Quantitative Trait Locus Mapping of Salt Tolerance in Wild Rice Oryza longistaminata
Abstract
:1. Introduction
2. Results
2.1. Phenotype Observation of the BIL Population under Salt Treatment
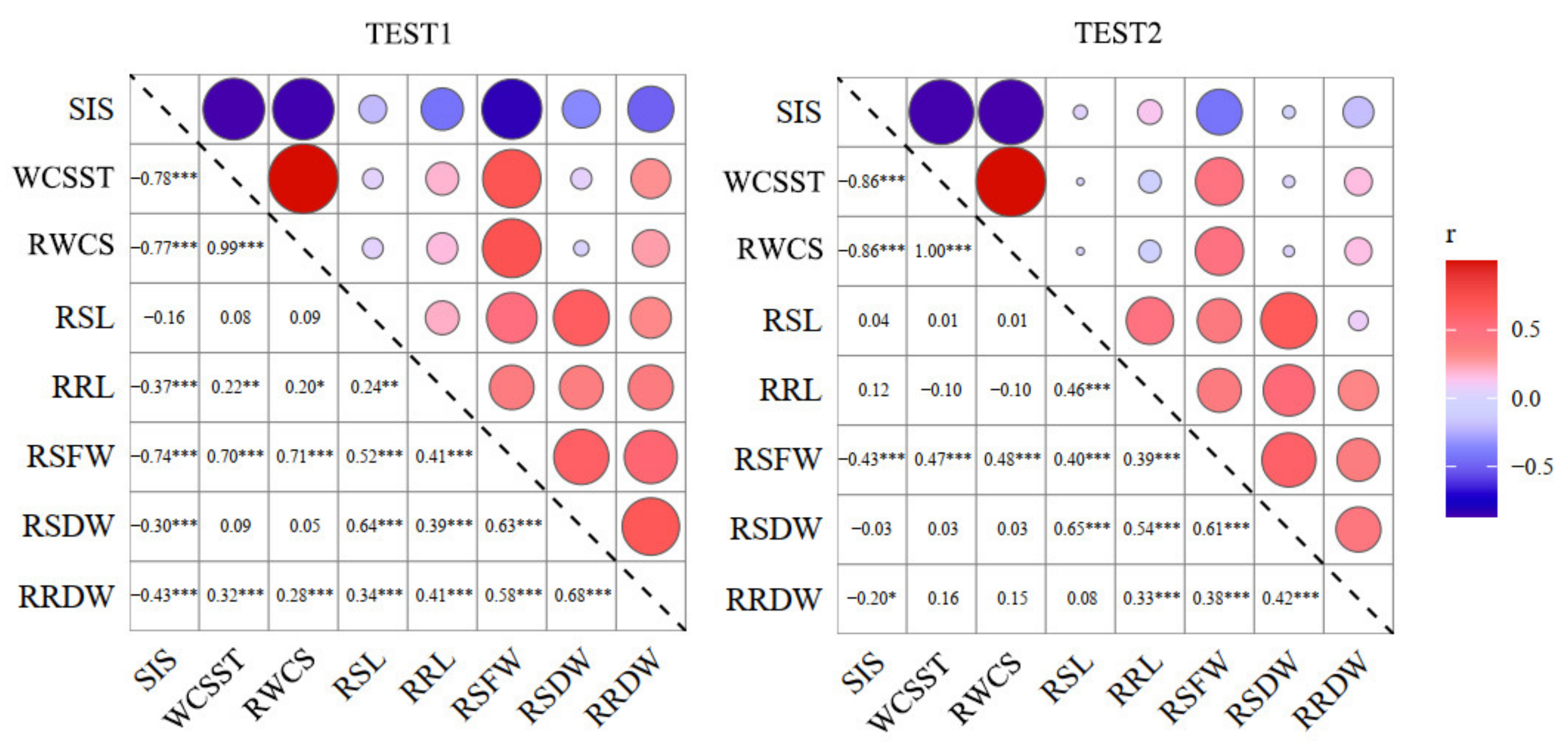
2.2. Correlation of the Salt-Tolerance-Related Traits
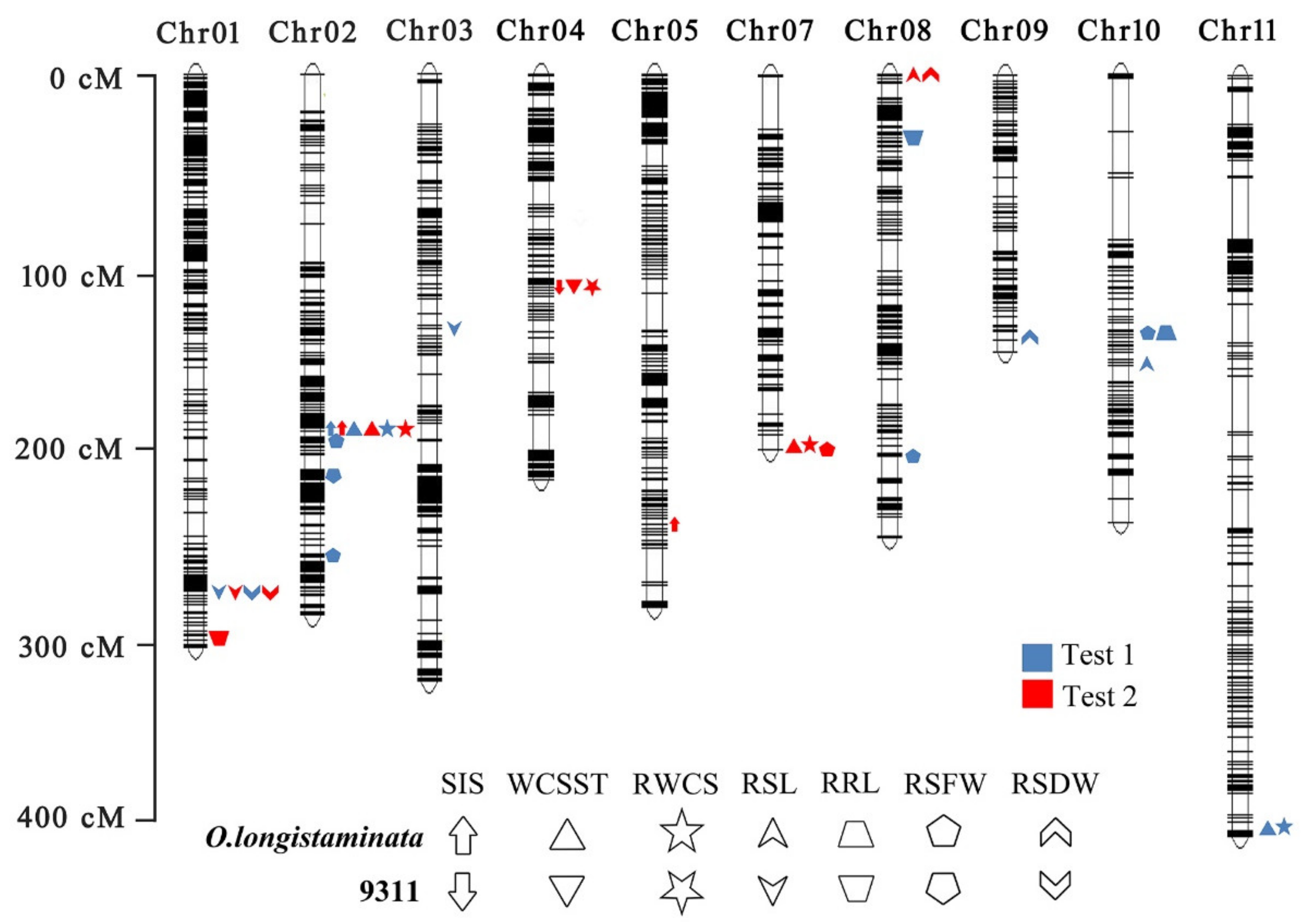
2.3. QTL Mapping of the Salt Tolerance of the O. longistaminata BIL Population
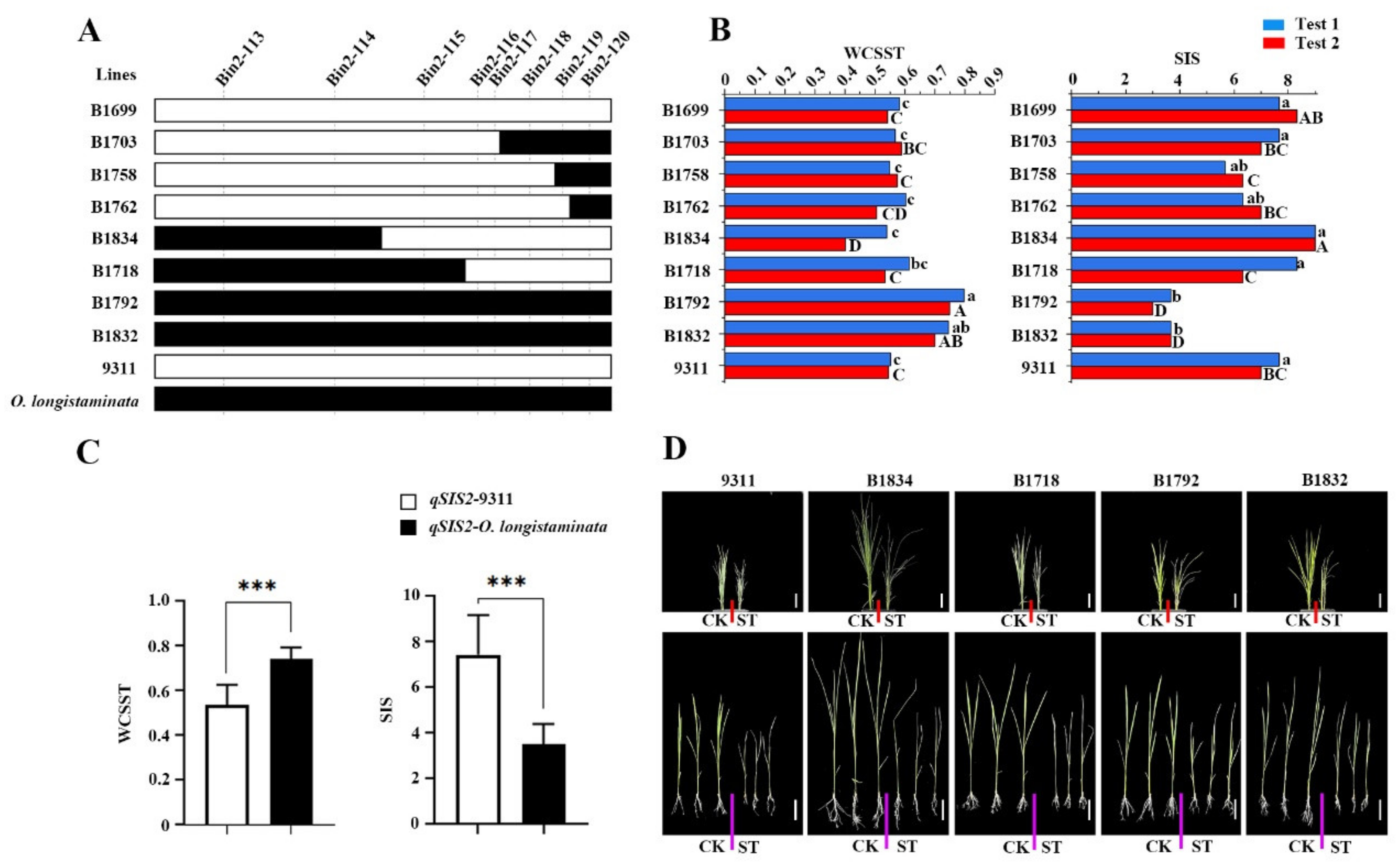
2.4. Confirmation of the QTLs for Salt Tolerance
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Salt Treatments
4.2. Phenotype Scores and Correlation Analysis
4.3. QTL Mapping
4.4. Real-Time Quantitative PCR
4.5. Gene Sequence Comparison and Protein Structure Prediction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SIS | salt injury score |
| WCSST | water content of seedlings under salt treatment |
| RWCS | relative water content of seedling |
| RSL | relative shoot length |
| RRL | relative root length |
| RSFW | relative shoot fresh weight |
| RSDW | relative shoot dry weight |
| RRDW | relative root dry weight |
| SD | standard deviation |
| CV | coefficient of variation |
References
- Hoang, T.M.L.; Tran, T.N.; Nguyen, T.K.T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of salinity stress tolerance in rice: Challenges and opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Wang, C.; Xue, Y.; Liu, X.; Chen, S.; Song, C.; Yang, Y.; Guo, Y. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 2019, 10, 1199. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Chu, Y.; Chen, H.; Li, X.; Wu, Q.; Jin, L.; Wang, G.; Huang, J.; Muday, G.K. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA–mediated regulatory pathway and ROS scavenging. PLoS Genet. 2018, 14, e1007662. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Gregorio, G.B.; Jain, R.K. QTL mapping for salinity tolerance in rice. Physol. Mol. Biol. Pla. 2007, 13, 87–99. [Google Scholar]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Zeng, L.; Wanamaker, S.I.; Mandal, J.; Xu, J.; Cui, X.; et al. Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol. 2005, 139, 822–835. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zou, D.T.; Luan, F.S.; Zhao, H.W.; Wang, J.G.; Liu, H.L.; Xie, D.W.; Su, D.Q.; Ma, J.; Liu, Z.L. Dynamic QTL analysis of the Na+ content, K+ content, and Na+/K+ ratio in rice roots during the field growth under salt stress. Biol. Plant. 2014, 58, 689–696. [Google Scholar] [CrossRef]
- Lei, L.; Zheng, H.; Bi, Y.; Yang, L.; Liu, H.; Wang, J.; Sun, J.; Zhao, H.; Li, X.; Li, J.; et al. Identification of a major QTL and candidate gene analysis of salt tolerance at the bud burst stage in rice (Oryza sativa L.) using QTL-Seq and RNA-seq. Rice 2020, 13, 55. [Google Scholar] [CrossRef]
- Lin, H.X.; Zhu, M.Z.; Yano, M.; Gao, J.P.; Liang, Z.W.; Su, W.A.; Hu, X.H.; Ren, Z.H.; Chao, D.Y. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Appl. Genet. 2004, 108, 253–260. [Google Scholar] [CrossRef]
- Tiwari, S.; Sl, K.; Kumar, V.; Singh, B.; Singh, N.K. Mapping QTLs for Salt Tolerance in Rice (Oryza sativa L.) by Bulked segregant analysis of recombinant inbred lines using 50K SNP Chip. PLoS ONE 2016, 11, e0153610. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.H.; Gao, J.P.; Li, L.G.; Cai, X.L.; Huang, W.; Chao, D.Y.; Zhu, M.Z.; Wang, Z.Y.; Luan, S.; Lin, H.X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef]
- Brar, D.S.; Khush, G.S. Alien introgression in rice. Plant Mol. Biol. 1997, 35, 35–47. [Google Scholar] [CrossRef]
- Ram, T.; Majumder, N.D.; Mishra, B.; Ansari, M.M.; Padmavathi, G. Introgression of broad-spectrum blast resistance gene(s) into cultivated rice (Oryza sativa ssp. indica) from wild rice O. rufipogon. Curr. Sci. India 2007, 92, 225–230. [Google Scholar]
- Khush, G.S. Origin, dispersal, cultivation and variation of rice. In Oryza: From Molecule to Plant; Sasaki, T., Moore, G., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 25–34. [Google Scholar]
- Fan, F.; Long, W.; Liu, M.; Yuan, H.; Pan, G.; Li, N.; Li, S. Quantitative trait locus mapping of the combining ability for yield-related traits in wild rice Oryza longistaminata. J. Ag.r Food. Chem. 2019, 67, 8766–8772. [Google Scholar] [CrossRef]
- Jin, J.; Long, W.; Wang, L.; Liu, X.; Pan, G.; Xiang, W.; Li, N.; Li, S. QTL Mapping of seed vigor of backcross inbred lines derived from Oryza longistaminata under artificial aging. Front. Plant. Sci. 2018, 9, 1909. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Fan, F.; Liu, M.; Long, W.; Yu, Y.; Yuan, H.; Pan, G.; Li, N.; Li, S.; Liu, J. Quantitative trait loci mapping of mineral element contents in brown rice using backcross inbred lines derived from Oryza longistaminata. Front. Plant. Sci. 2020, 11, 1229. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, Y.; Chen, K.; Shen, C.; Zhou, M. Identification of new QTL for salt tolerance from rice variety Pokkali. J. Agron. Crop. Sci. 2019, 206, 202–213. [Google Scholar] [CrossRef]
- Javed, M.A.; Huyop, F.Z.; Wagiran, A.; Salleh, F.M. Identification of QTLs for morph-physiological traits related to salinity tolerance at seedling stage in Indica rice. Procedia Environ. Sci. 2011, 8, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Nayyeripasand, L.; Garoosi, G.A.; Ahmadikhah, A. Genome-wide association study (GWAS) to identify salt-tolerance QTLs carrying novel candidate genes in rice during early vegetative stage. Rice 2021, 14, 9. [Google Scholar] [CrossRef]
- Thomson, M.J.; Ocampo, M.; Egdane, J.; Rahman, M.A.; Sajise, A.G.; Adorada, D.L.; Tumimbang-Raiz, E.; Blumwald, E.; Seraj, Z.I.; Singh, R.K. Characterizing the Saltol quantitative trait locus for salinity tolerance in rice. Rice 2010, 3, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.R.; Bagali, P.G.; Hittalmani, S.; Shashidhar, H.E. Molecular mapping of quantitative trait loci associated with seedling tolerance to salt stress in rice (Oryza sativa L.). Curr. Sci. India 2000, 78, 162–164. [Google Scholar]
- Yuan, J.; Wang, X.; Zhao, Y.; Khan, N.U.; Zhao, Z.; Zhang, Y.; Wen, X.; Tang, F.; Wang, F.; Li, Z. Genetic basis and identification of candidate genes for salt tolerance in rice by GWAS. Sci. Rep. 2020, 10, 9958. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Y.; Meng, L.; Hu, X.; Cui, Y.; Sun, Y.; Zhu, L.; Ali, J.; Xu, J.; Li, Z. Identification of salt-tolerant QTLs with strong genetic background effect using two sets of reciprocal introgression lines in rice. Genome 2012, 55, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tan, L.; Liu, F.; Cai, H.; Sun, C. Identification of quantitative trait loci associated with salt tolerance at seedling stage from Oryza rufipogon. J. Genet. Genom. 2011, 38, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Amoah, N.K.A.; Akromah, R.; Kena, A.W.; Manneh, B.; Dieng, I.; Bimpong, I.K. Mapping QTLs for tolerance to salt stress at the early seedling stage in rice (Oryza sativa L.) using a newly identified donor “Madina Koyo”. Euphytica 2020, 216, 156. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, L.; Xue, Y.; Zhang, Q.; Wang, L.; Shou, H. Overexpression of OsVP1 and OsNHX1 increases tolerance to drought and salinity in rice. J. Plant. Biol. 2010, 53, 444–452. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Du, F.; Wang, J.; Zhao, X.; Li, Z.; Wang, W.; Xu, J.; Fu, B. Comparative transcriptome and metabolome profiling reveal molecular mechanisms underlying OsDRAP1-mediated salt tolerance in rice. Sci. Rep. 2021, 11, 5166. [Google Scholar] [CrossRef]
- Höfer, R.; Briesen, I.; Beck, M.; Pinot, F.; Schreiber, L.; Franke, R. The arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid omega-hydroxylase involved in suberin monomer biosynthesis. J. Exp. Bot. 2008, 59, 2347–2360. [Google Scholar] [CrossRef] [Green Version]
- Nogueira do Amaral, M.; Arge, L.W.P.; Benitez, L.C.; Danielowski, R.; Ferreira da Silveira Silveira, S.; da Rosa Farias, D.; de Oliveira, A.C.; da Maia, L.C.; Braga, E.J.B. Comparative transcriptomics of rice plants under cold, iron, and salt stresses. Funct. Integr. Genom. 2016, 16, 567–579. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Vishal, B.; Ho, W.J.; Lok, F.C.J.; Lee, F.S.M.; Kumar, P.P. Regulation of a cytochrome P450 gene CYP94B1 by WRKY33 transcription factor controls apoplastic barrier formation in roots to confer salt tolerance. Plant Physiol. 2020, 184, 2199–2215. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory manual for physiological studies of rice. Int. Rice Res. Inst. 1971, 17, 62. [Google Scholar]
- Gregorio, G.; Senadhira, D.; Mendoza, R. Screening rice for salinity tolerance. Int. Rice Res. Inst. 1997, 22, 13. [Google Scholar]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef] [Green Version]


| Tests | Items | 9311 | BILs | ||
|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Range | |||
| Test 1 | SIS | 7.00 | 6.83 ± 1.45 | 21.24 | 3.00–9.00 |
| WCSST (%) | 55.22 | 62.09 ± 7.63 | 12.29 | 42.82–79.69 | |
| RWCS (%) | 64.40 | 72.78 ± 8.99 | 12.35 | 49.95–94.60 | |
| RSL (%) | 79.13 | 70.88 ± 7.64 | 10.78 | 53.25–89.91 | |
| RRL (%) | 120.71 | 96.43 ± 12.5 | 12.97 | 63.28–135.45 | |
| RSFW (%) | 25.35 | 28.89 ± 9.76 | 33.79 | 12.14–56.19 | |
| RSDW (%) | 78.90 | 70.06 ± 16.15 | 23.06 | 35.16–114.39 | |
| RRDW (%) | 81.73 | 77.28 ± 25.15 | 32.54 | 26.85–177.20 | |
| Test 2 | SIS | 7.00 | 6.71 ± 1.63 | 24.22 | 3.00–9.00 |
| WCSST (%) | 54.50 | 57.65 ± 6.52 | 11.30 | 40.23–77.33 | |
| RWCS (%) | 64.39 | 66.98 ± 7.51 | 11.21 | 47.32–89.48 | |
| RSL (%) | 79.15 | 78.63 ± 9.76 | 12.41 | 52.35–97.03 | |
| RRL (%) | 95.29 | 91.24 ± 12.46 | 13.66 | 61.72–117.00 | |
| RSFW (%) | 27.20 | 24.51 ± 6.98 | 28.46 | 12.30–44.70 | |
| RSDW (%) | 81.43 | 71.00 ± 13.95 | 19.65 | 42.04–114.39 | |
| RRDW (%) | 88.37 | 80.44 ± 18.77 | 23.33 | 35.76–139.68 | |
| Loci | Gene Names | Functional Annotation |
|---|---|---|
| qSIS2 | MH02t0465500 | NAC domain-containing protein 8 |
| MH02t0465600, MH02g0465700 | Beta-1,4-mannosyl-glycoprotein, 4-beta-N-acetylglucosaminyltransferase | |
| MH02t0465800 | hypothetical protein OsI_07895 | |
| MH02t0465900 | hypothetical protein OsI_07896 | |
| MH02t0466000 | unnamed protein product | |
| MH02g0466100 | Isocitrate dehydrogenase (NAD) regulatory subunit 1, mitochondrial | |
| MH02g0466200 | Hypothetical protein | |
| MH02g0466300 | Elongation factor Tu, chloroplastic | |
| MH02t0466400 | Putative eukaryotic translation initiation factor | |
| MH02t0466500 | High-affinity nitrate transporter 3.1 Partner protein for high-affinity nitrate transport OsNAR2.1 | |
| MH02t0466600 | Rhodanese-like domain-containing protein 11, chloroplastic | |
| MH02t0466700 | Nucleoporin GLE1 | |
| MH02t0466800 | Endoglucanase E1 | |
| MH02t0466900 | Cytochrome P450 86B1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Zhang, L.; Wei, X.; Wang, R.; Li, N.; Chen, G.; Fan, F.; Huang, S.; Li, J.; Li, S. Quantitative Trait Locus Mapping of Salt Tolerance in Wild Rice Oryza longistaminata. Int. J. Mol. Sci. 2022, 23, 2379. https://doi.org/10.3390/ijms23042379
Yuan L, Zhang L, Wei X, Wang R, Li N, Chen G, Fan F, Huang S, Li J, Li S. Quantitative Trait Locus Mapping of Salt Tolerance in Wild Rice Oryza longistaminata. International Journal of Molecular Sciences. 2022; 23(4):2379. https://doi.org/10.3390/ijms23042379
Chicago/Turabian StyleYuan, Lei, Licheng Zhang, Xiao Wei, Ruihua Wang, Nannan Li, Gaili Chen, Fengfeng Fan, Shaoying Huang, Jianxiong Li, and Shaoqing Li. 2022. "Quantitative Trait Locus Mapping of Salt Tolerance in Wild Rice Oryza longistaminata" International Journal of Molecular Sciences 23, no. 4: 2379. https://doi.org/10.3390/ijms23042379
APA StyleYuan, L., Zhang, L., Wei, X., Wang, R., Li, N., Chen, G., Fan, F., Huang, S., Li, J., & Li, S. (2022). Quantitative Trait Locus Mapping of Salt Tolerance in Wild Rice Oryza longistaminata. International Journal of Molecular Sciences, 23(4), 2379. https://doi.org/10.3390/ijms23042379







