Molecular and Cellular Mechanisms of M. tuberculosis and SARS-CoV-2 Infections—Unexpected Similarities of Pathogenesis and What to Expect from Co-Infection
Abstract
1. Introduction
2. Tuberculosis Diagnosis in the COVID-19 Pandemic
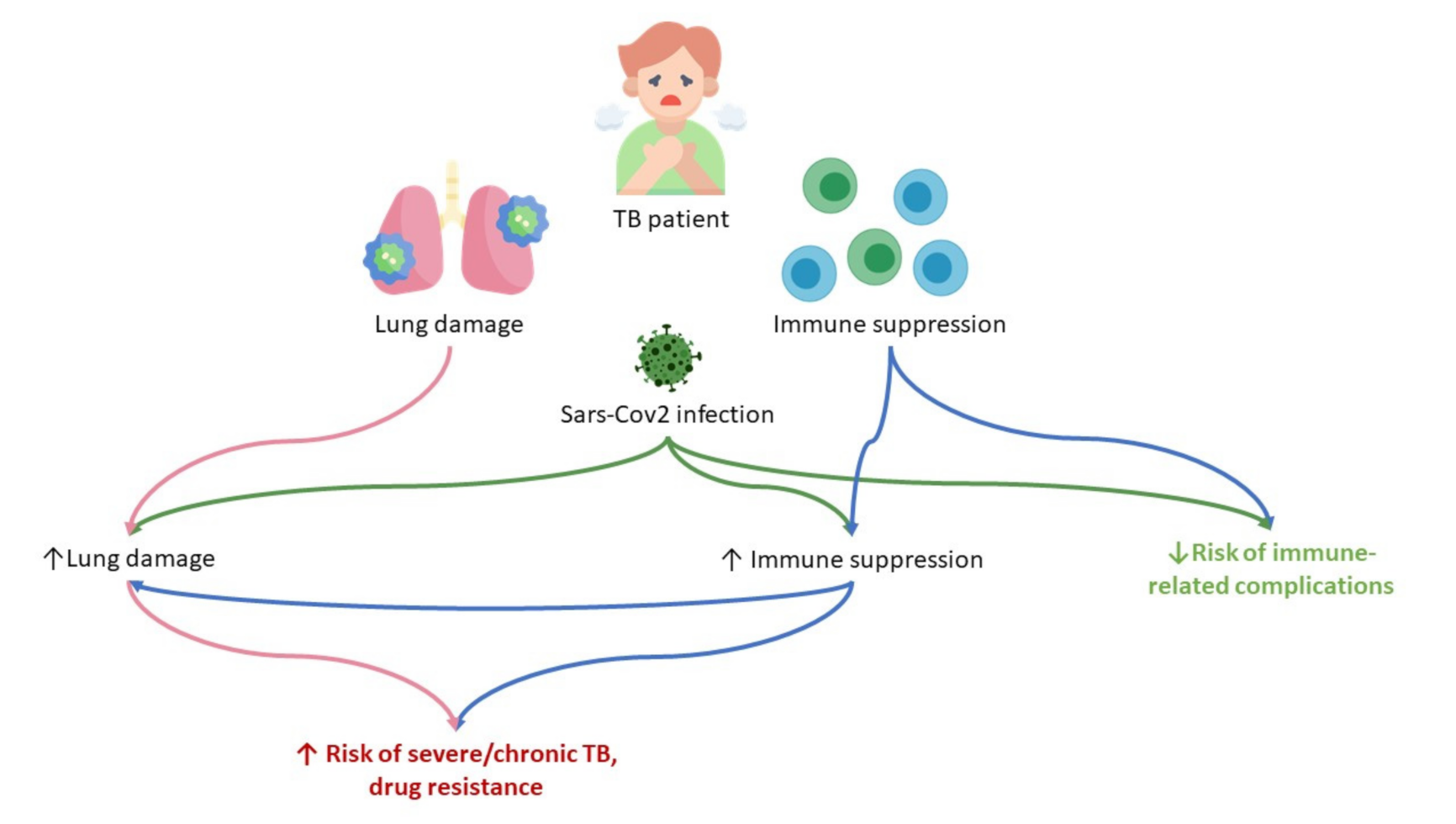
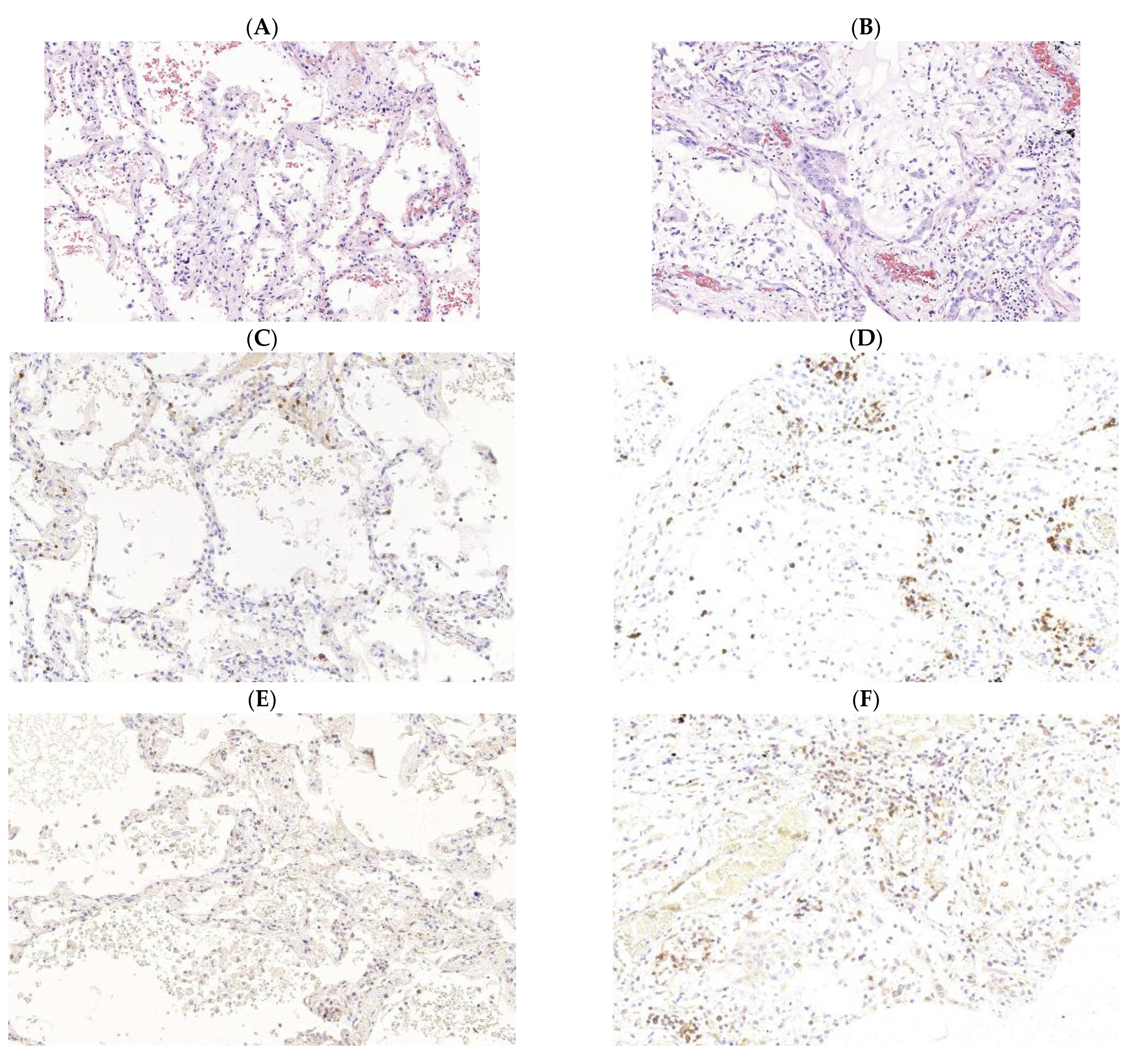
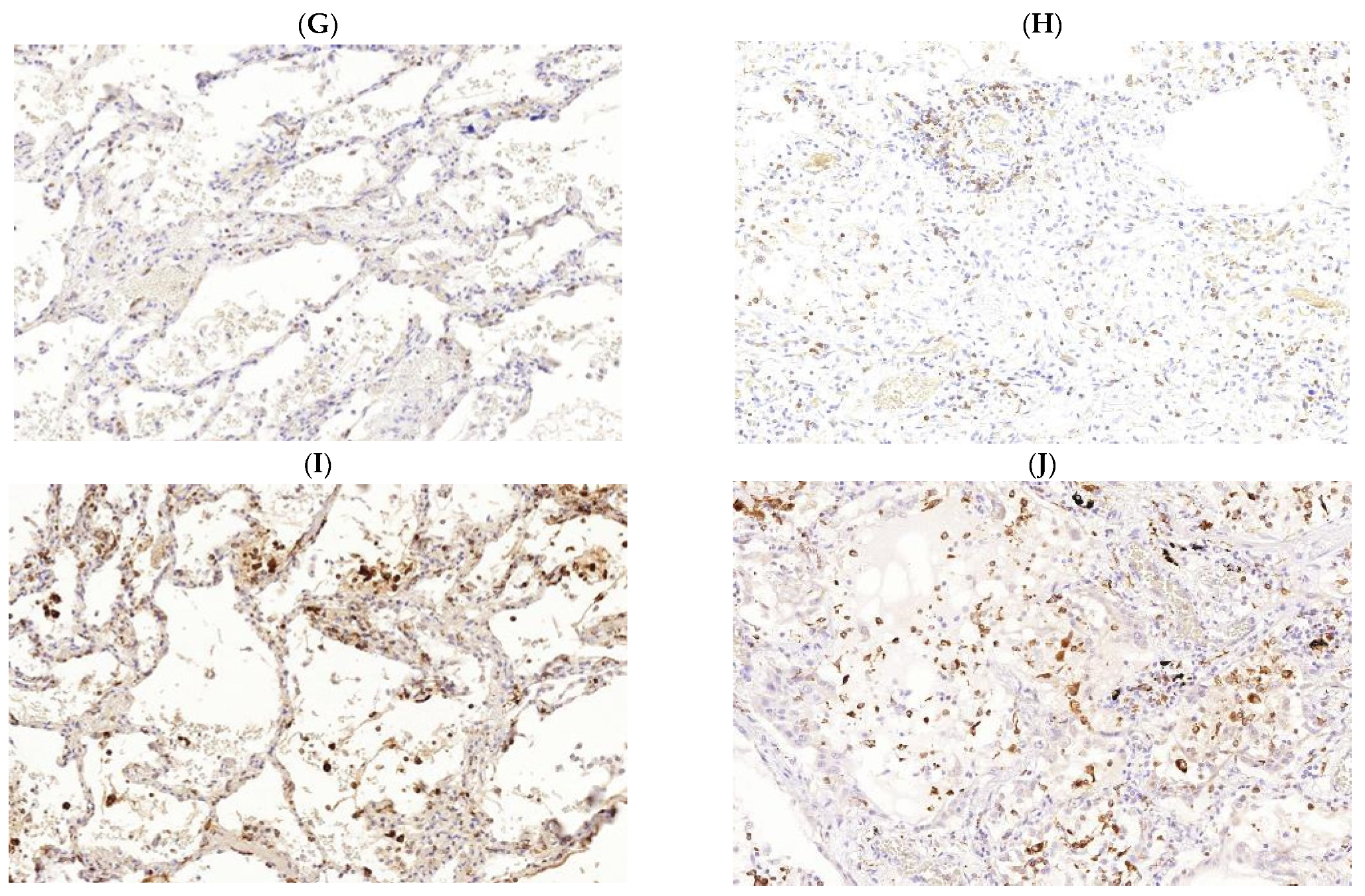
3. Pathogenesis of M. tuberculosis (Mtb) and SARS-CoV-2 Infections
4. Conclusions
- Fewer resources for the accurate diagnosis of new patients and management of already registered cases.
- Similar symptoms which complicate the diagnosis
- Pathogenetic aspects of exacerbation of tuberculosis infection in COVID-19.
- Poor understanding of immune mechanisms and changes due to COVID-19 and tuberculosis co-infection presents challenges in infection control and treatment of these diseases.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stochino, C.; Villa, S.; Zucchi, P.; Parravicini, P.; Gori, A.; Raviglione, M.C. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur. Respir. J. 2020, 56, 2001708. [Google Scholar] [CrossRef] [PubMed]
- Glaziou, P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. medRxiv 2020. [Google Scholar] [CrossRef]
- WHO Guidelines on Tuberculosis Infection Prevention and Control; WHO: Geneva, Switzerland, 2019; p. 265.
- World Health Organization. Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021; p. 57. ISBN 9789240037021. [Google Scholar]
- WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; 2019; p. 96. ISBN 978-92-4-155052-9.
- Guglielmetti, L.; Veziris, N.; Aubry, A.; Brossier, F.; Bernard, C.; Sougakoff, W.; Jarlier, V.; Robert, J. Risk factors for extensive drug resistance in multidrug-resistant tuberculosis cases: A case-case study. Int. J. Tuberc. Lung Dis. 2018, 22, 54–59. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2017; WHO: Geneva, Switzerland, 2017; p. 250. [Google Scholar]
- Al-Omari, A.; Alhuqbani, W.N.; Zaidi, Z.R.A.; Al-Subaie, F.M.; Alanoud, M.A.; Hindi, A.M.; Abogosh, K.A.; Alsharafi, A.A.; Alhuqbani, M.N.; Salih, S.; et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: A descriptive cross-sectional study. J. Infect. Public Health 2020, 13, 1639–1644. [Google Scholar] [CrossRef]
- Cantini, F.; Niccoli, L.; Matarrese, D.; Nicastri, E.; Stobbione, P.; Goletti, D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef]
- Hilda, J.N.; Das, S.; Tripathy, S.P.; Hanna, L.E. Role of neutrophils in tuberculosis: A bird’s eye view. Innate Immun. 2020, 26, 240–247. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Faqihi, F.; Alharthy, A.; Noor, A.; Balshi, A.; Balhamar, A.; Karakitsos, D. COVID-19 in a patient with active tuberculosis: A rare case-report RSS. Respir. Med. Case Rep. 2020, 31, 101146. [Google Scholar] [CrossRef]
- Goudiaby, M.S.; Gning, L.D.; Diagne, M.L.; Dia, B.M.; Rwezaura, H.; Tchuenche, J.M. Optimal control analysis of a COVID-19 and tuberculosis co-dynamics model. Inform. Med. Unlocked 2022, 28, 100849. [Google Scholar] [CrossRef]
- Chen, H.; Konglai Zhang, K. Insight into impact of COVID-19 epidemic on tuberculosis burden in China. Eur. Respir. J. 2020, 56, 2002710. [Google Scholar] [CrossRef]
- McQuaid, C.F.; McCreesh, N.; Read, J.M.; Sumner, T.; Houben, R.M.G.J.; White, R.G.; Harris, R.C. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur. Respir. J. 2020, 56, 2001718. [Google Scholar] [CrossRef] [PubMed]
- Cronin, A.M.; Railey, S.; Fortune, D.; Wegener, D.H.; Davis, J.B. Notes from the Field: Effects of the COVID-19 Response on Tuberculosis Prevention and Control Efforts—United States, March–April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Fleming, J.; Yu, Y.; Gu, Y.; Liu, C.; Fan, L.; Wang, X.; Cheng, M.; Bi, L.L.; et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. medRxiv 2020. [Google Scholar] [CrossRef]
- Kuday, D.A. Biomarkers and immunological tests. Experimental and clinical parallels of latent tuberculosis infection. Tuberc. Lung Dis. 2020, 98, 63–74. [Google Scholar] [CrossRef]
- Hogan, A.B.; Jewell, B.L.; Sherrard-Smith, E.; Vesga, J.F.; Watson, O.J.; Whittaker, C.; Hamlet, A.; Smith, J.A.; Winskill, P.; Verity, R.; et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: A modelling study. Lancet Glob. Health 2020, 8, e1132–e1141. [Google Scholar] [CrossRef]
- Comella, P.; De Souza, M.L.; Prat-Aymerich, C.; Dominguez, J. Impact of COVID-19 on Tuberculosis Control. Arch. Bronconeumol. 2021, 57, 5–6. [Google Scholar] [CrossRef] [PubMed]
- de Martino, M.; Lodi, L.; Galli, L.; Chiappini, E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front. Pediatr. 2019, 7, 350. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Lou, J.; Li, J.; Bo, L.; Zhu, K.; Wan, X.; Deng, X.; Cai, Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit. Care. 2010, 14, R220. [Google Scholar] [CrossRef]
- Venturini, E.; Lodi, L.; Francolino, I.; Ricci, S.; Chiappini, E.; de Martino, M.; Galli, L. CD3, CD4, CD8, CD19 and CD16/CD56 positive cells in tuberculosis infection and disease: Peculiar features in children. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419840241. [Google Scholar] [CrossRef]
- Parkash, O.; Agrawal, S.; Madhan Kumar, M. T regulatory cells: Achilles’ heel of Mycobacterium tuberculosis infection? Immunol. Res. 2015, 62, 386–398. [Google Scholar] [CrossRef]
- Martinot, A.J. Microbial offense vs host defense: Who controls the TB granuloma? Vet. Pathol. 2018, 55, 14–26. [Google Scholar] [CrossRef]
- Refai, A.; Gritli, S.; Barbouche, M.-R.; Essafi, M. Mycobacterium tuberculosis virulent factor ESAT-6 drives macrophage differentiation toward the pro-inflammatory M1 phenotype and subsequently switches it to the anti-inflammatory M2 phenotype. Front. Cell. Infect. Microbiol. 2018, 8, 327. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection—A review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Mello, F.C.Q.; D’Ambrosio, L.; Centis, R.; Dalcolmo, M.P.; Migliori, G.B. Tuberculosis and COVID-19, the new cursed duet: What differs between Brazil and Europe? J. Bras. Pneumol. 2021, 47, e20210044. [Google Scholar] [CrossRef]
- Tamuzi, J.L.; Ayele, B.T.; Shumba, C.S.; Adetokunboh, O.O.; Uwimana-Nicol, J.; Haile, Z.T. Implications of COVID-19 in high burden countries for HIV/TB A systematic review of evidence. BMC Infect. Dis. 2020, 20, 744. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Seminati, D.; Lucchini, A.; Foti, G.; Pagni, F. Exuberant Plasmocytosis in Bronchoalveolar Lavage Specimen of the First Patient Requiring Extracorporeal Membrane Oxygenation for SARS-CoV-2 in Europe. J. Thorac. Oncol. 2020, 15, e65–e66. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef]
- Ciceri, F.; Beretta, L.; Scandroglio, A.M.; Colombo, S.; Landoni, G.; Ruggeri, A.; Peccatori, J.; D’Angelo, A.; de Cobelli, F.; Rovere-Querini, P.; et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2020, 22, 95–97. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.; Sanchez, A.; Pirskanen, A.T. Hydrothermotherapy in prevention and treatment of mild to moderate cases of COVID-19. Med. Hypotheses 2021, 146, 110363. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, V.A.; Savchenko, A.A.; Kudryavtsev, I.V.; Kozlov, I.G.; Kudlay, D.A.; Prodeus, A.P.; Borisov, A.G. Clinical Immunology. In Krasnoyarsk; Polycor: Krasnoyarsk, Russia, 2020; 386p, ISBN 978-5-6044565-6-9. [Google Scholar]
- Kudryavtsev, I.V.; Serebriakova, M.K.; Starshinova, A.A.; Zinchenko, Y.S.; Basantsova, N.Y.; Belyaeva, E.N.; Pavlova, M.V.; Yablonskiy, P.K. Altered peripheral blood Th17 and follicular T-helper subsets in patients with pulmonary tuberculosis. Russ. J. Infect. Immun. 2019, 9, 304–314. [Google Scholar] [CrossRef]
- Shu, C.C.; Wu, M.F.; Wang, J.Y.; Lai, H.C.; Lee, L.N.; Chiang, B.L.; Yu, C.J. Decreased T helper 17 cells in tuberculosis is associated with increased percentages of programmed death ligand 1, T helper 2 and regulatory T cells. Respir. Res. 2017, 18, 128. [Google Scholar] [CrossRef]
- Wang, T.; Lv, M.; Qian, Q.; Nie, Y.; Yu, L.; Hou, Y. Increased frequencies of T helper type 17 cells in tuberculous pleural effusion. Tuberculosis 2011, 91, 231–237. [Google Scholar] [CrossRef]
- Dheda, K.; Chang, J.S.; Lala, S.; Huggett, J.F.; Zumla, A.; Rook, G.A. Gene expression of IL17 and IL23 in the lungs of patients with active tuberculosis. Thorax 2008, 63, 566–568. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Zhang, M.; Liao, M.; Graner, M.W.; Wu, C.; Yang, Q.; Liu, H.; Zhou, B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am. J. Respir. Crit. Care Med. 2010, 181, 734–742. [Google Scholar] [CrossRef]
- Scriba, T.J.; Kalsdorf, B.; Abrahams, D.A.; Isaacs, F.; Hofmeister, J.; Black, G.; Hassan, H.Y.; Wilkinson, R.J.; Walzl, G.; Gelderbloem, S.J.; et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 2008, 180, 1962–1970. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chin, C.H.; Liu, S.F.; Wu, C.C.; Tsen, C.C.; Wang, Y.H.; Chao, T.Y.; Lie, C.H.; Chen, C.J.; Wang, C.C.; et al. Prognostic values of serum IP-10 and IL-17 in patients with pulmonary tuberculosis. Dis. Markers 2011, 31, 101–110. [Google Scholar] [CrossRef]
- Gil-Etayo, F.J.; Suàrez-Fernández, P.; Cabrera-Marante, O.; Arroyo, D.; Garcinuño, S.; Naranjo, L.; Pleguezuelo, D.E.; Allende, L.M.; Mancebo, E.; Lalueza, A.; et al. T-Helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Front. Cell. Infect. Microbiol. 2021, 11, 624483. [Google Scholar] [CrossRef]
- Golovkin, A.; Kalinina, O.; Bezrukikh, V.; Aquino, A.; Zaikova, E.; Karonova, T.; Melnik, O.; Vasilieva, E.; Kudryavtsev, I. Imbalanced Immune Response of T-Cell and B-Cell Subsets in Patients with Moderate and Severe COVID-19. Viruses 2021, 13, 1966. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Nasillo, V.; Lusenti, B.; Riva, G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann. Hematol. 2020, 99, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Dai, Y.; Zheng, T.; Cheng, L.; Zhao, D.; Wang, H.; Liu, M.; Pei, H.; Jin, T.; Yu, D.; et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020, 130, 6588–6599. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Kalfaoglu, B.; Almeida-Santos, J.; Tye, C.A.; Satou, Y.; Ono, M. T-Cell Hyperactivation and Paralysis in Severe COVID-19 Infection Revealed by Single-Cell Analysis. Front. Immunol. 2020, 11, 589380. [Google Scholar] [CrossRef]
- Schultheiß, C.; Paschold, L.; Simnica, D.; Mohme, M.; Willscher, E.; von Wenserski, L.; Scholz, R.; Wieters, I.; Dahlke, C.; Tolosa, E.; et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease. Immunity 2020, 53, 442–455.e4. [Google Scholar] [CrossRef]
- Paulissen, S.M.; van Hamburg, J.P.; Dankers, W.; Lubberts, E. The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine 2015, 74, 43–53. [Google Scholar] [CrossRef]
- Wacleche, V.S.; Goulet, J.P.; Gosselin, A.; Monteiro, P.; Soudeyns, H.; Fromentin, R.; Jenabian, M.A.; Vartanian, S.; Deeks, S.G.; Chomont, N.; et al. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology 2016, 13, 59. [Google Scholar] [CrossRef]
- Monin, L.; Griffiths, K.L.; Slight, S.; Lin, Y.; Rangel-Moreno, J.; Khader, S.A. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 2015, 8, 1099–1109. [Google Scholar] [CrossRef]
- Jurado, J.O.; Pasquinelli, V.; Alvarez, I.B.; Peña, D.; Rovetta, A.I.; Tateosian, N.L.; Romeo, H.E.; Musella, R.M.; Palmero, D.; Chuluyán, H.E.; et al. IL-17 and IFN-γ expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J. Leukoc. Biol. 2012, 91, 991–1002. [Google Scholar] [CrossRef]
- Laing, A.G.; Lorenc, A.; Del Molino Del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Morita, R.; Schmitt, N.; Bentebibel, S.E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Sridhar, R.; Hanna, L.E.; Banurekha, V.V.; Nutman, T.B.; Babu, S. Decreased frequencies of circulating CD4+ T follicular helper cells associated with diminished plasma IL-21 in active pulmonary tuberculosis. PLoS ONE 2014, 9, e111098. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Massachusetts Consortium on Pathogen Readiness Specimen Working Group. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–157.e13. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Tan, H.X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef]
- Christensen, J.R.; Börnsen, L.; Ratzer, R.; Piehl, F.; Khademi, M.; Olsson, T.; Sørensen, P.S.; Sellebjerg, F. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS ONE 2013, 8, e578200. [Google Scholar] [CrossRef]
- Akiyama, M.; Suzuki, K.; Yamaoka, K.; Yasuoka, H.; Takeshita, M.; Kaneko, Y.; Kondo, H.; Kassai, Y.; Miyazaki., T.; Morita, R.; et al. Number of Circulating Follicular Helper 2 T Cells Correlates with IgG4 and Interleukin-4 Levels and Plasmablast Numbers in IgG4-Related Disease. Arthritis Rheumatol. 2015, 67, 2476–2481. [Google Scholar] [CrossRef]
- Kudryavtsev, I.; Serebriakova, M.; Starshinova, A.; Zinchenko, Y.; Basantsova, N.; Malkova, A.; Soprun, L.; Churilov, L.P.; Toubi, E.; Yablonskiy, P.; et al. Imbalance in B cell and T Follicular Helper Cell Subsets in Pulmonary Sarcoidosis. Sci. Rep. 2020, 10, 1059. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Wang, J.; Guo, L.; Liu, C.; Jiang, Y.; Wang, H.; Yang, S. Distribution of circulating T follicular helper cell subsets is altered in immunoglobulin A vasculitis in children. PLoS ONE 2017, 12, e0189133. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Shi, Y.; Wang, F.; Yang, H.; Han, S.; Bai, Y. Altered circulating T follicular helper cell subsets in patients with psoriasis vulgaris. Immunol. Lett. 2017, 181, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhong, M.; Yang, Q.; Hong, K.; Xia, J.; Li, X.; Liu, Y.; Chen, Y.Q.; Yang, J.; Huang, C.; et al. Alterations in Phenotypes and Responses of T Cells Within 6 Months of Recovery from COVID-19: A Cohort Study. Virol. Sin. 2021, 36, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Malkova, A.; Kudryavtsev, I.; Starshinova, A.; Kudlay, D.; Zinchenko, Y.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form. Pathogens 2021, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Canas, C.A. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med. Hypotheses 2020, 145, 110345. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Zhang, M.; Yang, C.X.; Zhang, N.; Wang, X.C.; Yang, X.P.; Dong, X.Q.; Zheng, Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef]
- Liu, W.D.; Wang, J.T.; Hung, C.C.; Chang, S.C. Accelerated progression of pulmonary tuberculosis in a COVID-19 patient after corticosteroid treatment. J. Microbiol. Immunol. Infect. 2021, in press. [Google Scholar] [CrossRef]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Gualano, G.; Vittozzi, P.; Nicastri, E.; Maffongelli, G.; Grifoni, A.; Sette, A.; et al. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int. J. Infect. Dis. 2021, 113 (Suppl. 1), S82–S87. [Google Scholar] [CrossRef]
- Bousquet, J.; Zuberbier, T.; Anto, J.M.; Iaccarino, G.; Czarlewski, W.; Anto, A.; Haahtela, T.; Akdis, C.A.; Blain, H.; Canonica, G.W.; et al. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin. Transl. Allergy 2020, 10, 16. [Google Scholar] [CrossRef]
- Pathak, L.; Gayan, S.; Pal, B.; Talukdar, J.; Bhuyan, S.; Sandhya, S.; Yeger, H.; Baishya, D.; Das, B. Coronavirus Activates an Altruistic Stem Cell-Mediated Defense Mechanism that Reactivates Dormant Tuberculosis: Implications in Coronavirus Disease 2019 Pandemic. Am. J. Pathol. 2021, 191, 1255–1268. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, M.; Chen, Y.; Shi, S.; Geng, J.; Tian, J. Association between tuberculosis and COVID-19 severity and mortality: A rapid systematic review and meta-analysis. J. Med. Virol. 2021, 93, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; Safdar, N.; Chandir, S.; Khan, U.; Khowaja, S.; Riaz, N.; Maniar, R.; Jaswal, M.; Khan, J.A.; Hussain, H. Tuberculosis control and care in the era of COVID-19. Health Policy Plan. 2020, 35, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Starshinova, A.A.; Kushnareva, E.A.; Malkova, A.M.; Dovgalyuk, I.F.; Kudlay, D.A. New coronavirus infection: Features of the clinical course, the possibility of diagnosis, treatment and prevention of infection in adults and children. Quest. Mod. Pediatr. 2020, 19, 123–131. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starshinova, A.A.; Kudryavtsev, I.; Malkova, A.; Zinchenko, U.; Karev, V.; Kudlay, D.; Glushkova, A.; Starshinova, A.Y.; Dominguez, J.; Villar-Hernández, R.; et al. Molecular and Cellular Mechanisms of M. tuberculosis and SARS-CoV-2 Infections—Unexpected Similarities of Pathogenesis and What to Expect from Co-Infection. Int. J. Mol. Sci. 2022, 23, 2235. https://doi.org/10.3390/ijms23042235
Starshinova AA, Kudryavtsev I, Malkova A, Zinchenko U, Karev V, Kudlay D, Glushkova A, Starshinova AY, Dominguez J, Villar-Hernández R, et al. Molecular and Cellular Mechanisms of M. tuberculosis and SARS-CoV-2 Infections—Unexpected Similarities of Pathogenesis and What to Expect from Co-Infection. International Journal of Molecular Sciences. 2022; 23(4):2235. https://doi.org/10.3390/ijms23042235
Chicago/Turabian StyleStarshinova, Anna A., Igor Kudryavtsev, Anna Malkova, Ulia Zinchenko, Vadim Karev, Dmitry Kudlay, Angela Glushkova, Anastasiya Y. Starshinova, Jose Dominguez, Raquel Villar-Hernández, and et al. 2022. "Molecular and Cellular Mechanisms of M. tuberculosis and SARS-CoV-2 Infections—Unexpected Similarities of Pathogenesis and What to Expect from Co-Infection" International Journal of Molecular Sciences 23, no. 4: 2235. https://doi.org/10.3390/ijms23042235
APA StyleStarshinova, A. A., Kudryavtsev, I., Malkova, A., Zinchenko, U., Karev, V., Kudlay, D., Glushkova, A., Starshinova, A. Y., Dominguez, J., Villar-Hernández, R., Dovgalyk, I., & Yablonskiy, P. (2022). Molecular and Cellular Mechanisms of M. tuberculosis and SARS-CoV-2 Infections—Unexpected Similarities of Pathogenesis and What to Expect from Co-Infection. International Journal of Molecular Sciences, 23(4), 2235. https://doi.org/10.3390/ijms23042235







