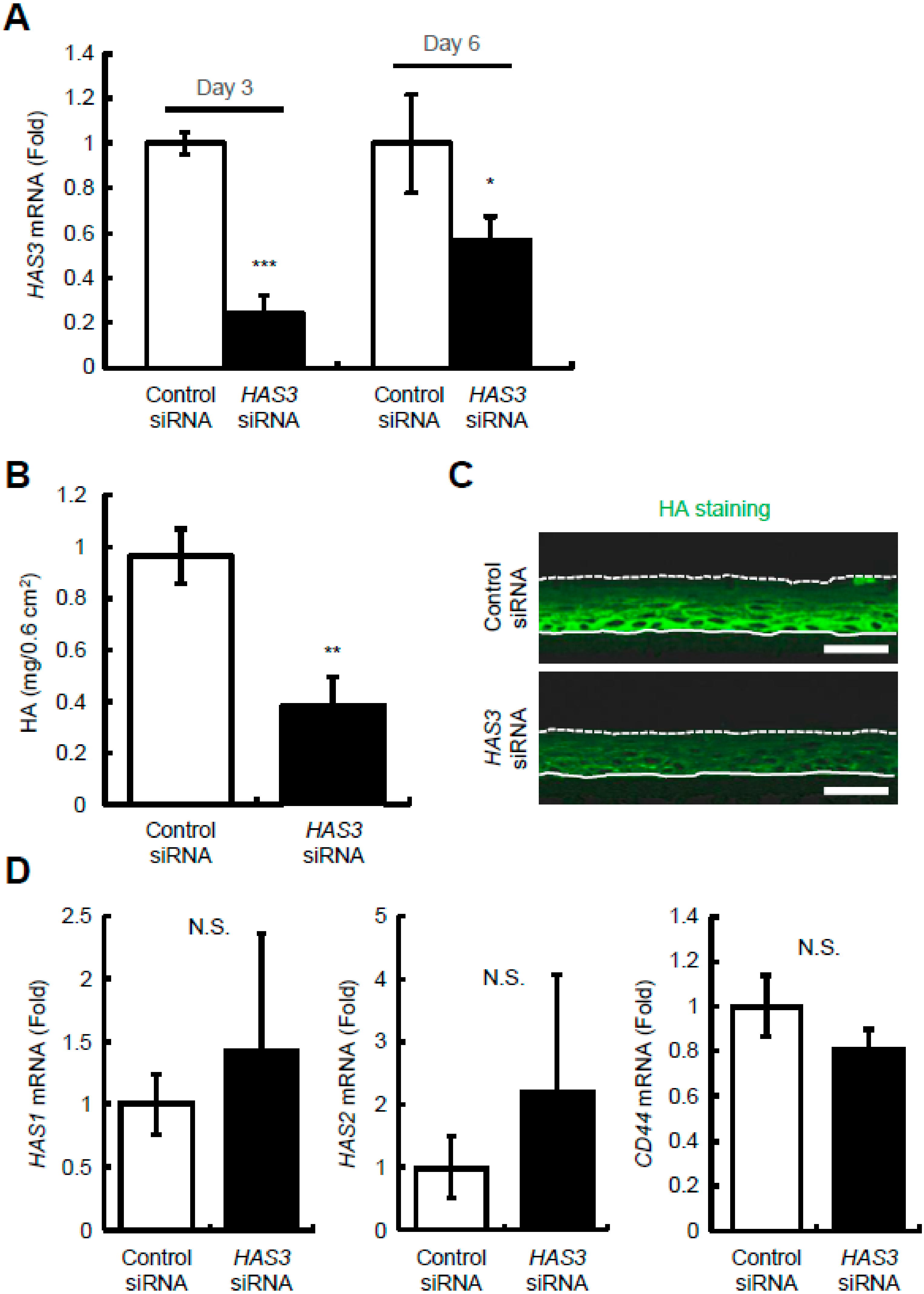
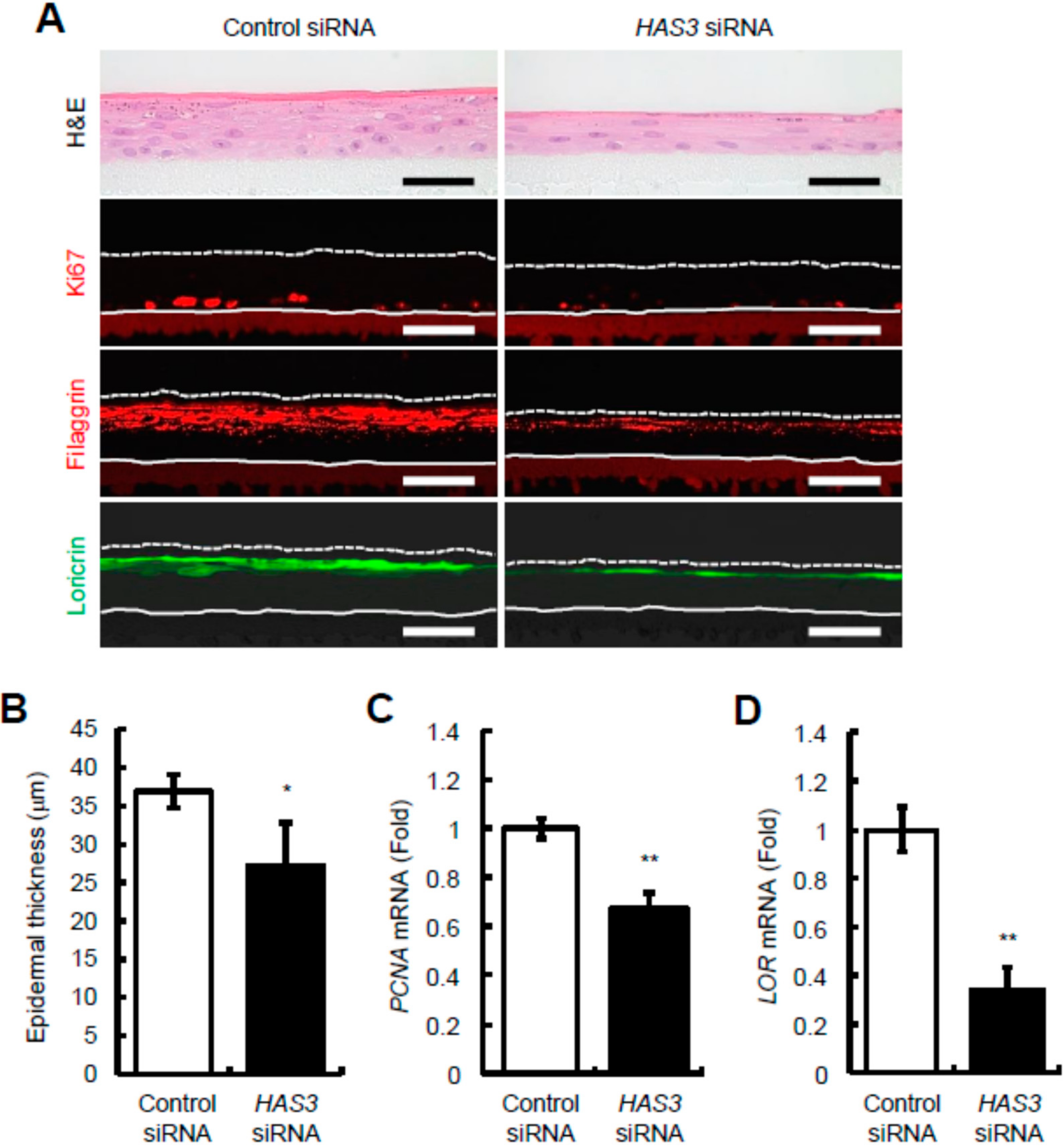
2.3. Reduction of HAS3-Mediated HA Production Decreases Epidermal Thickness and Keratinocyte Proliferation and Differentiation in Reconstructed Human Epidermal Equivalents
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Keratinocyte Cultures
4.3. Reconstruction of Full-Thickness Human Skin Equivalents
4.4. RNA Interference
4.5. Reconstruction of Human Epidermal Equivalents
4.6. Measurement of HA Content
4.7. Quantitative Real-Time PCR
4.8. Histological and Immunohistochemical Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Tammi, M.; Tammi, R. Distribution of Hyaluronan and Its CD44 Receptor in the Epithelia of Human Skin Appendages. Histochem. Cell Biol. 1992, 98, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Kimata, K. Expression Cloning and Molecular Characterization of HAS Protein, a Eukaryotic Hyaluronan Synthase. J. Biol. Chem. 1996, 271, 9875–9878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyjan, A.M.; Heldin, P.; Butcher, E.C.; Yoshino, T.; Briskin, M.J. Functional Cloning of the cDNA for a Human Hyaluronan Synthase. J. Biol. Chem. 1996, 271, 23395–23399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, K.; Yamaguchi, Y. Molecular Identification of a Putative Human Hyaluronan Synthase. J. Biol. Chem. 1996, 271, 22945–22948. [Google Scholar] [CrossRef] [Green Version]
- Weigel, P.H.; Hascall, V.C.; Tammi, M. Hyaluronan Synthases. J. Biol. Chem. 1997, 272, 13997–14000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rilla, K.; Siiskonen, H.; Spicer, A.P.; Hyttinen, J.M.T.; Tammi, M.I.; Tammi, R.H. Plasma Membrane Residence of Hyaluronan Synthase Is Coupled to Its Enzymatic Activity. J. Biol. Chem. 2005, 280, 31890–31897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayo, T.; Sugiyama, Y.; Takahashi, Y.; Ozawa, N.; Sakai, S.; Inoue, S.; Ishikawa, O.; Tamura, M. Hyaluronan Synthase 3 Regulates Hyaluronan Synthesis in Cultured Human Keratinocytes. J. Investig. Dermatol. 2002, 118, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Sayo, T.; Sakai, S.; Inoue, S. Synergistic Effect of N-Acetylglucosamine and Retinoids on Hyaluronan Production in Human Keratinocytes. Ski. Pharmacol. Physiol. 2004, 17, 77–83. [Google Scholar] [CrossRef]
- Kakizaki, I.; Itano, N.; Kimata, K.; Hanada, K.; Kon, A.; Yamaguchi, M.; Takahashi, T.; Takagaki, K. Up-Regulation of Hyalu-ronan Synthase Genes in Cultured Human Epidermal Keratinocytes by UVB Irradiation. Arch. Biochem. Biophys. 2008, 471, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sayo, T.; Sugiyama, Y.; Inoue, S. Lutein, a. Nonprovitamin A, Activates the Retinoic Acid Receptor to Induce HAS3-Dependent Hyaluronan Synthesis in Keratinocytes. Biosci. Biotechnol. Biochem. 2013, 77, 1282–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaisse, J.; Bourguignon, V.; De Vuyst, E.; de Rouvroit, C.L.; Nikkels, A.; Flamion, B.; Poumay, Y. Hyaluronan Metabolism in Human Keratinocytes and Atopic Dermatitis Skin Is Driven by a Balance of Hyaluronan Synthases 1 and 3. J. Investig. Dermatol. 2014, 134, 2174–2182. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Amagai, M. Dissecting the Formation, Structure and Barrier Function of the Stratum Corneum. Int. Immunol. Erratum in. 2015, 27, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Pasonen-Seppänen, S.; Karvinen, S.; Törrönen, K.; Hyttinen, J.M.; Jokela, T.; Lammi, M.; Tammi, M.I.; Tammi, R. EGF Upregulates, whereas TGF-β Downregulates, the Hyaluronan Synthases Has2 and Has3 in Organotypic Keratinocyte Cultures: Correlations with Epidermal Proliferation and Differentiation. J. Investig. Dermatol. 2003, 120, 1038–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karvinen, S.; Pasonen-Seppänen, S.; Hyttinen, J.M.; Pienimäki, J.-P.; Törrönen, K.; Jokela, T.A.; Tammi, M.I.; Tammi, R. Keratinocyte Growth Factor Stimulates Migration and Hyaluronan Synthesis in the Epidermis by Activation of Keratinocyte Hyaluronan Synthases 2 and 3. J. Biol. Chem. 2003, 278, 49495–49504. [Google Scholar] [CrossRef] [Green Version]
- Tammi, R.; Ripellino, J.A.; Margolis, R.U.; Maibach, H.I.; Tammi, M. Hyaluronate Accumulation in Human Epidermis Treated with Retinoic Acid in Skin Organ Culture. J. Investig. Dermatol. 1989, 92, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasonen-Seppänen, S.M.; Maytin, E.V.; Törrönen, K.J.; Hyttinen, J.M.T.; Hascall, V.C.; MacCallum, D.K.; Kultti, A.H.; Jokela, T.A.; Tammi, M.I.; Tammi, R.H. All-Trans Retinoic Acid-Induced Hyaluronan Production and Hyperplasia Are Partly Mediated by EGFR Signaling in Epidermal Keratinocytes. J. Investig. Dermatol. 2008, 128, 797–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rilla, K.; Pasonen-Seppänen, S.; Rieppo, J.; Tammi, M.; Tammi, R. The Hyaluronan Synthesis Inhibitor 4-Methylumbelliferone Prevents Keratinocyte Activation and Epidermal Hyperproliferation Induced by Epidermal Growth Factor. J. Investig. Dermatol. 2004, 123, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourguignon, L.Y.; Ramez, M.; Gilad, E.; Singleton, P.A.; Man, M.Q.; Crumrine, D.A.; Elias, P.M.; Feingold, K.R. Hyaluronan-CD44 Interaction Stimulates Keratinocyte Differentiation, Lamellar Body Formation/Secretion, and Permeability Barrier Homeostasis. J. Investig. Dermatol. 2006, 126, 1356–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akazawa, Y.; Yoshida, H.; Endo, Y.; Sugita, J.; Yakumaru, M.; Sayo, T. 1-Ethyl-β-N-Acetylglucosaminide Increases Hyaluronan Production in Human Keratinocytes by Being Converted to N-Acetylglucosamine via β-N-Acetylglucosaminidase-Dependent Manner. Biosci. Biotechnol. Biochem. 2021, 85, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Yoshida, H.; Akazawa, Y.; Yamazaki, K.; Ota, Y.; Sayo, T.; Takahashi, Y. Antiwrinkle Efficacy of 1-Ethyl-β-N-Acetylglucosaminide, an Inducer of Epidermal Hyaluronan Production. Skin Res. Technol. 2022, 28, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Yoshida, H.; Ota, Y.; Akazawa, Y.; Sayo, T.; Hanai, U.; Imagawa, K.; Sasaki, M.; Takahashi, Y. Accelerated Human Epidermal Turnover Driven by Increased Hyaluronan Production. J. Dermatol. Sci. 2020, 101, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, J.; Pendaries, V.; Hontoir, F.; De Glas, V.; Van Vlaender, D.; Simon, M.; de Rouvroit, C.L.; Poumay, Y.; Flamion, B. Hyaluronan Does Not Regulate Human Epidermal Keratinocyte Proliferation and Differentiation. J. Biol. Chem. 2016, 291, 6347–6358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourguignon, L.Y. Matrix Hyaluronan-Activated CD44 Signaling Promotes Keratinocyte Activities and Improves Abnormal Epidermal Functions. Am. J. Pathol. 2014, 184, 1912–1919. [Google Scholar] [CrossRef] [Green Version]
- Tammi, R.H.; Passi, A.G.; Rilla, K.; Karousou, E.; Vigetti, D.; Makkonen, K.; Tammi, M.I. Transcriptional and Post-Translational Regulation of Hyaluronan Synthesis. FEBS J. 2011, 278, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-P.; Pan, Y.-R.; Fu, C.-Y.; Chang, H.-Y. Down-Regulation of UDP-Glucose Dehydrogenase Affects Glycosaminoglycans Synthesis and Motility in HCT-8 Colorectal Carcinoma Cells. Exp. Cell Res. 2010, 316, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, I.; Kojima, K.; Takagaki, K.; Endo, M.; Kannagi, R.; Ito, M.; Maruo, Y.; Sato, H.; Yasuda, T.; Mita, S.; et al. A Novel Mechanism for the Inhibition of Hyaluronan Biosynthesis by 4-Methylumbelliferone. J. Biol. Chem. 2004, 279, 33281–33289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigetti, D.; Rizzi, M.; Viola, M.; Karousou, E.; Genasetti, A.; Clerici, M.; Bartolini, B.; Hascall, V.C.; De Luca, G.; Passi, A. The Effects of 4-Methylumbelliferone on Hyaluronan Synthesis, MMP2 Activity, Proliferation, and Motility of Human Aortic Smooth Muscle Cells. Glycobiology 2009, 19, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kultti, A.; Pasonen-Seppänen, S.; Jauhiainen, M.; Rilla, K.J.; Kärnä, R.; Pyöriä, E.; Tammi, R.H.; Tammi, M.I. 4-Methylumbelliferone Inhibits Hyaluronan Synthesis by Depletion of Cellular UDP-Glucuronic Acid and Downregulation of Hyaluronan Synthase 2 and 3. Exp. Cell Res. 2009, 315, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Yokoyama, Y.; Yoshida, H.; Imaizumi, T.; Mizunuma, H. 4-Methylumbelliferone Inhibits Ovarian Cancer Growth by Suppressing Thymidine Phosphorylase Expression. J. Ovarian Res. 2014, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Lopez, L.E.; Munoz, D.; Chi, A.; Shirodkar, S.P.; Lokeshwar, S.D.; Escudero, D.O.; Dhir, N.; Altman, N. An-titumor Activity of Hyaluronic Acid Synthesis Inhibitor 4-Methylumbelliferone in Prostate Cancer Cells. Cancer Res. 2010, 70, 2613–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urakawa, H.; Nishida, Y.; Wasa, J.; Arai, E.; Zhuo, L.; Kimata, K.; Kozawa, E.; Futamura, N.; Ishiguro, N. Inhibition of Hya-luronan Synthesis in Breast Cancer Cells by 4-Methylumbelliferone Suppresses Tumorigenicity in Vitro and Metastatic Lesions of Bone in Vivo. Int. J. Cancer 2012, 130, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, F.; Malvicini, M.; Garcia, M.G.; Rodriguez, A.; Atorrasagasti, C.; Kippes, N.; Buena, I.T.P.; Rizzo, M.M.; Bayo, J.; Aquino, J.B.; et al. Antitumor Effects of Hyaluronic Acid Inhibitor 4-Methylumbelliferone in an Orthotopic Hepatocellular Carcinoma Model in Mice. Glycobiology 2011, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Rizzi, M.; Moretto, P.; Deleonibus, S.; Dreyfuss, J.; Karousou, E.; Viola, M.; Clerici, M.; Hascall, V.C.; Ramoni, M.F.; et al. Glycosaminoglycans and Glucose Prevent Apoptosis in 4-Methylumbelliferone-Treated Human Aortic Smooth Muscle Cells. J. Biol. Chem. 2011, 286, 34497–34503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kage, M.; Tokudome, Y.; Matsunaga, Y.; Hariya, T.; Hashimoto, F. Effect of Hyaluronan Tetrasaccharides on Epidermal Dif-ferentiation in Normal Human Epidermal Keratinocytes. Int. J. Cosmet. Sci. 2014, 36, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Kage, M.; Tokudome, Y. Hyaluronan Tetrasaccharides Stimulate Ceramide Production through Upregulated mRNA Expression of Ceramide Synthesis-Associated Enzymes. Arch. Dermatol. Res. 2015, 308, 95–101. [Google Scholar] [CrossRef]
- Hascall, V.C.; Wang, A.; Tammi, M.; Oikari, S.; Tammi, R.; Passi, A.; Vigetti, D.; Hanson, R.W.; Hart, G.W. The Dynamic Me-tabolism of Hyaluronan Regulates the Cytosolic Concentration of UDP-GlcNAc. Matrix Biol. 2014, 35, 14–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Oh, J.-H.; Chung, J.H. Glycosaminoglycan and Proteoglycan in Skin Aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Zimmermann, D.R.; Dours-Zimmermann, M.T.; Schubert, M.; Bruckner-Tuderman, L. Versican Is Expressed in the Proliferating Zone in the Epidermis and in Association with the Elastic Network of the Dermis. J. Cell Biol. 1994, 124, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liu, F.; Tao, T.; Zhang, D.; Liu, X.; Zhu, G.; Xu, Z.; Ni, R.; Shen, A. Modification of P27 with O-Linked N-Acetylglucosamine Regulates Cell Proliferation in Hepatocellular Carcinoma. Mol. Carcinog. 2016, 56, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Bergós, J.; Tardio, L.; Larranaga-Vera, A.; Gómez, R.; Herrero-Beaumont, G.; Largo, R. The Increase in O-Linked N-Acetylglucosamine Protein Modification Stimulates Chondrogenic Differentiation Both in Vitro and in Vivo. J. Biol. Chem. 2012, 287, 33615–33628. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Mizofuchi, H.; Kobayashi, Y.; Tsuzuki, G.; Yamamoto, M.; Wada, S.; Kamemura, K. Terminal Differentiation Program of Skeletal Myogenesis Is Negatively Regulated by o-GlcNAc Glycosylation. Biochim. Biophys. Acta 2012, 1820, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Takahashi, I.; Tsuchiya, Y.; Hasegawa, M.; Kamemura, K. Characteristic Increase in Nucleocytoplasmic Protein Glycosylation by o-GlcNAc in 3T3-L1 Adipocyte Differentiation. Biochem. Biophys. Res. Commun. 2010, 398, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Delehedde, M.; Lyon, M.; Sergeant, N.; Rahmoune, H.; Fernig, D.G. Proteoglycans: Pericellular and Cell Surface Multireceptors that Integrate External Stimuli in the Mammary Gland. J. Mammary Gland Biol. Neoplasia 2001, 6, 253–273. [Google Scholar] [CrossRef]
- Perrot, G.; Colin-Pierre, C.; Ramont, L.; Proult, I.; Garbar, C.; Bardey, V.; Jeanmaire, C.; Mine, S.; Danoux, L.; Berthélémy, N.; et al. Decreased Expression of GPC1 in Human Skin Keratinocytes and Epidermis during Ageing. Exp. Gerontol. 2019, 126, 110693. [Google Scholar] [CrossRef]
- Ojeh, N.; Hiilesvuo, K.; Wärri, A.; Salmivirta, M.; Henttinen, T.; Määttä, A. Ectopic Expression of Syndecan-1 in Basal Epidermis Affects Keratinocyte Proliferation and Wound Re-Epithelialization. J. Investig. Dermatol. 2008, 128, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.-J.; Spicer, A.P.; Mascarenhas, M.M.; Yu, L.; Ochoa, C.; Garg, H.G.; Quinn, D.A. The Role of Hyaluronan Synthase 3 in Ventilator-induced Lung Injury. Am. J. Respir. Crit. Care Med. 2005, 172, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Miyoshi, S.; Mikami, T.; Koyama, H.; Kitazawa, M.; Takeoka, M.; Sano, K.; Amano, J.; Isogai, Z.; Niida, S.; et al. Hyaluronan Deficiency in Tumor Stroma Impairs Macrophage Trafficking and Tumor Neovascularization. Cancer Res. 2010, 70, 7073–7083. [Google Scholar] [CrossRef] [Green Version]
- Mack, J.A.; Feldman, R.J.; Itano, N.; Kimata, K.; Lauer, M.; Hascall, V.C.; Maytin, E.V. Enhanced Inflammation and Accelerated Wound Closure Following Tetraphorbol Ester Application or Full-Thickness Wounding in Mice Lacking Hyaluronan Synthases Has1 and Has. J. Investig. Dermatol. 2012, 132, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Gariboldi, S.; Palazzo, M.; Zanobbio, L.; Selleri, S.; Sommariva, M.; Sfondrini, L.; Cavicchini, S.; Balsari, A.; Rumio, C. Low Molecular Weight Hyaluronic Acid Increases the Self-Defense of Skin Epithelium by Induction of β-Defensin 2 via TLR2 and TLR. J. Immunol. 2008, 181, 2103–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.; Simon, J.C. Differential Regulation of Hyaluronan Metabolism in the Epidermal and Dermal Compartments of Human Skin by UVB Irradiation. J. Investig. Dermatol. 2007, 127, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauhala, L.; Jokela, T.; Kärnä, R.; Bart, G.; Takabe, P.; Oikari, S.; Tammi, M.I.; Pasonen-Seppänen, S.; Tammi, R.H. Extracellular ATP Activates Hyaluronan Synthase 2 (HAS2) in Epidermal Keratinocytes via P2Y2, Ca2+ Signaling, and MAPK Pathways. Biochem. J. 2018, 475, 1755–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ota, Y.; Yoshida, H.; Endo, Y.; Sayo, T.; Takahashi, Y. A Connecting Link between Hyaluronan Synthase 3-Mediated Hyaluronan Production and Epidermal Function. Int. J. Mol. Sci. 2022, 23, 2424. https://doi.org/10.3390/ijms23052424
Ota Y, Yoshida H, Endo Y, Sayo T, Takahashi Y. A Connecting Link between Hyaluronan Synthase 3-Mediated Hyaluronan Production and Epidermal Function. International Journal of Molecular Sciences. 2022; 23(5):2424. https://doi.org/10.3390/ijms23052424
Chicago/Turabian StyleOta, Yukiko, Hiroyuki Yoshida, Yoko Endo, Tetsuya Sayo, and Yoshito Takahashi. 2022. "A Connecting Link between Hyaluronan Synthase 3-Mediated Hyaluronan Production and Epidermal Function" International Journal of Molecular Sciences 23, no. 5: 2424. https://doi.org/10.3390/ijms23052424
APA StyleOta, Y., Yoshida, H., Endo, Y., Sayo, T., & Takahashi, Y. (2022). A Connecting Link between Hyaluronan Synthase 3-Mediated Hyaluronan Production and Epidermal Function. International Journal of Molecular Sciences, 23(5), 2424. https://doi.org/10.3390/ijms23052424





