How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants
Abstract
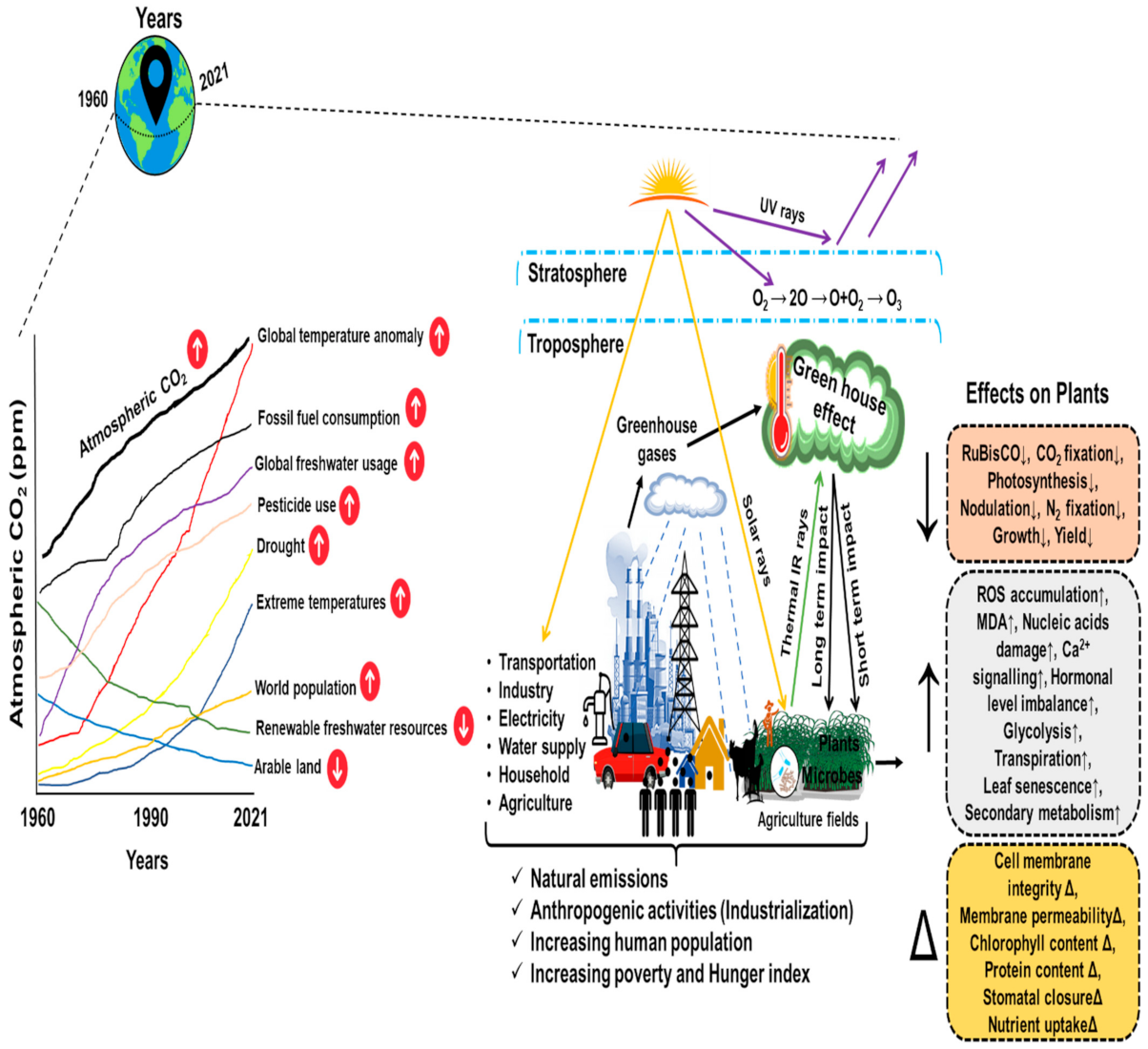
:1. Introduction
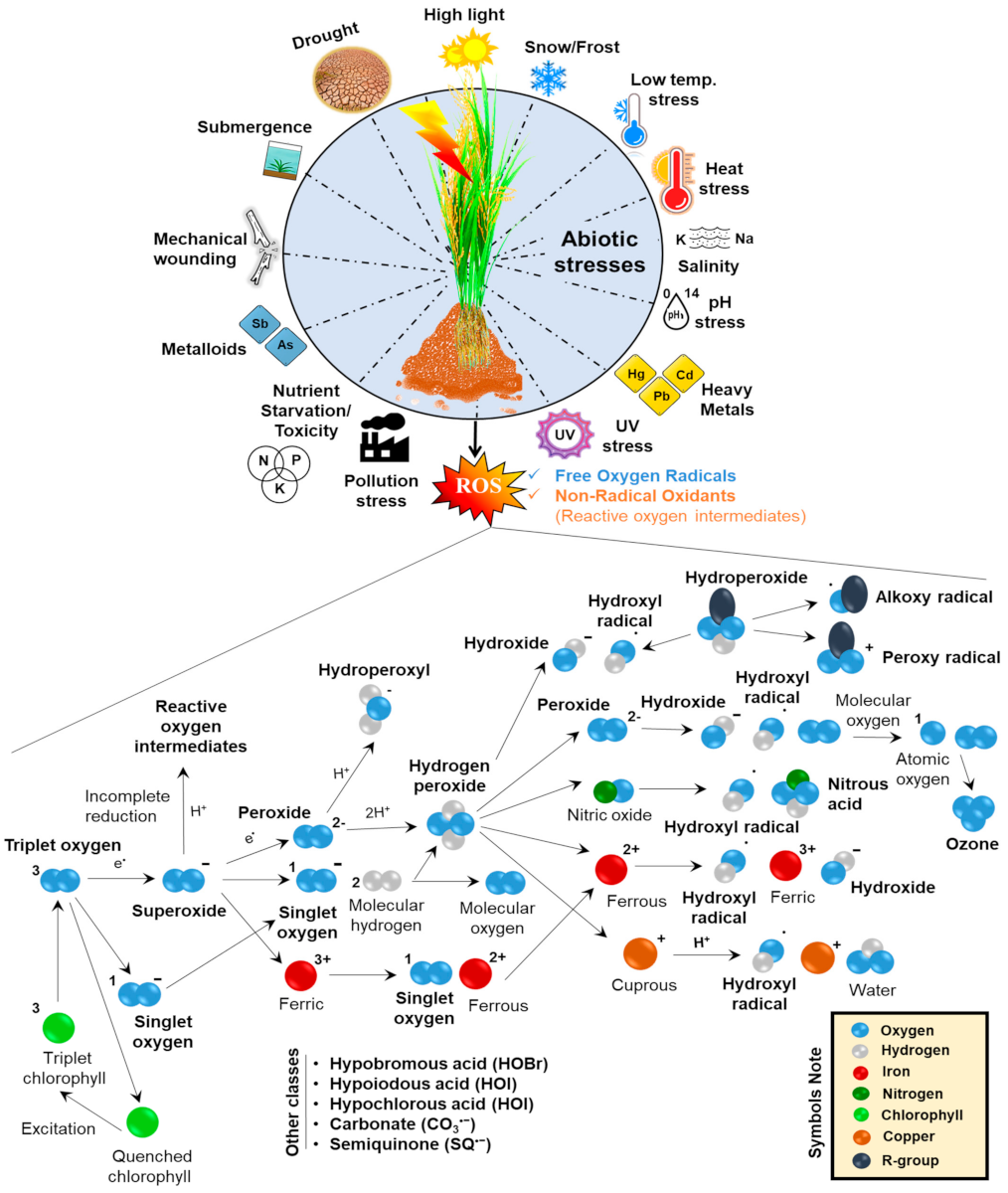
2. Insights into the Basic Mechanism of ROS Generation in Plants
3. Effects of ROS and Signaling in Different Cellular Compartments
3.1. ROS Generation and Signaling in Chloroplasts
3.2. ROS Generation and Signaling in Mitochondria
3.3. ROS Generation and Signaling in Other Cellular Compartments
4. Cytotoxic ROS Damage Cellular Structure and Function
5. Enzymatic Antioxidants for ROS Scavenging
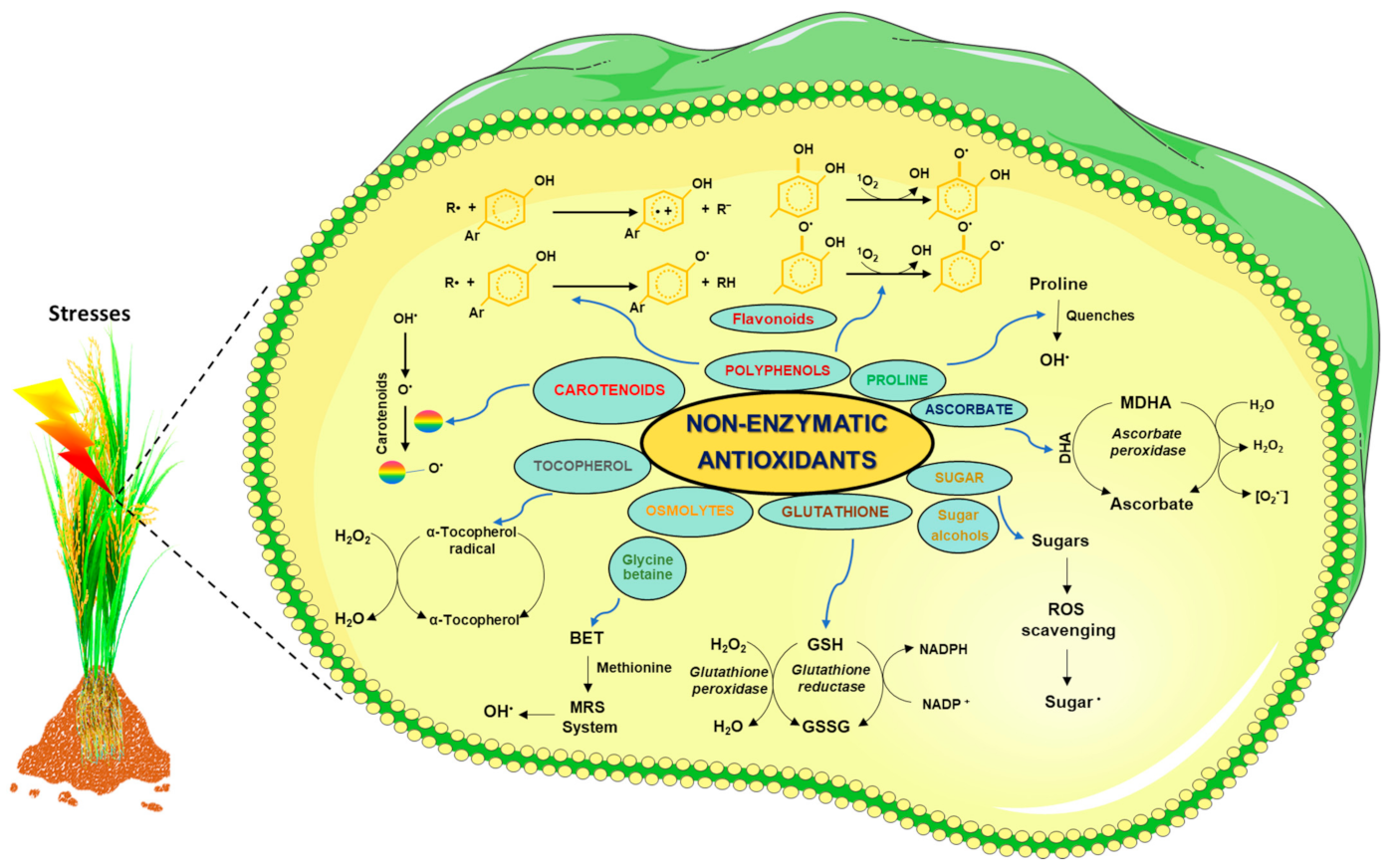
6. ROS Scavenging by Non-Enzymatic Antioxidants
7. Lower/Moderate Concentration of ROS Works as a Signal during Abiotic Stress
8. Function of RNA-Binding Proteins in ROS Scavenging
9. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mehta, S.; James, D.; Reddy, M.K. Omics Technologies for Abiotic Stress Tolerance in Plants: Current Status and Prospects. In Recent Approaches in Omics for Plant Resilience to Climate Change; Wani, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–34. [Google Scholar] [CrossRef]
- Mehta, S.; Singh, B.; Patra, A.; Islam, M. Databases: A Weapon from the Arsenal of Bioinformatics for Plant Abiotic Stress Research. In Recent Approaches in Omics for Plant Resilience to Climate Change; Wani, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 135–169. [Google Scholar] [CrossRef]
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic climate change has slowed global agricultural productivity growth. Nat. Clim. Change 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.; Saxena, R.; Orsat, V.; Raghavan, V. Effect of Climate Change on the Yield of Cereal Crops: A Review. Climate 2018, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Nogia, P.; Sidhu, G.K.; Mehrotra, R.; Mehrotra, S. Capturing atmospheric carbon: Biological and nonbiological methods. Int. J. Low-Carbon Technol. 2016, 11, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Abid, M.; Scheffran, J.; Schneider, U.A.; Ashfaq, M. Farmers’ perceptions of and adaptation strategies to climate change and their determinants: The case of Punjab province, Pakistan. Earth Syst. Dyn. 2015, 6, 225–243. [Google Scholar] [CrossRef] [Green Version]
- Zilli, M.; Scarabello, M.; Soterroni, A.C.; Valin, H.; Mosnier, A.; Leclère, D.; Havlík, P.; Kraxner, F.; Lopes, M.A.; Ramos, F.M. The impact of climate change on Brazil’s agriculture. Sci. Total Environ. 2020, 740, 139384. [Google Scholar] [CrossRef]
- Rahim, S.; Puay, T.G. The impact of climate on economic growth in Malaysia. J. Adv. Res. Bus. Manag. Stud. 2017, 6, 108–119. [Google Scholar]
- Kilicarslan, Z.; Dumrul, Y. Economic Impacts of Climate Change on Agriculture: Empirical Evidence From The ARDL Approach for Turkey. Pressacademia 2017, 6, 336–347. [Google Scholar] [CrossRef]
- WANG, J.; HUANG, J.; YANG, J. Overview of Impacts of Climate Change and Adaptation in China’s Agriculture. J. Integr. Agric. 2014, 13, 1–17. [Google Scholar] [CrossRef]
- Chandio, A.A.; Jiang, Y.; Rehman, A.; Rauf, A. Short and long-run impacts of climate change on agriculture: An empirical evidence from China. Int. J. Clim. Change Strateg. Manag. 2020, 12, 201–221. [Google Scholar] [CrossRef]
- Gupta, R.; Mishra, A. Climate change induced impact and uncertainty of rice yield of agro-ecological zones of India. Agric. Syst. 2019, 173, 1–11. [Google Scholar] [CrossRef]
- Pathak, T.; Maskey, M.; Dahlberg, J.; Kearns, F.; Bali, K.; Zaccaria, D. Climate Change Trends and Impacts on California Agriculture: A Detailed Review. Agronomy 2018, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.T.; Scrimgeour, F. Measuring the impact of climate change on agriculture in Vietnam: A panel Ricardian analysis. Agric. Econ. 2022, 53, 37–51. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Boutasknit, A.; Ben-Laouane, R.; Anli, M.; El Amerany, F.; Toubali, S.; Lahbouki, S.; Wahbi, S.; Meddich, A. Vulnerability of Oasis Agriculture to Climate Change in Morocco. In Research Anthology on Environmental and Societal Impacts of Climate Change; IGI Global: Hershey, PA, USA, 2022; pp. 1195–1219. [Google Scholar] [CrossRef]
- Affoh, R.; Zheng, H.; Dangui, K.; Dissani, B.M. The Impact of Climate Variability and Change on Food Security in Sub-Saharan Africa: Perspective from Panel Data Analysis. Sustainability 2022, 14, 759. [Google Scholar] [CrossRef]
- Mall, R.K.; Singh, R.; Gupta, A.; Srinivasan, G.; Rathore, L.S. Impact of climate change on Indian agriculture: A review. Clim. Change 2006, 78, 445–478. [Google Scholar] [CrossRef] [Green Version]
- Lal, S.K.; Kumar, S.; Sheri, V.; Mehta, S.; Varakumar, P.; Ram, B.; Borphukan, B.; James, D.; Fartyal, D.; Reddy, M.K. Seed Priming: An Emerging Technology to Impart Abiotic Stress Tolerance in Crop Plants. In Advances in Seed Priming; Rakshit, A., Singh, H., Eds.; Springer: Singapore, 2018; pp. 41–50. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Reactive Oxygen Species and Antioxidant Systems in Plants.; Springer: Singapore, 2017; ISBN 9789811052538. [Google Scholar]
- Khan, T.A.; Yusuf, M.; Ahmad, A.; Bashir, Z.; Saeed, T.; Fariduddin, Q.; Hayat, S.; Mock, H.-P.; Wu, T. Proteomic and physiological assessment of stress sensitive and tolerant variety of tomato treated with brassinosteroids and hydrogen peroxide under low-temperature stress. Food Chem. 2019, 289, 500–511. [Google Scholar] [CrossRef]
- Gao, J.-P.; Chao, D.-Y.; Lin, H.-X. Understanding Abiotic Stress Tolerance Mechanisms: Recent Studies on Stress Response in Rice. J. Integr. Plant Biol. 2007, 49, 742–750. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and Responding to Excess Light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Lamotte, O.; Bertoldo, J.B.; Besson-Bard, A.; Rosnoblet, C.; Aimé, S.; Hichami, S.; Terenzi, H.; Wendehenne, D. Protein S-nitrosylation: Specificity and identification strategies in plants. Front. Chem. 2014, 2, 114. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stresses and their classifications. Ukr. Biochem. J. 2015, 87, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Karpinska, B.; Zhang, K.; Rasool, B.; Pastok, D.; Morris, J.; Verrall, S.R.; Hedley, P.E.; Hancock, R.D.; Foyer, C.H. The redox state of the apoplast influences the acclimation of photosynthesis and leaf metabolism to changing irradiance. Plant Cell Environ. 2018, 41, 1083–1097. [Google Scholar] [CrossRef] [Green Version]
- Nath, O.; Singh, A.; Singh, I.K. In-Silico Drug discovery approach targeting receptor tyrosine kinase-like orphan receptor 1 for cancer treatment. Sci. Rep. 2017, 7, 1029. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [Green Version]
- Navrot, N.; Rouhier, N.; Gelhaye, E.; Jacquot, J.-P. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plant 2007, 129, 185–195. [Google Scholar] [CrossRef]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, R.; Turkan, I.; Uzilday, B.; Sekmen, A.H. Endoplasmic reticulum stress triggers ROS signalling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Vass, E. Hydroxyl radical production by ascorbate and hydrogen peroxide. Neurotox. Res. 2000, 2, 343–355. [Google Scholar] [CrossRef]
- Signorelli, S.; Coitiño, E.L.; Borsani, O.; Monza, J. Molecular Mechanisms for the Reaction Between • OH Radicals and Proline: Insights on the Role as Reactive Oxygen Species Scavenger in Plant Stress. J. Phys. Chem. B 2014, 118, 37–47. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Kröncke, K.-D.; Sies, H. Singlet oxygen-induced signaling effects in mammalian cells. Photochem. Photobiol. Sci. 2003, 2, 88–94. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- Hideg, É.; Barta, C.; Kálai, T.; Vass, I.; Hideg, K.; Asada, K. Detection of Singlet Oxygen and Superoxide with Fluorescent Sensors in Leaves Under Stress by Photoinhibition or UV Radiation. Plant Cell Physiol. 2002, 43, 1154–1164. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Breygina, M.; Klimenko, E. ROS and Ions in Cell Signaling during Sexual Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 9476. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Katano, K.; Suzuki, N. Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development. Plants 2021, 10, 1652. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Zhao, Y.; Xu, Q.; Wu, J.; Ma, H.; Guo, H.; Bai, L.; Zuo, J.; Zhou, J.-M.; et al. Regulation of mitochondrial NAD pool via NAD+ transporter 2 is essential for matrix NADH homeostasis and ROS production in Arabidopsis. Sci. China Life Sci. 2019, 62, 991–1002. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, H.; Zhou, J.-M.; Smith, S.M.; Li, J. Malate Circulation: Linking Chloroplast Metabolism to Mitochondrial ROS. Trends Plant Sci. 2020, 25, 446–454. [Google Scholar] [CrossRef] [Green Version]
- Locato, V.; Cimini, S.; De Gara, L. ROS and redox balance as multifaceted players of cross-tolerance: Epigenetic and retrograde control of gene expression. J. Exp. Bot. 2018, 69, 3373–3391. [Google Scholar] [CrossRef]
- Giacomelli, L.; Masi, A.; Ripoll, D.R.; Lee, M.J.; van Wijk, K.J. Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol. Biol. 2007, 65, 627–644. [Google Scholar] [CrossRef]
- Maruta, T.; Noshi, M.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. H2O2-triggered Retrograde Signaling from Chloroplasts to Nucleus Plays Specific Role in Response to Stress. J. Biol. Chem. 2012, 287, 11717–11729. [Google Scholar] [CrossRef] [Green Version]
- Maruta, T.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis Chloroplastic Ascorbate Peroxidase Isoenzymes Play a Dual Role in Photoprotection and Gene Regulation under Photooxidative Stress. Plant Cell Physiol. 2010, 51, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Awad, J.; Stotz, H.U.; Fekete, A.; Krischke, M.; Engert, C.; Havaux, M.; Berger, S.; Mueller, M.J. 2-Cysteine Peroxiredoxins and Thylakoid Ascorbate Peroxidase Create a Water-Water Cycle That Is Essential to Protect the Photosynthetic Apparatus under High Light Stress Conditions. Plant Physiol. 2015, 167, 1592–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broda, M.; Van Aken, O. Studying Retrograde Signaling in Plants. In Methods in Molecular Biology; Humana Press: Clifton, NJ, USA, 2018; Volume 1743, pp. 73–85. [Google Scholar]
- Lin, Y.-P.; Lee, T.; Tanaka, A.; Charng, Y. Analysis of an Arabidopsis heat-sensitive mutant reveals that chlorophyll synthase is involved in reutilization of chlorophyllide during chlorophyll turnover. Plant J. 2014, 80, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Carmody, M.; Crisp, P.A.; D’Alessandro, S.; Ganguly, D.; Gordon, M.; Havaux, M.; Albrecht-Borth, V.; Pogson, B.J. Uncoupling High Light Responses from Singlet Oxygen Retrograde Signaling and Spatial-Temporal Systemic Acquired Acclimation. Plant Physiol. 2016, 171, 1734–1749. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Apel, K. 1O2-Mediated and EXECUTER-Dependent Retrograde Plastid-to-Nucleus Signaling in Norflurazon-Treated Seedlings of Arabidopsis thaliana. Mol. Plant 2013, 6, 1580–1591. [Google Scholar] [CrossRef] [Green Version]
- Ugalde, J.M.; Fuchs, P.; Nietzel, T.; Cutolo, E.A.; Homagk, M.; Vothknecht, U.C.; Holuigue, L.; Schwarzländer, M.; Müller-Schüssele, S.J.; Meyer, A.J. Chloroplast-derived photo-oxidative stress causes changes in H2O2 and E GSH in other subcellular compartments. Plant Physiol. 2021, 186, 125–141. [Google Scholar] [CrossRef]
- Xiao, Y.; Savchenko, T.; Baidoo, E.E.K.; Chehab, W.E.; Hayden, D.M.; Tolstikov, V.; Corwin, J.A.; Kliebenstein, D.J.; Keasling, J.D.; Dehesh, K. Retrograde Signaling by the Plastidial Metabolite MEcPP Regulates Expression of Nuclear Stress-Response Genes. Cell 2012, 149, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for a SAL1-PAP Chloroplast Retrograde Pathway That Functions in Drought and High Light Signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef] [Green Version]
- Noren, L.; Kindgren, P.; Stachula, P.; Ruhl, M.; Eriksson, M.; Hurry, V.; Strand, A. Circadian and Plastid Signaling Pathways Are Integrated to Ensure Correct Expression of the CBF and COR Genes during Photoperiodic Growth. Plant Physiol. 2016, 171, 1392–1406. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The Roles of Environmental Factors in Regulation of Oxidative Stress in Plant. Biomed. Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef]
- Tikkanen, M.; Gollan, P.J.; Suorsa, M.; Kangasjärvi, S.; Aro, E.-M. STN7 Operates in Retrograde Signaling through Controlling Redox Balance in the Electron Transfer Chain. Front. Plant Sci. 2012, 3, 277. [Google Scholar] [CrossRef] [Green Version]
- Klein, P.; Seidel, T.; Stöcker, B.; Dietz, K.-J. The membrane-tethered transcription factor ANAC089 serves as redox-dependent suppressor of stromal ascorbate peroxidase gene expression. Front. Plant Sci. 2012, 3, 247. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, L.; Jin, H.; Chen, Q.; Chen, Z.; Xu, M. Lead-Induced Nitric Oxide Generation Plays a Critical Role in Lead Uptake by Pogonatherum crinitum Root Cells. Plant Cell Physiol. 2012, 53, 1728–1736. [Google Scholar] [CrossRef]
- Foyer, C.H.; Karpinska, B.; Krupinska, K. The functions of WHIRLY1 and REDOX-RESPONSIVE TRANSCRIPTION FACTOR 1 in cross tolerance responses in plants: A hypothesis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130226. [Google Scholar] [CrossRef] [Green Version]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY Transcription Factors: Molecular Regulation and Stress Responses in Plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef] [Green Version]
- Dietzel, L.; Gläßer, C.; Liebers, M.; Hiekel, S.; Courtois, F.; Czarnecki, O.; Schlicke, H.; Zubo, Y.; Börner, T.; Mayer, K.; et al. Identification of Early Nuclear Target Genes of Plastidial Redox Signals that Trigger the Long-Term Response of Arabidopsis to Light Quality Shifts. Mol. Plant 2015, 8, 1237–1252. [Google Scholar] [CrossRef] [Green Version]
- Virdi, K.S.; Wamboldt, Y.; Kundariya, H.; Laurie, J.D.; Keren, I.; Kumar, K.R.S.; Block, A.; Basset, G.; Luebker, S.; Elowsky, C.; et al. MSH1 Is a Plant Organellar DNA Binding and Thylakoid Protein under Precise Spatial Regulation to Alter Development. Mol. Plant 2016, 9, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Radwan, D.E.M.; Mohamed, A.K.; Fayez, K.A.; Abdelrahman, A.M. Oxidative stress caused by Basagran® herbicide is altered by salicylic acid treatments in peanut plants. Heliyon 2019, 5, e01791. [Google Scholar] [CrossRef] [Green Version]
- Steffens, B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front. Plant Sci. 2014, 5, 685. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.-H.; Zolman, B.K.; Bartel, B.; Lee, B.; Stevenson, B.; Agarwal, M.; Zhu, J.-K. Disruption of Arabidopsis CHY1 Reveals an Important Role of Metabolic Status in Plant Cold Stress Signaling. Mol. Plant 2009, 2, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wang, W.; Bittner, F.; Schmidt, N.; Berkey, R.; Zhang, L.; King, H.; Zhang, Y.; Feng, J.; Wen, Y.; et al. Dual and Opposing Roles of Xanthine Dehydrogenase in Defense-Associated Reactive Oxygen Species Metabolism in Arabidopsis. Plant Cell 2016, 28, 1108–1126. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, A.; Law, S.R.; Narsai, R.; Duncan, O.; Lee, J.-H.; Zhang, B.; Van Aken, O.; Radomiljac, J.D.; van der Merwe, M.; Yi, K.; et al. A Functional Antagonistic Relationship between Auxin and Mitochondrial Retrograde Signaling Regulates Alternative Oxidase1a Expression in Arabidopsis. Plant Physiol. 2014, 165, 1233–1254. [Google Scholar] [CrossRef] [Green Version]
- Gechev, T.; Petrov, V. Reactive Oxygen Species and Abiotic Stress in Plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem. 2020, 154, 657–664. [Google Scholar] [CrossRef]
- Gong, F.; Yao, Z.; Liu, Y.; Sun, M.; Peng, X. H2O2 response gene 1/2 are novel sensors or responders of H2O2 and involve in maintaining embryonic root meristem activity in Arabidopsis thaliana. Plant Sci. 2021, 310, 110981. [Google Scholar] [CrossRef]
- Farooq, M.A.; Zhang, X.; Zafar, M.M.; Ma, W.; Zhao, J. Roles of Reactive Oxygen Species and Mitochondria in Seed Germination. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Vanlerberghe, G.C. Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytol. 2017, 213, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feng, P.; Xu, X.; Guo, H.; Ma, J.; Chi, W.; Lin, R.; Lu, C.; Zhang, L. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2011, 2, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León, P.; Gregorio, J.; Cordoba, E. ABI4 and its role in chloroplast retrograde communication. Front. Plant Sci. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant 2018, 162. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef] [Green Version]
- Vanderauwera, S.; Vandenbroucke, K.; Inze, A.; van de Cotte, B.; Muhlenbock, P.; De Rycke, R.; Naouar, N.; Van Gaever, T.; Van Montagu, M.C.E.; Van Breusegem, F. AtWRKY15 perturbation abolishes the mitochondrial stress response that steers osmotic stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 20113–20118. [Google Scholar] [CrossRef] [Green Version]
- Martí, M.C.; Olmos, E.; Calvete, J.J.; Díaz, I.; Barranco-Medina, S.; Whelan, J.; Lázaro, J.J.; Sevilla, F.; Jiménez, A. Mitochondrial and Nuclear Localization of a Novel Pea Thioredoxin: Identification of Its Mitochondrial Target Proteins. Plant Physiol. 2009, 150, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Noguchi, K.; Motohashi, K.; Hisabori, T. Systematic Exploration of Thioredoxin Target Proteins in Plant Mitochondria. Plant Cell Physiol. 2013, 54, 875–892. [Google Scholar] [CrossRef] [Green Version]
- Gelhaye, E.; Rouhier, N.; Navrot, N.; Jacquot, J.P. The plant thioredoxin system. Cell. Mol. Life Sci. 2005, 62, 24–35. [Google Scholar] [CrossRef]
- Viola, I.L.; Güttlein, L.N.; Gonzalez, D.H. Redox Modulation of Plant Developmental Regulators from the Class I TCP Transcription Factor Family. Plant Physiol. 2013, 162, 1434–1447. [Google Scholar] [CrossRef] [Green Version]
- Calderón, A.; Ortiz-Espín, A.; Iglesias-Fernández, R.; Carbonero, P.; Pallardó, F.V.; Sevilla, F.; Jiménez, A. Thioredoxin (Trxo1) interacts with proliferating cell nuclear antigen (PCNA) and its overexpression affects the growth of tobacco cell culture. Redox Biol. 2017, 11, 688–700. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in Combating Abiotic Stresses Including Arsenic Detoxification in Plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [Green Version]
- Liebthal, M.; Maynard, D.; Dietz, K.-J. Peroxiredoxins and Redox Signaling in Plants. Antioxid. Redox Signal. 2018, 28, 609–624. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Garcia, L.; Welchen, E.; Gey, U.; Arce, A.L.; Steinebrunner, I.; Gonzalez, D.H. The cytochrome c oxidase biogenesis factor AtCOX17 modulates stress responses in Arabidopsis. Plant Cell Environ. 2016, 39, 628–644. [Google Scholar] [CrossRef]
- Chen, S.; Liu, A.; Zhang, S.; Li, C.; Chang, R.; Liu, D.; Ahammed, G.J.; Lin, X. Overexpression of mitochondrial uncoupling protein conferred resistance to heat stress and Botrytis cinerea infection in tomato. Plant Physiol. Biochem. 2013, 73, 245–253. [Google Scholar] [CrossRef]
- Barreto, P.; Yassitepe, J.E.C.T.; Wilson, Z.A.; Arruda, P. Mitochondrial Uncoupling Protein 1 Overexpression Increases Yield in Nicotiana tabacum under Drought Stress by Improving Source and Sink Metabolism. Front. Plant Sci. 2017, 8, 1836. [Google Scholar] [CrossRef]
- Dahal, K.; Martyn, G.D.; Vanlerberghe, G.C. Improved photosynthetic performance during severe drought in Nicotiana tabacum overexpressing a nonenergy conserving respiratory electron sink. New Phytol. 2015, 208, 382–395. [Google Scholar] [CrossRef]
- Su, T.; Li, W.; Wang, P.; Ma, C. Dynamics of Peroxisome Homeostasis and Its Role in Stress Response and Signaling in Plants. Front. Plant Sci. 2019, 10, 705. [Google Scholar] [CrossRef] [Green Version]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Howell, S.H. Managing the protein folding demands in the endoplasmic reticulum of plants. New Phytol. 2016, 211, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Kerchev, P.; Waszczak, C.; Lewandowska, A.; Willems, P.; Shapiguzov, A.; Li, Z.; Alseekh, S.; Mühlenbock, P.; Hoeberichts, F.A.; Huang, J.; et al. Lack of GLYCOLATE OXIDASE1, but Not GLYCOLATE OXIDASE2, Attenuates the Photorespiratory Phenotype of CATALASE2-Deficient Arabidopsis. Plant Physiol. 2016, 171, 1704–1719. [Google Scholar] [CrossRef]
- del Río, L.A.; López-Huertas, E. ROS Generation in Peroxisomes and its Role in Cell Signaling. Plant Cell Physiol. 2016, 57, pcw076. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [Green Version]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef] [Green Version]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. Singlet Oxygen in Plants: Generation, Detection, and Signaling Roles. Int. J. Mol. Sci. 2020, 21, 3237. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, B.-H.; Dellinger, M.; Cui, X.; Zhang, C.; Wu, S.; Nothnagel, E.A.; Zhu, J.-K. A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J. 2010, 63, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Endler, A.; Kesten, C.; Schneider, R.; Zhang, Y.; Ivakov, A.; Froehlich, A.; Funke, N.; Persson, S. A Mechanism for Sustained Cellulose Synthesis during Salt Stress. Cell 2015, 162, 1353–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plants. Curr. Sci. 2005, 89, 1113–1121. [Google Scholar]
- MILLER, G.; SUZUKI, N.; CIFTCI-YILMAZ, S.; MITTLER, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef] [Green Version]
- Savchenko, G.E.; Klyuchareva, E.A.; Abramchik, L.M.; Serdyuchenko, E.V. Effect of Periodic Heat Shock on the Inner Membrane System of Etioplasts. Russ. J. Plant Physiol. 2002, 49, 349–359. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, X.Y.; Li, F.; Luo, Y.; Wang, W. Overaccumulation of glycine betaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica 2010, 48, 117–126. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Heat Stress Injury in Relation to Membrane Lipid Peroxidation in Creeping Bentgrass. Crop. Sci. 2000, 40, 503. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches; Food Products Press: New York, NY, USA, 2005; ISBN 9781560229650. [Google Scholar]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef]
- Zhang, R.D.; Zhou, Y.F.; Yue, Z.X.; Chen, X.F.; Cao, X.; Xu, X.X.; Xing, Y.F.; Jiang, B.; Ai, X.Y.; Huang, R.D. Changes in photosynthesis, chloroplast ultrastructure, and antioxidant metabolism in leaves of sorghum under waterlogging stress. Photosynthetica 2019, 57, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Maestri, E.; Klueva, N.; Perrotta, C.; Gulli, M.; Nguyen, H.T.; Marmiroli, N. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol. Biol. 2002, 48, 667–681. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Zhou, J.; Zhou, Y.-H.; Yu, J.-Q. Role of Hormones in Plant Adaptation to Heat Stress. In Plant Hormones under Challenging Environmental Factors; Springer: Dordrecht, The Netherlands, 2016; pp. 1–21. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant 2003, 119, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Bela, K.; Horváth, E.; Gallé, Á.; Szabados, L.; Tari, I.; Csiszár, J. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.; Vargas, L. Defenses Against ROS in Crops and Weeds: The Effects of Interference and Herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Tavanti, T.R.; de Melo, A.A.; Moreira, L.D.K.; Sanchez, D.E.J.; dos Santos Silva, R.; da Silva, R.M.; dos Reis, A.R. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Chu, C.-C.; Lee, W.-C.; Guo, W.-Y.; Pan, S.-M.; Chen, L.-J.; Li, H.; Jinn, T.-L. A Copper Chaperone for Superoxide Dismutase That Confers Three Types of Copper/Zinc Superoxide Dismutase Activity in Arabidopsis. Plant Physiol. 2005, 139, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Medina, E.; Kim, S.-H.; Yun, M.; Choi, W.-G. Recapitulation of the Function and Role of ROS Generated in Response to Heat Stress in Plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef]
- Dumont, S.; Rivoal, J. Consequences of Oxidative Stress on Plant Glycolytic and Respiratory Metabolism. Front. Plant Sci. 2019, 10, 166. [Google Scholar] [CrossRef]
- Zhang, W.; Jeon, B.W.; Assmann, S.M. Heterotrimeric G-protein regulation of ROS signalling and calcium currents in Arabidopsis guard cells. J. Exp. Bot. 2011, 62, 2371–2379. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Samrana, S.; Zhang, Y.; Malik, Z.; Khan, M.D.; Zhu, S. Reduced Glutathione Protects Subcellular Compartments From Pb-Induced ROS Injury in Leaves and Roots of Upland Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Gill, R.; Hasanuzzaman, M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Tuteja, R.; Tuteja, N. Metal/metalloid stress tolerance in plants: Role of ascorbate, its redox couple, and associated enzymes. Protoplasma 2014, 251, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and Comparative Analysis of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Glutathione Peroxidase) in Selected Plants Employing Bioinformatics Approaches. Front. Plant Sci. 2016, 7, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passaia, G.; Spagnolo Fonini, L.; Caverzan, A.; Jardim-Messeder, D.; Christoff, A.P.; Gaeta, M.L.; de Araujo Mariath, J.E.; Margis, R.; Margis-Pinheiro, M. The mitochondrial glutathione peroxidase GPX3 is essential for H2O2 homeostasis and root and shoot development in rice. Plant Sci. 2013, 208, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. THE WATER-WATER CYCLE IN CHLOROPLASTS: Scavenging of Active Oxygens and Dissipation of Excess Photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Chen, G.; Asada, K. Ascorbate Peroxidase in Tea Leaves: Occurrence of Two Isozymes and the Differences in Their Enzymatic and Molecular Properties. Plant Cell Physiol. 1989, 30, 987–998. [Google Scholar] [CrossRef]
- Broad, R.C.; Bonneau, J.P.; Beasley, J.T.; Roden, S.; Sadowski, P.; Jewell, N.; Brien, C.; Berger, B.; Tako, E.; Glahn, R.P.; et al. Effect of Rice GDP-L-Galactose Phosphorylase Constitutive Overexpression on Ascorbate Concentration, Stress Tolerance, and Iron Bioavailability in Rice. Front. Plant Sci. 2020, 11, 595439. [Google Scholar] [CrossRef]
- Broad, R.C.; Bonneau, J.P.; Hellens, R.P.; Johnson, A.A.T. Manipulation of Ascorbate Biosynthetic, Recycling, and Regulatory Pathways for Improved Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 1790. [Google Scholar] [CrossRef] [Green Version]
- Koua, D.; Cerutti, L.; Falquet, L.; Sigrist, C.J.A.; Theiler, G.; Hulo, N.; Dunand, C. PeroxiBase: A database with new tools for peroxidase family classification. Nucleic Acids Res. 2009, 37, D261–D266. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, M.; Oono, Y.; Gusev, O.; Matsumoto, T.; Yazawa, T.; Levinskikh, M.A.; Sychev, V.N.; Bingham, G.E.; Wheeler, R.; Hummerick, M. Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol. 2014, 14, 4. [Google Scholar] [CrossRef] [Green Version]
- Salinas, A.E.; Wong, M.G. Glutathione S-transferases—A review. Curr. Med. Chem. 1999, 6, 279–309. [Google Scholar]
- Zhao, T.; Singhal, S.S.; Piper, J.T.; Cheng, J.; Pandya, U.; Clark-Wronski, J.; Awasthi, S.; Awasthi, Y.C. The Role of Human Glutathione S-Transferases hGSTA1-1 and hGSTA2-2 in Protection against Oxidative Stress. Arch. Biochem. Biophys. 1999, 367, 216–224. [Google Scholar] [CrossRef]
- Ologundudu, F. Antioxidant enzymes and non-enzymatic antioxidants as defense mechanism of salinity stress in cowpea (Vigna unguiculata L. Walp)—Ife brown and Ife bpc. Bull. Natl. Res. Cent. 2021, 45, 152. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Miyake, C.; Asada, K. Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate; hydrogen peroxide decomposes Compound I of ascorbate peroxidase. Plant Cell Physiol. 1996, 37, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Shibahara, T.; Inanaga, S.; Tanaka, K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2007, 225, 1255–1264. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The Multiple Roles of Ascorbate in the Abiotic Stress Response of Plants: Antioxidant, Cofactor, and Regulator. Front. Plant Sci. 2021, 12, 173. [Google Scholar] [CrossRef]
- Qi, C.; Lin, X.; Li, S.; Liu, L.; Wang, Z.; Li, Y.; Bai, R.; Xie, Q.; Zhang, N.; Ren, S.; et al. SoHSC70 positively regulates thermotolerance by alleviating cell membrane damage, reducing ROS accumulation, and improving activities of antioxidant enzymes. Plant Sci. 2019, 283, 385–395. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Zhu, Y.; Yang, X.; Zhang, K.; Xiao, Z.; Wang, H.; Zhao, J.; Zhang, L.; Li, G.; et al. Osa-miR398b boosts H 2 O 2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019, 222. [Google Scholar] [CrossRef] [Green Version]
- Karpinska, B.; Karlsson, M.; Schinkel, H.; Streller, S.; Süss, K.-H.; Melzer, M.; Wingsle, G. A Novel Superoxide Dismutase with a High Isoelectric Point in Higher Plants. Expression, Regulation, and Protein Localization. Plant Physiol. 2001, 126, 1668–1677. [Google Scholar] [CrossRef] [Green Version]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 2106. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Kumar, P. Stress amelioration response of glycine betaine and Arbuscular mycorrhizal fungi in sorghum under Cr toxicity. PLoS ONE 2021, 16, e0253878. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Marin, E.; Martínez, A. Carbohydrates and Their Free Radical Scavenging Capability: A Theoretical Study. J. Phys. Chem. B 2012, 116, 9668–9675. [Google Scholar] [CrossRef]
- Schneider, T.; Keller, F. Raffinose in Chloroplasts is Synthesized in the Cytosol and Transported across the Chloroplast Envelope. Plant Cell Physiol. 2009, 50, 2174–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Laxmi, A.; St. Martin, S.K.; Jang, J.-C. Global Transcription Profiling Reveals Multiple Sugar Signal Transduction Mechanisms in Arabidopsis. Plant Cell 2004, 16, 2128–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, F.; Kato, M.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. J. Exp. Bot. 2004, 56, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Savchenko, T.; Tikhonov, K. Oxidative Stress-Induced Alteration of Plant Central Metabolism. Life 2021, 11, 304. [Google Scholar] [CrossRef]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhao, X.; Zhu, H.; Paul, M.; Zu, Y.; Tang, Z. Exogenous trehalose largely alleviates ionic unbalance, ROS burst, and PCD occurrence induced by high salinity in Arabidopsis seedlings. Front. Plant Sci. 2014, 5, 570. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.M.; Loescher, W.H. [14C]-Assimilate translocation in the light and dark in celery (Apium graveokns) leaves of different ages. Physiol. Plant 1990, 79, 656–662. [Google Scholar] [CrossRef]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997, 113, 1177–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathor, P.; Borza, T.; Liu, Y.; Qin, Y.; Stone, S.; Zhang, J.; Hui, J.P.M.; Berrue, F.; Groisillier, A.; Tonon, T.; et al. Low Mannitol Concentrations in Arabidopsis thaliana Expressing Ectocarpus Genes Improve Salt Tolerance. Plants 2020, 9, 1508. [Google Scholar] [CrossRef]
- Noiraud, N.; Maurousset, L.; Lemoine, R. Transport of polyols in higher plants. Plant Physiol. Biochem. 2001, 39, 717–728. [Google Scholar] [CrossRef]
- Kolarovič, L.; Valentovič, P.; Luxová, M.; Gašparíková, O. Changes in antioxidants and cell damage in heterotrophic maize seedlings differing in drought sensitivity after exposure to short-term osmotic stress. Plant Growth Regul. 2009, 59, 21–26. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechmann, B. Subcellular distribution of ascorbate in plants. Plant Signal. Behav. 2011, 6, 360–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic Acid—The Little-Known Antioxidant in Woody Plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in Plants: Novel Concepts, New Perspectives, and Outstanding Issues. Antioxid. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef]
- Tausz, M.; Sircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Sato, M.; Ramarathnam, N.; Suzuki, Y.; Ohkubo, T.; Takeuchi, M.; Ochi, H. Varietal Differences in the Phenolic Content and Superoxide Radical Scavenging Potential of Wines from Different Sources. J. Agric. Food Chem. 1996, 44, 37–41. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.; Marín, M.; Recio, M. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Ohnishi, T. The significance of the study about the biological effects of solar ultraviolet radiation using the Exposed Facility on the International Space Station. Biol. Sci. Space 2004, 18, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.-R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific Roles of α- and γ-Tocopherol in Abiotic Stress Responses of Transgenic Tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef] [Green Version]
- Xiang, N.; Hu, J.; Wen, T.; Brennan, M.A.; Brennan, C.S.; Guo, X. Effects of temperature stress on the accumulation of ascorbic acid and folates in sweet corn ( Zea mays L.) seedlings. J. Sci. Food Agric. 2020, 100, 1694–1701. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Su, T.; Wang, P.; Li, H.; Zhao, Y.; Lu, Y.; Dai, P.; Ren, T.; Wang, X.; Li, X.; Shao, Q.; et al. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J. Integr. Plant Biol. 2018, 60. [Google Scholar] [CrossRef]
- Yang, Z.; Mhamdi, A.; Noctor, G. Analysis of catalase mutants underscores the essential role of CATALASE2 for plant growth and day length-dependent oxidative signalling. Plant Cell Environ. 2019, 42, 688–700. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Ren, H.; Zhao, T.; Li, Q.; Wang, S.; Zhang, Y.; Xiao, F.; Wang, X. BAK1 Mediates Light Intensity to Phosphorylate and Activate Catalases to Regulate Plant Growth and Development. Int. J. Mol. Sci. 2020, 21, 1437. [Google Scholar] [CrossRef] [Green Version]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Gohari, G.; Molaei, S.; Kheiry, A.; Ghafouri, M.; Razavi, F.; Lorenzo, J.M.; Juárez-Maldonado, A. Exogenous application of proline and L-cysteine alleviates internal browning and maintains eating quality of cold stored flat ‘maleki’ peach fruits. Horticulturae 2021, 7, 469. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Molaei, S.; Soleimani, A.; Rabiei, V.; Razavi, F. Impact of chitosan in combination with potassium sorbate treatment on chilling injury and quality attributes of pomegranate fruit during cold storage. J. Food Biochem. 2021, 45, e13633. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.-G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.-Q.; Liu, Y.-F.; Liu, P.; Lei, G.; He, S.-J.; Ma, B.; Zhang, W.-K.; Zhang, J.-S.; Chen, S.-Y. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J. 2010, 62, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, B.O.R.; Laxalt, A.M.; Riet, B.T.; van Schooten, B.; Merquiol, E.; Testerink, C.; Haring, M.A.; Bartels, D.; Munnik, T. Multiple PLDs Required for High Salinity and Water Deficit Tolerance in Plants. Plant Cell Physiol. 2009, 50, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Wang, X. Crosstalk between Phospholipase D and Sphingosine Kinase in Plant Stress Signaling. Front. Plant Sci. 2012, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Oda, T.; Hashimoto, H.; Kuwabara, N.; Akashi, S.; Hayashi, K.; Kojima, C.; Wong, H.L.; Kawasaki, T.; Shimamoto, K.; Sato, M.; et al. Structure of the N-terminal Regulatory Domain of a Plant NADPH Oxidase and Its Functional Implications. J. Biol. Chem. 2010, 285, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 193–198. [Google Scholar] [CrossRef]
- Costello, J.L.; Kershaw, C.J.; Castelli, L.M.; Talavera, D.; Rowe, W.; Sims, P.F.G.; Ashe, M.P.; Grant, C.M.; Hubbard, S.J.; Pavitt, G.D. Dynamic changes in eIF4F-mRNA interactions revealed by global analyses of environmental stress responses. Genome Biol. 2017, 18, 201. [Google Scholar] [CrossRef] [Green Version]
- Willems, P.; Mhamdi, A.; Stael, S.; Storme, V.; Kerchev, P.; Noctor, G.; Gevaert, K.; Van Breusegem, F. The ROS wheel: Refining ROS transcriptional footprints. Plant Physiol. 2016, 171, 1720–1733. [Google Scholar] [CrossRef] [Green Version]
- Delaney, K.J.; Xu, R.; Zhang, J.; Li, Q.Q.; Yun, K.-Y.; Falcone, D.L.; Hunt, A.G. Calmodulin Interacts with and Regulates the RNA-Binding Activity of an Arabidopsis Polyadenylation Factor Subunit. Plant Physiol. 2006, 140, 1507. [Google Scholar] [CrossRef] [Green Version]
- SCHMITZLINNEWEBER, C.; SMALL, I. Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 2008, 13, 663–670. [Google Scholar] [CrossRef]
- Chung, J.-S.; Zhu, J.-K.; Bressan, R.A.; Hasegawa, P.M.; Shi, H. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 2007, 53, 554–565. [Google Scholar] [CrossRef] [Green Version]
- de Jong, M.; van Breukelen, B.; Wittink, F.R.; Menke, F.L.H.; Weisbeek, P.J.; Ackerveken, G. Van den Membrane-associated transcripts in Arabidopsis; their isolation and characterization by DNA microarray analysis and bioinformatics. Plant J. 2006, 46, 708–721. [Google Scholar] [CrossRef]
- Monzingo, A.F.; Dhaliwal, S.; Dutt-Chaudhuri, A.; Lyon, A.; Sadow, J.H.; Hoffman, D.W.; Robertus, J.D.; Browning, K.S. The Structure of Eukaryotic Translation Initiation Factor-4E from Wheat Reveals a Novel Disulfide Bond. Plant Physiol. 2007, 143, 1504–1518. [Google Scholar] [CrossRef] [Green Version]
- Gallie, D.R. Eukaryotic Initiation Factor eIFiso4G1 and eIFiso4G2 Are Isoforms Exhibiting Distinct Functional Differences in Supporting Translation in Arabidopsis. J. Biol. Chem. 2016, 291, 1501–1513. [Google Scholar] [CrossRef] [Green Version]
- Waszczak, C.; Akter, S.; Eeckhout, D.; Persiau, G.; Wahni, K.; Bodra, N.; Van Molle, I.; De Smet, B.; Vertommen, D.; Gevaert, K.; et al. Sulfenome mining in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2014, 111, 11545–11550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akter, S.; Huang, J.; Bodra, N.; De Smet, B.; Wahni, K.; Rombaut, D.; Pauwels, J.; Gevaert, K.; Carroll, K.; Van Breusegem, F.; et al. DYn-2 Based Identification of Arabidopsis Sulfenomes. Mol. Cell. Proteom. 2015, 14, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.; Köster, T.; Nolte, C.; Weinholdt, C.; Lewinski, M.; Grosse, I.; Staiger, D. Adaptation of iCLIP to plants determines the binding landscape of the clock-regulated RNA-binding protein AtGRP7. Genome Biol. 2017, 18, 204. [Google Scholar] [CrossRef] [Green Version]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.-F.; Tsai, M.-Y.; Lu, C.-A.; Wu, S.-J.; Yeh, C.-H. The roles of Arabidopsis HSFA2, HSFA4a, and HSFA7a in the heat shock response and cytosolic protein response. Bot. Stud. 2018, 59, 15. [Google Scholar] [CrossRef]
- Boraiah, K.M.; Basavaraj, P.S.; Harisha, C.B.; Kochewad, S.A.; Khapte, P.S.; Bhendarkar, M.P.; Kakade, V.D.; Rane, J.; Kulshreshtha, N.; Pathak, H. Abiotic Stress Tolerant Crop Varieties, Livestock Breeds and Fish Species. Tech. Bull. 2021, 32, 1–83. [Google Scholar]


| Country | Population in 2020 (in Millions) | World Population Share (in %) | People Living in Water-Scarce Areas (in %) | Water Stress (in Millions) | Water Scarcity (in Millions) | Absolute Scarcity (in Millions) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2020 | 2030 | 2010 | 2020 | 2030 | 2010 | 2020 | 2030 | ||||
| China | 1439.3 | 18.47 | 36 | 140.6 | 143.8 | 142.6 | 175.2 | 185.4 | 185.2 | 310.1 | 311.4 | 322.2 |
| India | 1380 | 17.70 | 33 | 108.4 | 153.6 | 168.9 | 193.1 | 201.2 | 232.2 | 216.8 | 262.6 | 298.1 |
| United States | 331.1 | 4.25 | 24 | 25.7 | 29.8 | 33.4 | 25.9 | 29.4 | 34.3 | 45.9 | 53.2 | 61.7 |
| Indonesia | 273.5 | 3.51 | 36 | 37.4 | 34.7 | 36.2 | 22 | 51.2 | 54.2 | 33.4 | 44.4 | 47.8 |
| Pakistan | 220.8 | 2.83 | 47 | 12.7 | 3.7 | 3.9 | 7.5 | 8.5 | 10.4 | 67.5 | 89.9 | 105.7 |
| Brazil | 212.5 | 2.73 | 31 | 13.1 | 18.5 | 24 | 15.3 | 12.1 | 13.8 | 31.8 | 54.37 | 57.9 |
| Nigeria | 206.1 | 2.64 | 19 | 28.7 | 40.9 | 28.3 | 15.3 | 19.8 | 52.6 | 12.4 | 18.7 | 27 |
| Bangladesh | 164.6 | 2.11 | 33 | 14.9 | 14.1 | 15.4 | 39.1 | 28.7 | 18.7 | 6 | 24.8 | 39.7 |
| Russia | 145.9 | 0.87 | 19 | 7.4 | 7.8 | 7.8 | 9.1 | 11.9 | 11.7 | 12.9 | 15.2 | 15.4 |
| Mexico | 128.9 | 1.65 | 52 | 6.1 | 7.2 | 14.1 | 6.9 | 9.6 | 10.2 | 45.9 | 55.1 | 60.6 |
| Japan | 126.4 | 1.62 | 25 | 14.6 | 12.9 | 12.8 | 11.5 | 8.4 | 6.8 | 28.4 | 22.5 | 22.6 |
| Ethiopia | 114.9 | 1.47 | 19 | 12.2 | 19.8 | 21.6 | 8.5 | 14.2 | 25.1 | 5.2 | 5.9 | 10 |
| Philippines | 109.2 | 1.41 | 26 | 5 | 12.9 | 16 | 9.3 | 3.3 | 3.7 | 18.2 | 26.2 | 29.6 |
| Egypt | 102.3 | 1.31 | 49 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 31 | 47.1 | 53 |
| Vietnam | 97.3 | 1.25 | 4 | 1 | 4.3 | 3.5 | 0 | 0 | 0 | 3.7 | 3.9 | 4.2 |
| Abiotic Stress | Enzymatic Component | Enzymes Involved | Cellular Compartments | ROS Scavenged | Mode of Action | References |
|---|---|---|---|---|---|---|
| Salinity, Drought, Heat, Cold | SOD | FeSOD (FSD1) FeSOD (FSD2) FeSOD (FSD3) Cu/ZnSOD (CSD1) Cu/ZnSOD (CSD2) Cu/ZnSOD (CSD3) MnSOD (MSD1) | Chloroplast Chloroplast Chloroplast Chloroplast Cytoplasm Peroxisome Mitochondria | O2. | O2 + O2. + 2H+ ↓ H2O2 + O2 | [137,138,139] |
| Salinity, Drought, Heat, Cold | CAT | CAT1, CAT2, CAT3 | Peroxisome, Chloroplast, Mitochondria, Glyoxysomes, Cytosol | H2O2 | 2H2O2 ↓ 2H2O + O2 | [139,140] |
| Drought, Salinity, Extreme temperatures, Heavy metals, High light | APX | APX1, APX2 APX3 APX4 APX5 APX6 APX7 Stomatal APX Thylakoid APX | Cytoplasm Chloroplast Peroxisome Chloroplast Peroxisome Chloroplast Mitochondria Cytoplasm Mitochondria Chloroplast Chloroplast | H2O2 | H2O2 + 2AsA ↓ 2H2O + 2MDHA | [141,142] |
| Salinity, Osmotic stress, O3 | MDHAR | MDHAR1 MDHAR2 MDHAR3 MDHAR4 MDHAR5 | Chloroplast, Mitochondria Cytoplasm Cytoplasm Mitochondria Cytoplasm Cytoplasm | H2O2 | MDHA + NAD(P)H + H+ ↓ AsA + NAD(P)+ | [131,142] |
| Salinity, Heavy metals | DHAR | DHAR1 DHAR2 DHAR3 DHAR4 DHAR5 | Chloroplast, Mitochondria Cytoplasm Cytoplasm, Chloroplast Cytoplasm, Chloroplast Cytoplasm, Chloroplast | H2O2 | DHA + 2GSH ↓ AsA + 2GSSG | [27,131] |
| Salinity, Heavy metals, Drought, Low temperature | GR | GR1 GR2 | Cytoplasm Chloroplast Mitochondria | H2O2 | GSSG + NAD(P)H ↓ 2GSH + NAD(P)+ | [131] |
| Salinity, Cold, Drought | GPX | GPX1, GPX7 GPX2, GPX8 GPX3 GPX4 GPX5 Phospholipid GPX6 | Chloroplast Chloroplast, Cytoplasm Mitochondria Cytoplasm ER Chloroplast, Mitochondria | H2O2 | H2O2 + 2GSH ↓ 2H2O + GSSG | [131,136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants. Int. J. Mol. Sci. 2022, 23, 1995. https://doi.org/10.3390/ijms23041995
Singh A, Mehta S, Yadav S, Nagar G, Ghosh R, Roy A, Chakraborty A, Singh IK. How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants. International Journal of Molecular Sciences. 2022; 23(4):1995. https://doi.org/10.3390/ijms23041995
Chicago/Turabian StyleSingh, Archana, Sahil Mehta, Sunita Yadav, Garima Nagar, Rajgourab Ghosh, Amit Roy, Amrita Chakraborty, and Indrakant K. Singh. 2022. "How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants" International Journal of Molecular Sciences 23, no. 4: 1995. https://doi.org/10.3390/ijms23041995
APA StyleSingh, A., Mehta, S., Yadav, S., Nagar, G., Ghosh, R., Roy, A., Chakraborty, A., & Singh, I. K. (2022). How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants. International Journal of Molecular Sciences, 23(4), 1995. https://doi.org/10.3390/ijms23041995








