What Worth the Garlic Peel
Abstract
:1. Introduction
2. Results
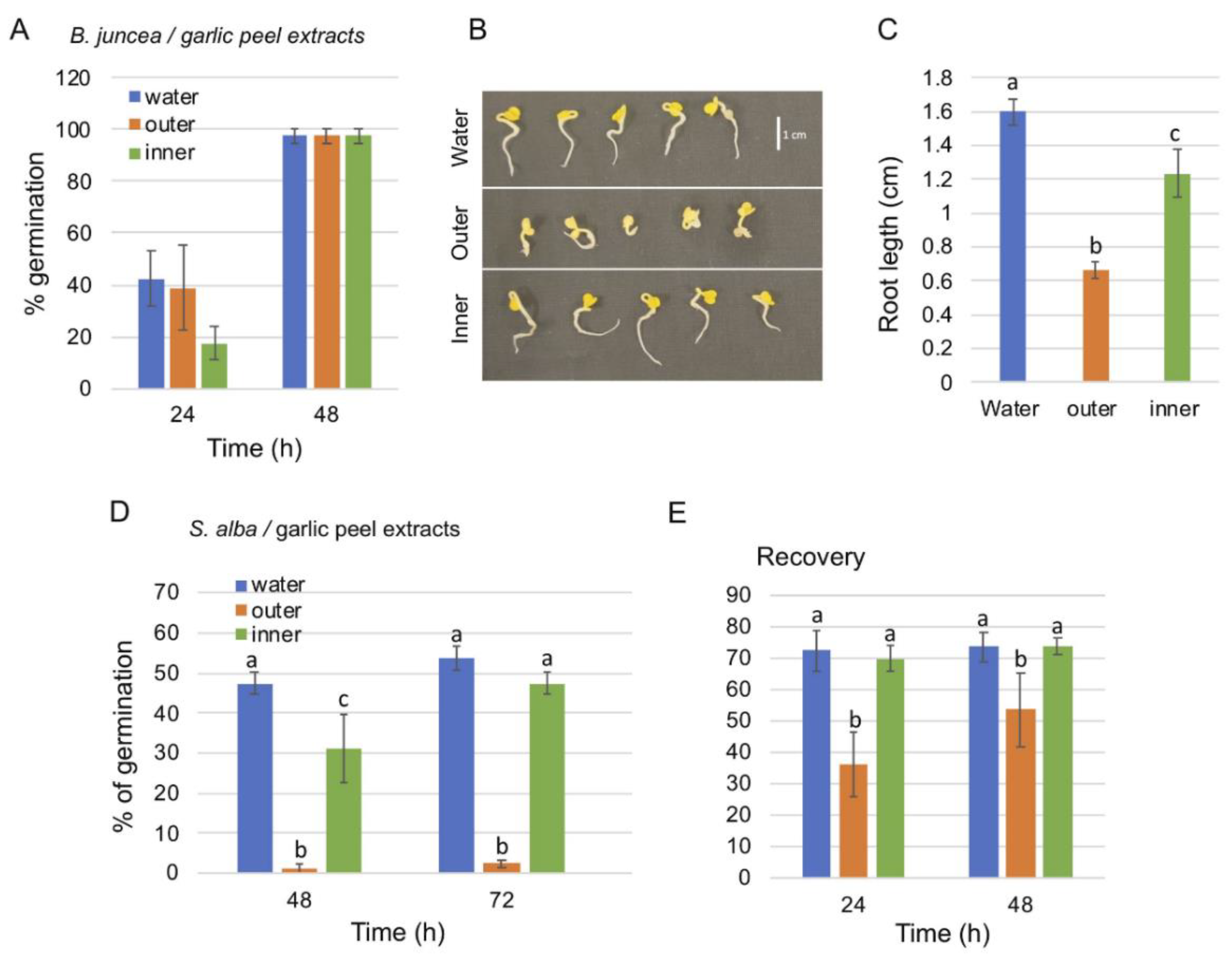
2.1. Effect of Garlic Peel Extracts on Seed Germination
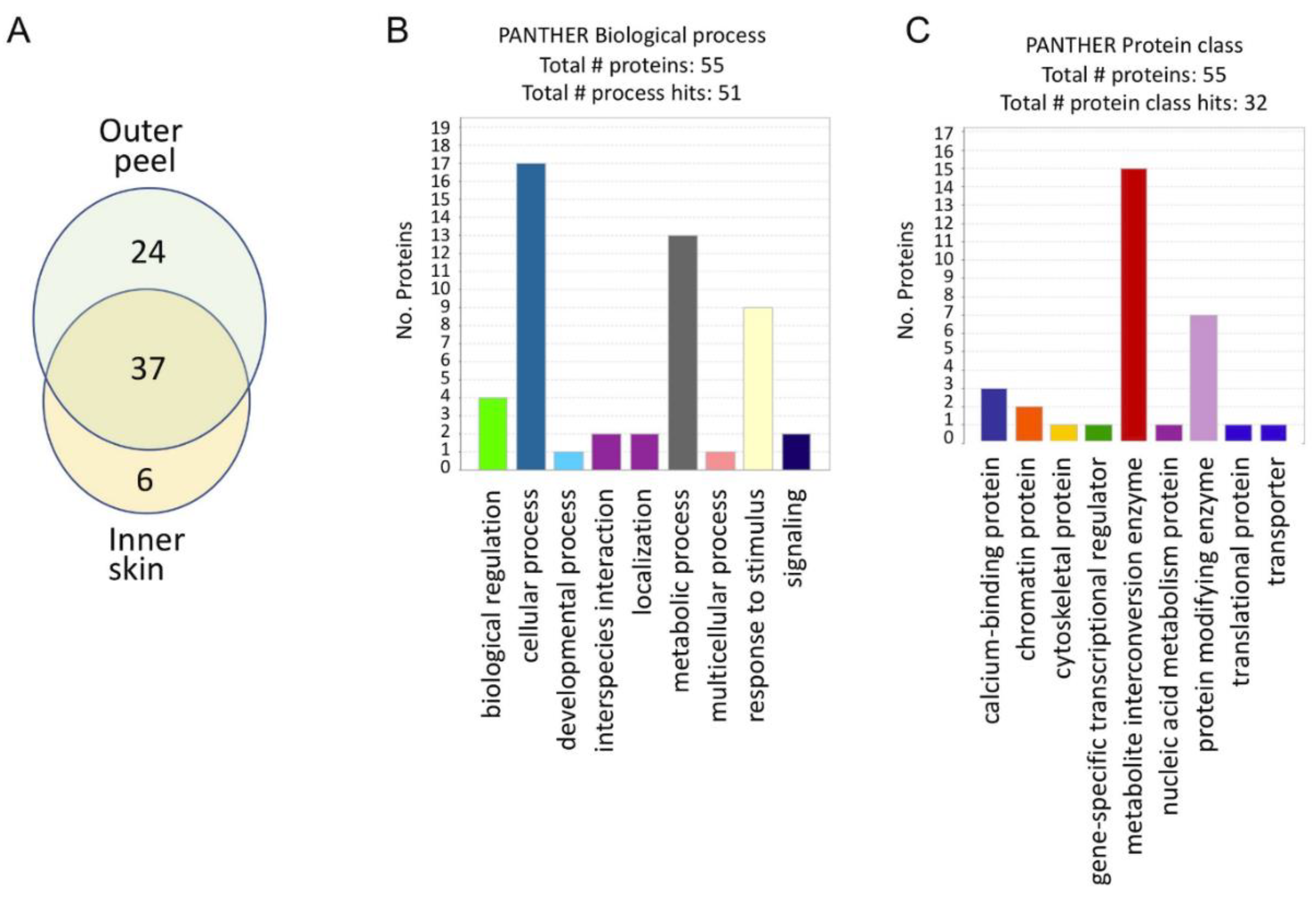
2.2. Garlic Peels Function as a Protein Storage Entity
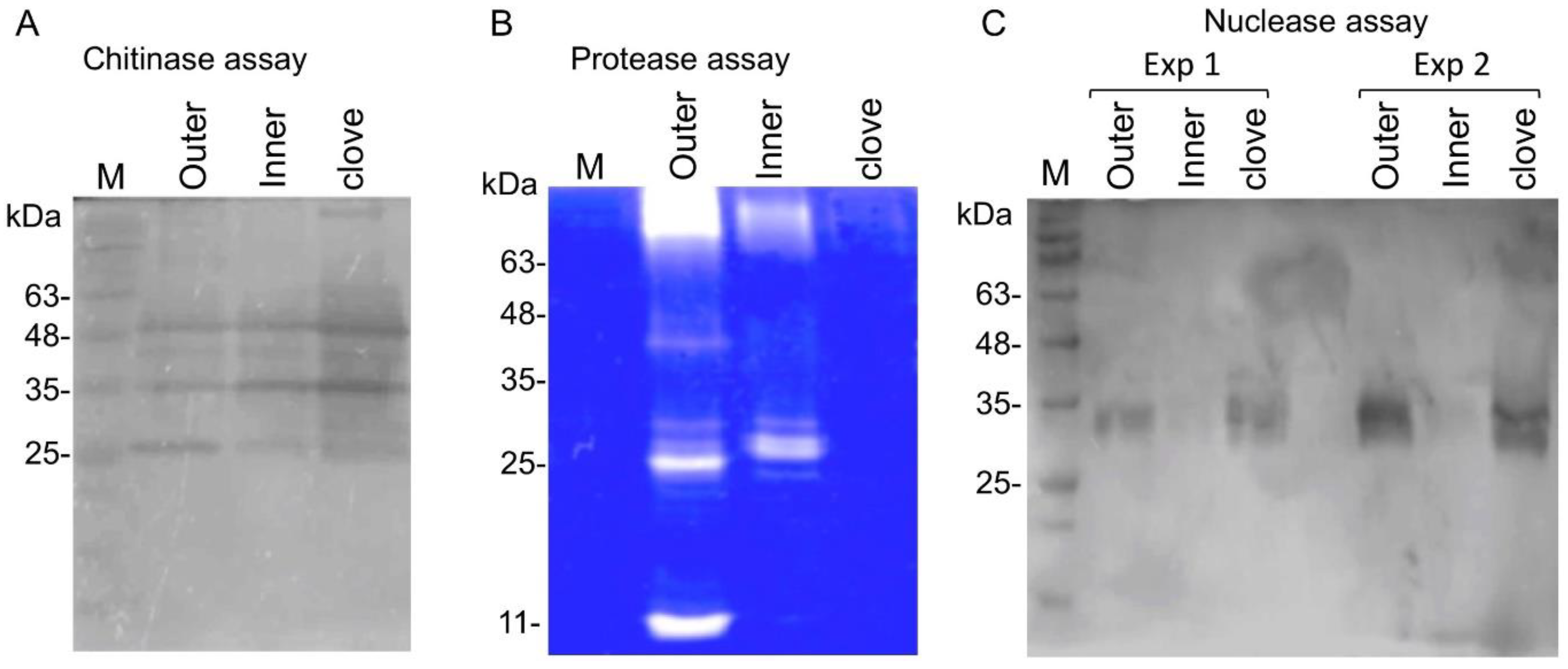
2.3. Garlic Peel Proteins Retain Their Enzymatic Activities
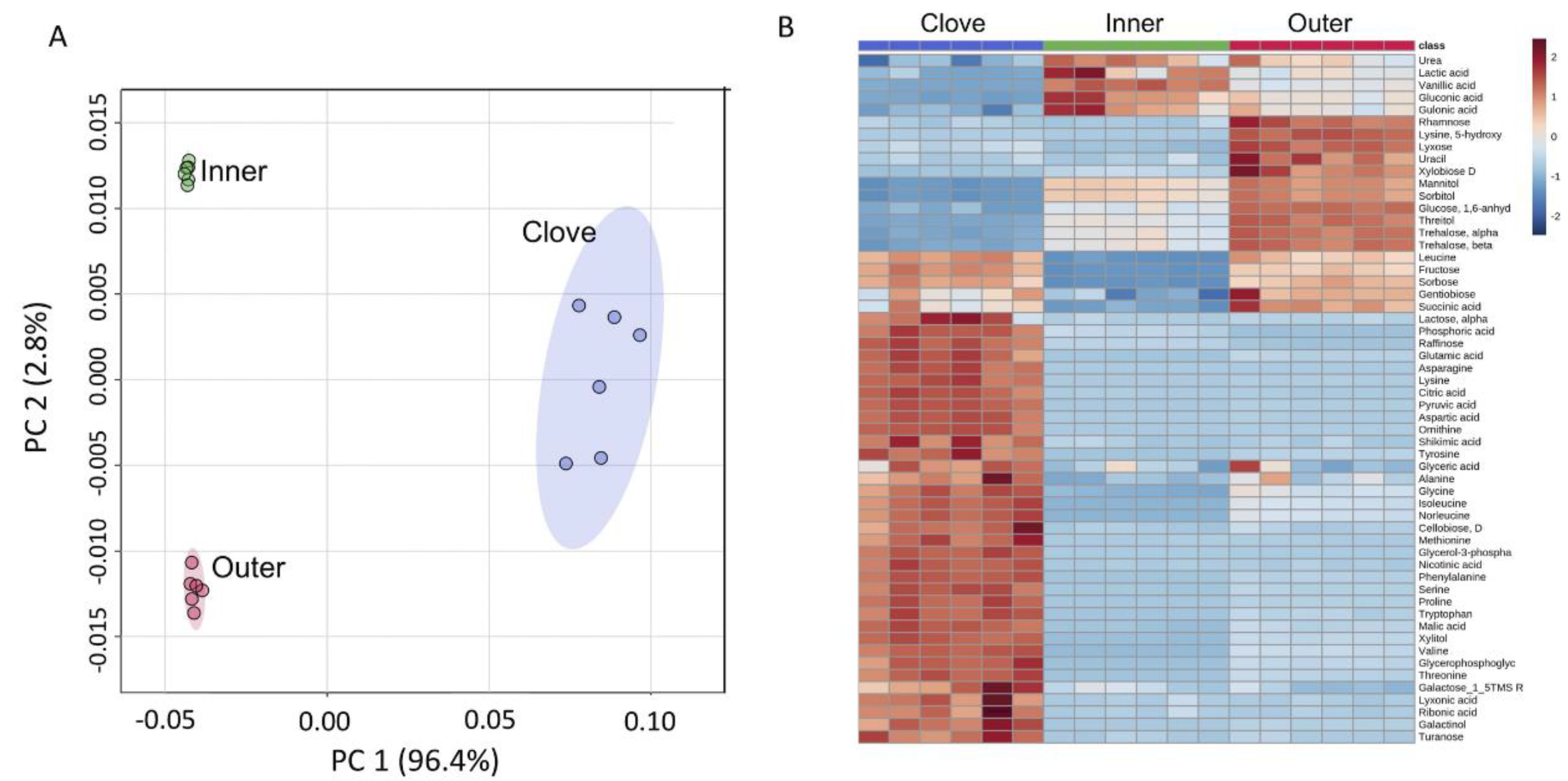
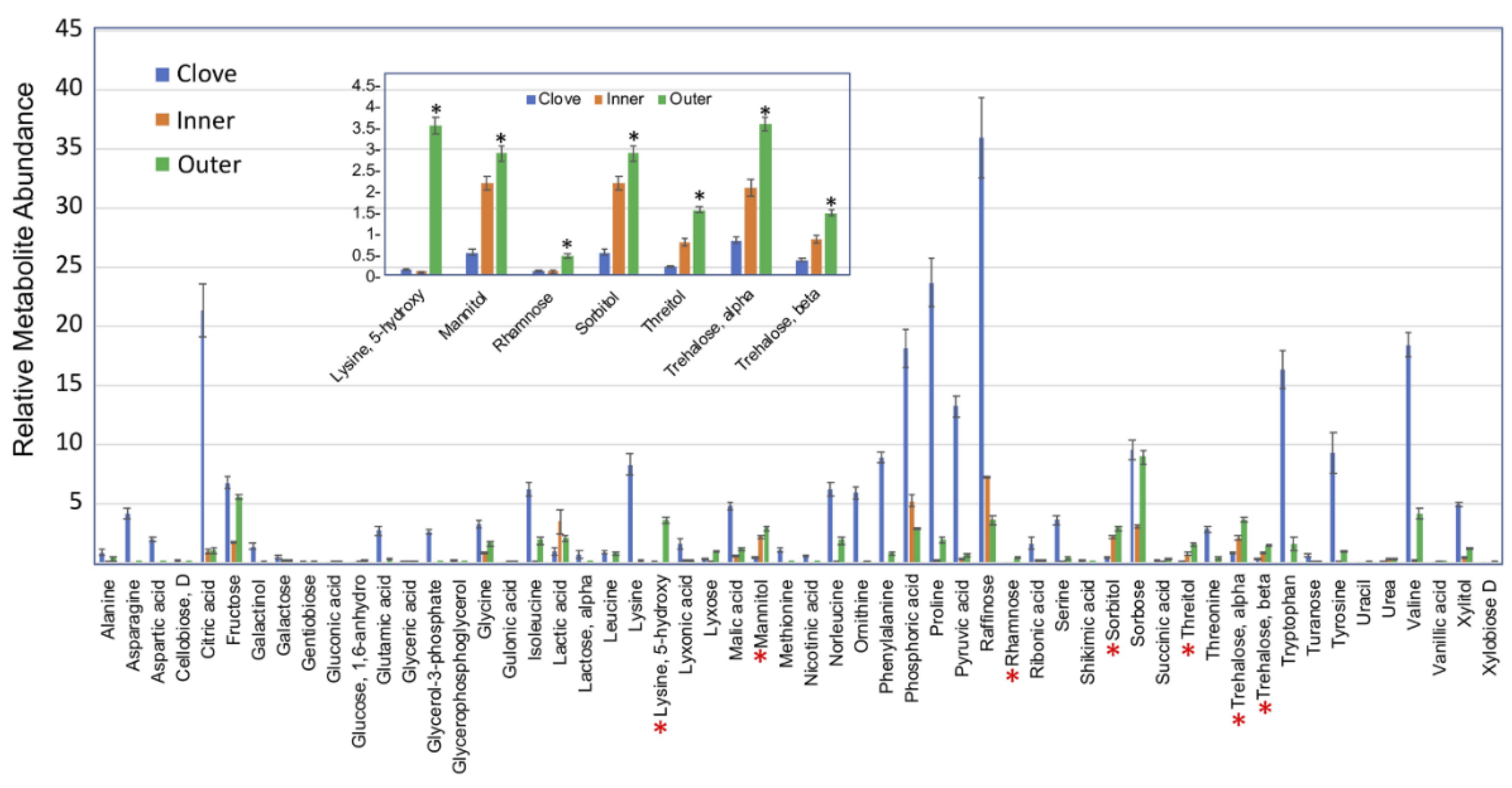
2.4. Primary Metabolites in Garlic Peels
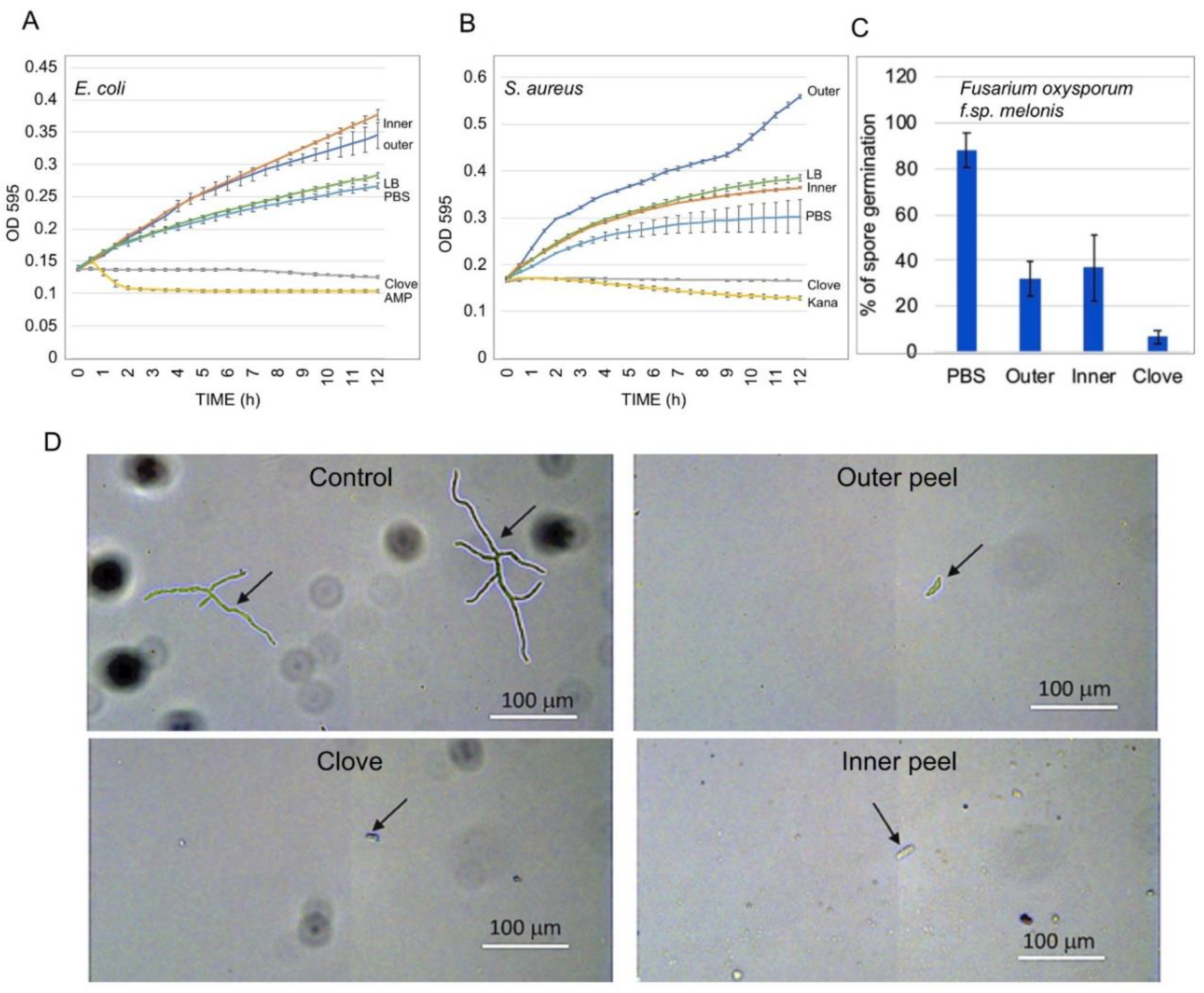
2.5. Effect of Garlic Peel Extracts on Microbial Growth
3. Discussion
4. Materials and Methods
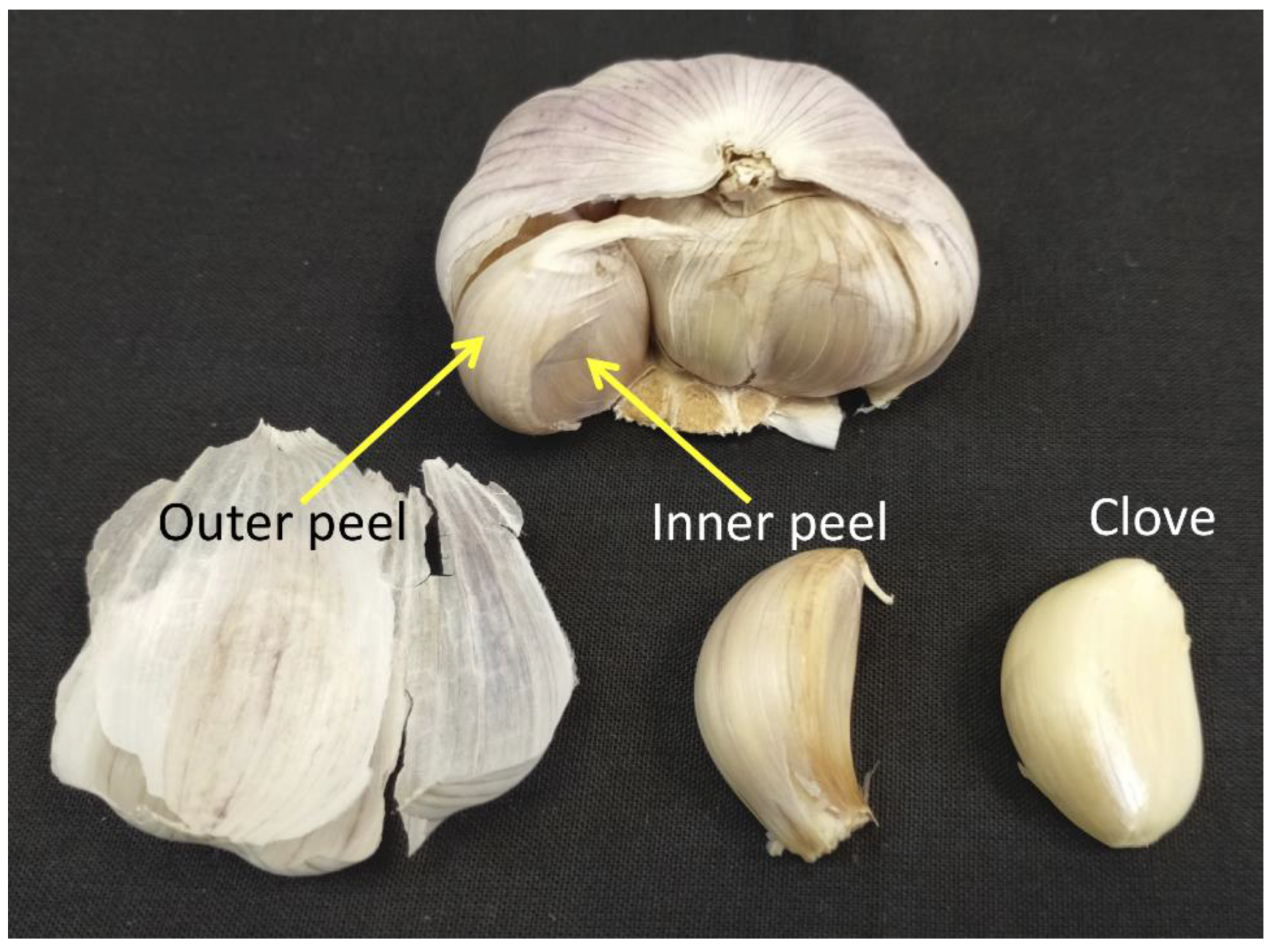
4.1. Collection and Preparation of Samples
4.2. Germination Assays
4.3. Proteome Analysis
4.4. In-Gel Chitinase Assay
4.5. In-Gel Protease Assay
4.6. In-Gel Nuclease Assay
4.7. Metabolite Analysis
4.8. Bacterial Growth Assay
4.9. Fungal Spore Germination Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksson, O. Evolution of seed size and biotic seed dispersal in angiosperms: Paleoecological and neoecological evidence. Int. J. Plant Sci. 2008, 169, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Howe, F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence—A genomics approach. Plant Biotechnol. J. 2002, 1, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yun, H.W.; Jun, J.Y.; Yu, D.L.; Fa, F.S. Protein degradation and nitrogen remobilization during leaf senescence. J. Plant Biol. 2008, 51, 11–19. [Google Scholar] [CrossRef]
- Raviv, B.; Granot, G.; Chalifa-Caspi, V.; Grafi, G. The dead, hardened floral bracts of dispersal units of wild wheat function as storage for active hydrolases and in enhancing seedling vigor. PLoS ONE 2017, 12, e0177537. [Google Scholar] [CrossRef] [Green Version]
- Godwin, J.; Raviv, B.; Grafi, G. Dead pericarps of dry fruits function as long-term storage for active hydrolytic enzymes and other substances that affect germination and microbial growth. Plants 2017, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Raviv, B.; Godwin, J.; Granot, G.; Grafi, G. The dead can nurture: Novel insights into the function of dead organs enclosing embryos. Int. J. Mol. Sci. 2018, 19, 2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadka, J.; Raviv, B.; Swetha, B.; Grandhi, R.; Singiri, J.R.; Novoplansky, N.; Gutterman, Y.; Galis, I.; Huang, Z.; Grafi, G. Maternal environment alters dead pericarp biochemical properties of the desert annual plant Anastatica hierochuntica L. PLoS ONE 2020, 15, e0237045. [Google Scholar] [CrossRef]
- Grafi, G. Dead but not dead end: Multifunctional role of dead organs enclosing embryos in seed biology. Int. J. Mol. Sci. 2020, 21, 8024. [Google Scholar] [CrossRef]
- Singiri, J.R.; Swetha, B.; Persi, N.S.; Grafi, G. Differential response to single and combined salt and heat stresses: Impact on accumulation of proteins and metabolites in dead pericarps of Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7076. [Google Scholar] [CrossRef]
- Sun, W.; Van Montagu, M.; Verbruggen, N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta. 2002, 1577, 1–9. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins—Evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raviv, B.; Khadka, J.; Swetha, B.; Singiri, J.R.; Grandhi, R.; Shapira, E.; Novoplansky, N.; Gutterman, Y.; Galis, I.; Sternberg, M.; et al. Extreme drought alters progeny dispersal unit properties of winter wild oat (Avena sterilis L.). Planta 2020, 252, 77. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.T.; Schuman, G.E. Seedbed ecology of winterfat: Fruits versus threshed seeds. J. Range Manag. 1983, 36, 387. [Google Scholar] [CrossRef]
- Ueno, K.; Miyoshi, K. Difference of optimum germination temperature of seeds of intact and dehusked japonica rice during seed development. Euphytica 2005, 143, 271–275. [Google Scholar] [CrossRef]
- Hu, X.W.; Wang, Y.R.; Wu, Y.P. Effects of the pericarp on imbibition, seed germination, and seedling establishment in seeds of Hedysarum scoparium Fisch. et Mey. Ecol. Res. 2009, 24, 559–564. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Elgabra, M.; Mosa, K.A.; Fakhry, A.; Soliman, S. Roles of hardened husks and membranes surrounding Brachypodium hybridum grains on germination and seedling growth. Plants 2019, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Klimes, L.; Klimesova, J.; Hendriks, R.; van Groenendael, J. Clonal plant architecture: A comparative analysis of form and function. In The Ecology and Evolution of Clonal Plants; De Kroon, H., Van Groenendael, J., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1979; pp. 1–29. [Google Scholar]
- Rivlin, R.S. Historical perspective on the use of garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [CrossRef] [Green Version]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar]
- Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical characteristics and antimicrobial activity of Australian grown garlic (Allium sativum L.) Cultivars. Foods 2019, 8, 358. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, M.; Ryu, K.; Yoshida, J.; Ide, N.; Kodera, Y.; Sasaoka, T.; Rosen, R.T. Identification of six phenylpropanoids from garlic skin as major antioxidants. J. Agric. Food Chem. 2003, 51, 7313–7317. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.P.; Mushayamaha, T.; Thomas, P.D. PANTHER version 16: A revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef]
- Harris, J.C.; Cottrell, S.L.; Plummer, S.; Lloyd, D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 2001, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Evenari, M. Germination inhibitors. Bot. Rev. 1949, 15, 153–194. [Google Scholar] [CrossRef]
- Kushima, M.; Kakuta, H.; Kosemura, S.; Yamamura, S.; Yamada, K.; Yokotani-Tomita, K.; Hasegawa, K. An allelopathic substance exuded from germinating watermelon seeds. Plant Growth Regul. 1998, 25, 1–4. [Google Scholar] [CrossRef]
- Ohno, S.; Tomita-Yokotani, K.; Kosemura, S.; Node, M.; Suzuki, T.; Amano, M.; Yasui, K.; Goto, T.; Yamamura, S.; Hasegawa, K. A species-selective allelopathic substance from germinating sunflower (Helianthus annuus L.) seeds. Phytochemistry 2001, 56, 577–581. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Galiana, E.; Bonnet, P.; Conrod, S.; Keller, H.; Panabières, F.; Ponchet, M.; Poupet, A.; Ricci, P. RNase activity prevents the growth of a fungal pathogen in tobacco leaves and increases upon induction of systemic acquired resistance with elicitin. Plant Physiol. 1997, 115, 1557–1567. [Google Scholar] [CrossRef] [Green Version]
- Hugot, K.; Ponchet, M.; Marais, A.; Ricci, P.; Galiana, E. A tobacco S-like RNase inhibits hyphal elongation of plant pathogens. Mol. Plant Microbe Interact. 2002, 15, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Van Der Hoorn, R.A.L. Plant proteases: From phenotypes to molecular mechanisms. Annu. Rev. Plant Biol. 2008, 59, 191–223. [Google Scholar] [CrossRef] [Green Version]
- Bertini, L.; Caporale, C.; Testa, M.; Proietti, S.; Caruso, C. Structural basis of the antifungal activity of wheat PR4 proteins. FEBS Lett. 2009, 583, 2865–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Sharma, K.P.; Gaur, R.K.; Gupta, V.K. Role of chitinase in plant defense. Asian J. Biochem. 2011, 6, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Raviv, B.; Aghajanyan, L.; Granot, G.; Makover, V.; Frenkel, O.; Gutterman, Y.; Grafi, G. The dead seed coat functions as a long-term storage for active hydrolytic enzymes. PLoS ONE 2017, 12, e0181102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swetha, B.; Singiri, J.R.; Novoplansky, N.; Grandhi, R.; Srinivasan, J.; Khadka, J.; Galis, I.; Grafi, G. Single and combined salinity and heat stresses impact yield and dead pericarp priming activity. Plants 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Su, Z.; Xu, W.; Sun, H.X.; Gao, J.F.; Tu, D.F.; Ren, C.H.; Zhang, Z.J.; Cao, H.G. Garlic skin induces shifts in the rumen microbiome and metabolome of fattening lambs. Animal 2021, 15, 100216. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, G.; Xiong, H.; Qin, H.; Zhang, H.; Sun, Y.; Dong, X.; Lei, Y.; Zhao, Y.; Zhao, Z. Effects of mixing garlic skin on fermentation quality, microbial community of high-moisture Pennisetum hydridum Silage. Front. Microbiol. 2021, 12, 770591. [Google Scholar] [CrossRef]
- Berdi, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar]
- Stoop, J.M.H.; Williamson, J.D.; Pharr, D.M. Mannitol metabolism in plants: A method for coping with stress. Trends Plant Sci. 1996, 1, 139–144. [Google Scholar] [CrossRef]
- Briens, M.; Larher, F. Sorbitol accumulation in Plantaginaceae; further evidence for a function in stress tolerance. Z. Pflanzenphysiol. 1983, 110, 447–458. [Google Scholar] [CrossRef]
- Theerakulpisut, P.; Gunnula, W. Exogenous sorbitol and trehalose mitigated salt stress damage in salt-sensitive but not salt-tolerant rice seedlings. Asian. J. Crop Sci. 2012, 4, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Stutte, G.W. The role of carbohydrates in active osmotic adjustment in apple under water stress. J. Am. Soc. Hortic. Sci. 2019, 117, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Lo Bianco, R.; Rieger, M.; Sung, S.J.S. Effect of drought on sorbitol and sucrose metabolism in sinks and sources of peach. Physiol. Plant. 2000, 108, 71–78. [Google Scholar] [CrossRef]
- Ranney, T.G.; Bassuk, N.L.; Whitlow, T.H. Osmotic adjustment and solute constituents in leaves and roots of water-stressed cherry (Prunus) trees. J. Am. Soc. Hortic. Sci. 2019, 116, 684–688. [Google Scholar] [CrossRef]
- Aguayo, M.F.; Ampuero, D.; Mandujano, P.; Parada, R.; Muñoz, R.; Gallart, M.; Altabella, T.; Cabrera, R.; Stange, C.; Handford, M. Sorbitol dehydrogenase is a cytosolic protein required for sorbitol metabolism in Arabidopsis thaliana. Plant Sci. 2013, 205, 63–75. [Google Scholar] [CrossRef]
- Drennan, P.M.; Smith, M.T.; Goldsworthy, D.; van Staden, J. The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius Welw. J. Plant Physiol. 1993, 142, 493–496. [Google Scholar] [CrossRef]
- Albini, F.M.; Murelli, C.; Patritti, G.; Rovati, M.; Zienna, P.; Finzi, P.V. Low-molecular weight substances from the resurrection plant Sporobolus stapfianus. Phytochemistry 1994, 37, 137–142. [Google Scholar] [CrossRef]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef] [Green Version]
- Iordachescu, M.; Imai, R. Trehalose biosynthesis in response to abiotic stresses. J. Integr. Plant Biol. 2008, 50, 1223–1229. [Google Scholar] [CrossRef]
- Walters, K.R., Jr.; Pan, Q.; Serianni, A.S.; Duman, J.G. Cryoprotectant biosynthesis and the selective accumulation of threitol in the freeze-tolerant Alaskan beetle, Upis ceramboides. J. Biol. Chem. 2009, 284, 16822–16831. [Google Scholar] [CrossRef] [Green Version]
- Trudel, J.; Asselin, A. Detection of chitin deacetylase activity after polyacrylamide gel electrophoresis. Anal. Biochem. 1990, 189, 249–253. [Google Scholar] [CrossRef]
- Solomon, M.; Belenghi, B.; Delledonne, M.; Menachem, E.; Levine, A. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 1999, 11, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Sugiyama, R.H.; Dekker, C.A. Activity staining of nucleolytic enzymes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis: Use of aqueous isopropanol to remove detergent from gels. Anal. Biochem. 1982, 120, 267–275. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Reshef, N.; Fait, A.; Agam, N. Grape berry position affects the diurnal dynamics of its metabolic profile. Plant Cell Environ. 2019, 42, 1897–1912. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 10–14. [Google Scholar] [CrossRef]
- Patton, T.; Barrett, J.; Brennan, J.; Moran, N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J. Microbiol. Methods 2006, 64, 84–95. [Google Scholar] [CrossRef]
- Masood, S.; Rehman, A.U.; Ihsan, M.A.; Shahzad, K.; Sabir, M.; Alam, S.; Ahmed, W.; Shah, Z.H.; Alghabari, F.; Mehmood, A.; et al. Antioxidant potential and α-glucosidase inhibitory activity of onion (Allium cepa L.) peel and bulb extracts. Braz. J. Biol. 2021, 83, 00264. [Google Scholar] [CrossRef]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Gazzerro, P.; Tucci, M.; Di Sanzo, R.; Carabetta, S.; Campone, L.; Russo, M.; Rastrelli, L. Onion Peel: Turning a food waste into a resource. Antioxidants 2021, 10, 304. [Google Scholar] [CrossRef]
- Ildar, G.; Shaikhiev, I.G.; Kraysman, N.V.; Sverguzova, S.V. Use of garlic processing by-products to remove pollutants from aqueous media. Bioint. Res. Appl. Chem. 2022, 12, 4518–4528. [Google Scholar]
| Biological Process/Response to Stimulus | |||
|---|---|---|---|
| ID | Protein | Inner | Outer |
| A0A0Q3KZX7 | CHITINASE | P | P |
| A0A0Q3RUL2 | CHITINASE | P | P |
| A0A2K2D641 | PEROXIDASE 64 | A | P |
| D7NLB8 | PEROXIDASE 1 | P | P |
| I1H6 × 1 | SHOCK COGNATE 70 KDA PROTEIN | P | P |
| I1HEK5 | CALCIUM-BINDING PROTEIN CML9 | A | P |
| I1HIQ7 | HEAT SHOCK COGNATE 71 KDA PROTEIN | P | P |
| I1HWC7 | HEAT SHOCK 70 KDA PROTEIN BIP1 | A | P |
| I1IAA5 | PEROXIREDOXIN-4 | A | P |
| Prtein class/protein modifying enzyme | |||
| ID | Protein name | ||
| A0A0Q3INI3 | SERINE PROTEASE | A | P |
| I1GNN5 | CYSTEINE PROTEASE | A | P |
| I1HWA5 | SERINE PROTEASE | A | P |
| I1I5B9 | SERINE PROTEASE | A | P |
| I1IQ27 | CYSTEINE PROTEASE | P | P |
| I1ITB7 | SERINE PROTEASE | A | P |
| I1J2L7 | CYSTEINE PROTEASE | P | P |
| A0A0Q3H6A6 | SERINE PROTEASE | A | P |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singiri, J.R.; Swetha, B.; Ben-Natan, A.; Grafi, G. What Worth the Garlic Peel. Int. J. Mol. Sci. 2022, 23, 2126. https://doi.org/10.3390/ijms23042126
Singiri JR, Swetha B, Ben-Natan A, Grafi G. What Worth the Garlic Peel. International Journal of Molecular Sciences. 2022; 23(4):2126. https://doi.org/10.3390/ijms23042126
Chicago/Turabian StyleSingiri, Jeevan R., Bupur Swetha, Arava Ben-Natan, and Gideon Grafi. 2022. "What Worth the Garlic Peel" International Journal of Molecular Sciences 23, no. 4: 2126. https://doi.org/10.3390/ijms23042126
APA StyleSingiri, J. R., Swetha, B., Ben-Natan, A., & Grafi, G. (2022). What Worth the Garlic Peel. International Journal of Molecular Sciences, 23(4), 2126. https://doi.org/10.3390/ijms23042126







