Lycium barbarum Polysaccharides and Capsaicin Inhibit Oxidative Stress, Inflammatory Responses, and Pain Signaling in Rats with Dextran Sulfate Sodium-Induced Colitis
Abstract
:1. Introduction
2. Results
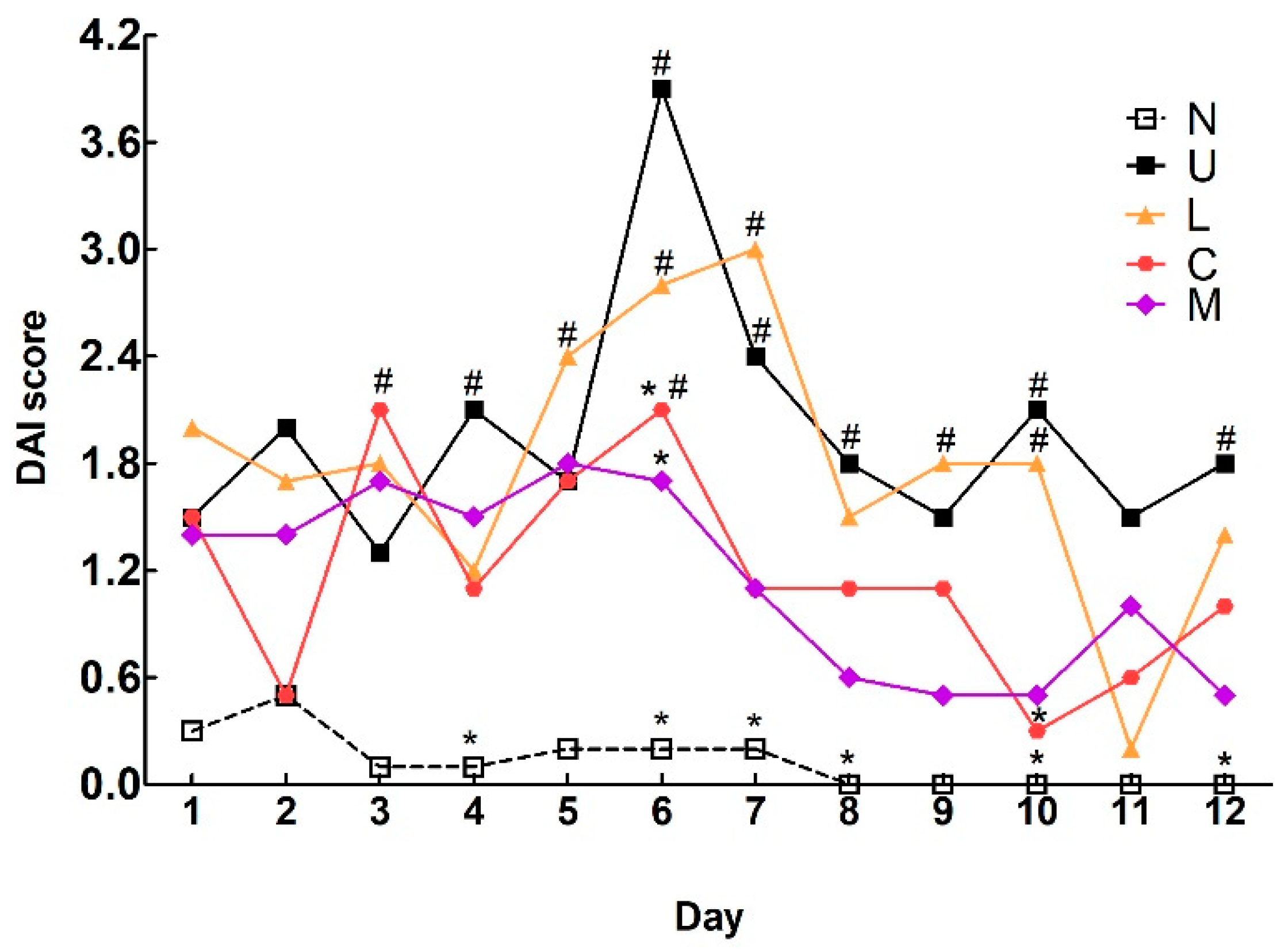
2.1. Effects of LBP and CAP on Body Weight and Colitis Symptoms
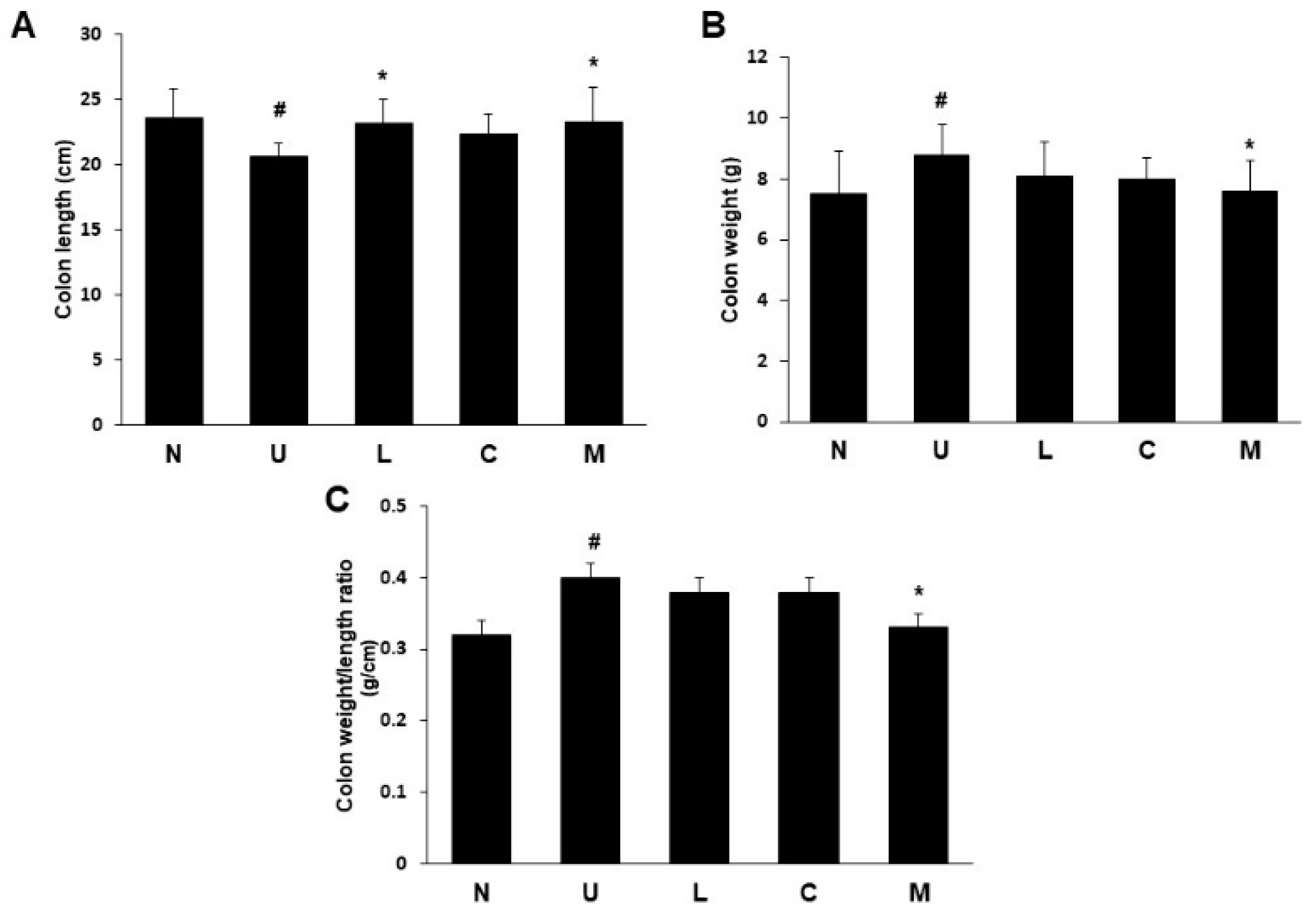
2.2. Effects of LBP and CAP on Colon Length and Weight
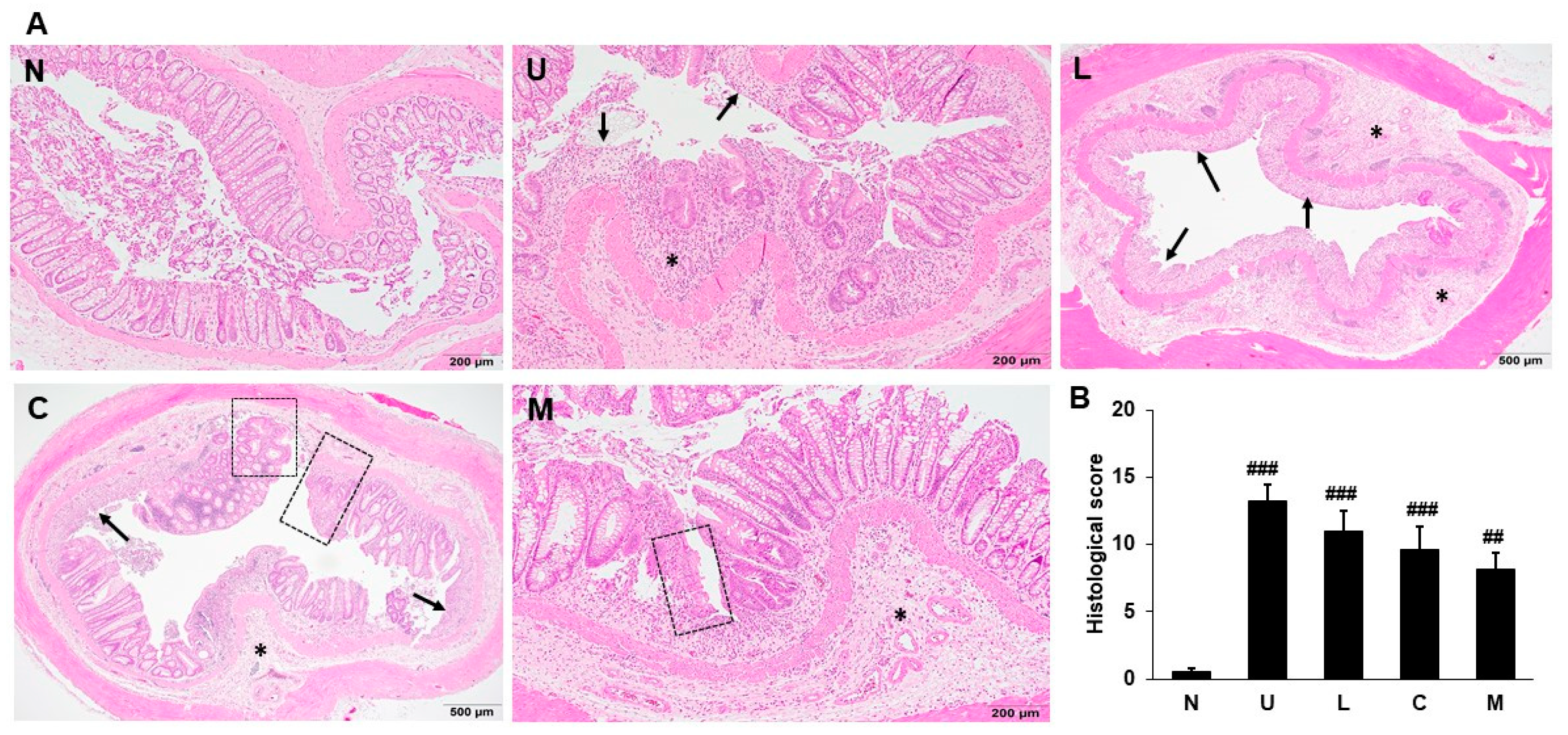
2.3. Effects of LBP and CAP on Histopathological Changes
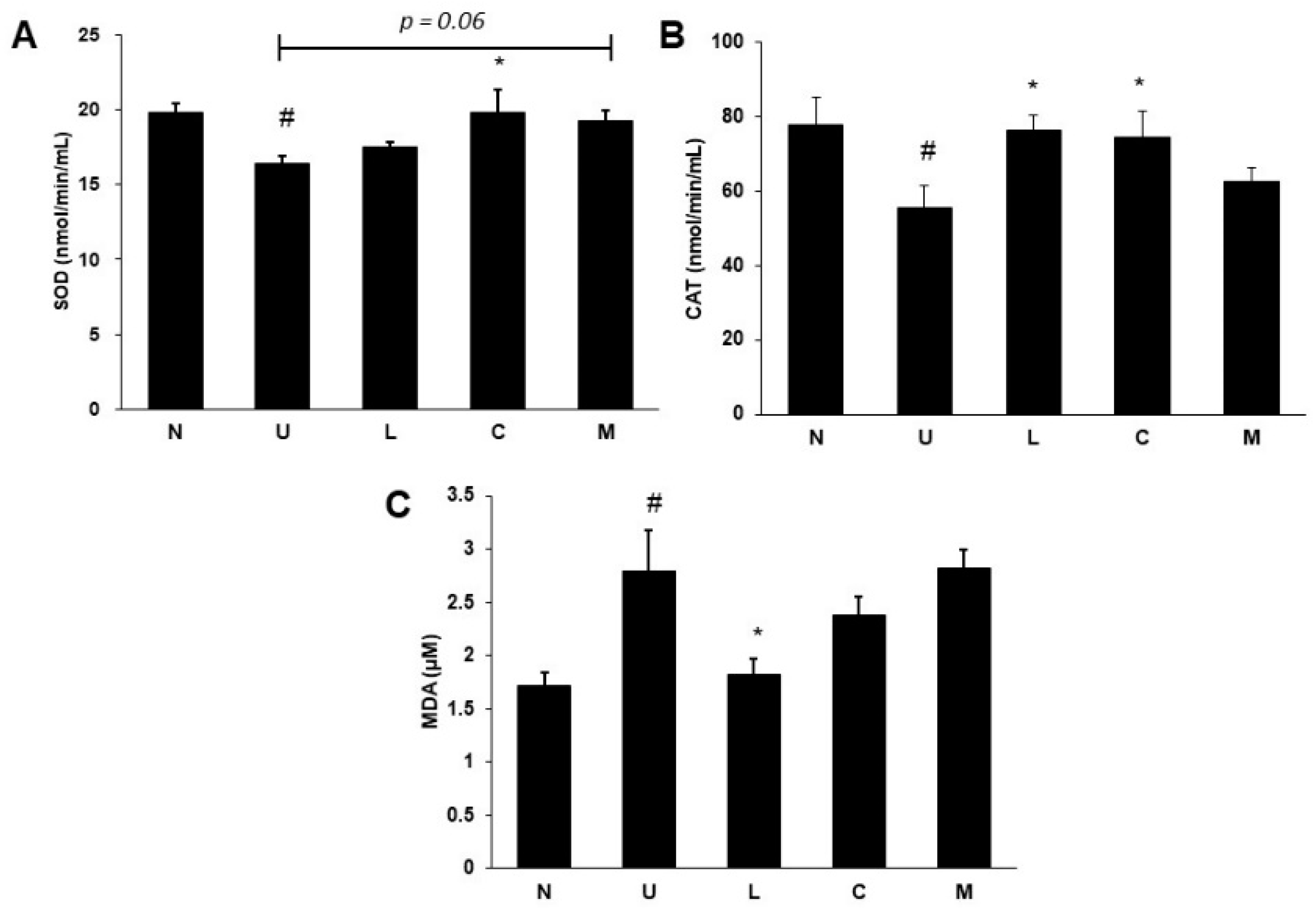
2.4. Effects of LBP and CAP on Antioxidative Enzymes and Oxidative Marker
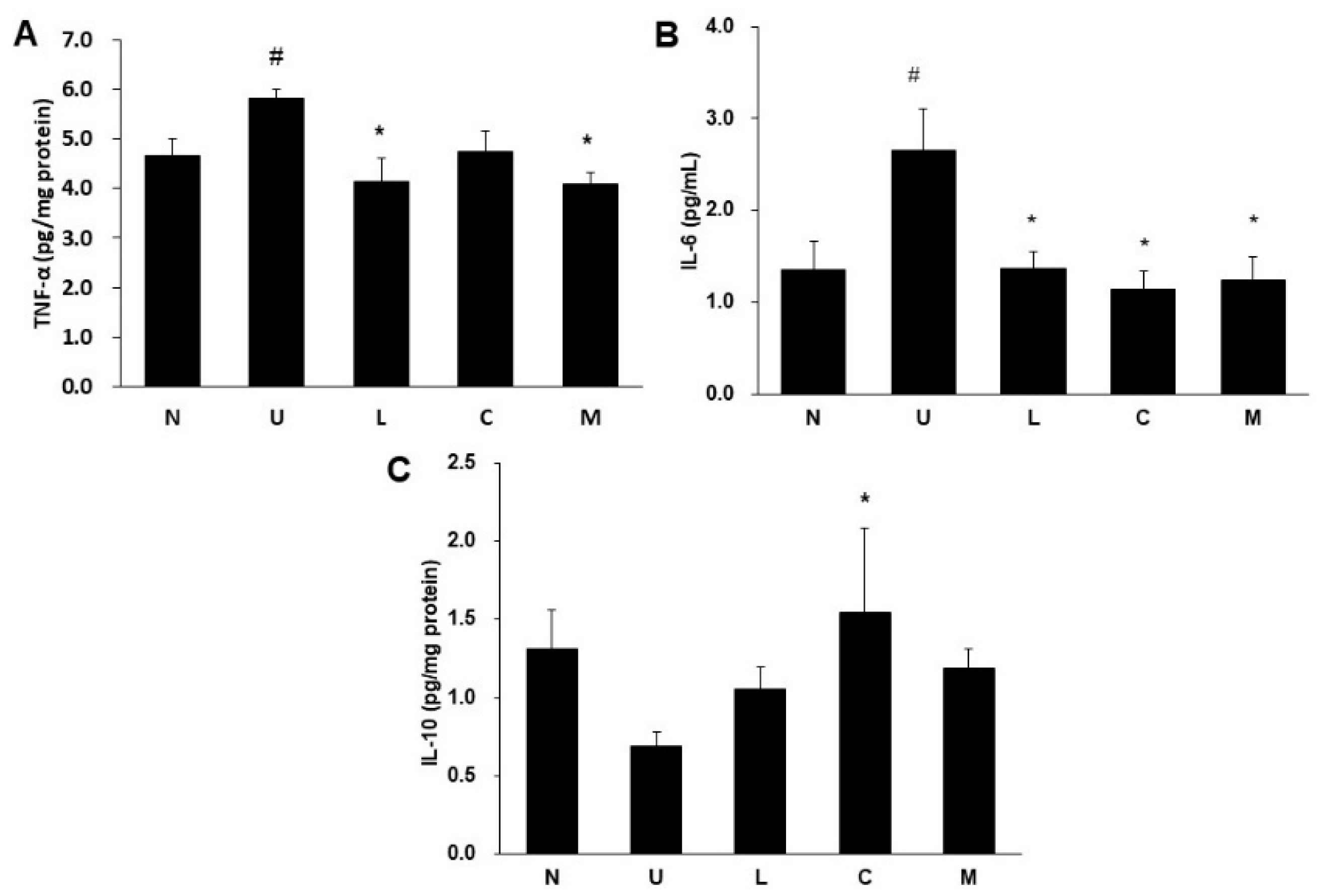
2.5. Effects of LBP and CAP on Inflammatory Cytokines and Cyclooxygenase-2 (COX-2) Protein
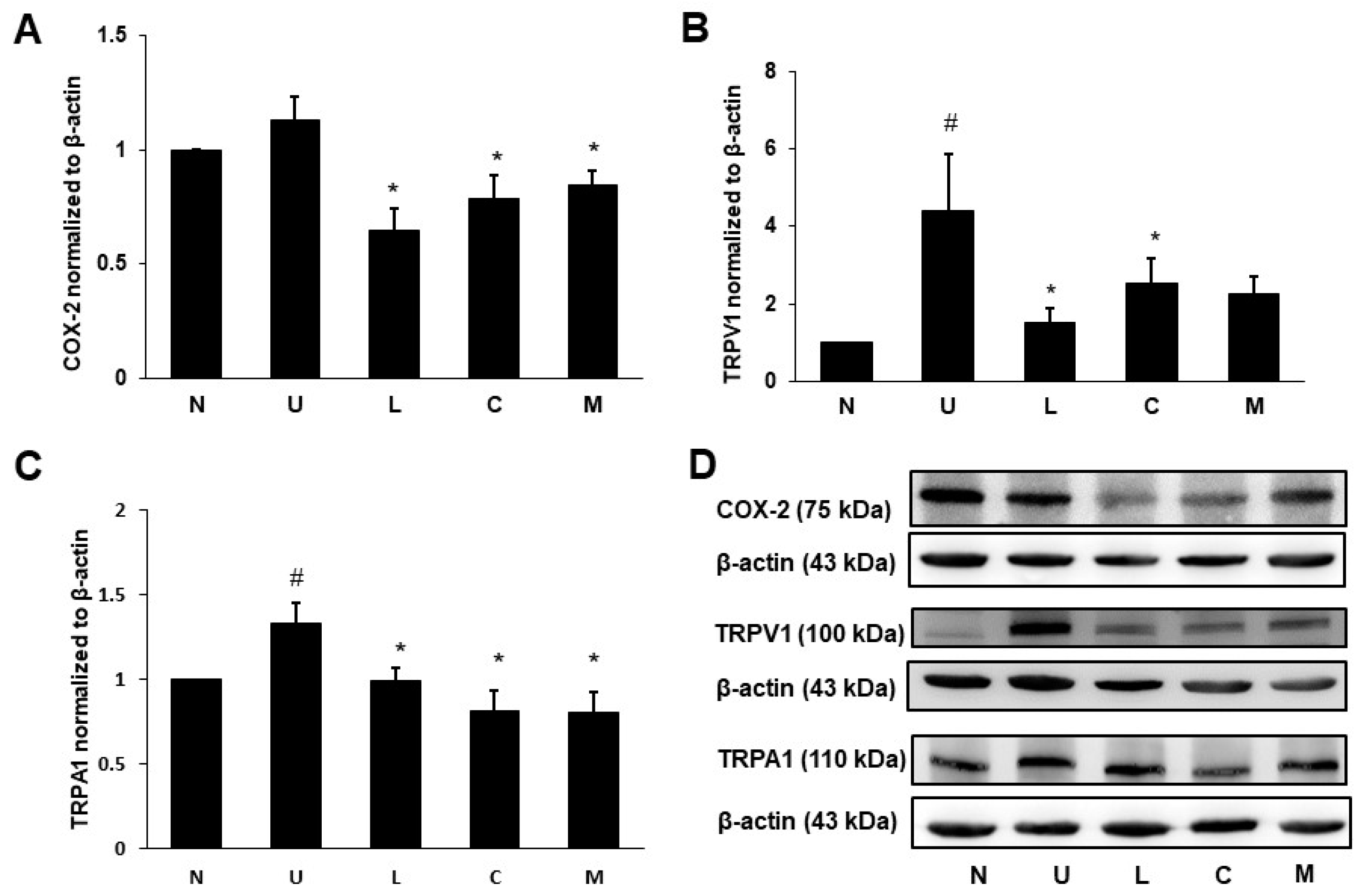
2.6. Effects of LBP and CAP on Pain Signaling Protein Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Treatments
4.4. Histological Evaluation of Colonic Ulceration
4.5. Measurement of Serum SOD, CAT, and MDA
4.6. Measurement of Proinflammatory and Anti-Inflammatory Cytokines
4.7. Electrophoresis and Western Blot for COX-2 and Pain Signaling Proteins
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Yen, H.-H.; Weng, M.-T.; Tung, C.-C.; Wang, Y.-T.; Chang, Y.T.; Chang, C.-H.; Shieh, M.-J.; Wong, J.-M.; Wei, S.-C. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: A nationwide populationbased study. Intest. Res. 2019, 17, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerling, B.J.; Dagnelie, P.C.; Badart-Smook, A.; Russel, M.G.; Stockbrügger, R.W.; Brummer, R.J. Diet as a risk factor for the development of ulcerative colitis. Am. J. Gastroenterol. 2000, 95, 1008–1013. [Google Scholar] [CrossRef]
- Carbonnel, F.; Jantchou, P.; Monnet, E.; Cosnes, J. Environmental risk factors in Crohn’s disease and ulcerative colitis: An update. Gastroenterol. Clin. Biol. 2009, 33, S145–S157. [Google Scholar] [CrossRef]
- Cosnes, J. Smoking, physical activity, nutrition and lifestyle: Environmental factors and their impact on IBD. Dig. Dis. 2010, 28, 411–417. [Google Scholar] [CrossRef]
- Nikkhah-Bodaghi, M.; Maleki, I.; Agah, S.; Hekmatdoost, A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement. Ther. Med. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Ajayi, B.O.; Adedara, I.A.; Farombi, E.O. Pharmacological activity of 6-gingerol in dextran sulphate sodium-induced ulcerative colitis in BALB/c mice. Phytother. Res. 2015, 29, 566–572. [Google Scholar] [CrossRef]
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 778–788. [Google Scholar] [CrossRef]
- Vermeulen, W.; De Man, J.G.; De Schepper, H.U.; Bult, H.; Moreels, T.G.; Pelckmans, P.A.; De Winter, B.Y. Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur. J. Pharmacol. 2013, 698, 404–412. [Google Scholar] [CrossRef]
- Xu, C.-T.; Meng, S.-Y.; Pan, B.-R. Drug therapy for ulcerative colitis. World J. Gastroenterol. 2004, 10, 2311–2317. [Google Scholar] [CrossRef]
- Li, R.; Kim, M.H.; Sandhu, A.K.; Gao, C.; Gu, L. Muscadine grape (Vitis rotundifolia) or wine phytochemicals reduce intestinal inflammation in mice with dextran sulfate sodium-induced colitis. J. Agric. Food Chem. 2017, 65, 769–776. [Google Scholar] [CrossRef]
- Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Xie, J.-H.; Tang, W.; Jin, M.-L.; Li, J.-E.; Xie, M.-Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Reyes-Escogido Mde, L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [Green Version]
- Hayman, M.; Kam, P.C. Capsaicin: A review of its pharmacology and clinical applications. Cuur. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin-the major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, N.; Shim, Y.K.; Choi, Y.J.; Nam, R.H.; Choi, Y.J.; Ham, M.H.; Suh, J.H.; Lee, S.M.; Lee, C.M.; et al. Adequate dextran sodium sulfate-induced colitis model in mice and effective outcome measurement method. J. Cancer Prev. 2015, 20, 260–267. [Google Scholar] [CrossRef]
- Boussenna, A.; Cholet, J.; Goncalves-Mendes, N.; Joubert-Zakeyh, J.; Fraisse, D.; Vasson, M.P.; Texier, O.; Felgines, C. Polyphenol-rich grape pomace extracts protect against dextran sulfate sodium-induced colitis in rats. J. Sci. Food Agric. 2016, 96, 1260–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, M.; Guanhua, L.; Zhanhai, Y.; Guang, C.; Xuan, Z. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 2009, 113, 872–877. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhao, G.X.; He, L.L.; Wang, Z.; Zibrila, A.I.; Niu, B.C.; Gong, H.Y.; Xu, J.N.; Soong, L.; Li, C.F.; et al. Lycium barbarum polysaccharides inhibit ischemia/reperfusion-induced myocardial injury via the Nrf2 antioxidant pathway. Toxicol. Rep. 2021, 8, 657–667. [Google Scholar] [CrossRef]
- Mendivil, E.J.; Sandoval-Rodriguez, A.; Zuñiga-Ramos, L.M.; Santos-Garcia, A.; Armendariz-Borunda, J. Capsaicin and sulforaphane prevent experimental liver fibrosis via upregulation of peroxisome proliferator-activated receptor gamma and nuclear factor (erythroid-derived 2)-like 2. J. Funct. Foods 2019, 52, 382–388. [Google Scholar] [CrossRef]
- Hur, S.J.; Kang, S.H.; Jung, H.S.; Kim, S.C.; Jeon, H.S.; Kim, I.H.; Lee, J.D. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr. Res. 2012, 32, 801–816. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef]
- Zhang, D.K.; Cheng, L.N.; Huang, X.L.; Shi, W.; Xiang, J.Y.; Gan, H.T. Tetrandrine ameliorates dextran-sulfate-sodium-induced colitis in mice through inhibition of nuclear factor-κB activation. Int. J. Colorectal Dis. 2009, 24, 5–12. [Google Scholar] [CrossRef]
- Li, W.; Gao, M.; Han, T. Lycium barbarum polysaccharides ameliorate intestinal barrier dysfunction and inflammation through the MLCK-MLC signaling pathway in Caco-2 cells. Food Funct. 2020, 11, 3741–3748. [Google Scholar] [CrossRef]
- Kang, Y.; Xue, Y.; Du, M.; Zhu, M.J. Preventive effects of Goji berry on dextran-sulfate-sodium-induced colitis in mice. J. Nutr. Biochem. 2017, 40, 70–76. [Google Scholar] [CrossRef]
- Kim, C.S.; Kawada, T.; Kim, B.S.; Han, I.S.; Choe, S.Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IκB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Melgar, S.; Yeung, M.M.; Bas, A.; Forsberg, G.; Suhr, O.; Oberg, A.; Hammarstrom, S.; Danielsson, A.; Hammarstrom, M.L. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin. Exp. Immunol. 2003, 134, 127–137. [Google Scholar] [CrossRef]
- Toyoda, T.; Shi, L.; Takasu, S.; Cho, Y.M.; Kiriyama, Y.; Nishikawa, A.; Ogawa, K.; Tatematsu, M.; Tsukamoto, T. Anti-inflammatory effects of capsaicin and piperine on Helicobacter pylori-induced chronic gastritis in mongolian gerbils. Helicobacter 2016, 21, 131–142. [Google Scholar] [CrossRef]
- Zhou, Y.; Guan, X.; Zhu, W.; Liu, Z.; Wang, X.; Yu, H.; Wang, H. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 211–219. [Google Scholar] [CrossRef]
- Nevius, E.; Srivastava, P.K.; Basu, S. Oral ingestion of capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunol. 2012, 5, 76–86. [Google Scholar] [CrossRef]
- Hussein, A.H.; Freund, J.; Reimund, J.; Shams, A.; Yamine, M.; Leone, A.; Jurjus, A. Enteropathogenic E. coli sustains iodoacetamide-induced ulcerative colitis-like colitis in rats: Modulation of IL-1β, IL-6, TNF-α, COX-2, and apoptosis. J. Biol. Regul. Homeost. Agents 2012, 26, 515–526. [Google Scholar]
- Hegazi, R.A.F.; Saad, R.S.; Mady, H.; Matarese, L.E.; O’Keefe, S.; Kandil, H.M. Dietary fatty acids modulate chronic colitis, colitis-associated colon neoplasia and COX-2 expression in IL-10 knockout mice. Nutrition 2006, 22, 275–282. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Engel, M.A.; Becker, C.; Reeh, P.W.; Neurath, M.F. Role of sensory neurons in colitis: Increasing evidence for a neuroimmune link in the gut. Inflamm. Bowel Dis. 2011, 17, 1030–1033. [Google Scholar] [CrossRef]
- Szitter, I.; Pozsgai, G.; Sandor, K.; Elekes, K.; Kemeny, A.; Perkecz, A.; Szolcsanyi, J.; Helyes, Z.; Pinter, E. The role of transient receptor potential vanilloid 1 (TRPV1) receptors in dextran sulfate-induced colitis in mice. J. Mol. Neurosci. 2010, 42, 80–88. [Google Scholar] [CrossRef]
- De Schepper, H.U.; De Man, J.G.; Ruyssers, N.E.; Deiteren, A.; Van Nassauw, L.; Timmermans, J.P.; Martinet, W.; Herman, A.G.; Pelckmans, P.A.; De Winter, B.Y. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G245–G253. [Google Scholar] [CrossRef] [Green Version]
- Engel, M.A.; Leffler, A.; Niedermirtl, F.; Babes, A.; Zimmermann, K.; Filipović, M.R.; Izydorczyk, I.; Mirjam, E.; Kichko, T.I.; Mueller-Tribbensee, S.M.; et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology 2011, 141, 1346–1358. [Google Scholar] [CrossRef]
- Csekő, K.; Beckers, B.; Keszthelyi, D.; Helyes, Z. Role of TRPV1 and TRPA1 ion channels in inflammatory bowel diseases: Potential therapeutic targets? Pharmaceuticals 2019, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Kulczyński, B.; Gramza-Michałowska, A. Goji berry (Lycium barbarum): Composition and health effects—A review. Pol. Int. J. Food Sci. 2016, 66, 67–76. [Google Scholar] [CrossRef]
- Xie, J.; Liu, Y.; Chen, B.; Zhang, G.; Ou, S.; Luo, J.; Peng, X. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 2019, 63, 1559. [Google Scholar] [CrossRef]
| N | U | L | C | M | |
|---|---|---|---|---|---|
| Body weight (g) | |||||
| Week 0 | 229.4 ± 5.9 | 226.3 ± 4.3 | 231.2 ± 4.0 | 229.9 ± 5.6 | 234.7 ± 5.6 |
| Week 1 | 272.6 ± 9.1 | 267.6 ± 7.9 | 277.7 ± 9.5 | 274.5 ± 8.2 | 277.2 ± 9.4 |
| Week 3 | 351.6 ± 20.9 | 336.3 ± 17.5 | 350.0 ± 19.7 | 341.9 ± 14.8 | 353.9 ± 16.2 * |
| Week 4 | 379.4 ± 27.7 | 357.4 ± 22.7 # | 370.6 ± 22.5 | 362.5 ± 20.6 | 381.2 ± 17.6 * |
| Food intake (g/d) | |||||
| Week 1 | 27.8 ± 1.3 | 29.5 ± 2.2 | 30.1 ± 2.6 | 29.7 ± 1.9 | 29.0 ± 3.4 |
| Week 4 | 28.2 ± 1.8 | 29.2 ± 3.3 | 28.8 ± 2.3 | 28.8 ± 2.2 | 27.8 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-S.; Lian, Y.Z.; Chen, W.-C.; Chang, C.-C.; Tinkov, A.A.; Skalny, A.V.; Chao, J.C.-J. Lycium barbarum Polysaccharides and Capsaicin Inhibit Oxidative Stress, Inflammatory Responses, and Pain Signaling in Rats with Dextran Sulfate Sodium-Induced Colitis. Int. J. Mol. Sci. 2022, 23, 2423. https://doi.org/10.3390/ijms23052423
Chen Y-S, Lian YZ, Chen W-C, Chang C-C, Tinkov AA, Skalny AV, Chao JC-J. Lycium barbarum Polysaccharides and Capsaicin Inhibit Oxidative Stress, Inflammatory Responses, and Pain Signaling in Rats with Dextran Sulfate Sodium-Induced Colitis. International Journal of Molecular Sciences. 2022; 23(5):2423. https://doi.org/10.3390/ijms23052423
Chicago/Turabian StyleChen, Yu-Shan, Yu Zhi Lian, Wen-Chao Chen, Chun-Chao Chang, Alexey A. Tinkov, Anatoly V. Skalny, and Jane C.-J. Chao. 2022. "Lycium barbarum Polysaccharides and Capsaicin Inhibit Oxidative Stress, Inflammatory Responses, and Pain Signaling in Rats with Dextran Sulfate Sodium-Induced Colitis" International Journal of Molecular Sciences 23, no. 5: 2423. https://doi.org/10.3390/ijms23052423
APA StyleChen, Y.-S., Lian, Y. Z., Chen, W.-C., Chang, C.-C., Tinkov, A. A., Skalny, A. V., & Chao, J. C.-J. (2022). Lycium barbarum Polysaccharides and Capsaicin Inhibit Oxidative Stress, Inflammatory Responses, and Pain Signaling in Rats with Dextran Sulfate Sodium-Induced Colitis. International Journal of Molecular Sciences, 23(5), 2423. https://doi.org/10.3390/ijms23052423







