Association of Tim-3/Gal-9 Axis with NLRC4 Inflammasome in Glioma Malignancy: Tim-3/Gal-9 Induce the NLRC4 Inflammasome
Abstract
:1. Introduction
2. Methods
2.1. Description of TCGA Data
2.2. Correlation Analysis and Correlation Coefficient Hierarchical Clustering
2.3. Gene Ontology Enrichment Analysis
2.4. Survival Analysis
2.5. Patient Samples
2.6. Glioma Cells
2.7. Transfection
2.8. Immunohistochemistry
2.9. Flow Cytometry
2.10. TUNEL Assay
2.11. FLICAcaspase−1 Assay
2.12. Programmed Cell Death Analysis
2.13. ELISA
2.14. qRT-PCR
2.15. Protein–Protein Interaction Analysis
2.16. TF Prediction and Kinase-TF Axis Analysis
2.17. Statistical Analysis
3. Results
3.1. Tim-3/Gal-9 Are Significantly Correlated with the NLRC4 Inflammasome in Glioma
3.2. Tim-3/Gal-9 and NLRC4 Inflammasome Expression Is Associated with Poor Survival in Patients with Glioma
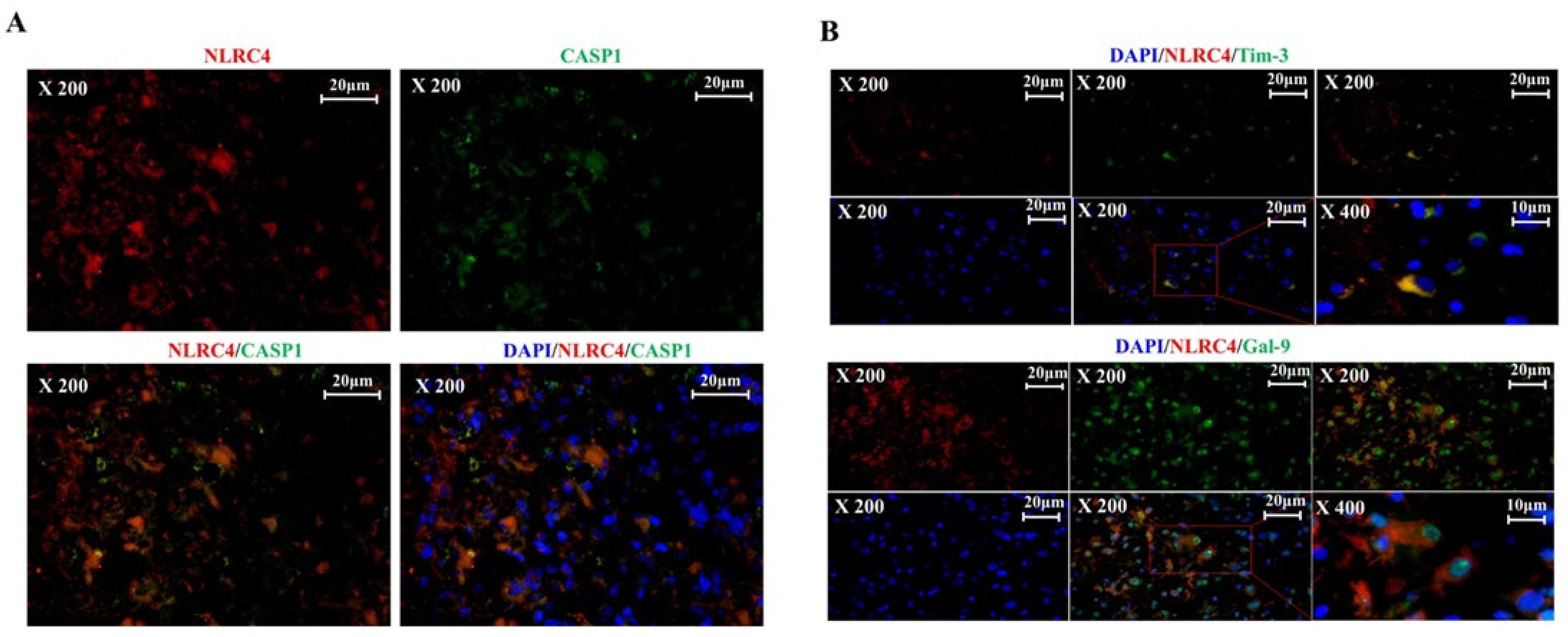
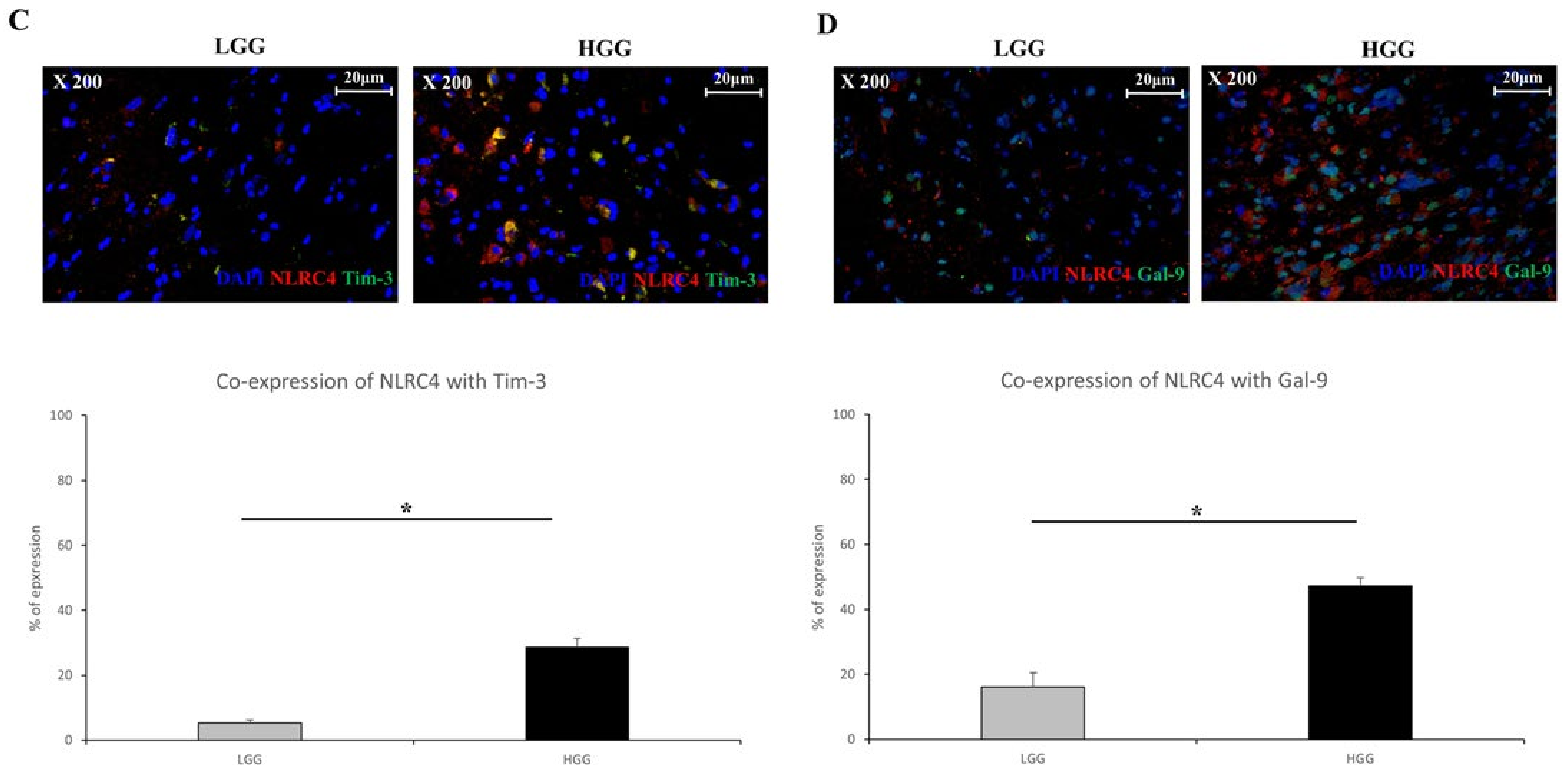
3.3. Tim-3/Gal-9 and NLRC4 Inflammasome Are Upregulated in High-Grade Glioma Tissues
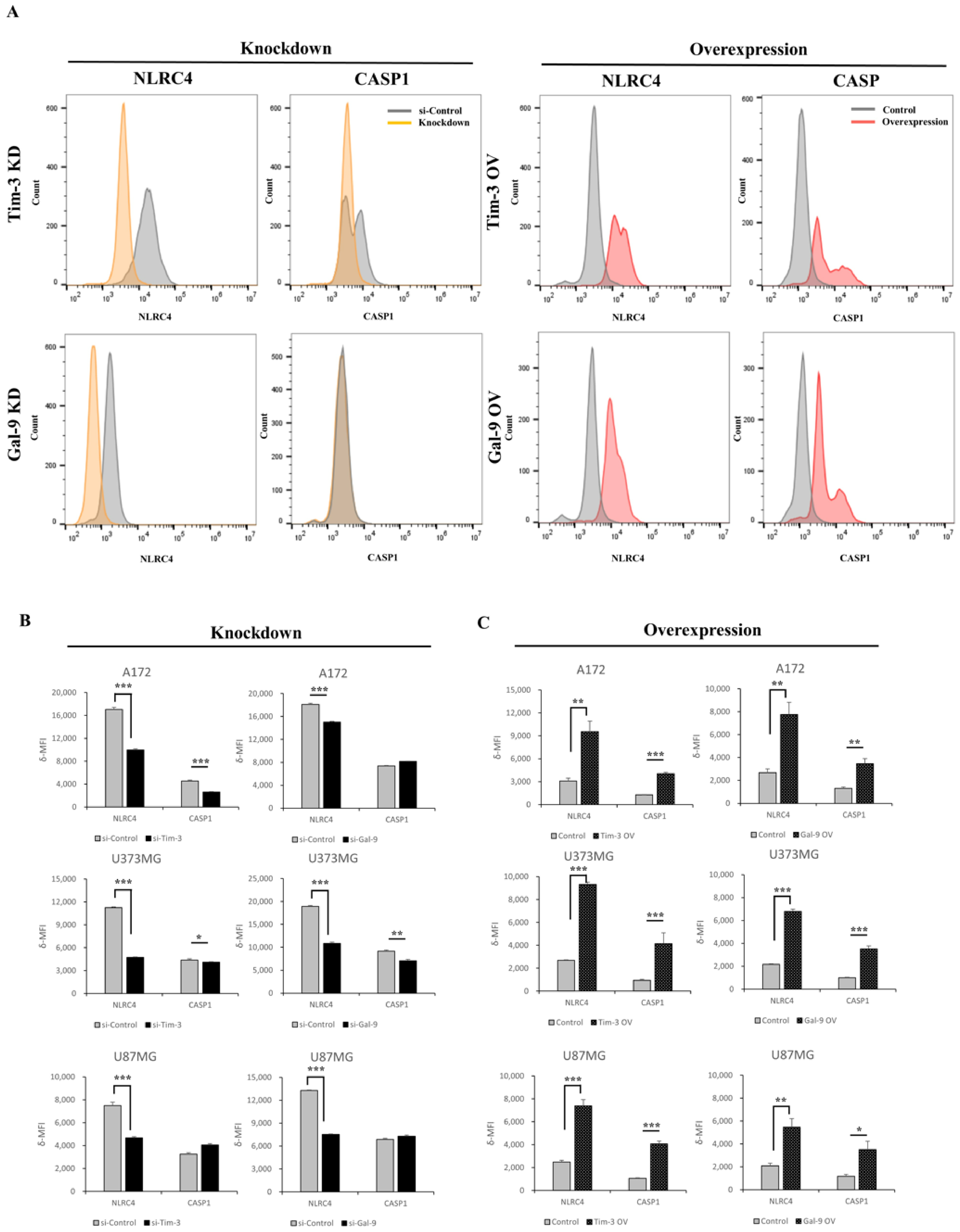
3.4. Tim-3/Gal-9 Regulate the Expression of NLRC4 Inflammasome-Associated Molecules in Glioma
3.5. Tim-3/Gal-9 Are Correlated with Caspase-1 Activation and Induces Programmed Cell Death in Glioma
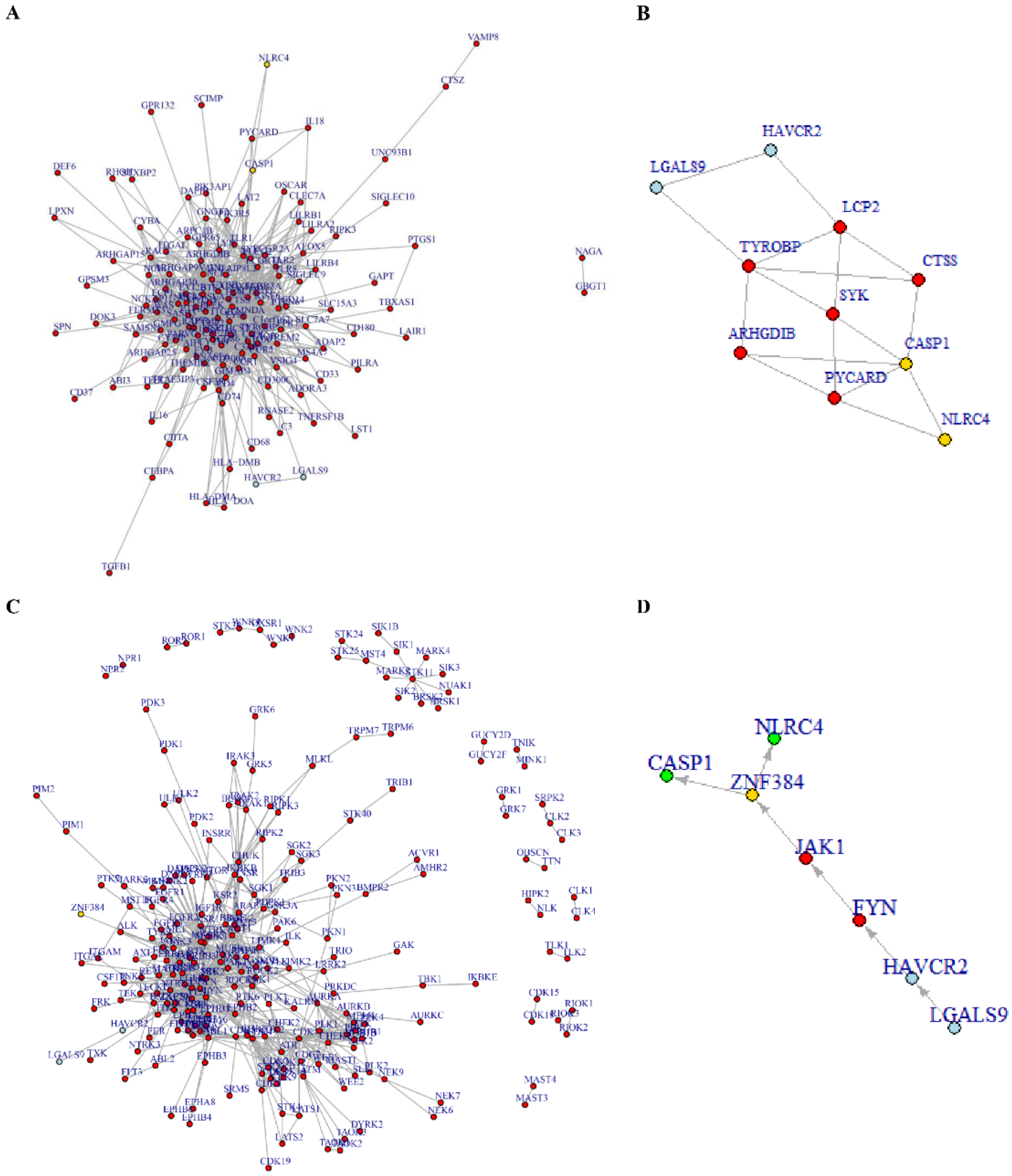
3.6. Tim-3/Gal-9-NLRC4 Inflammasome Network Landscape Determined by PPI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, P.Y.; Reardon, D.A. Progress in glioma diagnosis, classification and treatment. Nat. Rev. Neurol. 2016, 12, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Shervington, A. Chemoresistance in gliomas. Mol. Cell. Biochem. 2008, 312, 71–80. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Boussiotis, V.A.; Charest, A. Immunotherapies for malignant glioma. Oncogene 2018, 37, 1121–1141. [Google Scholar] [CrossRef]
- Rolle, C.E.; Sengupta, S.; Lesniak, M.S. Mechanisms of Immune Evasion by Gliomas. In Glioma: Immunotherapeutic Approaches; Yamanaka, R., Ed.; Springer: New York, NY, USA, 2012; pp. 53–76. [Google Scholar]
- Nørøxe, D.S.; Poulsen, H.S.; Lassen, U. Hallmarks of glioblastoma: A systematic review. ESMO Open 2016, 1, e000144. [Google Scholar] [CrossRef]
- Yang, C.; Wen, H.-B.; Zhao, Y.-H.; Huang, W.-H.; Wang, Z.-F.; Li, Z.-Q. Systemic Inflammatory Indicators as Prognosticators in Glioblastoma Patients: A Comprehensive Meta-Analysis. Front. Neurol. 2020, 11, 580101. [Google Scholar] [CrossRef] [PubMed]
- Kimbara, S.; Kondo, S. Immune checkpoint and inflammation as therapeutic targets in pancreatic carcinoma. World J. Gastroenterol. 2016, 22, 7440–7452. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Galon, J.; Fridman, W.-H.; Smyth, M.J. From mice to humans: Developments in cancer immunoediting. J. Clin. Investig. 2015, 125, 3338–3346. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.F.; Carter, T.J.; Ottaviani, D.; Mulholland, P. Harnessing the immune system in glioblastoma. Br. J. Cancer 2018, 119, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, M.; Guo, Y.; Zhong, Y.; He, Z.; Xu, Y.; Zou, J. Immune response in glioma’s microenvironment. Innov. Surg. Sci. 2021, 5, 115–125. [Google Scholar] [CrossRef]
- Anderson, A.C. Tim-3: An Emerging Target in the Cancer Immunotherapy Landscape. Cancer Immunol. Res. 2014, 2, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Wang, Z.; Zhang, C.; Liu, X.; Cai, J.; Wang, Z.; Hu, H.; Wu, F.; Bao, Z.; Liu, Y.; et al. Molecular and clinical characterization of TIM-3 in glioma through 1,024 samples. Oncoimmunology 2017, 6, e1328339. [Google Scholar] [CrossRef]
- Kim, H.-S.; Chang, C.Y.; Yoon, H.J.; Kim, K.S.; Koh, H.S.; Kim, S.S.; Lee, S.-J.; Kane, L.P.; Park, E.J. Glial TIM-3 modulates immune responses in the brain tumor microenvironment. Cancer Res. 2020, 80, 1833–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandel, S.; Adhikary, P.; Li, G.; Cheng, K. The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy. Cancer Lett. 2021, 510, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, H.; Yu, L.; Liu, M.; Zuo, Z.; Han, Q.; Zhang, J.; Tian, Z.; Zhang, C. Intestinal Lamina Propria CD4 + T Cells Promote Bactericidal Activity of Macrophages via Galectin-9 and Tim-3 Interaction during Salmonella enterica Serovar Typhimurium Infection. Infect. Immun. 2018, 86, e00769-17. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, P.; Sada-Ovalle, I.; Beladi, S.; Anderson, A.C.; Dardalhon, V.; Hotta, C.; Kuchroo, V.K.; Behar, S.M. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 2010, 207, 2343–2354. [Google Scholar] [CrossRef]
- Dixon, K.O.; Tabaka, M.; Schramm, M.A.; Xiao, S.; Tang, R.; Dionne, D.; Anderson, A.C.; Rozenblatt-Rosen, O.; Regev, A.; Kuchroo, V.K. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature 2021, 595, 101–106. [Google Scholar] [CrossRef]
- Wang, W.; Qin, Y.; Song, H.; Wang, L.; Jia, M.; Zhao, C.; Gong, M.; Zhao, W. Galectin-9 Targets NLRP3 for Autophagic Degradation to Limit Inflammation. J. Immunol. 2021, 206, 2692–2699. [Google Scholar] [CrossRef]
- Lim, J.; Kim, M.J.; Park, Y.; Ahn, J.W.; Hwang, S.J.; Moon, J.-S.; Cho, K.G.; Kwack, K. Upregulation of the NLRC4 inflammasome contributes to poor prognosis in glioma patients. Sci. Rep. 2019, 9, 7895. [Google Scholar] [CrossRef] [Green Version]
- Galvão, R.P.; Zong, H. Inflammation and Gliomagenesis: Bi-Directional Communication at Early and Late Stages of Tumor Progression. Curr. Pathobiol. Rep. 2013, 1, 19–28. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.-D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Cancer 2019, 19, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Karki, R.; Man, S.M.; Kanneganti, T.-D. Inflammasomes and Cancer. Cancer Immunol. Res. 2017, 5, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhang, J.; Lai, X.; Chen, M. Tim-3 exacerbates kidney ischaemia/reperfusion injury through the TLR-4/NF-κB signalling pathway and an NLR-C4 inflammasome activation. Clin. Exp. Immunol. 2018, 193, 113–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, D.; Balça-Silva, J.; Da Graça, G.C.; Wanjiru, C.M.; Macharia, L.W.; Nascimento, C.P.; Roque, N.R.; Coelho-Aguiar, J.M.; Pereira, C.M.; Dos Santos, M.F.; et al. Microglia/Astrocytes–Glioblastoma Crosstalk: Crucial Molecular Mechanisms and Microenvironmental Factors. Front. Cell. Neurosci. 2018, 12, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuura, A.; Tsukada, J.; Mizobe, T.; Higashi, T.; Mouri, F.; Tanikawa, R.; Yamauchi, A.; Hirashima, M.; Tanaka, Y. Intracellular galectin-9 activates inflammatory cytokines in monocytes. Genes Cells 2009, 14, 511–521. [Google Scholar] [CrossRef]
- Song, F.; Ma, Y.; Bai, X.-Y.; Chen, X. The Expression Changes of Inflammasomes in the Aging Rat Kidneys. J. Gerontol. Ser. A 2016, 71, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, Y.; Otsuka, K.; Arimochi, H.; Tsukumo, S.-I.; Yasutomo, K. Distinct Roles of IL-1β and IL-18 in NLRC4-Induced Autoinflammation. Front. Immunol. 2020, 11, 591713. [Google Scholar] [CrossRef]
- Qin, S.; Dong, B.; Yi, M.; Chu, Q.; Wu, K. Prognostic Values of TIM-3 Expression in Patients With Solid Tumors: A Meta-Analysis and Database Evaluation. Front. Oncol. 2020, 10, 1288. [Google Scholar] [CrossRef]
- Zhou, E.; Huang, Q.; Wang, J.; Fang, C.; Yang, L.; Zhu, M.; Chen, J.; Chen, L.; Dong, M. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8018. [Google Scholar] [PubMed]
- Kim, J.E.; Patel, M.; Mangraviti, A.; Kim, E.S.; Theodros, D.; Velarde, E.; Liu, A.; Sankey, E.W.; Tam, A.; Xu, H.; et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin. Cancer Res. 2017, 23, 124–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandao, M.; Simon, T.; Critchley, G.; Giamas, G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 2019, 67, 779–790. [Google Scholar] [CrossRef]
- Roca, H.; Jones, J.D.; Purica, M.C.; Weidner, S.; Koh, A.J.; Kuo, R.; Wilkinson, J.E.; Wang, Y.; Daignault-Newton, S.; Pienta, K.J.; et al. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J. Clin. Investig. 2017, 128, 248–266. [Google Scholar] [CrossRef]
- Ucker, D.S.; Levine, J.S. Exploitation of Apoptotic Regulation in Cancer. Front. Immunol. 2018, 9, 241. [Google Scholar] [CrossRef]
- Gadiyar, V.; Lahey, K.C.; Calianese, D.; DeVoe, C.; Mehta, D.; Bono, K.; Desind, S.; Davra, V.; Birge, R.B. Cell Death in the Tumor Microenvironment: Implications for Cancer Immunotherapy. Cells 2020, 9, 2207. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.A.; Canna, S.W. The NLRC4 Inflammasome. Immunol. Rev. 2018, 281, 115–123. [Google Scholar] [CrossRef]
- Chen, K.W.; Groß, C.J.; Sotomayor, F.V.; Stacey, K.; Tschopp, J.; Sweet, M.; Schroder, K. The Neutrophil NLRC4 Inflammasome Selectively Promotes IL-1β Maturation without Pyroptosis during Acute Salmonella Challenge. Cell Rep. 2014, 8, 570–582. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Elahi, S.; Mashhouri, S.; Ye, C. Gout presenting as a chronic inflammatory arthritis from immune checkpoint inhibitors: Case series. Rheumatology 2021, 60, e441–e443. [Google Scholar] [CrossRef]
- Zhai, Y.; Celis-Gutierrez, J.; Voisinne, G.; Mori, D.; Girard, L.; Burlet-Schiltz, O.; de Peredo, A.G.; Roncagalli, R.; Malissen, B. Opposing regulatory functions of the TIM3 (HAVCR2) signalosome in primary effector T cells as revealed by quantitative interactomics. Cell. Mol. Immunol. 2021, 18, 1581–1583. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2019, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.; Park, J.; Kim, S.; Hwang, S.; Sung, K.; Lee, J.-E.; Yang, S.; Cho, K.; Lee, S.; Moon, J.-S.; et al. Association of Tim-3/Gal-9 Axis with NLRC4 Inflammasome in Glioma Malignancy: Tim-3/Gal-9 Induce the NLRC4 Inflammasome. Int. J. Mol. Sci. 2022, 23, 2028. https://doi.org/10.3390/ijms23042028
Sim J, Park J, Kim S, Hwang S, Sung K, Lee J-E, Yang S, Cho K, Lee S, Moon J-S, et al. Association of Tim-3/Gal-9 Axis with NLRC4 Inflammasome in Glioma Malignancy: Tim-3/Gal-9 Induce the NLRC4 Inflammasome. International Journal of Molecular Sciences. 2022; 23(4):2028. https://doi.org/10.3390/ijms23042028
Chicago/Turabian StyleSim, JeongMin, JeongMan Park, Suwan Kim, Sojung Hwang, KyoungSu Sung, Jung-Eun Lee, SeungHo Yang, Kyunggi Cho, SungHwan Lee, Jong-Seok Moon, and et al. 2022. "Association of Tim-3/Gal-9 Axis with NLRC4 Inflammasome in Glioma Malignancy: Tim-3/Gal-9 Induce the NLRC4 Inflammasome" International Journal of Molecular Sciences 23, no. 4: 2028. https://doi.org/10.3390/ijms23042028
APA StyleSim, J., Park, J., Kim, S., Hwang, S., Sung, K., Lee, J.-E., Yang, S., Cho, K., Lee, S., Moon, J.-S., Ahn, J., & Lim, J. (2022). Association of Tim-3/Gal-9 Axis with NLRC4 Inflammasome in Glioma Malignancy: Tim-3/Gal-9 Induce the NLRC4 Inflammasome. International Journal of Molecular Sciences, 23(4), 2028. https://doi.org/10.3390/ijms23042028





