Glucose-6-Phosphate Dehydrogenase, Redox Homeostasis and Embryogenesis
Abstract
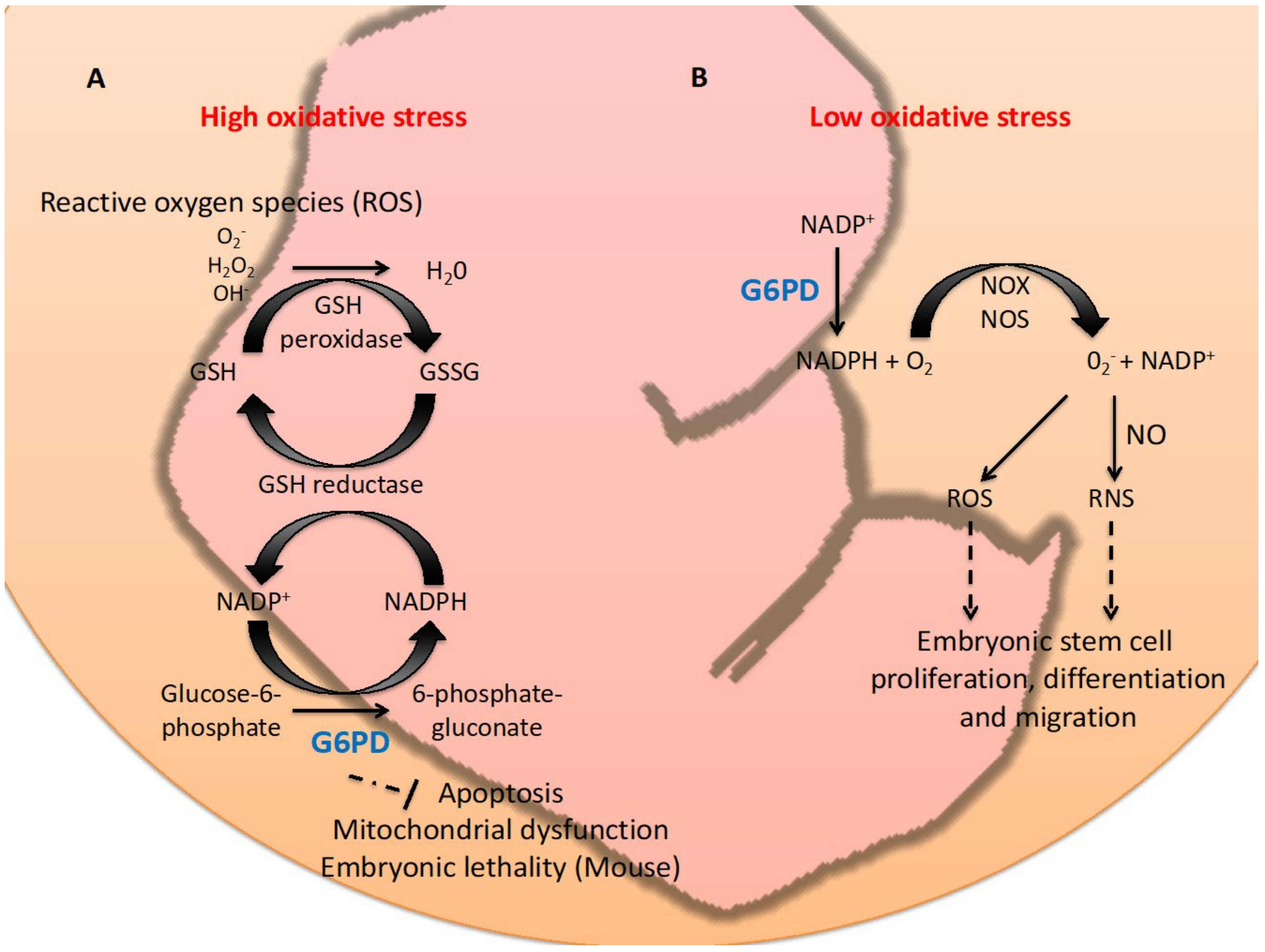
1. Introduction
2. The Role of Redox in Humans and Other Species
3. G6PD Studies in Embryonic Stem Cells and Animal Models
3.1. Embryonic Stem Cells
3.2. Mouse
3.3. Zebrafish
3.4. Nematode
4. The Redox Role of G6PD in Cellular Migration and Differentiation during Embryogenesis
4.1. Involvement of G6PD in Cell Migration
4.2. Contribution of G6PD to Cell Differentiation
5. Embryogenesis Regulated by Other Redox-Related Genes, Transcription Factors, MicroRNAs, Growth Factors and Signaling Pathways
5.1. Anti-Oxidant Enzymes
5.2. Transcription Factors
5.3. Redox-Related Post-Transcriptional Controls
5.4. Growth Factors and Redox Modulation
5.5. Redox Status and Signaling Pathways
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Menezo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Timme-Laragy, A.R.; Hahn, M.E.; Hansen, J.M.; Rastogi, A.; Roy, M.A. Redox stress and signaling during vertebrate embryonic development: Regulation and responses. Semin. Cell Dev. Biol. 2018, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Jones, D.P.; Harris, C. The Redox Theory of Development. Antioxid. Redox Signal. 2020, 32, 715–740. [Google Scholar] [CrossRef]
- Yang, H.C.; Wu, Y.H.; Liu, H.Y.; Stern, A.; Chiu, D.T. What has passed is prolog: New cellular and physiological roles of G6PD. Free Radic. Res. 2016, 50, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Wu, Y.H.; Yen, W.C.; Liu, H.Y.; Hwang, T.L.; Stern, A.; Chiu, D.T. The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells 2019, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Serpillon, S.; Floyd, B.C.; Gupte, R.S.; George, S.; Kozicky, M.; Neito, V.; Recchia, F.; Stanley, W.; Wolin, M.S.; Gupte, S.A. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H153–H162. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Cap, A.; Scribner, A.W.; Stanton, R.C.; Loscalzo, J. Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. FASEB J. 2001, 15, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.J.; Hung, I.J.; Chow, C.K.; Stern, A.; Chao, S.S.; Chiu, D.T. Impaired production of nitric oxide, superoxide, and hydrogen peroxide in glucose 6-phosphate-dehydrogenase-deficient granulocytes. FEBS Lett. 1998, 436, 411–414. [Google Scholar] [CrossRef]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 2005, 3, 43. [Google Scholar] [CrossRef]
- Matsui, M.; Oshima, M.; Oshima, H.; Takaku, K.; Maruyama, T.; Yodoi, J.; Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev. Biol. 1996, 178, 179–185. [Google Scholar] [CrossRef]
- Nonn, L.; Williams, R.R.; Erickson, R.P.; Powis, G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 2003, 23, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Jakupoglu, C.; Przemeck, G.K.; Schneider, M.; Moreno, S.G.; Mayr, N.; Hatzopoulos, A.K.; de Angelis, M.H.; Wurst, W.; Bornkamm, G.W.; Brielmeier, M.; et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol. Cell. Biol. 2005, 25, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Hirao, F.; Sakamoto, T.; Sekine, K.; Mizukura, Y.; Saito, M.; Kitamoto, T.; Hayasaka, M.; Hanaoka, K.; Nakagawa, Y. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 2003, 305, 278–286. [Google Scholar] [CrossRef]
- Iuchi, Y.; Kaneko, T.; Matsuki, S.; Ishii, T.; Ikeda, Y.; Uchida, K.; Fujii, J. Carbonyl stress and detoxification ability in the male genital tract and testis of rats. Histochem. Cell Biol. 2004, 121, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Harris, C. Glutathione during emb.bryonic development. Biochim. Biophys. Acta 2015, 1850, 1527–1542. [Google Scholar] [CrossRef]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr. Top. Microbiol. Immunol. 2017, 403, 143–170. [Google Scholar] [CrossRef]
- Hitchler, M.J.; Domann, F.E. An epigenetic perspective on the free radical theory of development. Free Radic. Biol. Med. 2007, 43, 1023–1036. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Coffman, J.A.; Su, Y.H. Redox regulation of development and regeneration. Curr. Opin. Genet. Dev. 2019, 57, 9–15. [Google Scholar] [CrossRef]
- Knoll, A.H.; Sperling, E.A. Oxygen and animals in Earth history. Proc. Natl. Acad. Sci. USA 2014, 111, 3907–3908. [Google Scholar] [CrossRef]
- Miseta, A.; Csutora, P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol. Biol. Evol. 2000, 17, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Ofman, G.; Tipple, T.E. Thiol-Redox Regulation in Lung Development and Vascular Remodeling. Antioxid Redox Signal. 2019, 31, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Feng, Z.; Peterson, A.L.; Carr, J.F.; Lu, X.; Zhao, H.; Ji, X.; Zhao, Y.Y.; De Paepe, M.E.; Dennery, P.A.; et al. The pentose phosphate pathway mediates hyperoxia-induced lung vascular dysgenesis and alveolar simplification in neonates. JCI Insight 2021, 6, e137594. [Google Scholar] [CrossRef]
- Yang, H.C.; Cheng, M.L.; Ho, H.Y.; Chiu, D.T. The microbicidal and cytoregulatory roles of NADPH oxidases. Microbes Infect. 2011, 13, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.; Bellot, G.L.; Lemoine, A.; Brenner, C. Redox signaling in the pathogenesis of human disease and the regulatory role of autophagy. Int. Rev. Cell. Mol. Biol. 2020, 352, 189–214. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Johnston, A.D.; Ebert, P.R. The Redox System in C. elegans, a Phylogenetic Approach. J. Toxicol. 2012, 2012, 546915. [Google Scholar] [CrossRef]
- Lim, P.L.; Liu, J.; Go, M.L.; Boelsterli, U.A. The mitochondrial superoxide/thioredoxin-2/Ask1 signaling pathway is critically involved in troglitazone-induced cell injury to human hepatocytes. Toxicol. Sci. 2008, 101, 341–349. [Google Scholar] [CrossRef]
- Pedrajas, J.R.; Kosmidou, E.; Miranda-Vizuete, A.; Gustafsson, J.A.; Wright, A.P.; Spyrou, G. Identification and functional characterization of a novel mitochondrial thioredoxin system in Saccharomyces cerevisiae. J. Biol. Chem. 1999, 274, 6366–6373. [Google Scholar] [CrossRef]
- Isermann, K.; Liebau, E.; Roeder, T.; Bruchhaus, I. A peroxiredoxin specifically expressed in two types of pharyngeal neurons is required for normal growth and egg production in Caenorhabditis elegans. J. Mol. Biol. 2004, 338, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Kanzok, S.M.; Fechner, A.; Bauer, H.; Ulschmid, J.K.; Muller, H.M.; Botella-Munoz, J.; Schneuwly, S.; Schirmer, R.; Becker, K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science 2001, 291, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.D.; McCormack, G.; Page, A.P. Protein disulfide isomerase activity is essential for viability and extracellular matrix formation in the nematode Caenorhabditis elegans. Dev. Biol. 2007, 308, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, P.P.; Sonati, F.; Rivi, R.; Mason, P.; Grosveld, F.; Luzzatto, L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995, 14, 5209–5215. [Google Scholar] [CrossRef]
- Fico, A.; Paglialunga, F.; Cigliano, L.; Abrescia, P.; Verde, P.; Martini, G.; Iaccarino, I.; Filosa, S. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004, 11, 823–831. [Google Scholar] [CrossRef]
- Paglialunga, F.; Fico, A.; Iaccarino, I.; Notaro, R.; Luzzatto, L.; Martini, G.; Filosa, S. G6PD is indispensable for erythropoiesis after the embryonic-adult hemoglobin switch. Blood 2004, 104, 3148–3152. [Google Scholar] [CrossRef]
- Filosa, S.; Fico, A.; Paglialunga, F.; Balestrieri, M.; Crooke, A.; Verde, P.; Abrescia, P.; Bautista, J.M.; Martini, G. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem. J. 2003, 370, 935–943. [Google Scholar] [CrossRef]
- Longo, L.; Vanegas, O.C.; Patel, M.; Rosti, V.; Li, H.; Waka, J.; Merghoub, T.; Pandolfi, P.P.; Notaro, R.; Manova, K.; et al. Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO J. 2002, 21, 4229–4239. [Google Scholar] [CrossRef]
- Perez-Crespo, M.; Ramirez, M.A.; Fernandez-Gonzalez, R.; Rizos, D.; Lonergan, P.; Pintado, B.; Gutierrez-Adan, A. Differential sensitivity of male and female mouse embryos to oxidative induced heat-stress is mediated by glucose-6-phosphate dehydrogenase gene expression. Mol. Reprod. Dev. 2005, 72, 502–510. [Google Scholar] [CrossRef]
- Patrinostro, X.; Carter, M.L.; Kramer, A.C.; Lund, T.C. A model of glucose-6-phosphate dehydrogenase deficiency in the zebrafish. Exp. Hematol. 2013, 41, 697–710.e692. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.H.; Lee, Y.H.; Shih, H.Y.; Chen, S.H.; Cheng, Y.C.; Tsun-Yee Chiu, D. Glucose-6-phosphate dehydrogenase is indispensable in embryonic development by modulation of epithelial-mesenchymal transition via the NOX/Smad3/miR-200b axis. Cell Death Dis. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Babb, S.G.; Marrs, J.A. E-cadherin regulates cell movements and tissue formation in early zebrafish embryos. Dev. Dyn. 2004, 230, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, X.; Adams, I.R.; Yuan, P. A tight control of Rif1 by Oct4 and Smad3 is critical for mouse embryonic stem cell stability. Cell Death Dis. 2015, 6, e1588. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.; Yu, M.; Wu, H.; Ai, Z.; Wu, Y.; Liu, H.; Du, J.; Guo, Z.; Zhang, Y. Retinoic Acid Induces Embryonic Stem Cell Differentiation by Altering Both Encoding RNA and microRNA Expression. PLoS ONE 2015, 10, e0132566. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Chen, T.L.; Wu, Y.H.; Cheng, K.P.; Lin, Y.H.; Cheng, M.L.; Ho, H.Y.; Lo, S.J.; Chiu, D.T. Glucose 6-phosphate dehydrogenase deficiency enhances germ cell apoptosis and causes defective embryogenesis in Caenorhabditis elegans. Cell Death Dis. 2013, 4, e616. [Google Scholar] [CrossRef]
- Chen, T.L.; Yang, H.C.; Hung, C.Y.; Ou, M.H.; Pan, Y.Y.; Cheng, M.L.; Stern, A.; Lo, S.J.; Chiu, D.T. Impaired embryonic development in glucose-6-phosphate dehydrogenase-deficient Caenorhabditis elegans due to abnormal redox homeostasis induced activation of calcium-independent phospholipase and alteration of glycerophospholipid metabolism. Cell Death Dis. 2017, 8, e2545. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Yu, H.; Ma, T.H.; Tjong, W.Y.; Stern, A.; Chiu, D.T. tert-Butyl Hydroperoxide (tBHP)-Induced Lipid Peroxidation and Embryonic Defects Resemble Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency in C. elegans. Int. J. Mol. Sci. 2020, 21, 8688. [Google Scholar] [CrossRef]
- Almeida, A.S.; Soares, N.L.; Sequeira, C.O.; Pereira, S.A.; Sonnewald, U.; Vieira, H.L.A. Improvement of neuronal differentiation by carbon monoxide: Role of pentose phosphate pathway. Redox Biol. 2018, 17, 338–347. [Google Scholar] [CrossRef]
- Harris, J.M.; Esain, V.; Frechette, G.M.; Harris, L.J.; Cox, A.G.; Cortes, M.; Garnaas, M.K.; Carroll, K.J.; Cutting, C.C.; Khan, T.; et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood 2013, 121, 2483–2493. [Google Scholar] [CrossRef]
- Hernandez-Garcia, D.; Wood, C.D.; Castro-Obregon, S.; Covarrubias, L. Reactive oxygen species: A radical role in development? Free Radic Biol Med. 2010, 49, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Hom, J.R.; Quintanilla, R.A.; Hoffman, D.L.; de Mesy Bentley, K.L.; Molkentin, J.D.; Sheu, S.S.; Porter, G.A., Jr. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev. Cell 2011, 21, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, M.; Chen, C.; Mai, M.; Huang, J.; Zhu, P. Roles of Reactive Oxygen Species in Cardiac Differentiation, Reprogramming, and Regenerative Therapies. Oxid. Med. Cell Longev. 2020, 2020, 2102841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Heims-Waldron, D.; Bezzerides, V.; Guatimosim, S.; Guo, Y.; Gu, F.; Zhou, P.; Lin, Z.; Ma, Q.; et al. Mitochondrial Cardiomyopathy Caused by Elevated Reactive Oxygen Species and Impaired Cardiomyocyte Proliferation. Circ. Res. 2018, 122, 74–87. [Google Scholar] [CrossRef]
- Kanda, Y.; Hinata, T.; Kang, S.W.; Watanabe, Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011, 89, 250–258. [Google Scholar] [CrossRef]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef]
- Horwitz, R.; Webb, D. Cell migration. Curr. Biol. 2003, 13, R756–R759. [Google Scholar] [CrossRef]
- Scarpa, E.; Mayor, R. Collective cell migration in development. J. Cell Biol. 2016, 212, 143–155. [Google Scholar] [CrossRef]
- Wang, G.; Chen, B.Z.; Wang, C.J.; Zhang, J.; Gao, L.R.; Chuai, M.; Bao, Y.; Yang, X. Ethanol exposure leads to disorder of blood island formation in early chick embryo. Reprod. Toxicol. 2017, 73, 96–104. [Google Scholar] [CrossRef]
- Berndt, C.; Poschmann, G.; Stuhler, K.; Holmgren, A.; Brautigam, L. Zebrafish heart development is regulated via glutaredoxin 2 dependent migration and survival of neural crest cells. Redox Biol. 2014, 2, 673–678. [Google Scholar] [CrossRef]
- Syal, S.; Ng, C.; Kim, Y.; Janbieh, J.; Govind, S.; Deshpande, G. Reactive oxygen species signaling in primordial germ cell development in Drosophila embryos. Genesis 2020, 58, e23362. [Google Scholar] [CrossRef] [PubMed]
- Keller, G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev. 2005, 19, 1129–1155. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell 2008, 132, 661–680. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Zeydel, M. gamma-Glutamyl transpeptidase and glutathione in aging IMR-90 fibroblasts and in differentiating 3T3 L1 preadipocytes. Arch. Biochem. Biophys. 1982, 214, 260–267. [Google Scholar] [CrossRef]
- Li, J.; Stouffs, M.; Serrander, L.; Banfi, B.; Bettiol, E.; Charnay, Y.; Steger, K.; Krause, K.H.; Jaconi, M.E. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol. Biol. Cell. 2006, 17, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Xiao, Z.; Wang, Y.; Wang, H. Human embryonic development: From peri-implantation to gastrulation. Trends Cell Biol. 2022, 32, 18–29. [Google Scholar] [CrossRef]
- Gerri, C.; Menchero, S.; Mahadevaiah, S.K.; Turner, J.M.A.; Niakan, K.K. Human Embryogenesis: A Comparative Perspective. Annu. Rev. Cell Dev. Biol. 2020, 36, 411–440. [Google Scholar] [CrossRef]
- Pera, M.F.; Rossant, J. The exploration of pluripotency space: Charting cell state transitions in peri-implantation development. Cell Stem Cell 2021, 28, 1896–1906. [Google Scholar] [CrossRef]
- Reynolds, L.P.; McLean, K.J.; McCarthy, K.L.; Diniz, W.J.S.; Menezes, A.C.B.; Forcherio, J.C.; Scott, R.R.; Borowicz, P.P.; Ward, A.K.; Dahlen, C.R.; et al. Nutritional Regulation of Embryonic Survival, Growth, and Development. Adv. Exp. Med. Biol. 2022, 1354, 63–76. [Google Scholar] [CrossRef]
- Laurent, L.C. MicroRNAs in embryonic stem cells and early embryonic development. J. Cell. Mol. Med. 2008, 12, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Balin, A.K. Oxidative influence on development and differentiation: An overview of a free radical theory of development. Free Radic. Biol. Med. 1989, 6, 631–661. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.T.; Carlson, E.J.; Melov, S.; Ursell, P.C.; Olson, J.L.; Noble, L.J.; Yoshimura, M.P.; Berger, C.; Chan, P.H.; et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995, 11, 376–381. [Google Scholar] [CrossRef]
- Allu, A.D.; Soja, A.M.; Wu, A.; Szymanski, J.; Balazadeh, S. Salt stress and senescence: Identification of cross-talk regulatory components. J. Exp. Bot. 2014, 65, 3993–4008. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.M.; Koo, H.C.; Rosenfeld, W. Oxygen affects human endothelial cell proliferation by inactivation of fibroblast growth factors. Am. J. Physiol. 1992, 263, L370–L375. [Google Scholar] [CrossRef] [PubMed]
- Ufer, C.; Wang, C.C.; Borchert, A.; Heydeck, D.; Kuhn, H. Redox control in mammalian embryo development. Antioxid. Redox Signal. 2010, 13, 833–875. [Google Scholar] [CrossRef]
- Sekiba, K.; Yoshioka, T. Changes of lipid peroxidation and superoxide dismutase activity in the human placenta. Am. J. Obstet. Gynecol. 1979, 135, 368–371. [Google Scholar] [CrossRef]
- Takehara, Y.; Yoshioka, T.; Sasaki, J. Changes in the levels of lipoperoxide and antioxidant factors in human placenta during gestation. Acta Med. Okayama 1990, 44, 103–111. [Google Scholar] [CrossRef]
- Celotto, A.M.; Liu, Z.; Vandemark, A.P.; Palladino, M.J. A novel Drosophila SOD2 mutant demonstrates a role for mitochondrial ROS in neurodevelopment and disease. Brain Behav. 2012, 2, 424–434. [Google Scholar] [CrossRef]
- Borchert, A.; Wang, C.C.; Ufer, C.; Schiebel, H.; Savaskan, N.E.; Kuhn, H. The role of phospholipid hydroperoxide glutathione peroxidase isoforms in murine embryogenesis. J. Biol. Chem. 2006, 281, 19655–19664. [Google Scholar] [CrossRef]
- Li, L. The Relevance of Mammalian Peroxiredoxins to the Gametogenesis, Embryogenesis, and Pregnancy Outcomes. Reprod. Sci. 2017, 24, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Fakruzzaman, M.; Ghanem, N.; Bang, J.I.; Ha, A.N.; Lee, K.L.; Sohn, S.H.; Wang, Z.; Lee, D.S.; Kong, I.K. Effect of peroxiredoxin II on the quality and mitochondrial activity of pre-implantation bovine embryos. Anim. Reprod. Sci. 2015, 159, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.R.; Stewart, B.; Peek, J.C.; Shelling, A.N.; Cree, L.M. Assessing embryo quality by combining non-invasive markers: Early time-lapse parameters reflect gene expression in associated cumulus cells. Hum. Reprod. 2015, 30, 1850–1860. [Google Scholar] [CrossRef][Green Version]
- Radyuk, S.N.; Michalak, K.; Klichko, V.I.; Benes, J.; Rebrin, I.; Sohal, R.S.; Orr, W.C. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem. J. 2009, 419, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Buggisch, M.; Ateghang, B.; Ruhe, C.; Strobel, C.; Lange, S.; Wartenberg, M.; Sauer, H. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J. Cell Sci. 2007, 120, 885–894. [Google Scholar] [CrossRef]
- Jang, J.; Wang, Y.; Kim, H.S.; Lalli, M.A.; Kosik, K.S. Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells 2014, 32, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, M.; Halabian, R.; Gharehbaghian, A.; Amirizadeh, N.; Jahanian-Najafabadi, A.; Roushandeh, A.M.; Roudkenar, M.H. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperones 2012, 17, 553–565. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Huang, Y.; Zhang, Y.; Liu, W.; Wang, G.; Zhang, Q.; Wang, G.; Yang, Y.; Li, H.; et al. NRF2 overexpression in mesenchymal stem cells induces stem-cell marker expression and enhances osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2017, 491, 228–235. [Google Scholar] [CrossRef]
- Lin, Y.; Sui, L.C.; Wu, R.H.; Ma, R.J.; Fu, H.Y.; Xu, J.J.; Qiu, X.H.; Chen, L. Nrf2 inhibition affects cell cycle progression during early mouse embryo development. J. Reprod. Dev. 2018, 64, 49–55. [Google Scholar] [CrossRef]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Ghosh, S.; Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 1995, 376, 167–170. [Google Scholar] [CrossRef]
- Hilberg, F.; Aguzzi, A.; Howells, N.; Wagner, E.F. c-jun is essential for normal mouse development and hepatogenesis. Nature 1993, 365, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.S.; van Lingen, B.; Papaioannou, V.E.; Spiegelman, B.M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993, 7, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Wang, Z.Q.; Jochum, W.; Fetka, I.; Elliott, C.; Wagner, E.F. Placental vascularisation requires the AP-1 component fra1. Development 2000, 127, 4937–4948. [Google Scholar] [CrossRef] [PubMed]
- Karreth, F.; Hoebertz, A.; Scheuch, H.; Eferl, R.; Wagner, E.F. The AP1 transcription factor Fra2 is required for efficient cartilage development. Development 2004, 131, 5717–5725. [Google Scholar] [CrossRef] [PubMed]
- Schorpp-Kistner, M.; Wang, Z.Q.; Angel, P.; Wagner, E.F. JunB is essential for mammalian placentation. EMBO J. 1999, 18, 934–948. [Google Scholar] [CrossRef]
- Dunwoodie, S.L. The role of hypoxia in development of the Mammalian embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef]
- Compernolle, V.; Brusselmans, K.; Franco, D.; Moorman, A.; Dewerchin, M.; Collen, D.; Carmeliet, P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc. Res. 2003, 60, 569–579. [Google Scholar] [CrossRef]
- Amarilio, R.; Viukov, S.V.; Sharir, A.; Eshkar-Oren, I.; Johnson, R.S.; Zelzer, E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 2007, 134, 3917–3928. [Google Scholar] [CrossRef]
- Ream, M.; Ray, A.M.; Chandra, R.; Chikaraishi, D.M. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R583–R595. [Google Scholar] [CrossRef]
- L’Honore, A.; Commere, P.H.; Ouimette, J.F.; Montarras, D.; Drouin, J.; Buckingham, M. Redox regulation by Pitx2 and Pitx3 is critical for fetal myogenesis. Dev. Cell 2014, 29, 392–405. [Google Scholar] [CrossRef]
- Lozano-Velasco, E.; Vallejo, D.; Esteban, F.J.; Doherty, C.; Hernandez-Torres, F.; Franco, D.; Aranega, A.E. A Pitx2-MicroRNA Pathway Modulates Cell Proliferation in Myoblasts and Skeletal-Muscle Satellite Cells and Promotes Their Commitment to a Myogenic Cell Fate. Mol. Cell. Biol. 2015, 35, 2892–2909. [Google Scholar] [CrossRef]
- Ufer, C.; Wang, C.C.; Fahling, M.; Schiebel, H.; Thiele, B.J.; Billett, E.E.; Kuhn, H.; Borchert, A. Translational regulation of glutathione peroxidase 4 expression through guanine-rich sequence-binding factor 1 is essential for embryonic brain development. Genes Dev. 2008, 22, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Michiue, T.; Terabayashi, T.; Yukita, A.; Danno, H.; Asashima, M.; Miki, H. Nucleoredoxin regulates the Wnt/planar cell polarity pathway in Xenopus. Genes Cells 2008, 13, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Michiue, T.; Asashima, M.; Miki, H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat. Cell Biol. 2006, 8, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Miki, H. Redox regulation of Wnt signalling via nucleoredoxin. Free Radic. Res. 2010, 44, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive Oxygen Species and Their Involvement in Red Blood Cell Damage in Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef] [PubMed]
- Lequarre, A.S.; Feugang, J.M.; Malhomme, O.; Donnay, I.; Massip, A.; Dessy, F.; Van Langendonckt, A. Expression of Cu/Zn and Mn superoxide dismutases during bovine embryo development: Influence of in vitro culture. Mol. Reprod. Dev. 2001, 58, 45–53. [Google Scholar] [CrossRef]
- Kably Ambe, A.; Ruiz Anguas, J.; Carballo Mondragon, E.; Corona de Lau, C.; Karchmer Krivitsky, S. Correlation between follicle levels of superoxide dismutase and oocyte quality, fertilization rates and embryo development. Ginecol. Obstet. Mex. 2004, 72, 335–344. [Google Scholar] [PubMed]
- Nonogaki, T.; Noda, Y.; Narimoto, K.; Umaoka, Y.; Mori, T. Effects of superoxide dismutase on mouse in vitro fertilization and embryo culture system. J. Assist. Reprod. Genet. 1992, 9, 274–280. [Google Scholar] [CrossRef]
- Lee, H.; Ismail, T.; Kim, Y.; Chae, S.; Ryu, H.Y.; Lee, D.S.; Kwon, T.K.; Park, T.J.; Kwon, T.; Lee, H.S. Xenopus gpx3 Mediates Posterior Development by Regulating Cell Death during Embryogenesis. Antioxidants 2020, 9, 1265. [Google Scholar] [CrossRef]
- Elshazzly, M.; Lopez, M.J.; Reddy, V.; Caban, O. Embryology, Central Nervous System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Olguin-Albuerne, M.; Moran, J. Redox Signaling Mechanisms in Nervous System Development. Antioxid. Redox Signal. 2018, 28, 1603–1625. [Google Scholar] [CrossRef] [PubMed]
- Petriv, O.I.; Rachubinski, R.A. Lack of peroxisomal catalase causes a progeric phenotype in Caenorhabditis elegans. J. Biol. Chem. 2004, 279, 19996–20001. [Google Scholar] [CrossRef] [PubMed]
- Miller-Pinsler, L.; Wells, P.G. Embryonic catalase prot.tects against ethanol embryopathies in acatalasemic mice and transgenic human catalase-expressing mice in embryo culture. Toxicol. Appl. Pharmacol. 2015, 287, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Wells, P.G. Altered methanol embryopathies in embryo culture with mutant catalase-deficient mice and transgenic mice expressing human catalase. Toxicol. Appl. Pharmacol. 2011, 252, 55–61. [Google Scholar] [CrossRef]
- Abramov, J.P.; Wells, P.G. Embryonic catalase protects against endogenous and phenytoin-enhanced DNA oxidation and embryopathies in acatalasemic and human catalase-expressing mice. FASEB J. 2011, 25, 2188–2200. [Google Scholar] [CrossRef]
- Abramov, J.P.; Wells, P.G. Embryoprotective role of endogenous catalase in acatalasemic and human catalase-expressing mouse embryos exposed in culture to developmental and phenytoin-enhanced oxidative stress. Toxicol. Sci. 2011, 120, 428–438. [Google Scholar] [CrossRef]
- Elhiti, M.; Stasolla, C. Transduction of Signals during Somatic Embryogenesis. Plants 2022, 11, 178. [Google Scholar] [CrossRef]
- Seminotti, B.; Grings, M.; Tucci, P.; Leipnitz, G.; Saso, L. Nuclear Factor Erythroid-2-Related Factor 2 Signaling in the Neuropathophysiology of Inherited Metabolic Disorders. Front. Cell Neurosci. 2021, 15, 785057. [Google Scholar] [CrossRef]
- Gacesa, R.; Dunlap, W.C.; Barlow, D.J.; Laskowski, R.A.; Long, P.F. Rising levels of atmospheric oxygen and evolution of Nrf2. Sci. Rep. 2016, 6, 27740. [Google Scholar] [CrossRef]
- Reddy, N.M.; Kleeberger, S.R.; Yamamoto, M.; Kensler, T.W.; Scollick, C.; Biswal, S.; Reddy, S.P. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol. Genom. 2007, 32, 74–81. [Google Scholar] [CrossRef]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and Metabolic Regulator of Stem Cell State and Function. Trends Mol. Med. 2020, 26, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Menyiy, N.; Oumeslakht, L.; El Allam, A.; Balahbib, A.; Rauf, A.; Muhammad, N.; Kuznetsova, E.; Derkho, M.; Thiruvengadam, M.; et al. Preclinical and Clinical Antioxidant Effects of Natural Compounds against Oxidative Stress-Induced Epigenetic Instability in Tumor Cells. Antioxidants 2021, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, Y.; Ikeda, T.; Saito-Takatsuji, H.; Yonekura, H. Emerging Role of AP-1 Transcription Factor JunB in Angiogenesis and Vascular Development. Int. J. Mol. Sci. 2021, 22, 2804. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Cerychova, R.; Pavlinkova, G. HIF-1, Metabolism, and Diabetes in the Embryonic and Adult Heart. Front. Endocrinol. 2018, 9, 460. [Google Scholar] [CrossRef]
- Sheflin, L.G.; Zou, A.P.; Spaulding, S.W. Androgens regulate the binding of endogenous HuR to the AU-rich 3′UTRs of HIF-1alpha and EGF mRNA. Biochem. Biophys. Res. Commun. 2004, 322, 644–651. [Google Scholar] [CrossRef]
- Galban, S.; Kuwano, Y.; Pullmann, R., Jr.; Martindale, J.L.; Kim, H.H.; Lal, A.; Abdelmohsen, K.; Yang, X.; Dang, Y.; Liu, J.O.; et al. RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1alpha. Mol. Cell. Biol. 2008, 28, 93–107. [Google Scholar] [CrossRef]
- Yang, R.; Weber, D.J.; Carrier, F. Post-transcriptional regulation of thioredoxin by the stress inducible heterogenous ribonucleoprotein A18. Nucleic Acids Res. 2006, 34, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Badoiu, S.C.; Stanescu, S., II; Totan, A.R.; Stefani, C.; Greabu, M. Growth Factors, Reactive Oxygen Species, and Metformin-Promoters of the Wound Healing Process in Burns? Int. J. Mol. Sci. 2021, 22, 9512. [Google Scholar] [CrossRef] [PubMed]
- Heppner, D.E.; van der Vliet, A. Redox-dependent regulation of epidermal growth factor receptor signaling. Redox Biol. 2016, 8, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Rafikova, O.; Rafikov, R.; Kangath, A.; Qu, N.; Aggarwal, S.; Sharma, S.; Desai, J.; Fields, T.; Ludewig, B.; Yuan, J.X.; et al. Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension. Free Radic. Biol. Med. 2016, 95, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Portilho, N.; Pelajo-Machado, M. Mechanism of hematopoiesis and vasculogenesis in mouse placenta. Placenta 2018, 69, 140–145. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Vriz, S. Redox signalling in development and regeneration. Semin. Cell Dev. Biol. 2018, 80, 1–2. [Google Scholar] [CrossRef]
- Guo, Y.L.; Chakraborty, S.; Rajan, S.S.; Wang, R.; Huang, F. Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-renewal. Stem Cells Dev. 2010, 19, 1321–1331. [Google Scholar] [CrossRef]
- Forsyth, N.R.; Musio, A.; Vezzoni, P.; Simpson, A.H.; Noble, B.S.; McWhir, J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells 2006, 8, 16–23. [Google Scholar] [CrossRef]
- Harvey, A.J.; Rathjen, J.; Yu, L.J.; Gardner, D.K. Oxygen modulates human embryonic stem cell metabolism in the absence of changes in self-renewal. Reprod. Fertil. Dev. 2016, 28, 446–458. [Google Scholar] [CrossRef]
- Tepekoy, F.; Akkoyunlu, G.; Demir, R. The role of Wnt signaling members in the uterus and embryo during pre-implantation and implantation. J. Assist. Reprod. Genet. 2015, 32, 337–346. [Google Scholar] [CrossRef]
- Covarrubias, L.; Hernandez-Garcia, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregon, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Deng, Y.; Su, G.; Enninful, A.; Guo, C.C.; Tebaldi, T.; Zhang, D.; Kim, D.; Bai, Z.; et al. High-Spatial-Resolution Multi-Omics Sequencing via Deterministic Barcoding in Tissue. Cell 2020, 183, 1665–1681.e1618. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Hisata, K.; Foster, S.; Morita, S.; Nishitsuji, K.; Oulhen, N.; Tominaga, H.; Wessel, G. A single-cell RNA-seq analysis of Brachyury-expressing cell clusters suggests a morphogenesis-associated signal center of oral ectoderm in sea urchin embryos. Dev. Biol. 2022, 483, 128–142. [Google Scholar] [CrossRef]
- Palinkas, H.L.; Racz, G.A.; Gal, Z.; Hoffmann, O.I.; Tihanyi, G.; Rona, G.; Gocza, E.; Hiripi, L.; Vertessy, B.G. CRISPR/Cas9-Mediated Knock-Out of dUTPase in Mice Leads to Early Embryonic Lethality. Biomolecules 2019, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Del Rio, A.; Signore, F.; Umani Ronchi, F.; Marinelli, E.; Zaami, S. CRISPR-Cas and Its Wide-Ranging Applications: From Human Genome Editing to Environmental Implications, Technical Limitations, Hazards and Bioethical Issues. Cells 2021, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Erwood, S.; Gu, B. Embryo-Based Large Fragment Knock-in in Mammals: Why, How and What’s Next. Genes 2020, 11, 140. [Google Scholar] [CrossRef] [PubMed]

| Type | Name | Model | Functions | Experimental Protocols | References |
|---|---|---|---|---|---|
| Enzymatic/anti-oxidant enzyme | SODs | Human | The SOD activity in placenta protects embryos from lipid peroxidation. | Biochemical analysis | [77,78] |
| Enzymatic/anti-oxidant enzyme | MnSOD | Mouse Drosophila | Maintains cardiac function and neonatal survival. Prevents abnormal brain morphology and axonal aberrations during neurodevelopment. | Mouse: biochemical analysis. Drosophila: lifespan analysis, brain/neuron morphology | [73,79] |
| Enzymatic/anti-oxidant enzyme | GPx4 | Mouse | Male reproduction and early embryonic development. Initiates gastrulation and develops embryonic cavities | siRNA knockdown. Molecular biology/morphology analysis. | [13,16,80] |
| Enzymatic/anti-oxidant enzyme | Prx | Human Cow Drosophila C. elegans | Stimulates blastocyst development and increases embryonic mitochondrial activity. Support embryogenesis. | Molecular biology/biochemical/life span analysis. | [31,81,82,83,84] |
| Enzymatic/anti-oxidant enzyme | Trx/TrxR | Mouse | Support embryonic viability and embryogenesis. | Knockout mice. Molecular biology/morphology analysis. | [10,11,12,13] |
| Enzymatic/pro-oxidant enzyme | NOX | Mouse | Promotes cardiac differentiation, cardiomyogenesis, and neonatal cardiac cell growth. | shRNA/siRNA knockdown. Biochemical/molecular biology/morphology analysis. | [66,85] |
| Non-enzymatic/transcription factor | Nrf2 | Human Mouse | Enhances oxidative stress resistance. Controls self-renewal and pluripotency of ES cells. Supports embryo cleavage and blastocyst formation. Maintains stemness and survival under oxidative stress. | siRNA knockdown and CRISPR-mediated ectopic gene expression | [86,87,88,89] |
| Non-enzymatic/transcription factor | NF-κB | Mouse | Embryonic and liver parenchymal cell survival | Knockout mice coupled with histological/molecular biology analysis. | [90] |
| Non-enzymatic/transcription factor | AP-1 | Mouse | Embryonic survival, liver erythropoiesis, hepatogenesis, development of the placenta and yolk sac, chondrocytes and extraembryonic tissues | Knockout mice coupled with biochemical/molecular biology/morphology analyses. | [91,92,93,94,95] |
| Non-enzymatic/transcription factor | HIF-1 | Human mouse | Modulates vascular development (VEGF) in hypoxia. Trophoblast proliferation and differentiation, morphogenesis of the developing heart, chondrogenesis and myocardial development. | Knockout mice. Molecular biology/morphology analysis. | [96,97,98,99] |
| Non-enzymatic/transcription factor | Pitx2, Pitx3 | Mouse | Anti-oxidant defense in fetal myogenesis | Knockout/knockdown and ectopic gene expression. Metabolomics/molecular biology analysis. | [100] |
| Non-enzymatic/post-transcriptional control | Pitx-miRNA (mir-15b, mir -23b, mir-106b and mir-503) pathway | Mouse | Modulates cell proliferation and cell fate of skeletal-muscle stem cells (satellite cells) | Knockout/knockdown and ectopic gene expression. Bioinformatics/molecular biology analysis. | [101] |
| Non-enzymatic/post-transcriptional control | Grsf-1 | Mouse | Modulates brain development by recruiting GPx4 mRNA to translationally active polysome fractions by targeting 5′ untranslated region (UTR) of GPx4 mRNAs. | Bioinformatics/biochemical analysis | [102] |
| Signaling pathway | Wnt signaling (Nrx and Dvl) | Human C. aethiops Mouse Xenopus | Senses ROS levels to activate or inactivate Wnt signaling for directing embryo gastrulation. | siRNA knockdown and microinjection-mediated ectopic gene expression research. Biochemical/molecular biology/morphology analysis. | [103,104,105] |
| Signaling pathway | PPP pathway (including G6PD) | Mouse Zebrafish C. elegans | Maintains redox homeostasis during embryogenesis. Mediates abnormal lung development in hyperoxia in the neonatal mice. | Knockout/siRNA or morpholino knockdown. Metabolomics/biochemical/molecular biology/morphology analysis. | [23,34,35,36,37,38,39,40,41,42,43,44,46,47,48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-H.; Tjong, W.-Y.; Yang, H.-C.; Liu, H.-Y.; Stern, A.; Chiu, D.T.-Y. Glucose-6-Phosphate Dehydrogenase, Redox Homeostasis and Embryogenesis. Int. J. Mol. Sci. 2022, 23, 2017. https://doi.org/10.3390/ijms23042017
Chen P-H, Tjong W-Y, Yang H-C, Liu H-Y, Stern A, Chiu DT-Y. Glucose-6-Phosphate Dehydrogenase, Redox Homeostasis and Embryogenesis. International Journal of Molecular Sciences. 2022; 23(4):2017. https://doi.org/10.3390/ijms23042017
Chicago/Turabian StyleChen, Po-Hsiang, Wen-Ye Tjong, Hung-Chi Yang, Hui-Ya Liu, Arnold Stern, and Daniel Tsun-Yee Chiu. 2022. "Glucose-6-Phosphate Dehydrogenase, Redox Homeostasis and Embryogenesis" International Journal of Molecular Sciences 23, no. 4: 2017. https://doi.org/10.3390/ijms23042017
APA StyleChen, P.-H., Tjong, W.-Y., Yang, H.-C., Liu, H.-Y., Stern, A., & Chiu, D. T.-Y. (2022). Glucose-6-Phosphate Dehydrogenase, Redox Homeostasis and Embryogenesis. International Journal of Molecular Sciences, 23(4), 2017. https://doi.org/10.3390/ijms23042017







