Flavonoids—Natural Gifts to Promote Health and Longevity
Abstract
:1. Introduction
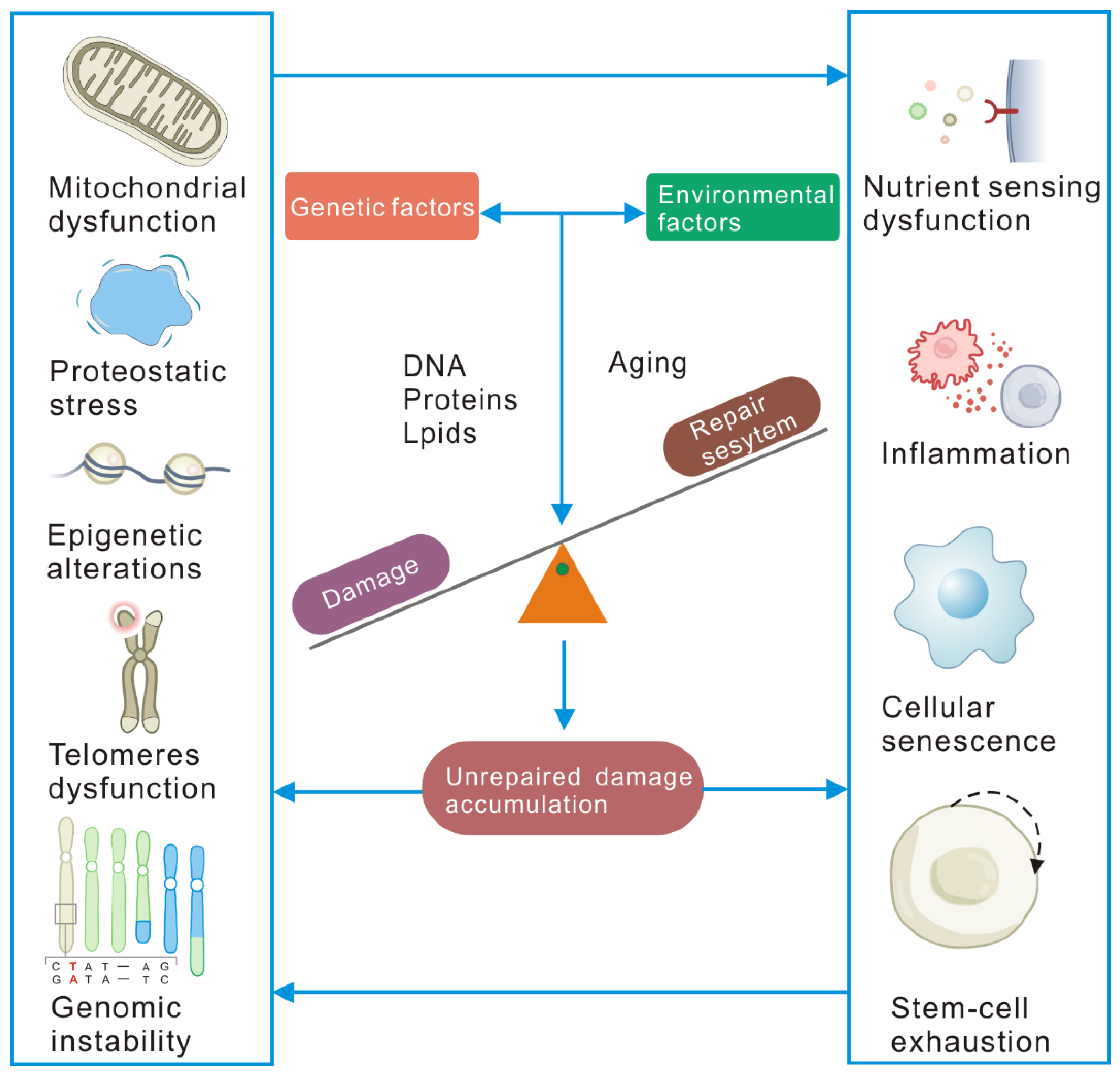
2. Cellular Senescence Is Driven by Unrepaired Damage
2.1. DNA Damage and Repair
2.2. Protein Damage
2.3. Lipid Damage
2.4. Molecular, Cellular, and Systemic Consequences of Unrepaired Damage Accumulation
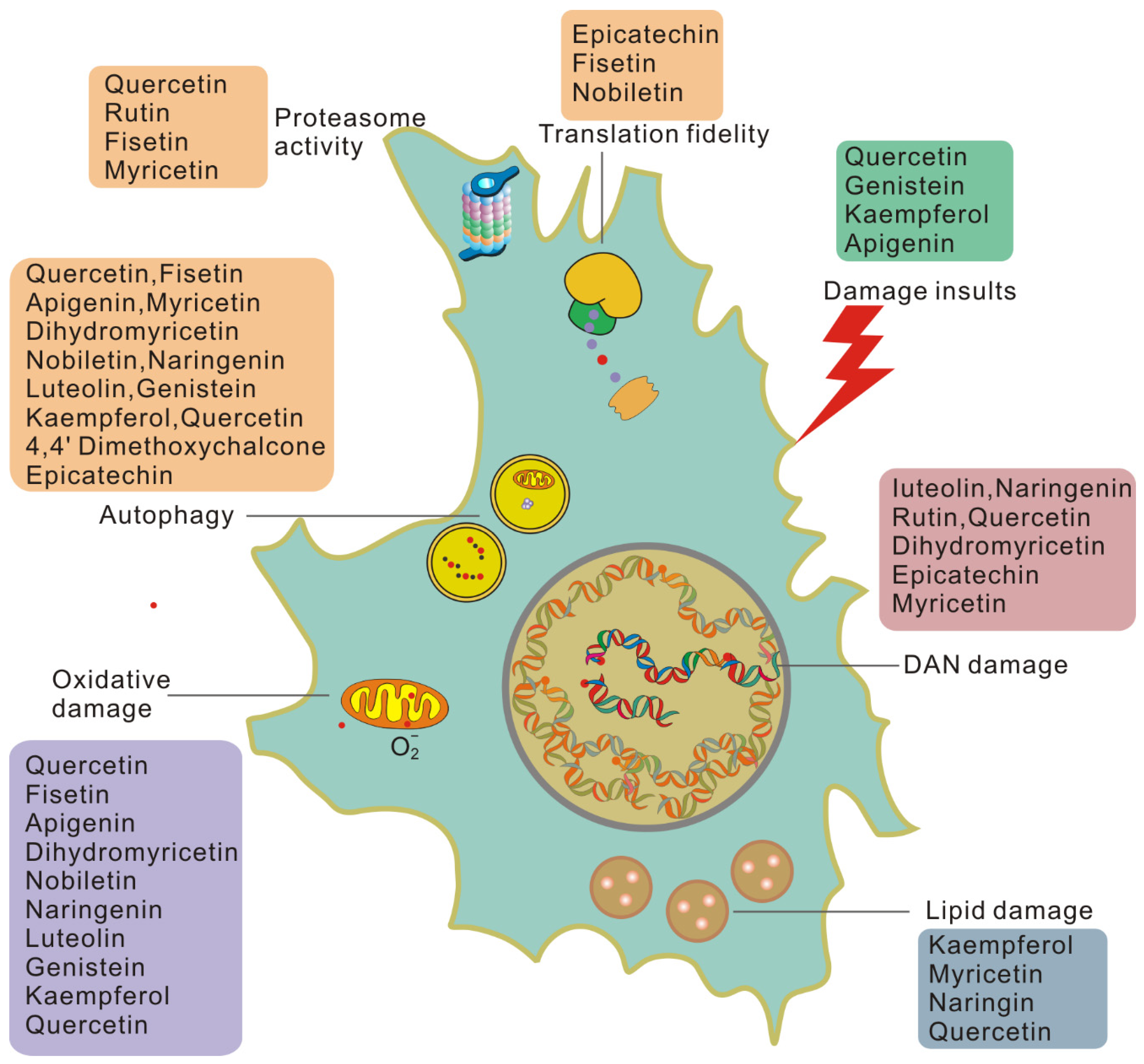
3. Flavonoid Compounds Serve as Anti-Aging Agents
3.1. Senolytic Flavonoids
3.2. Senomorphic Flavonoids
3.3. Another Antisenescence Activity of Flavonoids
4. Benefits of Flavonoids in Attenuating Aging Damage
5. Clinical Applications of Flavonoid on Aging
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Demmitt, B.; Nath, R.; Brunet, A. The Genetics of Aging: A Vertebrate Perspective. Cell 2019, 177, 200–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, B.; Berger, S.; Brunet, A.; Campisi, J.; Cuervo, A.; Epel, E.; Franceschi, C.; Lithgow, G.; Morimoto, R.; Pessin, J.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.; Belwal, T.; Efferth, T.; Farooqi, A.; Sanches-Silva, A.; Vacca, R.; Nabavi, S.; Khan, F.; Prasad Devkota, H.; Barreca, D.; et al. Targeting epigenetics in cancer: Therapeutic potential of flavonoids. Crit. Rev. Food Sci. Nutr. 2021, 61, 1616–1639. [Google Scholar] [CrossRef]
- Rufino, A.; Costa, V.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Hickson, L.; Langhi Prata, L.; Bobart, S.; Evans, T.; Giorgadze, N.; Hashmi, S.; Herrmann, S.; Jensen, M.; Jia, Q.; Jordan, K.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Justice, J.; Nambiar, A.; Tchkonia, T.; LeBrasseur, N.; Pascual, R.; Hashmi, S.; Prata, L.; Masternak, M.; Kritchevsky, S.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Yousefzadeh, M.; Zhu, Y.; McGowan, S.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.; Melos, K.; Pirtskhalava, T.; Inman, C.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Burton, M.; Rytych, J.; Amin, R.; Johnson, R. Dietary Luteolin Reduces Proinflammatory Microglia in the Brain of Senescent Mice. Rejuvenation Res. 2016, 19, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Vijg, J. Do DNA Double-Strand Breaks Drive Aging? Mol. Cell 2016, 63, 729–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperka, T.; Wang, J.; Rudolph, K. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Rieckher, M.; Garinis, G.; Schumacher, B. Molecular pathology of rare progeroid diseases. Trends Mol. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Vijg, J. Measuring genome instability in aging—A mini-review. Gerontology 2012, 58, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Pugh, J.; Foster, S.; Sukhina, A.; Petravic, J.; Uhrlaub, J.; Padilla-Torres, J.; Hayashi, T.; Nakachi, K.; Smithey, M.; Nikolich-Žugich, J. Acute systemic DNA damage in youth does not impair immune defense with aging. Aging Cell 2016, 15, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Tse, K.; Herrup, K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech. Ageing Dev. 2017, 161, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Petr, M.A.; Tulika, T.; Carmona-Marin, L.M.; Scheibye-Knudsen, M. Protecting the Aging Genome. Trends Cell Biol. 2020, 30, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Azpurua, J.; Ke, Z.; Chen, I.; Zhang, Q.; Ermolenko, D.; Zhang, Z.; Gorbunova, V.; Seluanov, A. Naked mole-rat has increased translational fidelity compared with the mouse, as well as a unique 28S ribosomal RNA cleavage. Proc. Natl. Acad. Sci. USA 2013, 110, 17350–17355. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Miguel, V.; Lujan, C.; Espie-Caullet, T.; Martinez-Martinez, D.; Moore, S.; Backes, C.; Gonzalez, S.; Galimov, E.; Brown, A.; Halic, M.; et al. Increased fidelity of protein synthesis extends lifespan. Cell Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
- Xilouri, M.; Stefanis, L. Chaperone mediated autophagy in aging: Starve to prosper. Ageing Res. Rev. 2016, 32, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Endicott, S.; Boynton, D.; Beckmann, L.; Miller, R. Long-lived mice with reduced growth hormone signaling have a constitutive upregulation of hepatic chaperone-mediated autophagy. Autophagy 2021, 17, 612–625. [Google Scholar] [CrossRef]
- Koyuncu, S.; Loureiro, R.; Lee, H.; Wagle, P.; Krueger, M.; Vilchez, D. Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature 2021, 596, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Sitte, N.; Merker, K.; Grune, T.; von Zglinicki, T. Lipofuscin accumulation in proliferating fibroblasts in vitro: An indicator of oxidative stress. Exp. Gerontol. 2001, 36, 475–486. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.; Bindewald-Wittich, A.; Spaide, R.; Wolf, S.; Sadda, S.; Holz, F. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef]
- Galanos, P.; Vougas, K.; Walter, D.; Polyzos, A.; Maya-Mendoza, A.; Haagensen, E.; Kokkalis, A.; Roumelioti, F.; Gagos, S.; Tzetis, M.; et al. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat. Cell Biol. 2016, 18, 777–789. [Google Scholar] [CrossRef]
- Myrianthopoulos, V.; Evangelou, K.; Vasileiou, P.; Cooks, T.; Vassilakopoulos, T.; Pangalis, G.; Kouloukoussa, M.; Kittas, C.; Georgakilas, A.; Gorgoulis, V. Senescence and senotherapeutics: A new field in cancer therapy. Pharmacol. Ther. 2019, 193, 31–49. [Google Scholar] [CrossRef]
- Reeg, S.; Grune, T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid. Redox Signal. 2015, 23, 239–255. [Google Scholar] [CrossRef] [Green Version]
- Moreno-García, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef]
- Russo, G.; Landi, R.; Pezone, A.; Morano, A.; Zuchegna, C.; Romano, A.; Muller, M.; Gottesman, M.; Porcellini, A.; Avvedimento, E. DNA damage and Repair Modify DNA methylation and Chromatin Domain of the Targeted Locus: Mechanism of allele methylation polymorphism. Sci. Rep. 2016, 6, 33222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, E.; Scheibye-Knudsen, M.; Brace, L.; Kassahun, H.; SenGupta, T.; Nilsen, H.; Mitchell, J.; Croteau, D.; Bohr, V. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 2014, 157, 882–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, S.; Sato, S.; Fukuda, T.; Tada, N.; Uchiyama, Y.; Tanaka, K.; Hattori, N. Loss of Parkin contributes to mitochondrial turnover and dopaminergic neuronal loss in aged mice. Neurobiol. Dis. 2020, 136, 104717. [Google Scholar] [CrossRef] [PubMed]
- Edifizi, D.; Nolte, H.; Babu, V.; Castells-Roca, L.; Mueller, M.M.; Brodesser, S.; Kruger, M.; Schumacher, B. Multilayered Reprogramming in Response to Persistent DNA Damage in C. elegans. Cell Rep. 2017, 20, 2026–2043. [Google Scholar] [CrossRef] [Green Version]
- Tsakiri, E.; Iliaki, K.; Höhn, A.; Grimm, S.; Papassideri, I.; Grune, T.; Trougakos, I. Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free Radic. Biol. Med. 2013, 65, 1155–1163. [Google Scholar] [CrossRef]
- Rodier, F.; Muñoz, D.; Teachenor, R.; Chu, V.; Le, O.; Bhaumik, D.; Coppé, J.; Campeau, E.; Beauséjour, C.; Kim, S.; et al. DNA-SCARS: Distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 2011, 124, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Na, H.; Pyo, J.; Kim, Y.; Yoo, M. Age- and oxidative stress-induced DNA damage in Drosophila intestinal stem cells as marked by Gamma-H2AX. Exp. Gerontol. 2012, 47, 401–405. [Google Scholar] [CrossRef]
- Wang, C.; Jurk, D.; Maddick, M.; Nelson, G.; Martin-Ruiz, C.; von Zglinicki, T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009, 8, 311–323. [Google Scholar] [CrossRef]
- Ermolaeva, M.; Segref, A.; Dakhovnik, A.; Ou, H.; Schneider, J.; Utermöhlen, O.; Hoppe, T.; Schumacher, B. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 2013, 501, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Shibazaki, A.; Ono, R.; Kaisho, T. HSP70 mediates degradation of the p65 subunit of nuclear factor κB to inhibit inflammatory signaling. Sci. Signal. 2014, 7, ra119. [Google Scholar] [CrossRef] [PubMed]
- Sunjaya, A.; Sunjaya, A. Targeting ageing and preventing organ degeneration with metformin. Diabetes Metab. 2021, 47, 101203. [Google Scholar] [CrossRef] [PubMed]
- Pyo, I.; Yun, S.; Yoon, Y.; Choi, J.; Lee, S. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, J.; Elia, A.; Carroll, V.A.; Moumen, A. DNA damage-induced S and G2/M cell cycle arrest requires mTORC2-dependent regulation of Chk1. Oncotarget 2015, 6, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ding, J.; Köhnlein, K.; Urban, N.; Ori, A.; Villavicencio-Lorini, P.; Walentek, P.; Klotz, L.; Hollemann, T.; Pfirrmann, T. The GID ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan. Autophagy 2020, 16, 1618–1634. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Evans, S.; Fielder, E.; Victorelli, S.; Kruger, P.; Salmonowicz, H.; Weigand, B.; Patel, A.; Pirtskhalava, T.; Inman, C.; et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 2021, 20, e13296. [Google Scholar] [CrossRef]
- Chen, T.; Shen, L.; Yu, J.; Wan, H.; Guo, A.; Chen, J.; Long, Y.; Zhao, J.; Pei, G. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell 2011, 10, 908–911. [Google Scholar] [CrossRef]
- Perrott, K.; Wiley, C.; Desprez, P.; Campisi, J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. GeroScience 2017, 39, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, E.; Kim, D.; Yu, B.; Chung, H. Kaempferol modulates pro-inflammatory NF-kappaB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 2010, 32, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Gutierrez, D.; Zimmermann, A.; Kainz, K.; Pietrocola, F.; Chen, G.; Maglioni, S.; Schiavi, A.; Nah, J.; Mertel, S.; Beuschel, C.; et al. The flavonoid 4,4′-dimethoxychalcone promotes autophagy-dependent longevity across species. Nat. Commun. 2019, 10, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, Y.; Zeng, Y.; Xu, J.; Xu, X. Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoemice: Role of SIRT1. Phytomed. Int. J. Phytother. Phytopharm. 2021, 81, 153412. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Sen, S.; Chatterjee, R.; Roy, D.; James, J.; Thirumurugan, K. Context- and dose-dependent modulatory effects of naringenin on survival and development of Drosophila melanogaster. Biogerontology 2016, 17, 383–393. [Google Scholar] [CrossRef]
- Da Pozzo, E.; Costa, B.; Cavallini, C.; Testai, L.; Martelli, A.; Calderone, V.; Martini, C. The Citrus Flavanone Naringenin Protects Myocardial Cells against Age-Associated Damage. Oxidative Med. Cell. Longev. 2017, 2017, 9536148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Kim, J.; Choi, H. Genistein-induced LKB1-AMPK activation inhibits senescence of VSMC through autophagy induction. Vasc. Pharmacol. 2016, 81, 75–82. [Google Scholar] [CrossRef]
- Kim, J.; Uehara, Y.; Choi, Y.; Ha, Y.; Ye, B.; Yu, B.; Chung, H. Mechanism of attenuation of pro-inflammatory Ang II-induced NF-κB activation by genistein in the kidneys of male rats during aging. Biogerontology 2011, 12, 537–550. [Google Scholar] [CrossRef]
- Bonet-Costa, V.; Herranz-Pérez, V.; Blanco-Gandía, M.; Mas-Bargues, C.; Inglés, M.; Garcia-Tarraga, P.; Rodriguez-Arias, M.; Miñarro, J.; Borras, C.; Garcia-Verdugo, J.; et al. Clearing Amyloid-β through PPARγ/ApoE Activation by Genistein is a Treatment of Experimental Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2016, 51, 701–711. [Google Scholar] [CrossRef]
- Büchter, C.; Ackermann, D.; Havermann, S.; Honnen, S.; Chovolou, Y.; Fritz, G.; Kampkötter, A.; Wätjen, W. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int. J. Mol. Sci. 2013, 14, 11895–11914. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Lee, D.; Ryu, H.; Choi, B.; Go, Y.; Lee, N.; Lee, D.; Son, H.; Jeon, J.; Kim, S.; et al. Myricetin improves endurance capacity and mitochondrial density by activating SIRT1 and PGC-1α. Sci. Rep. 2017, 7, 6237. [Google Scholar] [CrossRef]
- Fan, X.; Zeng, Y.; Fan, Z.; Cui, L.; Song, W.; Wu, Q.; Gao, Y.; Yang, D.; Mao, X.; Zeng, B.; et al. DrosophilaDihydromyricetin promotes longevity and activates the transcription factors FOXO and AOP in. Aging 2020, 13, 460–476. [Google Scholar] [CrossRef]
- Qian, J.; Wang, X.; Cao, J.; Zhang, W.; Lu, C.; Chen, X. Dihydromyricetin attenuates D-galactose-induced brain aging of mice via inhibiting oxidative stress and neuroinflammation. Neurosci. Lett. 2021, 756, 135963. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Coria, H.; Mendoza-Rojas, M.; Arrieta-Cruz, I.; López-Valdés, H. Preclinical Research of Dihydromyricetin for Brain Aging and Neurodegenerative Diseases. Front. Pharmacol. 2019, 10, 1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Sanchez, I.; Mansour, C.; Navarrete-Yañez, V.; Ayala-Hernandez, M.; Guevara, G.; Castillo, C.; Loredo, M.; Bustamante, M.; Ceballos, G.; Villarreal, F. (-)-Epicatechin induced reversal of endothelial cell aging and improved vascular function: Underlying mechanisms. Food Funct. 2018, 9, 4802–4813. [Google Scholar] [CrossRef]
- Si, H.; Wang, X.; Zhang, L.; Parnell, L.; Admed, B.; LeRoith, T.; Ansah, T.; Zhang, L.; Li, J.; Ordovás, J.; et al. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 965–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarrete-Yañez, V.; Garate-Carrillo, A.; Rodriguez, A.; Mendoza-Lorenzo, P.; Ceballos, G.; Calzada-Mendoza, C.; Hogan, M.; Villarreal, F.; Ramirez-Sanchez, I. Effects of (-)-epicatechin on neuroinflammation and hyperphosphorylation of tau in the hippocampus of aged mice. Food Funct. 2020, 11, 10351–10361. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.; Guillory, B.; Zhao, Z.; Garcia, J.; Koike, N.; Lee, C.; Takahashi, J.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, D.; Thirumurugan, K. Longevity-promoting efficacies of rutin in high fat diet fed Drosophila melanogaster. Biogerontology 2020, 21, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, S.; Feng, T.; Dong, J.; Li, Y.; Li, H. Rutin protects against aging-related metabolic dysfunction. Food Funct. 2016, 7, 1147–1154. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Zhang, H.; Su, Y.; Zhou, W.; Zhang, Z.; Wang, S.; Xu, P.; Wang, Y.; Liu, R. Rutin inhibits amylin-induced neurocytotoxicity and oxidative stress. Food Funct. 2015, 6, 3296–3306. [Google Scholar] [CrossRef]
- Sun, K.; Xiang, L.; Ishihara, S.; Matsuura, A.; Sakagami, Y.; Qi, J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH1 gene expression. Biosci. Biotechnol. Biochem. 2012, 76, 640–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elavarasan, J.; Velusamy, P.; Ganesan, T.; Ramakrishnan, S.; Rajasekaran, D.; Periandavan, K. Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J. Pharm. Pharmacol. 2012, 64, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Ji, S.; Li, M.; Zheng, S.; Zhou, X.; Guo, H.; Deng, S.; Zhu, J.; Li, D.; Xie, Z. DrosophilaTheaflavin-regulated Imd condensates control intestinal homeostasis and aging. iScience 2021, 24, 102150. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, M.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.; Cai, D.; Luo, X. Reducing Hypothalamic Stem Cell Senescence Protects against Aging-Associated Physiological Decline. Cell Metab. 2020, 31, 534–548.e535. [Google Scholar] [CrossRef] [PubMed]
- Gasek, N.; Kuchel, G.; Kirkland, J.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Dookun, E.; Passos, J.F.; Arthur, H.M.; Richardson, G.D. Therapeutic Potential of Senolytics in Cardiovascular Disease. Cardiovasc. Drugs Ther. 2020. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.; Misra, A.; Netto, J.; Tchkonia, T.; Kirkland, J. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 2021, 200, 111591. [Google Scholar] [CrossRef]
- Camell, C.; Yousefzadeh, M.; Zhu, Y.; Langhi Prata, L.; Huggins, M.; Pierson, M.; Zhang, L.; O’Kelly, R.; Pirtskhalava, T.; Xun, P.; et al. Senolytics reduce coronavirus-related mortality in old mice. Science 2021, 373, eabe4832. [Google Scholar] [CrossRef]
- Sang, Y.; Zhang, F.; Wang, H.; Yao, J.; Chen, R.; Zhou, Z.; Yang, K.; Xie, Y.; Wan, T.; Ding, H. Apigenin exhibits protective effects in a mouse model of d-galactose-induced aging via activating the Nrf2 pathway. Food Funct. 2017, 8, 2331–2340. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef]
- Liu, M.; Guo, H.; Li, Z.; Zhang, C.; Zhang, X.; Cui, Q.; Tian, J. Molecular Level Insight Into the Benefit of Myricetin and Dihydromyricetin Uptake in Patients With Alzheimer’s Diseases. Front. Aging Neurosci. 2020, 12, 601603. [Google Scholar] [CrossRef]
- Man, G.; Mauro, T.; Zhai, Y.; Kim, P.; Cheung, C.; Hupe, M.; Crumrine, D.; Elias, P.; Man, M. Topical hesperidin enhances epidermal function in an aged murine model. J. Investig. Dermatol. 2015, 135, 1184–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Zhang, H.; Guan, X.; Zhou, Z. Saccharomyces CerevisiaeThe Anti-Aging Potential of Neohesperidin and Its Synergistic Effects with Other Citrus Flavonoids in Extending Chronological Lifespan of BY4742. Molecules 2019, 24, 4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Lo, C.; Pan, M.; Lai, C.; Ho, C. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, G.; Tang, S.; Liu, H.; Hu, Y.; Shang, P. viaTheaflavin protects chondrocytes against apoptosis and senescence regulating Nrf2 and ameliorates murine osteoarthritis. Food Funct. 2021, 12, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.; Fetoui, H.; Fakhfakh, F.; Keskes, L. Caffeic acid and quercetin protect erythrocytes against the oxidative stress and the genotoxic effects of lambda-cyhalothrin in vitro. Hum. Exp. Toxicol. 2012, 31, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, A.; Raciti, M.; Cucina, A.; Bizzarri, M.; Di Renzo, L. Quercetin affects Hsp70/IRE1α mediated protection from death induced by endoplasmic reticulum stress. Oxidative Med. Cell. Longev. 2015, 2015, 645157. [Google Scholar] [CrossRef] [Green Version]
- Ng, A.; Nin, D.; Fong, J.; Venkataraman, D.; Chen, C.; Khan, M. Therapeutic targeting of nuclear receptor corepressor misfolding in acute promyelocytic leukemia cells with genistein. Mol. Cancer Ther. 2007, 6, 2240–2248. [Google Scholar] [CrossRef] [Green Version]
- Tsai, F.; Lin, C.; Lai, C.; Lan, Y.; Lai, C.; Hung, C.; Hsueh, K.; Lin, T.; Chang, H.; Wan, L.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef]
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin restricts FMDV infection and inhibits viral IRES driven translational activity. Viruses 2015, 7, 1613–1626. [Google Scholar] [CrossRef] [Green Version]
- Kootstra, A. Protection from UV-B-induced DNA damage by flavonoids. Plant Mol. Biol. 1994, 26, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Esser, P.; Simon-Haarhaus, B.; Martin, S.; Lademann, J.; Schempp, C. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Darband, S.; Sadighparvar, S.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Rahimi, Y.; Mobaraki, K.; Majidinia, M. Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci. 2020, 253, 117584. [Google Scholar] [CrossRef]
- Dalcin, A.; Roggia, I.; Felin, S.; Vizzotto, B.; Mitjans, M.; Vinardell, M.; Schuch, A.; Ourique, A.; Gomes, P. UVB photoprotective capacity of hydrogels containing dihydromyricetin nanocapsules to UV-induced DNA damage. Colloids Surf. B Biointerfaces 2021, 197, 111431. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Haza, A.; García, A.; Morales, P. Myricetin, quercetin, (+)-catechin and (-)-epicatechin protect against N-nitrosamines-induced DNA damage in human hepatoma cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2009, 23, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Nachtergael, A.; Ouedraogo, M.; Belayew, A.; Duez, P. Effects of chemopreventive natural products on non-homologous end-joining DNA double-strand break repair. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2014, 768, 33–41. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yu, H.; Li, M.; Hang, L.; Xu, X. Apigenin Protects Mouse Retina against Oxidative Damage by Regulating the Nrf2 Pathway and Autophagy. Oxidative Med. Cell. Longev. 2020, 2020, 9420704. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Garg, G.; Rizvi, S. Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sci. 2018, 193, 171–179. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Peng, L.; Tian, X.; Qiu, X.; Cao, H.; Yang, Q.; Liao, R.; Yan, F. Dihydromyricetin protects HUVECs of oxidative damage induced by sodium nitroprusside through activating PI3K/Akt/FoxO3a signalling pathway. J. Cell. Mol. Med. 2019, 23, 4829–4838. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Chen, F.; Liu, M.; Ning, L.; Yan, Y.; Shang, Z.; Huang, S.; Tu, C. Nobiletin mitigates hepatocytes death, liver inflammation, and fibrosis in a murine model of NASH through modulating hepatic oxidative stress and mitochondrial dysfunction. J. Nutr. Biochem. 2021, 100, 108888. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, D.; Ahmed, M.; Al-Rejaie, S.; Alhomida, A.; Ola, M. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Hu, W.; Hung, M.; Ou, H.; Huang, S.; Hsu, P.; Day, C.; Lin, K.; Viswanadha, V.; Kuo, W.; et al. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr. Metab. Cardiovasc. Dis. NMCD 2020, 30, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zheng, L.; Wang, Y.; Huang, J.; Yang, Z.; Yue, Z.; Guo, B. Genistein exhibits therapeutic potential for PCOS mice the ER-Nrf2-Foxo1-ROS pathway. Food Funct. 2021, 12, 8800–8811. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Jiang, H.; Yong, N.X.; Piao, X.; Kim, N.H. Kaempferol attenuates mitochondrial dysfunction and oxidative stress induced by H2O2 during porcine embryonic development. Theriogenology 2019, 135, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yi, P.; Yi, M.; Tong, X.; Cheng, X.; Yang, J.; Hu, Y.; Peng, W. Protective Effect of Quercetin against HO-Induced Oxidative Damage in PC-12 Cells: Comprehensive Analysis of a lncRNA-Associated ceRNA Network. Oxidative Med. Cell. Longev. 2020, 2020, 6038919. [Google Scholar] [CrossRef]
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 2008, 283, 3050–3058. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Liu, Y.; Chen, L.; Zhu, Y.; Xiao, X.; Wang, D.; Zhu, Y. Nobiletin prevents cadmium-induced neuronal apoptosis by inhibiting reactive oxygen species and modulating JNK/ERK1/2 and Akt/mTOR networks in rats. Neurol. Res. 2018, 40, 211–220. [Google Scholar] [CrossRef]
- Fan, Q.W.; Nicolaides, T.P.; Weiss, W.A. Inhibiting 4EBP1 in Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann-Stroissnigg, H.; Ling, Y.; Zhao, J.; McGowan, S.; Zhu, Y.; Brooks, R.; Grassi, D.; Gregg, S.; Stripay, J.; Dorronsoro, A.; et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef]
- Joshi, V.; Mishra, R.; Upadhyay, A.; Amanullah, A.; Poluri, K.; Singh, S.; Kumar, A.; Mishra, A. Polyphenolic flavonoid (Myricetin) upregulated proteasomal degradation mechanisms: Eliminates neurodegenerative proteins aggregation. J. Cell. Physiol. 2019, 234, 20900–20914. [Google Scholar] [CrossRef] [PubMed]
- Martín-Aragón, S.; Jiménez-Aliaga, K.L.; Benedí, J.; Bermejo-Bescós, P. Neurohormetic responses of quercetin and rutin in a cell line over-expressing the amyloid precursor protein (APPswe cells). Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 1285–1294. [Google Scholar] [CrossRef]
- Maher, P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch. Biochem. Biophys. 2008, 476, 139–144. [Google Scholar] [CrossRef]
- Brimson, J.; Prasanth, M.; Malar, D.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Plant Polyphenols for Aging Health: Implication from Their Autophagy Modulating Properties in Age-Associated Diseases. Pharmaceuticals 2021, 14, 982. [Google Scholar] [CrossRef]
- Qiu, P.; Dong, Y.; Li, B.; Kang, X.; Gu, C.; Zhu, T.; Luo, Y.; Pang, M.; Du, W.; Ge, W. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol. Lett. 2017, 274, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Xu, X.; Zhou, S.; Chen, Y.; Ding, G.; Cao, L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis. 2019, 10, 142. [Google Scholar] [CrossRef]
- Varshney, R.; Gupta, S.; Roy, P. Cytoprotective effect of kaempferol against palmitic acid-induced pancreatic β-cell death through modulation of autophagy via AMPK/mTOR signaling pathway. Mol. Cell. Endocrinol. 2017, 448, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Meng, Z.; Chen, Y.; Yu, L.; Gao, B.; Zheng, Y.; Guan, S. Apigenin induced autophagy and stimulated autophagic lipid degradation. Food Funct. 2020, 11, 9208–9215. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, R.; Lv, H.; Song, L.; Xue, X.; Wu, L. Inhibitive Effect of Luteolin on Sevoflurane-Induced Neurotoxicity through Activation of the Autophagy Pathway by HMOX1. ACS Chem. Neurosci. 2021, 12, 3314–3322. [Google Scholar] [CrossRef]
- Ahsan, A.; Sharma, V.; Wani, A.; Chopra, M. Naringenin Upregulates AMPK-Mediated Autophagy to Rescue Neuronal Cells From β-Amyloid Evoked Neurotoxicity. Mol. Neurobiol. 2020, 57, 3589–3602. [Google Scholar] [CrossRef]
- Dai, B.; Zhong, T.; Chen, Z.; Chen, W.; Zhang, N.; Liu, X.; Wang, L.; Chen, J.; Liang, Y. Myricetin slows liquid-liquid phase separation of Tau and activates ATG5-dependent autophagy to suppress Tau toxicity. J. Biol. Chem. 2021, 297, 101222. [Google Scholar] [CrossRef] [PubMed]
- Kampkötter, A.; Gombitang Nkwonkam, C.; Zurawski, R.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef]
- Kampkötter, A.; Nkwonkam, C.; Zurawski, R.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Mrvová, N.; Škandík, M.; Bezek, Š.; Račková, L. Protective Effect of Semisynthetic and Natural Flavonoid on Aged Rat Microglia-enriched Cultures. Neurotox. Res. 2019, 36, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Naia, L.; Pinho, C.; Dentoni, G.; Liu, J.; Leal, N.; Ferreira, D.; Schreiner, B.; Filadi, R.; Fão, L.; Connolly, N.; et al. Neuronal cell-based high-throughput screen for enhancers of mitochondrial function reveals luteolin as a modulator of mitochondria-endoplasmic reticulum coupling. BMC Biol. 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef] [PubMed]

| Flavonoids | Structure | Targets | Activity | Lifespan Extension | Reference |
|---|---|---|---|---|---|
| Senolytic | |||||
| Quercetin | 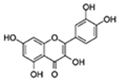 | Numerous (including PI3K) | ↓Senescent human cells in vitro; ↓SASP and hepatic steatosis in mice; ↓AD in mice; ↓Insulin resistance in obese mice; ↓Physical dysfunction in human IPF patients; ↓Anxiety in obese mice; ↑Exercise capacity; ↑Renal function in obese mice | 15–60% lifespan in mice and C. elegans | [6,46,47] |
| Fisetin |  | PI3K/AKT/mTOR | ↓Senescent human cells in vitro; ↓Senescent cells and SASP in progeroid and aged mice in vivo; ↓Age-related pathology; ↑Lifespan of wild type in aged mice | ~13% in mice, 23% in Drosophila, and 55% in Saccharomyces cerevisiae | [9,48] |
| Luteolin | 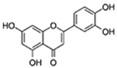 | ROS, PGE2, COX2 | ↓Senescent human cells and SASP in vitro | No data | [10] |
| Senomorphic | |||||
| Apigenin | 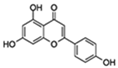 | NF-κB p65 subunit IκB | ↓SASP in fibroblasts; ↓SASP in the kidney of age rats; ↓Age-related skeletal muscle atrophy | No data | [49] |
| Kaempferol |  | IRAK1/IkBα/NF-κB p65 | ↓SASP in fibroblasts; ↓ROS; ↓AGEs | No data | [50] |
| Others with antisenescence activity | |||||
| 4,4′Dimethoxychalcone | 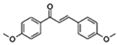 | Autophagy GATA transcription factors | ↓Cell senescence; ↑Health span | Approximately 20% increase in Drosophila and C. elegans | [51] |
| Naringenin | 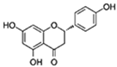 | SIRT1/LKB1/PGC1α/NF-κB | ↓Cardiac markers of aging-induced damage; ↓ROS | 22.62% increase in females of Drosophila | [52,53,54] |
| Genistein | 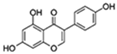 | NF-κB p38 | ↓Proinflammatory genes expression; ↓Cell senescence; ↑Parameters of cognition in AD | No data | [55,56,57] |
| Myricetin | 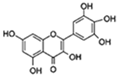 | FOXO SIRT1/PGC-1α | ↓HMW-Aβ-induced neurotoxicity; ↑Mitochondrial function | 32.9% in increase C. elegans | [58,59] |
| Dihydromyricetin | 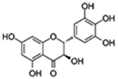 | FOXO/ AOP Autophagy | ↓Oxidative stress and inflammation-related senescence; ↓Gut dysfunction; ↑Motor and cognitive behavior | 16.07% increase in Drosophila | [60,61,62] |
| Epicatechin | 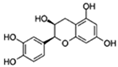 | Autophagy | ↓Cell senescence; ↓Skeletal muscle degeneration; ↑Brain function | 7.1% increase in C. elegans | [63,64,65] |
| Nobiletin | 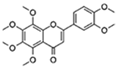 | Target RORs | ↓ROS; ↓Metabolic disease; ↑Circadian rhythms | 1 month longer at median lifespan in mouse | [66,67] |
| Rutin | 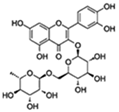 | Insulin/IGF1 Autophagy | ↓ROS and proinflammatory cytokines (TNF-α and IL-1β); ↓Aging-related metabolic dysfunction; ↑ATGs, Foxo | 32% increase HFD Drosophila | [68,69,70] |
| Hesperidin | 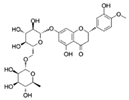 | Nrf2 | ↓ROS; ↑Activity of antioxidant enzymes | Extends the repilicative lifespan of the yeast | [71,72] |
| Theaflavin | 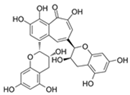 | Nrf2 | ↓Stem cell senescence; ↑Intestinal homeostasis | Lifespan increase in Drosophila | [73,74] |
| Flavonoid Therapy | Indication | Dose and Duration | Trial |
|---|---|---|---|
| Quercetin (Q) + Dasatinib (D) | Alzheimer’s disease | Q (1000 mg/day) + D (100 mg/day) administered orally for 2 consecutive days every 15 days (2 days on drug, 13 days off) for 6 cycles | NCT04785300 |
| Intermittent D + Q administered for 2 days on/14 days off for 12 weeks (6 cycles) | NCT04063124 | ||
| Age-related osteoporosis | D (100 mg/2 days) plus Q (1000 mg/day last for 3 days) taken orally on an intermittent schedule (starting every 28 days) over 20 weeks, resulting in five total dosing periods throughout the entire intervention | NCT04313634 | |
| Accelerated-ageing-like state post bone marrow transplantation | Q (1000 mg/day) + D (100 mg/day) administered orally for 3 consecutive days | NCT02652052 | |
| Diabetic kidney disease | Q (1000 mg/day) + D (100 mg/day) administered orally for 3 consecutive days | NCT02848131 | |
| Epigenetic aging | 500 mg Q and 50 mg D oral capsules on Monday, Tuesday, and Wednesday (3 days in a row) for 6 months | NCT04946383 | |
| Fisetin | Age-related osteoporosis | 20 mg/kg/day for three consecutive days, taken orally on an intermittent schedule (starting every 28 days) over 20 weeks, resulting in five total dosing periods throughout the entire intervention | NCT04313634 |
| Elderly syndrome | 20 mg/kg/day, orally for 2 consecutive days | NCT03675724 | |
| Elderly syndrome in old women | 20/mg/kg/day, orally for 2 consecutive days, for 2 consecutive months | NCT03430037 | |
| Osteoarthritis | Administered orally at 20 mg/kg for 2 consecutive days, followed by 28 days off, then 2 more consecutive days | NCT04210986 | |
| Oral fisetin 20 mg/kg taken for 10 days total | NCT04815902 | ||
| Diabetic and chronic kidney disease | 20 mg/kg/day, orally for 2 consecutive days | NCT03325322 | |
| COVID-19 in hospitalized patients | 20 mg/kg/day, orally for 2 consecutive days | NCT04476953 | |
| COVID-19 in outpatients | 20 mg/kg/day oral for 4 days | NCT04771611 | |
| Coronavirus disease 2019 (COVID-19) in nursing home patients | 20 mg/kg/day, orally for 2 consecutive days | NCT04537299 | |
| Genistein | Alzheimer’s disease | 60 mg of genistein BID for 360 days | NCT01982578 |
| Metabolic syndrome | Genistein capsules of 25 mg each, 50 mg/day | NCT04105023 | |
| Rutin | Type 2 diabetes mellitus | Rutin 60 mg in combination with vitamin C 160 mg three times daily in addition to usual antidiabetic treatment for 8 weeks. | NCT03437902 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Fan, Z.; Yang, Z.; Huang, T.; Tong, Y.; Yang, D.; Mao, X.; Yang, M. Flavonoids—Natural Gifts to Promote Health and Longevity. Int. J. Mol. Sci. 2022, 23, 2176. https://doi.org/10.3390/ijms23042176
Fan X, Fan Z, Yang Z, Huang T, Tong Y, Yang D, Mao X, Yang M. Flavonoids—Natural Gifts to Promote Health and Longevity. International Journal of Molecular Sciences. 2022; 23(4):2176. https://doi.org/10.3390/ijms23042176
Chicago/Turabian StyleFan, Xiaolan, Ziqiang Fan, Ziyue Yang, Tiantian Huang, Yingdong Tong, Deying Yang, Xueping Mao, and Mingyao Yang. 2022. "Flavonoids—Natural Gifts to Promote Health and Longevity" International Journal of Molecular Sciences 23, no. 4: 2176. https://doi.org/10.3390/ijms23042176
APA StyleFan, X., Fan, Z., Yang, Z., Huang, T., Tong, Y., Yang, D., Mao, X., & Yang, M. (2022). Flavonoids—Natural Gifts to Promote Health and Longevity. International Journal of Molecular Sciences, 23(4), 2176. https://doi.org/10.3390/ijms23042176






