Impact of Collagen Crosslinking on Dislocated Human Shoulder Capsules—Effect on Structural and Mechanical Properties
Abstract
:1. Introduction
2. Results
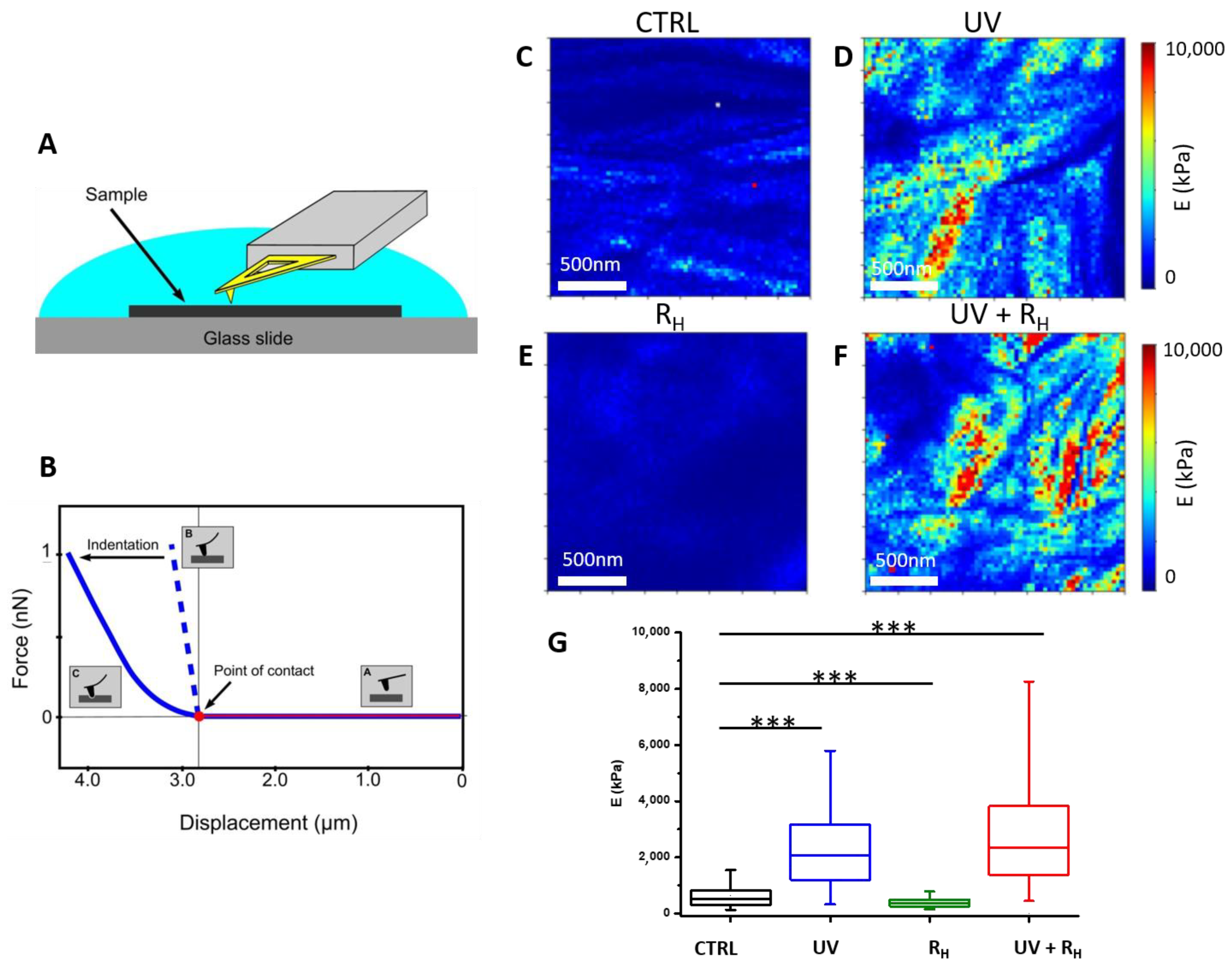
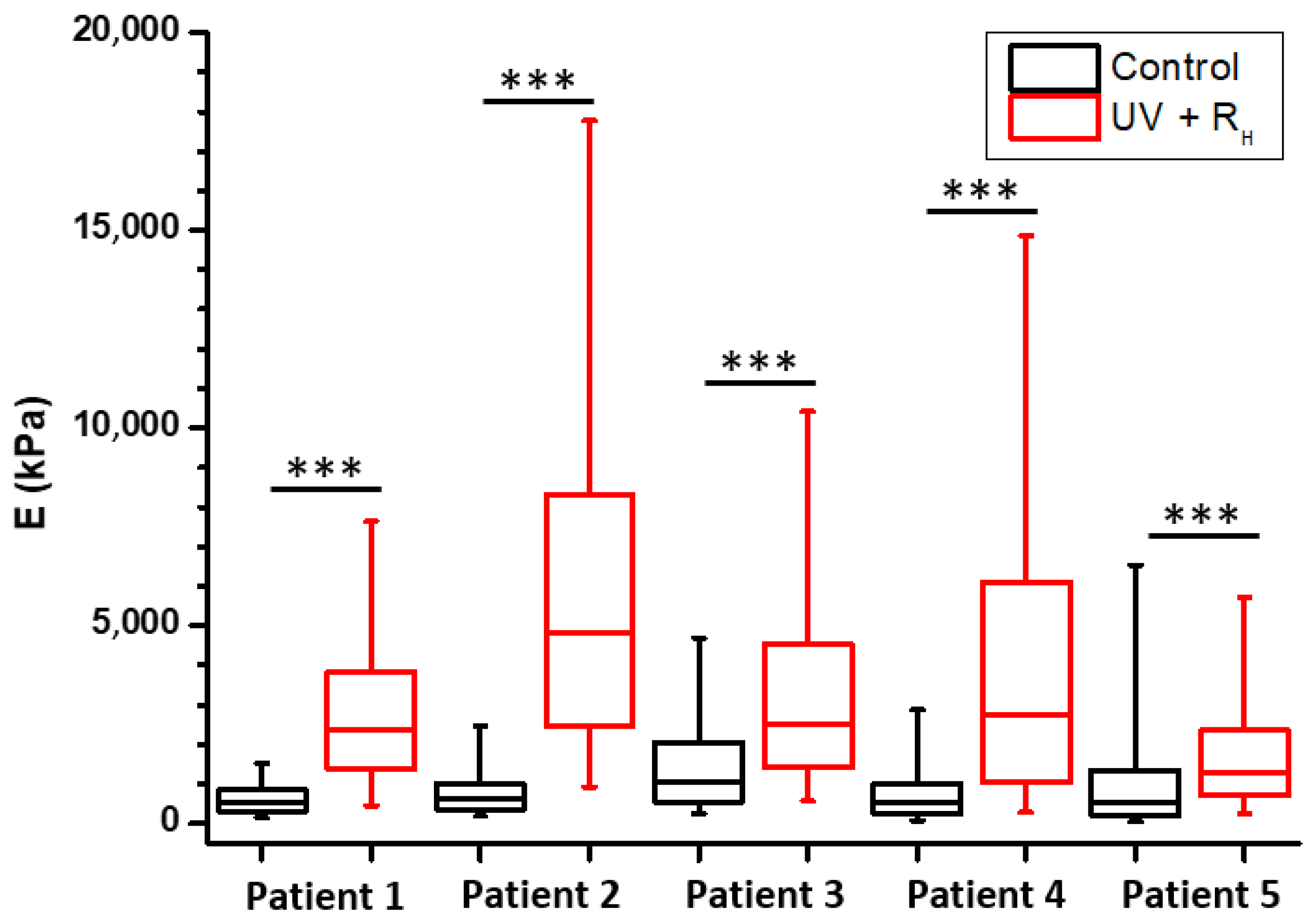
2.1. Increased Capsule Stiffness after Crosslinking Procedure
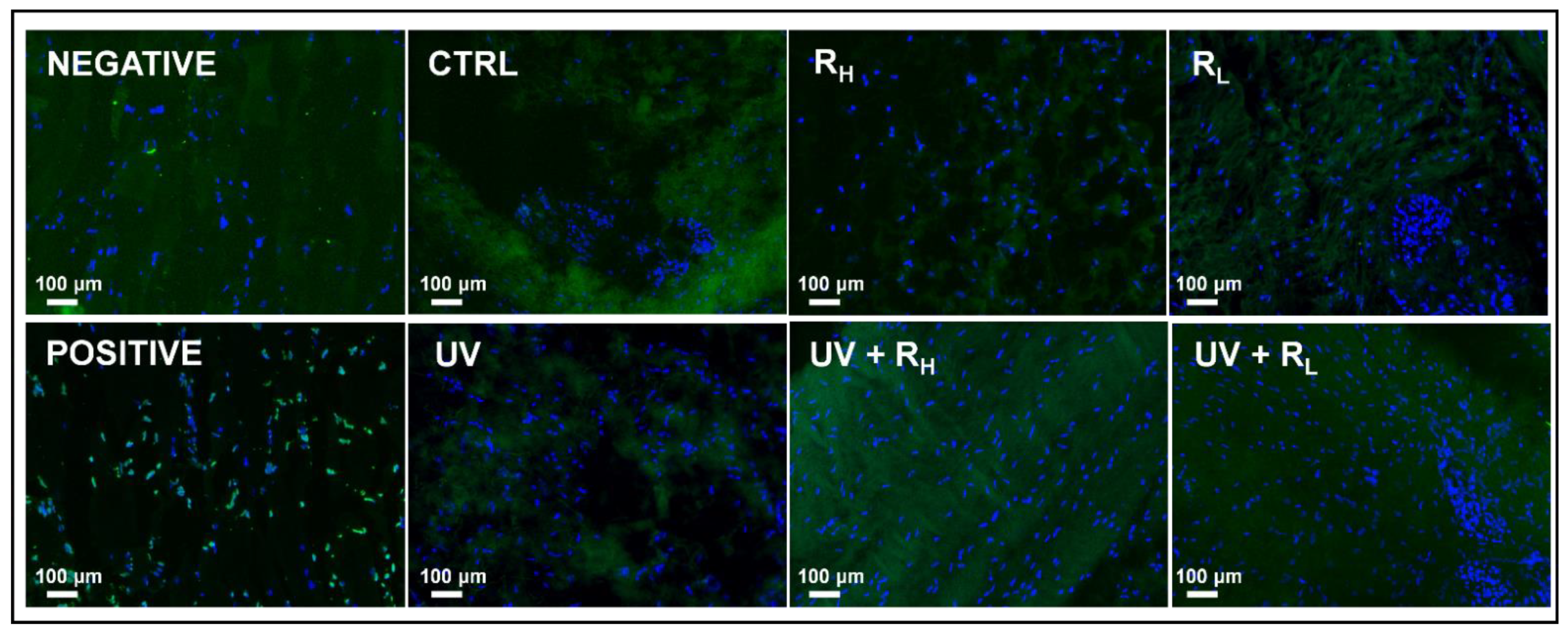
2.2. Preserved Morphological Characteristics of Capsules and Cell Viability after Crosslinking Procedure
3. Discussion
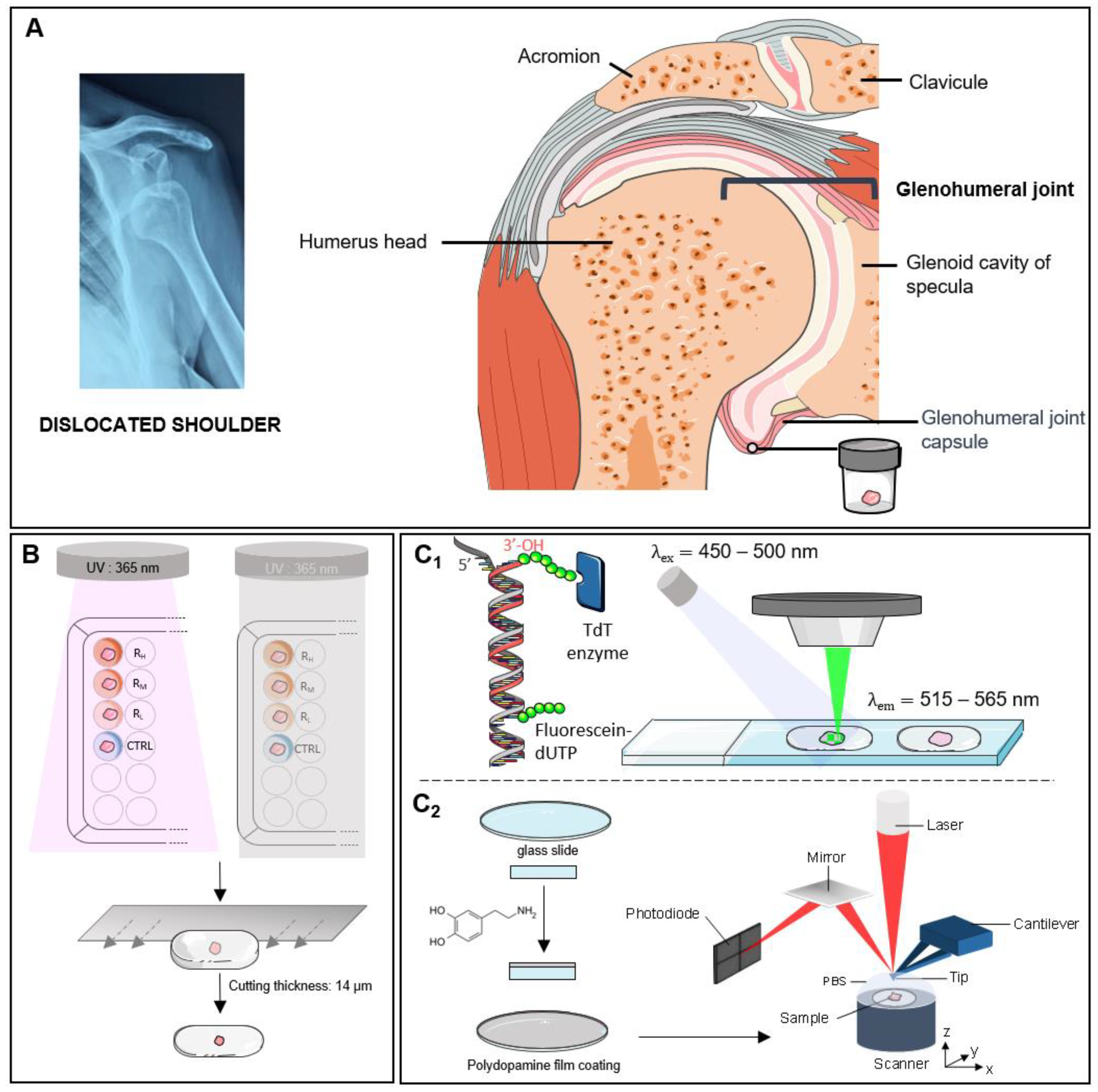
4. Materials and Methods
4.1. Sample Preparation
4.2. Cell Death Detection
4.3. Atomic Force Microscopy
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Competing Interests Statement
References
- Thomazeau, H.; Langlais, T.; Hardy, A.; Curado, J.; Herisson, O.; Mouton, J.; Charousset, C.; Courage, O.; French Arthroscopy Society; Nourissat, G. Long-Term, Prospective, Multicenter Study of Isolated Bankart Repair for a Patient Selection Method Based on the Instability Severity Index Score. Am. J. Sports Med. 2019, 47, 1057–1061. [Google Scholar] [CrossRef]
- Jobe, F.W.; Pink, M. The Athlete’s Shoulder. J. Hand Ther. 1994, 7, 107–110. [Google Scholar] [CrossRef]
- Miniaci, A.; Codsi, M.J. Thermal Capsulorrhaphy for the Treatment of Shoulder Instability. Am. J. Sports Med. 2006, 34, 1356–1363. [Google Scholar] [CrossRef]
- Levy, O.; Wilson, M.; Williams, H.; Bruguera, J.A.; Dodenhoff, R.; Sforza, G.; Copeland, S. Thermal Capsular Shrinkage for Shoulder Instability: Mid-Term Longitudinal Outcome Study. J. Bone Joint Surg. Br. 2001, 83, 640–645. [Google Scholar] [CrossRef]
- Rodeo, S.A.; Suzuki, K.; Yamauchi, M.; Bhargava, M.; Warren, R.F. Analysis of Collagen and Elastic Fibers in Shoulder Capsule in Patients with Shoulder Instability. Am. J. Sports Med. 1998, 26, 634–643. [Google Scholar] [CrossRef]
- Debski, R.E.; Moore, S.M.; Mercer, J.L.; Sacks, M.S.; McMahon, P.J. The Collagen Fibers of the Anteroinferior Capsulolabrum Have Multiaxial Orientation to Resist Shoulder Dislocation. J. Shoulder Elbow Surg. 2003, 12, 247–252. [Google Scholar] [CrossRef]
- Browe, D.P.; Voycheck, C.A.; McMahon, P.J.; Debski, R.E. Changes to the Mechanical Properties of the Glenohumeral Capsule during Anterior Dislocation. J. Biomech. 2014, 47, 464–469. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen Fibril Formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Tao, X.; Zhang, J.; Li, Z.; Xu, Y.; Wang, Y.; Zhang, C.; Mu, G. A Review of Collagen Cross-Linking in Cornea and Sclera. J. Ophthalmol. 2015, 2015, 289467. [Google Scholar] [CrossRef] [Green Version]
- Stolz, M.; Gottardi, R.; Raiteri, R.; Miot, S.; Martin, I.; Imer, R.; Staufer, U.; Raducanu, A.; Düggelin, M.; Baschong, W.; et al. Early Detection of Aging Cartilage and Osteoarthritis in Mice and Patient Samples Using Atomic Force Microscopy. Nat. Nanotechnol. 2009, 4, 186–192. [Google Scholar] [CrossRef]
- Andriotis, O.G.; Manuyakorn, W.; Zekonyte, J.; Katsamenis, O.L.; Fabri, S.; Howarth, P.H.; Davies, D.E.; Thurner, P.J. Nanomechanical Assessment of Human and Murine Collagen Fibrils via Atomic Force Microscopy Cantilever-Based Nanoindentation. J. Mech. Behav. Biomed. Mater. 2014, 39, 9–26. [Google Scholar] [CrossRef]
- Han, L.; Grodzinsky, A.J.; Ortiz, C. Nanomechanics of the Cartilage Extracellular Matrix. Annu. Rev. Mater. Res. 2011, 41, 133–168. [Google Scholar] [CrossRef] [Green Version]
- Fullwood, N.J.; Hammiche, A.; Pollock, H.M.; Hourston, D.J.; Song, M. Atomic Force Microscopy of the Cornea and Sclera. Curr. Eye Res. 1995, 14, 529–535. [Google Scholar] [CrossRef]
- Revenko, I.; Sommer, F.; Minh, D.T.; Garrone, R.; Franc, J.-M. Atomic Force Microscopy Study of the Collagen Fibre Structure. Biol. Cell 1994, 80, 67–69. [Google Scholar] [CrossRef]
- Bozec, L.; van der Heijden, G.; Horton, M. Collagen Fibrils: Nanoscale Ropes. Biophys. J. 2007, 92, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Gutsmann, T.; Fantner, G.E.; Venturoni, M.; Ekani-Nkodo, A.; Thompson, J.B.; Kindt, J.H.; Morse, D.E.; Fygenson, D.K.; Hansma, P.K. Evidence That Collagen Fibrils in Tendons Are Inhomogeneously Structured in a Tubelike Manner. Biophys. J. 2003, 84, 2593–2598. [Google Scholar] [CrossRef] [Green Version]
- Miyagawa, A.; Kobayashi, M.; Fujita, Y.; Hamdy, O.; Hirano, K.; Nakamura, M.; Miyake, Y. Surface Ultrastructure of Collagen Fibrils and Their Association With Proteoglycans in Human Cornea and Sclera by Atomic Force Microscopy and Energy-Filtering Transmission Electron Microscopy. Cornea 2001, 20, 651–656. [Google Scholar] [CrossRef]
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical Properties of Collagen Fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Beauvais, M.; Degabriel, T.; Aissaoui, N.; Dupres, V.; Colaço, E.; El Kirat, K.; Domingos, R.F.; Brouri, D.; Pradier, C.; Spadavecchia, J.; et al. Supramolecular Self-Assembly and Organization of Collagen at Solid/Liquid Interface: Effect of Spheroid- and Rod-Shaped TiO2 Nanocrystals. Adv. Mater. Interfaces 2019, 6, 1900195. [Google Scholar] [CrossRef]
- Gutsmann, T.; Fantner, G.E.; Kindt, J.H.; Venturoni, M.; Danielsen, S.; Hansma, P.K. Force Spectroscopy of Collagen Fibers to Investigate Their Mechanical Properties and Structural Organization. Biophys. J. 2004, 86, 3186–3193. [Google Scholar] [CrossRef] [Green Version]
- Erickson, B.; Fang, M.; Wallace, J.M.; Orr, B.G.; Les, C.M.; Banaszak Holl, M.M. Nanoscale Structure of Type I Collagen Fibrils: Quantitative Measurement of D-Spacing. Biotechnol. J. 2013, 8, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, A.S.; Kraft, S.; Edelhauser, H.F.; Kidder, G.W.; Lundquist, R.R.; Bradshaw, H.E.; Dedeic, Z.; Dionne, M.J.C.; Clement, E.M.; Conrad, G.W. Mechanisms of Corneal Tissue Cross-Linking in Response to Treatment with Topical Riboflavin and Long-Wavelength Ultraviolet Radiation (UVA). Investig. Ophthalmol. Vis. Sci. 2010, 51, 129–138. [Google Scholar] [CrossRef]
- Uemura, R.; Miura, J.; Ishimoto, T.; Yagi, K.; Matsuda, Y.; Shimizu, M.; Nakano, T.; Hayashi, M. UVA-Activated Riboflavin Promotes Collagen Crosslinking to Prevent Root Caries. Sci. Rep. 2019, 9, 1252. [Google Scholar] [CrossRef] [Green Version]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical Crosslinking of Collagen Fibers: Comparison of Ultraviolet Irradiation and Dehydrothermal Treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef]
- Lew, D.-H.; Liu, P.H.-T.; Orgill, D.P. Optimization of UV Cross-Linking Density for Durable and Nontoxic Collagen GAG Dermal Substitute. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 51–56. [Google Scholar] [CrossRef]
- Lampi, M.C.; Reinhart-King, C.A. Targeting Extracellular Matrix Stiffness to Attenuate Disease: From Molecular Mechanisms to Clinical Trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef] [Green Version]
- Fratzl, P. Collagen: Structure and Mechanics; Springer: New York, NY, USA, 2008; ISBN 978-0-387-73905-2. [Google Scholar]
- Marturano, J.E.; Arena, J.D.; Schiller, Z.A.; Georgakoudi, I.; Kuo, C.K. Characterization of Mechanical and Biochemical Properties of Developing Embryonic Tendon. Proc. Natl. Acad. Sci. USA 2013, 110, 6370–6375. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hughes, R.; Mullin, N.; Hawkins, R.J.; Holen, I.; Brown, N.J.; Hobbs, J.K. Mechanical Heterogeneity in the Bone Microenvironment as Characterized by Atomic Force Microscopy. Biophys. J. 2020, 119, 502–513. [Google Scholar] [CrossRef]
- Fessel, G.; Gerber, C.; Snedeker, J.G. Potential of Collagen Cross-Linking Therapies to Mediate Tendon Mechanical Properties. J. Shoulder Elbow Surg. 2012, 21, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Nourissat, G.; Vigan, M.; Hamonet, C.; Doursounian, L.; Deranlot, J. Diagnosis of Ehlers-Danlos Syndrome after a First Shoulder Dislocation. J. Shoulder Elbow Surg. 2018, 27, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Giménez, A.; Uriarte, J.J.; Vieyra, J.; Navajas, D.; Alcaraz, J. Elastic Properties of Hydrogels and Decellularized Tissue Sections Used in Mechanobiology Studies Probed by Atomic Force Microscopy. Microsc. Res. Tech. 2017, 80, 85–96. [Google Scholar] [CrossRef] [PubMed]

| Plate | Sample | Treatment | Solution |
|---|---|---|---|
| Control condition | CTRL | Control Riboflavin | PBS 1X |
| RH | Riboflavin 2.5% | Riboflavin 2.5% in PBS 1X | |
| RM | Riboflavin 1.0% | Riboflavin 1.0% in PBS 1X | |
| RL | Riboflavin 0.1% | Riboflavin 0.1% in PBS 1X | |
| UV condition | UV | Control UV | PBS 1X |
| UV + RH | Riboflavin 2.5% | Riboflavin 2.5% in PBS 1X | |
| UV + RM | Riboflavin 1.0% | Riboflavin 1.0% in PBS 1X | |
| UV + RL | Riboflavin 0.1% | Riboflavin 0.1% in PBS 1X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornette, P.; Jaabar, I.L.; Dupres, V.; Werthel, J.-D.; Berenbaum, F.; Houard, X.; Landoulsi, J.; Nourissat, G. Impact of Collagen Crosslinking on Dislocated Human Shoulder Capsules—Effect on Structural and Mechanical Properties. Int. J. Mol. Sci. 2022, 23, 2297. https://doi.org/10.3390/ijms23042297
Cornette P, Jaabar IL, Dupres V, Werthel J-D, Berenbaum F, Houard X, Landoulsi J, Nourissat G. Impact of Collagen Crosslinking on Dislocated Human Shoulder Capsules—Effect on Structural and Mechanical Properties. International Journal of Molecular Sciences. 2022; 23(4):2297. https://doi.org/10.3390/ijms23042297
Chicago/Turabian StyleCornette, Pauline, Ilhem Lilia Jaabar, Vincent Dupres, Jean-David Werthel, Francis Berenbaum, Xavier Houard, Jessem Landoulsi, and Geoffroy Nourissat. 2022. "Impact of Collagen Crosslinking on Dislocated Human Shoulder Capsules—Effect on Structural and Mechanical Properties" International Journal of Molecular Sciences 23, no. 4: 2297. https://doi.org/10.3390/ijms23042297
APA StyleCornette, P., Jaabar, I. L., Dupres, V., Werthel, J.-D., Berenbaum, F., Houard, X., Landoulsi, J., & Nourissat, G. (2022). Impact of Collagen Crosslinking on Dislocated Human Shoulder Capsules—Effect on Structural and Mechanical Properties. International Journal of Molecular Sciences, 23(4), 2297. https://doi.org/10.3390/ijms23042297






